Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.108731
Revised: June 16, 2025
Accepted: August 12, 2025
Published online: September 24, 2025
Processing time: 147 Days and 22.7 Hours
Gliomas are the most common primary tumors of the central nervous system; among them, glioblastoma multiforme stands out as the most aggressive and lethal subtype, characterized by high therapeutic resistance and frequent recur
Core Tip: Mesoporous silica nanoparticles (MSNs) offer a promising delivery platform for glioma treatment due to their large surface area, tunable pore sizes, and high drug-loading capacity. By functionalizing their surfaces with tumor-specific ligands, MSNs enhance targeted uptake by glioma cells, minimizing off-target toxicity. They can also respond to both endogenous (e.g., acidic pH, redox conditions) and exogenous (e.g., magnetic fields, ultrasound) stimuli for controlled, localized drug release. These properties enable MSNs to overcome blood-brain barrier challenges and improve treatment outcomes while reducing side effects.
- Citation: Abdel-Maksoud YT, Abdelhaseb AH, Abdo AAE, Kamel AM, Elsebay MT, Attia MS. Responsive mesoporous silica nanocarriers in glioma therapy: A step forward in overcoming biological barriers. World J Clin Oncol 2025; 16(9): 108731
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/108731.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.108731
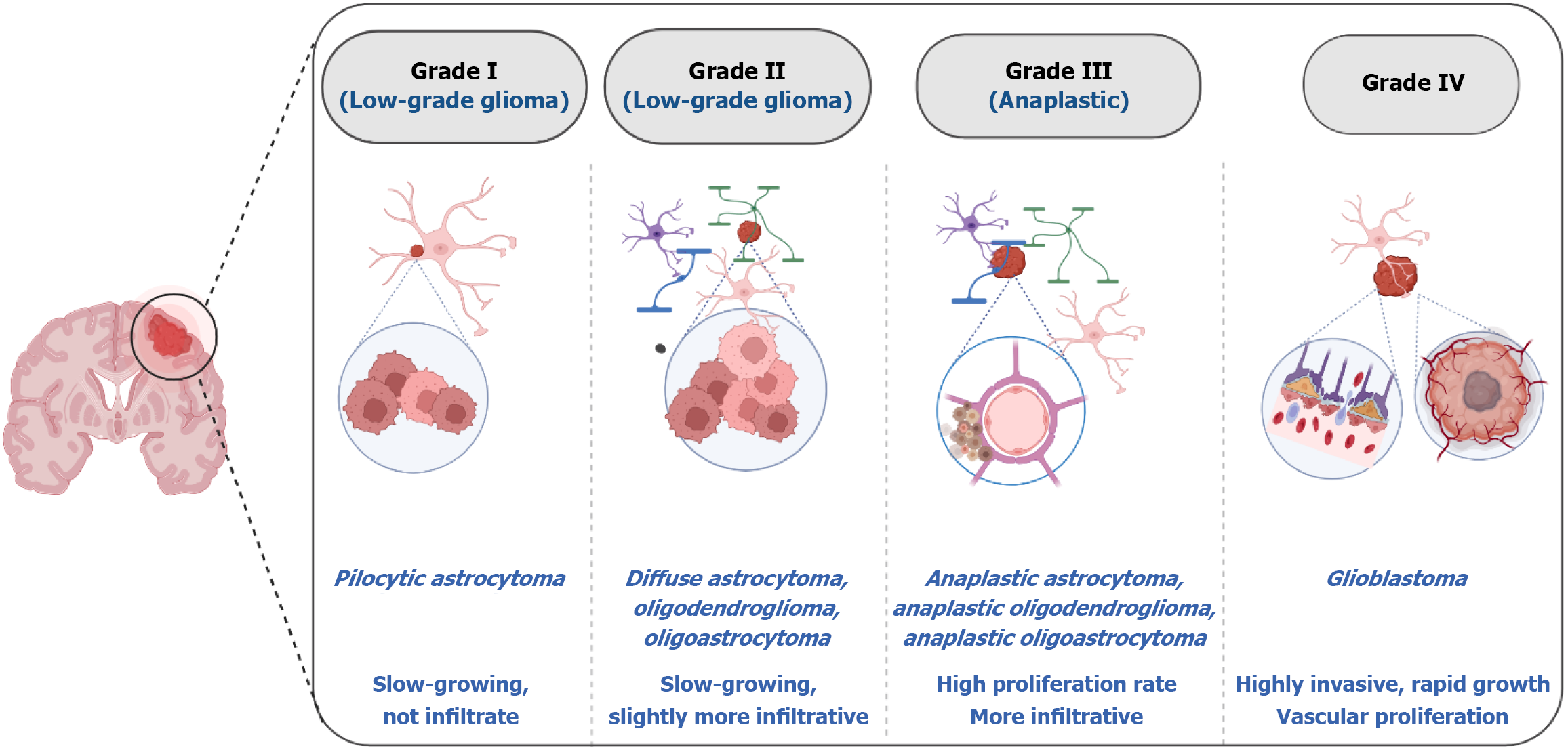
Gliomas are among the most prevalent and malignant brain tumors affecting the central nervous system. They account for approximately 46% of intracranial tumors[1]. Annually, there are approximately six new cases with glioma for every 100000 individuals, and the prognosis depends on the patient's characteristics and the type of tumor[2,3]. Gliomas are classified by the World Health Organization into four grades according to their histological characteristics and level of malignancy[4]. Grade I gliomas, such as pilocytic astrocytomas, are typically the least aggressive, remain localized and are surgically resectable. The growth rate of grade II gliomas, such as diffuse astrocytomas, is slow, but they are infiltrative, posing complications to complete removal, and they can sometimes progress to higher grades[5]. Among grade III gliomas, anaplastic astrocytomas demonstrate higher mitotic activity and have an increased recurrence potential. Glioblastomas (GBMs) are the most aggressive and invasive forms of gliomas (grade IV) and are marked by quick proliferation and serious complications (Figure 1)[6].
GBM accounts for 57% of all gliomas and almost half of all malignant central nervous system tumors[7-10], where the tumor develops rapidly and infiltrates nearby brain tissue, complicating its resection. As a result of its heterogeneous and invasive nature, along with immune evasion abilities, this type of cancer is resistant to several standard therapeutic approaches[11]. GBM is driven by complex genetic mutations, among them the epidermal growth factor receptor (EGFR) overexpression, which accelerates cell proliferation[12,13]. Additionally, apoptosis-resistant pathways like PI3K/Akt/mTOR are activated by the loss of tumor suppressor genes (PTEN, TP53)[14,15]. Additionally, the tumor microenvironment (TME) produces several elements that further complicate GBM therapy, along with the abnormal blood-brain barrier (BBB) that limits drug access to the tumor[16-18]. Thus despite advances in therapeutic interventions, median survival for GBM patients remains around 15 months, with a 5-year survival rate below 6.8%[19].
There are a variety of treatments available to treat glioma, which target the tumor's growth, relieve symptoms, and improve survival rates. Surgical intervention is initially implemented, especially in cases of low-grade gliomas or when the tumor is accessible[20,21]. When treating GBM, surgery is also effective in reducing the tumor burden prior to further chemotherapy and radiation[21-23]. In high-grade gliomas, targeted therapy and immunotherapy are often used together to achieve better outcomes. In current medical practice, several chemotherapies have been approved by the FDA for GBM, including temozolomide, bevacizumab, and carmustine[24]. In 2023, Dabrafenib plus Trametinib was approved for patients with grade 2 gliomas harboring a BRAF V600E mutation[25]. Meanwhile, in 2024, Vorasidenib has been approved for patients with grade 2 gliomas displaying an IDH1 or IDH2 mutation[26]. Recent developments in glioma treatments entail exploring new approaches such as tumor-treating fields, approved by the FDA in 2015 for patients with newly diagnosed GBM[27,28]. Tumor treating fields are often used in combination with temozolomide, which has been shown to further improve survival rates for recurrent GBM[24,29]. Several other experimental approaches are currently undergoing gliomas trials that involve gene therapy, such as restoring tumor suppressor genes (e.g., p53) or introducing genes to make tumor cells more responsive to other treatments[30,31]. Also, vaccine-based immunotherapies like DCVax® and rindopepimut are designed to provoke body immunity specifically against target tumor antigens[24,30].
Designing drug delivery systems that can cross the BBB and target tumor cells selectively while minimizing systemic toxicity is among the key hurdles in managing gliomas. Multiple nanotechnological advances have taken place in recent years, attempting to address these issues. In the exploration of nanocarriers, mesoporous silica nanoparticles (MSNs) have emerged as promising nanoplatforms for cancer therapy, possessing unique characteristics, including a large surface area, adjustable pore sizes, high loading capacity, and versatility[32-35]. The functionalized surface of these particles allows targeted delivery via ligands, enhancing tumor cell specificity while reducing off-target effects[36-38]. MSNs have the potential to improve drug solubility, targetability, and transport across the biological barriers like the BBB. Surface PEGylation of MSNs prolongs circulation time, while functionalizing MSNs with targeting ligands like angiopep-2 promotes brain tissue targeting[39,40]. Peptide decoration of MSNs such as CREKA or NGR enabled drug delivery to GBM cells without harming healthy cells[23]. These properties make MSNs an attractive candidate for overcoming the limitations of current glioma treatments[23]. Herein, we present glioma therapy challenges and explore the potential of MSNs as novel drug delivery systems, highlighting how they can improve therapeutic targetability across the BBB and deliver chemotherapeutic agents to glioma cells more effectively (Figure 1).
The molecular and cellular complexity of gliomas renders them difficult to diagnose and treat, which is why there is an unmet need for progress in glioma research[41]. Intratumoral heterogeneity poses a major barrier to accurate diagnosis and effective therapy, as GBMs are characterized by genetic diversity and cellular plasticity[42,43]. Although computed tomography and magnetic resonance imaging remain standard tools for diagnosis and monitoring[44], they often fail to distinguish primary tumors from metastases, differentiate true progression from pseudoprogression, or fully capture tumor heterogeneity[45-47].
Surgical resection is the first-line intervention, but the highly invasive nature of glioma cells into surrounding brain tissue makes complete removal nearly hopeless without compromising vital neurological functions[48]. Consequently, invasive cells evading resection contribute to tumor recurrence and poor patient outcomes. Postoperative treatments such as radiotherapy and chemotherapy are standard, yet both face considerable limitations. Radiotherapy efficacy is under
A further challenge complicating glioma therapy is that the TME promotes tumor progression, therapeutic resistance, and immune evasion[8]. Additionally, glioma stem cells (GSCs), a distinct subpopulation capable of self-renewal and differentiation, play a pivotal role in driving resistance, recurrence, and treatment failure[41,43]. Other challenges include activation of aberrant signaling pathways and intrinsic drug resistance mechanisms[50], which further strengthens GBM’s resilience against conventional therapies and discussed as follows.
While there have been significant advances in brain tumor treatment research, the survival rate of GBM remains poor, with the majority of patients surviving less than 15 months after diagnosis[51]. A significant reason why conventional and immunotherapeutic approaches fail is the highly immunosuppressive nature of the TME, which compromise anti-tumor immune responses[52]. The TME in GBM is distinct from that of other solid tumors due to the restrictive nature of the BBB, which limits immune cell infiltration. These challenges coupled with underlying immunosuppressive mechanisms, contributes to tumor progression and resistance to therapy[53].
The TME of GBM is characterized by hypoxia, which is diminished oxygen availability due to excessive tumor growth and inadequate vascularization[54]. A key role of hypoxia is to activate hypoxia-inducible factors, which then recruit and promote immunosuppressive cells[55]. Hypoxia-inducible factor signaling induces the release of immunosuppressive cytokines, including transforming growth factor β (TGF-β) and interleukin-10 (IL-10), which suppress antitumor immune responses[56]. The immunosuppression driven by hypoxia is further exacerbated by the infiltration of myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and regulatory T cells. Together, these immune cells compromise T-cell responses, aiding tumors to evade immunity[57]. Furthermore, hypoxia causes immune cells to undergo metabolic reprogramming, shifting to more immunosuppressive phenotypes which limits their cytotoxic acti
Among the immune cells contributing to immunosuppression, MDSCs play a central role in disrupting anti-tumor immunity in GBM. These cells exist in two primary subtypes, monocytic MDSCs and polymorphonuclear MDSCs[59]. Monocytic MDSCs primarily mediate immunosuppression through the secretion of TGF-β and IL-10, which inhibit T-cell activation and proliferation[57]. Meanwhile, polymorphonuclear MDSCs exert their immunosuppressive effects through the production of reactive oxygen species (ROS), which further impair T-cell responses. In addition to MDSCs, TAMs, particularly those exhibiting an M2-like phenotype, play a crucial role in sustaining the immunosuppressive TME[60]. M2-like TAMs secrete TGF-β, IL-10, and arginase-1, which depletes arginine, a critical nutrient required for T-cell function[61]. Moreover, these macrophages express immune checkpoint molecules, such as programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen-4 Ligands, which inhibit T-cell activation and promote immune tolerance[62]. TAMs also recruit additional immunosuppressive cells, including Tregs and MDSCs, amplifying the overall suppression of anti-tumor immunity[61].
Moreover, cancer-associated fibroblasts reinforce the immunosuppressive environment within the TME by remodeling the extracellular matrix (ECM). In particular, those expressing fibroblast activation protein-α form a dense ECM, limiting immune cell infiltration into the tumor core[63]. ECM serves not only as a physical barrier, but also as a signaling hub that regulates immune cell function thanks to the presence of various structural proteins and glycoproteins. In particular, ECM components interact with immune checkpoint molecules and dampen T-cell activation[64]. Aside from this, these fibroblasts secrete TGF-β, further compromising immune responses and enhancing tumor progression[65].
Adding to these challenges, galectins specifically galectin (Gal)-1, Gal-3, and Gal-9 play a role in GBM immunosuppression. These glycoproteins contribute to T-cell apoptosis, drive macrophage polarization toward an M2-like phenotype, and enhance immune checkpoint signaling[66]. Further, Gal-3 binds to PD-1, reinforcing T-cell exhaustion and impairing immune-mediated tumor clearance.
Furthermore, dendritic cells, essential for initiating immune responses, are notably impaired in GBM. Tumor-derived factors such as 2-hydroxyglutarate and fibrinogen-like protein 2 disrupt dendritic cells' maturation and function, leading to reduced antigen presentation and ineffective T-cell priming. In patients with isocitrate dehydrogenase mutations, the accumulation of 2-hydroxyglutarate impairs dendritic cells differentiation and antigen presentation, weakening anti-tumor immunity[67].
Additionally, neutrophils contribute to immune evasion through the formation of neutrophil extracellular traps (NETs), networks of DNA, histones, and proteins that capture and suppress immune cells. In GBM, these NETs promote a hypercoagulable state, enhance glioma invasion, and interfere with anti-tumor immune responses by engaging the HMGB1/RAGE/IL-8 signaling axis, further exacerbating immunosuppression[68].
Another contributor to immune evasion is the release of exosomes and extracellular vesicles by GBM cells. These vesicles carry immunosuppressive microRNAs that modulate immune cell behavior, reinforcing an immune-tolerant microenvironment. These tumor-derived exosomal microRNAs can regulate immune cells, weakening host immune functions and contributing to glioma cell genesis, immunosuppression, and immune escape[69]. Altogether, the interplay of immunosuppressive cells, galectin-mediated immune modulation, dendritic cell dysfunction, NET networks, ECM remodeling, and exosome-mediated communication establishes a formidable immunosuppressive barrier in gliomas. Understanding these intricate interactions is essential for developing effective strategies to overcome glioma-induced immune resistance and improve patient outcomes.
The BBB is a highly selective and dynamic neurovascular interface that maintains central nervous system homeostasis by restricting the entry of circulating pathogens, immune cells, and the vast majority of therapeutics[70-72]. Structurally, the BBB is composed of endothelial cells joined by tight junctions, and surrounded by pericytes and astrocytic end feet. This controls the access of essential metabolites through the BBB while actively excluding xenobiotics through a network of efflux transporters, including P-glycoprotein[73-75], which is a significant hurdle to brain-targeting therapeutics[76]. Although focal disruption of the BBB is observed in tumor cores, large regions of diffusely infiltrating glioma cells remain protected by intact vasculature, leading to heterogeneous and subtherapeutic drug exposure. Moreover, macromolecular agents, such as monoclonal antibodies, siRNA, and gene-editing vectors, are virtually excluded[77]. This calls for precision-targeted delivery platforms and spatiotemporally controlled barrier modulation to overcome the physicochemical and biological constraints imposed by the BBB.
As GBM progresses, pathological angiogenesis and vascular remodeling contribute to the formation of the blood-tumor barrier (BTB), which arises from aberrant vasculature and elevated interstitial fluid pressure within the TME[71,78]. While the BTB is more permissive than the intact BBB, marked by fenestrated endothelium, disrupted tight junctions, and irregular pericyte distribution, this increased permeability is spatially heterogeneous, predominately occuring in necrotic tumor cores. The BTB exhibits several distinct features from the normal BBB, including irregular vascular architecture, enlarged endothelial gaps, elevated permeability to macromolecules, and overexpression of specific transporters and receptors[79,80]. In contrast, peripheral and infiltrative regions often retain BBB-like integrity, impeding therapeutic distribution.
Moreover, elevated interstitial fluid pressure and chaotic vessel morphology further hinder convective transport. Tumor-derived factors such as vascular endothelial growth factor and matrix metalloproteinases aggravate barrier dysfunction, impeding uniform drug access[81,82]. Furthermore, receptor-mediated interactions between glioma cells and BBB-associated ECM components, via integrins and receptor tyrosine kinases such as EGFR and PDGFR, drive barrier dysfunction and invasive progression[83,84]. Despite its pathologic permeability, the BTB remains a critical impediment to consistent therapeutic exposure, emphasizing the necessity for drug delivery systems capable of pene
Glioma continues to be a challenging malignancy to treat, largely because of the presence of GSCs within TME. This distinct subpopulation plays a key role in resistance, recurrence, and tumor progression[85]. GSCs contribute to tumorigenesis, as well as to the pronounced intratumoral heterogeneity and cellular plasticity that characterize GBM[86,87]. These cells are key drivers of glioma progression due to their ability to self-renew, differentiate, and adapt to changes in both genetic and microenvironmental signals[30,88]. GSCs' resistance to conventional therapies is further driven by epi
Beyond intrinsic therapy resistance, GSC heterogeneity plays a pivotal role in immune evasion, dynamically reshaping the TME to foster a "cold tumor" phenotype characterized by low T-cell infiltration and high immunosuppression[94]. This immunosuppressive environment is further reinforced by the secretion of factors such as TGF-β, IL-10, and prostaglandin E2, recruiting immunosuppressive regulatory T cells, M2 macrophages, and MDSCs. GSCs upregulate immune checkpoint ligands like PD-L1 to inhibit cytotoxic T-cell activity and downregulate MHC class I and II molecules, reducing tumor antigen visibility to T cells[94,95].
GBM is characterized by dysregulation of key pathways, including the PI3K/AKT/mTOR, RAS/MAPK, retinoblastoma, Wnt/β-catenin, Notch, Hippo, receptor tyrosine kinases like EGFR and TGF-β signaling cascades, which collectively drive tumor growth, invasion, stemness, and resistance to therapy[96]. EGFR overexpression and EGFRvIII mutation notably activate PI3K/AKT and RAS/MAPK pathways, promoting oncogenic signaling and metabolic reprogramming, including enhanced lipid and cholesterol biosynthesis[97-102]. Additionally, the crosstalk among pathways, such as between AKT/mTOR and ERK, enables compensatory activation, limiting monotherapy efficacy. Epigenetic modulators like histone deacetylase 8 also sustain resistance through inactivating p53, while upregulating O6-methylguanine-DNA methyltransferase expression[103]. Recent strategies combining inhibitors of EGFR, PI3K/mTOR, and mevalonate pathways have shown synergistic efficacy, suppressing stemness markers like c-Myc and SOX2[103,104]. This led to restoring p53 function and sensitizing tumors to chemotherapy in preclinical models, offering promising avenues for overcoming GBM resistance[105].
Over the past decade, various MSN types have been developed to enhance drug delivery efficiency, specificity, and tolerability. In preclinical studies, MSNs have succeeded in crossing the BBB, a major obstacle to brain cancer therapy, thanks to rational surface engineering and ligand-mediated transport mechanisms. In the following subsections, we explore in detail how various MSN formulations are applied to glioma therapy, along with the tactics implemented to facilitate the entry of these agents through the BBB.
MSNs used in glioma therapy exist in several structural forms with tailored surface modifications to enhance specificity, drug delivery, and therapeutic response. Common variants include MSNs, magnetic silica/poly (lactic-co-glycolic acid), and magnetic graphene-based MSNs. Conventional MSNs, such as MCM-41 and SBA-15, offer a versatile, promising nanoplatform with large surface areas and pore volumes, which enable high drug-loading capacities and controlled release of therapeutic agents directly to tumor cells[106-109]. MCM-41, with its ability to induce ROS, can amplify cytotoxic effects, especially when combined with agents like zero-valent iron[110], while SBA-15’s structure allows for enhanced control over drug release, helping maintain therapeutic concentrations within tumor sites[111]. Despite the inability of these materials to cross the BBB, tuned surface decoration can allow them to pass to the target. Furthermore, their multifunctional nature enables potential integration with imaging agents, allowing clinicians to monitor treatment by directly visualizing the tumor[112].
Additionally, surface modification with polymeric materials, such as dendrimers, can help enhance cellular uptake and increase therapeutic efficacy[113]. Another advantage of surface-modified MSNs and specific types of MSNs, such as dendritic MSNs, is that they can form bioconjugates with proteins and other macromolecules. In view of this, there has been recent interest in their potential use as gene delivery agents, such as siRNA and miRNA, for cancer therapy[114].
Further, hollow MSNs (HMSNs) represent a unique subset thanks to their characteristic hollow core design that enables a high drug-loading capacity, allowing larger therapeutic doses to be delivered directly to tumor cells[115]. HMSNs may also regulate a drug's release based on TME-related conditions, such as redox and pH changes, which maximize therapeutic effectiveness and minimize systemic toxicity[116]. Further, HMSNs are particularly appealing as clinical candidates, given that they possess a lower silica content than conventional MSNs; in turn, they are more biocompatible with reduced toxicity in sensitive tissues like the brain[117].
Another advanced, multifunctional silica-based nanostructure entails core-shell MSNs, which contain a solid silica core wrapped in a mesoporous-supported shell. The multifunctional properties of these core-shell nano-silica include enhanced loading, controlled release, and the ability to cross the BBB, along with loading synergistic combinations of chemotherapeutics and photodynamic agents[118]. Further, they may be equipped with magnetic elements for externally guided targeting, or they may even be loaded with photosensitizers to produce ROS upon light activation, adding alternative treatment modalities to available glioma treatments[119]. Together, these advancements position MSNs as up-and-coming platforms for treating gliomas.
In view of the highly specialized endothelial architecture of the BBB and its low transcytosis rates and active efflux mechanisms, this barrier represents a significant impediment to brain drug delivery. Consequently, systemic treatments for brain tumors and neurological diseases are constrained by this neurovascular interface. MSNs have shown the ability to circumvent these barriers based on their ordered mesoporous architecture and abundant surface silanol groups, which enable precise surface engineering with polymers, targeted ligands, and stimuli-responsive moieties (Table 1). Through strategic surface engineering, MSNs can be endowed with properties that promote receptor-mediated transcytosis, evade immune clearance, and maintain colloidal stability in the cerebrovascular milieu. In this section, we explore the mechanistic role of MSNs in breaching the BBB for potential neuro-oncological applications.
| Ref. | Design | MSN size | Cargo | Surface modifications | Transport of MSNs | Primary findings |
| Chen et al[120] | Zebrafish model and cell culture | 50, 200 nm | Doxorubicin | PEG; TMAC | Protein corona-mediated transcytosis | Negatively charged (approximately -40 mV), 50 nm MSNs were shown to cross the BBB via a protein corona-mediated mechanism involving afamin, basigin, and ApoE |
| Baghirov et al[122] | In vitro BBB model and in vivo mouse imaging | 50 (spherical), 300 approximately 100 nm (rod) | -- | PEG-PEI copolymer | Transcellular (transcytosis) | PEG-PEI enhanced cellular uptake, and MSNs were detectable in brain vasculature with low cytotoxicity |
| Shadmani et al[125] | In vitro and in vivo | Approximately 50 nm | Methotrexate | TAT peptide | Energy-dependent endocytosis (expected) | TAT-functionalized MSNs improved methotrexate delivery, increasing the brain-to-plasma ratio |
| Mo et al[38] | In vitro | 40 nm | Doxorubicin | PEI-cRGD peptide | Integrin receptor-mediated | cRGD-conjugated MSNs show enhanced BBB penetration and glioblastoma targeting |
| Bouchoucha et al[121] | In vitro and in vivo | 50, 160 nm | Gd-chelate (MRI contrast agent) | PEG; Ri7 antibody; Gd chelate | Receptor-mediated (antibody) | Ri7-MSNs (50 nm) had targeted brain endothelium, were MRI-visible, and showed in vivo brain accumulation |
| Shevtsov et al[110] | In vitro and in vivo (Wistar rat glioma model) | 100 to 150 nm × 250 nm (length) | Fe (0) (Core) | -- | -- | Fe-loaded MSNs accumulated in glioma and showed a dose-dependent toxicity |
| Orlando et al[153] | In vitro and ex vivo | 30, 250 nm | -- | APTES modified (MSN-NH2) | Size/protein corona-dependent | MSNs showed size-dependent toxicity, with 30 nm particles being less toxic, and protein corona exerted a key role in modulating cell interactions |
Particle size effect: MSNs cross the BBB primarily via size- and surface chemistry-dependent mechanisms, with strong evidence supporting the role of receptor-mediated transcytosis and protein corona-assisted transport. For example, Chen et al[120] showed that 50 nm PEGylated MSNs with a negative surface charge were able to cross the BBB in a zebrafish model, while larger (200 nm) or positively charged counterparts did not. The study revealed that the protein corona formed around these MSNs included proteins, such as apolipoprotein E, afamin, and basigin, known to facilitate transcytosis through the BBB. Similarly, Mo et al[38] reported that Doxorubicin-loaded MSNs (40 nm) conjugated with cyclic RGD (cRGD) peptides exhibited enhanced BBB penetration and preferential accumulation in glioblastoma cells due to integrin-mediated uptake, underscoring the importance of ligand-directed targeting in crossing the BBB. Further, this system reduced human glioma tumor spheroid (U87) growth to 42.8% after 5 days, compared to 48.4% for 20 nm, 64.4% for 80 nm, whereas the control spheroids grew by 258%.
The engineering of MSNs allows them to better adapt to BBB applications than other nanobiomaterials due to their fine-tuned characteristics in size, shape, and surface modifications. The study by Bouchoucha et al[121] demonstrated that MSNs of 50 nm conjugated with Ri7 antisera targeting the transferrin receptor were more readily absorbed by brain microvascular endothelial cells in vivo compared with particles of 160 nm. Furthermore, Baghirov et al[122] employed a copolymer (PEG-polyethyleneimine) coating on 50 nm MSNs, which promoted cellular uptake and transcellular transport, though parenchymal penetration remained limited. These findings highlight how specific and feasible MSN design characteristics are when compared with other nanoparticles. These nanoparticles can achieve effective brain targeting by combining optimal size with the right ligands like transferrin, TAT, or cRGD, without compromising drug loading capacity.
Active targeting: Among the most effective strategies for other nanoparticles targeting glioblastoma are those leveraging receptor-mediated nanoparticle delivery across the BBB, particularly through the transferrin and lactoferrin receptor pathways. For example, Clark and Davis[123] used PEG-coated gold nanoparticles with an acid-cleavable transferrin linkage, achieving a 10-fold increase in brain accumulation compared to non-cleavable versions and reaching approximately 1% of the injected dose in the brain within 12 hours in vivo, significantly enhancing parenchymal delivery. Moreover, Ye et al[124] developed cyclodextrin nanoparticles (approximately 93 nm) linked to lactoferrin via a PEG spacer, which showed a 7-fold higher brain area under the curve from 0-2 hours compared to non-targeted nanoparticles.
The biofunctionality and targeting efficiency of MSNs are strongly enhanced by surface modifications, such as peptides (e.g., NGR, cRGD, R8-PNA), polymers (polydopamine), and ligands (folic acid, transferrin). These conjugations were shown to enhance cellular uptake and drug accumulation, often under 100 nm in size, which is optimal for endocytosis and potential BBB permeability. For example, Mo et al[38] demonstrated strong BBB penetration with 40 nm MSNs modified with cRGD peptides. Shadmani et al[125] showed that MSNs functionalized with TAT peptide improved methotrexate delivery to the brain, with a higher brain-to-plasma ratio, further highlighting how functionalization and delivery strategy together dictate tissue-level compatibility and therapeutic outcomes.
Another effective surface-engineering strategy for MSNs in glioblastoma treatment is brain-targeting ligands such as angiopep-2 for facilitated receptor-mediated transport. Angiopep-2-conjugated, paclitaxel- and doxorubicin-loaded poly (lactic-co-glycolic acid)-modified MSNs exhibited potent anticancer activity (IC50 = 0.026 μg/mL) and enhanced BBB penetration[126]. In another study, Angiopep-2-modified lipid-coated MSNs loaded with paclitaxel demonstrated superior BBB penetration (10.74%), enhanced glioma targeting efficiency by 2-fold, and prolonged survival in glioma-bearing rats[39].
Interaction with plasma proteins: When MSNs enter the systemic circulation, they undergo immediate and dynamic interactions with plasma proteins, forming a protein corona that fundamentally redefines their biological fate, influencing their pharmacokinetics, biodistribution, immune recognition, and capacity to traverse the BBB[127,128]. The composition and structure of the protein corona are dictated by the physicochemical properties of the nanoparticles and the sur
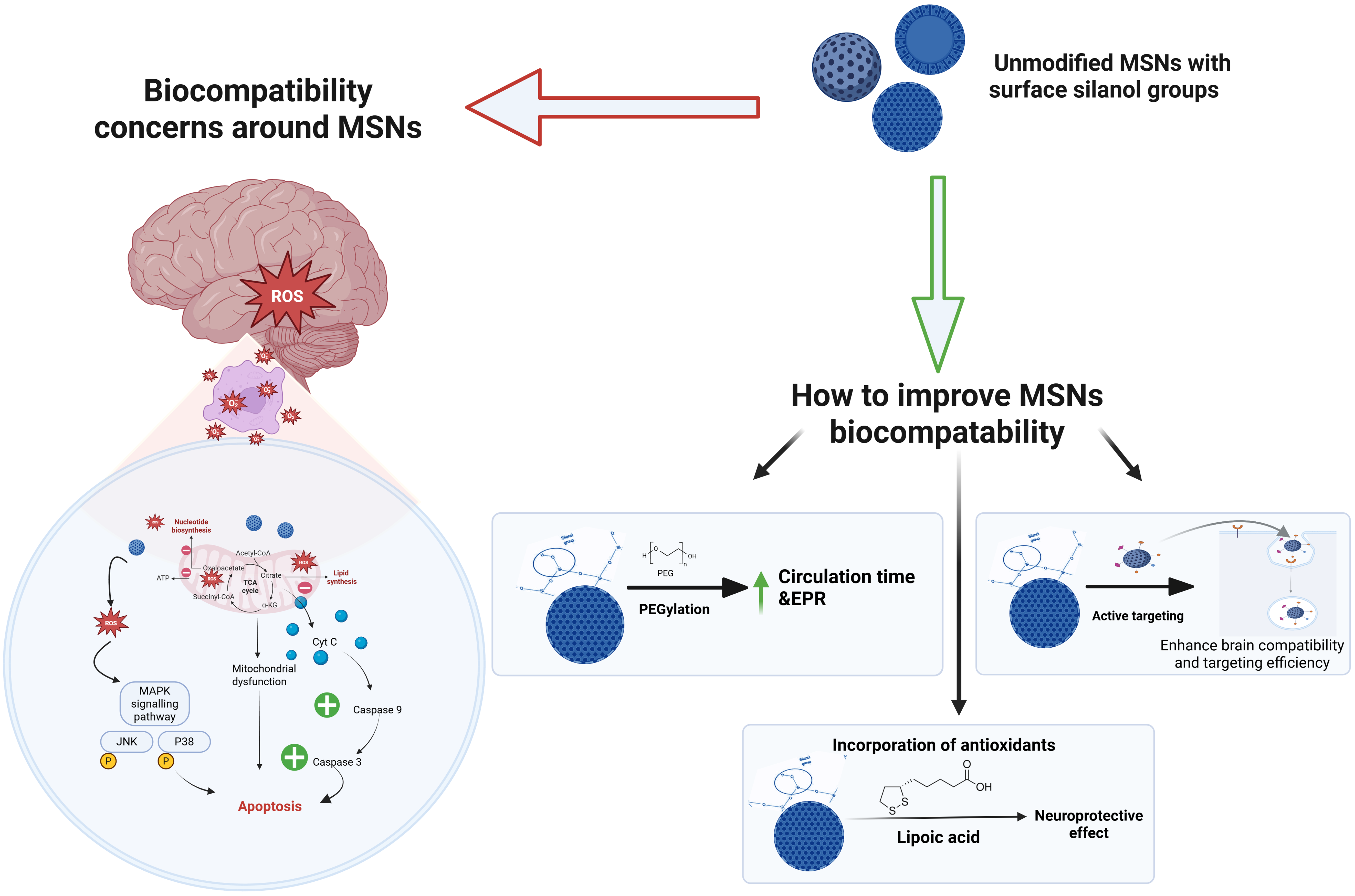
While MSNs are potential nanocarriers for brain delivery due to their ability to cross the BBB, this property also raises concerns about adverse reaction, including oxidative stress, mitochondrial dysfunction, apoptosis, and cognitive decline. These concerns about MSNs' safety emerge from previous in vivo studies, which have further shown that MSNs can induce oxidative stress, activating the NF-κB pathway and leading to inflammation and fibrosis in organs such as the heart, lungs, liver, and kidneys[132-135]. Intravenous administration of MSNs (20 mg/kg/day) caused hepatic injury, increased glycolysis, pentose phosphate pathway activity, and glutathione synthesis, while reducing mitochondrial energy metabolism[135]. Although oral administration at ten times the dose resulted in milder toxicity, MSNs’ cytotoxicity, mainly from the interaction of surface silanol groups with cell membranes, remains a significant issue[133], highlighting the importance of surface modifications to enhance biocompatibility. It has been shown that the physicochemical characteristics of MSNs contribute significantly to concerns about their toxicity[132-135], such as their size, shape, surface charge, and surface modification[136].
Further, unmodified MSNs with exposed silanol groups tend to interact strongly with biological membranes, parti
| Ref. | Factors affecting hemolysis | Hemolysis range | Key findings |
| Yu et al[137] | Size, shape, porosity | 10-500 (significant hemolysis at high doses) | Porosity and shape affected hemolysis |
| Paula et al[138] | Size, pore, surface charge | -- | Protein corona and positive charge lowered hemolysis |
| Joglekar et al[148] | Morphology (spherical/tubular) | Minimal up to 500 µg/mL | Spherical MSNs showed lower hemolysis |
| Martínez-Carmona et al[165] | Protein interaction | EC50: 8-28 µg/mL (protein-dependent) | Protein corona suppressed hemolysis dose-dependently |
| Yildirim et al[176] | Surface functionalization with ionic, polar, neutral, and hydrophobic groups | Approximately 100-500 µg/mL | Functionalization with ionic groups reduced hemolysis |
Cell uptake of MSNs is size-dependent and is mainly the result of endocytosis, phagocytosis, or pinocytosis. When MSNs are internalized, they often reside in acidic endosomes or lysosomes, where their acidic pH triggers the release of drugs via the nanopores[139]. MSNs are thereafter biodegraded through the hydrolysis of the silica meso-architecture with the aid of water molecules' interaction with the superficial silanol groups on MSNs' exteriors. This leads to hydrogen bond forma
Building on MSNs’ metabolic pathway, the evaluation of their chronic effects remains a significant concern as it can easily build up in the vital organs, resulting in undesired biological reactions and toxicity[143]. Despite this concern, evidence on cumulative toxicity is still sparse and primarily limited to short-term effects. However, chronic exposure to MSNs has been reported to activate inflammatory pathways such as NF-κB and JAK2/STAT3, which are associated with tissue degeneration and fibrosis[144]. Moreover, long-term exposure to functionalized MSNs induced cellular transformation without persistent DNA damage, indicating moderate tumorigenic potential lower than that of some approved drugs[145]. Since cumulative toxicity and long-term safety have yet to be adequately evaluated, MSNs might likely be confined within the experimental/preclinical phase.
Particle size: MSNs exhibit size-dependent cytotoxicity and inflammatory potential, with smaller MSNs (100 nm) showing heightened toxicity, while larger particles (> 100 nm), especially those with a 600 nm diameter, demonstrate markedly reduced toxicity, highlighting the importance of size optimization for biocompatibility[146]. Kim et al[136] also reported that smaller MSNs (20 nm) were cytotoxic and induced damage to intracellular organelles, nuclear fragmen
Particle shape: Based on the evaluation of the different shapes of MSNs, spherical MSNs had superior biocompatibility, with minimal toxicity, and no noticeable changes in biochemical markers or organ functions. Rod-shaped MSNs and needle-like MSNs exhibited more toxicity, with elevated creatinine levels, suggesting potential kidney stress. In spite of the slight increase in creatinine in all particle shapes, indicative of kidney stress, no severe systemic toxicity or organ failure was observed in any of the groups, as indicated by the stable levels of liver, kidney, and other organ biomarkers[132]. In addition, Joglekar et al[148] reported that spherical MSNs showed minimal hemolytic activity when concentrations were as high as 500 μg/mL, while tubular MSNs exhibited a more disruptive effect.
Furthermore, different characteristics were reported between nanospheres and nanorods in terms of hepatic and renal clearance. However, nanospheres exhibited greater effects on biliary and renal markers, which indicated potential dysfunction, while nanorods experienced slower excretion rates without significant toxic effects[149]. The biocompatibility of MSNs is good regardless of their shape, and PEGylation further enhances their biocompatibility by reducing reticuloendothelial capture and lowering clearance rates.
Surface modifications: MSNs exhibit varying toxicity levels that are subject to surface modification. In an effort to mitigate the cytotoxicity of pristine MSNs, Sun et al[150] modified MSNs with sulfhydryl (MSN-SH) groups and lipoic acid, as well as lipoic acid (Figure 2). Pristine MSNs exhibited high cytotoxicity (IC50 = 29.8 μg/mL) with increased ROS and mitochondrial damage. Meanwhile, the thiol modification significantly reduced cytotoxicity, with an inhibitory rate below 30% at 400 μg/mL, owing to the antioxidant properties of the thiol group (SH), which lowered ROS levels and reduced mitochondrial dysfunction. The modified MSNs exhibited the most significant protective effects, with minimal ROS generation and mitochondrial damage even at 400 μg/mL. It also showed the lowest apoptotic rate (2.1%), com
Modifying MSNs with polyethylene glycol-tannic acid significantly reduced their cytotoxicity and increased their biocompatibility. PEG provided the "stealth" effect, and prevented protein adsorption and nonspecific cellular uptake, thereby reducing immune responses and off-target effects (Figure 2). Meanwhile, tannic acid was introduced for its antioxidant and anti-inflammatory properties to further protect tissues from drug-induced damage. This modification also improved pharmacokinetics by extending circulation time, enhancing drug retention, and tumor accumulation[131]. Additionally, Lin and Haynes[151] found that PEGylated MSNs eliminated hemolytic activity, indicating improved biocompatibility through surface shielding. Similarly, Rogers et al[152] adopted the biomimetic surface design by coating large-pore MSNs with lipid bilayers mimicking red blood cell membranes to significantly improve the hemocompati
Concentration: The concentration of MSNs plays a critical role in their biocompatibility, as higher doses may induce cytotoxic effects, inflammation, or oxidative stress, whereas lower concentrations are often better tolerated. For instance, Orlando et al[153] reported that 30 nm MSNs were less toxic than 250 nm particles, and that toxicity was dose-dependent, with negligible toxicity observed at concentrations below 0.25 mg/mL. This finding highlights how even physically optimized MSNs may induce toxicity when administered at high doses. Similarly, Baghirov et al[122] reported low cytotoxicity for both spherical (50 nm) and rod-shaped MSNs (300 × 100 nm), but this safety was contingent on controlled concentration.
Other factors: Biocompatibility of MSNs is not solely dictated by their size, shape, concentration, surface charge, and surface modification, but is also influenced by their concentration, production methods, and administration routes that together determine their overall biological performance and safety profile. The synthesis and functionalization process used to decorate MSNs likewise takes part in determining their safety and efficacy. Differences in surfactant (template), cross-linking agents, solvent systems, and template removal steps (e.g., calcination, sonication, chemical etching) can potentially leave behind harmful residuals or alter surface silanol group density, both of which are key contributors to unintended inflammatory responses or cellular stress[154]. Furthermore, administration routes and in vivo exposure conditions heavily influence MSN biodistribution, clearance, and therefore biocompatibility[155]. Intravenous injection was reported to yield widespread organ distribution, whereas oral administration led to gut and liver accumulation, and intramuscular and hypodermic routes caused local inflammation with low absorption[156] (Figure 2; Table 2).
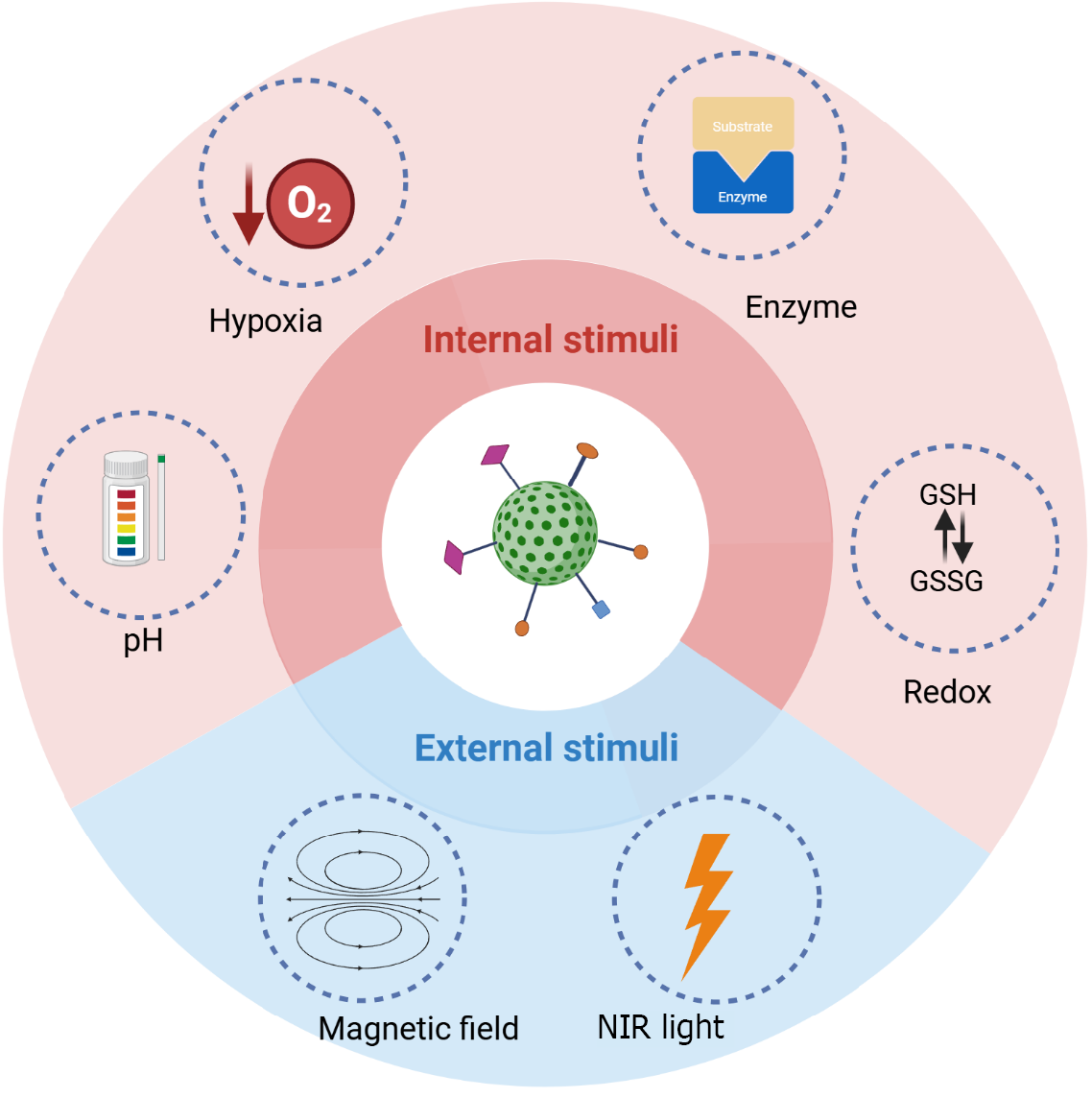
The surface of MSNs can be designed to be sensitive to both internal and external triggers for precise drug delivery and therapy in glioma treatment. Internal triggers, such as the elevated glutathione levels or acidic pH found in the TME, can activate the release of therapeutic agents from the mesoporous matrix directly within tumor cells[157]. External triggers, including light or magnetic fields, can be used to externally control the release or activation of drug-loaded MSNs, offering enhanced spatial and temporal control over treatment delivery (Figure 3).
Near-infrared: Near-infrared (NIR)-responsive MSNs can enhance the therapeutic efficacy of GBM therapy thanks to their superior tissue penetration and low scattering properties[158]. Light-triggered systems have been shown to increase drug release efficiency under NIR stimulation as well as improve cytotoxicity against GBM cells, demonstrating their potential for more effective and localized cancer treatment[159]. MSNs were shown to allow controlled, site-specific drug release when loaded with doxorubicin and functionalized with a NIR-responsive gatekeeper. Upon NIR irradiation, the gatekeeper underwent structural changes, triggering the precise release of doxorubicin at the GBM site.
Therefore, Wang et al[160] developed a multifunctional MSN-based delivery system combining chemotherapy with photothermal therapy for glioma treatment. The system comprised a mesoporous silica-coated graphene nanosheet functionalized with a targeting peptide and PEG. When loaded with doxorubicin, the resulting system exhibited heat-responsive, pH-sensitive, and sustained drug release properties. When graphene nanosheets were present in 50 g/mL concentration, NIR-induced photothermal reactions increased solution temperatures to over 50 °C within 2 minutes, and doxorubicin release was increased from 5.9% to 17% at pH 5.0. Thus, MSNs with NIR exposure showed the highest glioma cell death rates, with a calculated combination index of 0.5 indicating strong synergistic effects. Moreover, when MSNs were loaded with cisplatin and aluminium chloride phthalocyanine for combined chemo and photo-dynamic therapy[161], they were designed to release cisplatin for chemotherapy, while phthalocyanine was activated by light to produce ROS for enhanced cancer cell killing. The dual therapy demonstrated synergistic effects, showing significantly better efficacy, precision, and reduced drug resistance than either treatment alone.
Furthermore, in 2020, Reddy et al[162] applied MSNs coated with upconversion nanoparticles to develop a targeted NIR light-induced drug delivery system. These upconversion nanoparticles were employed to convert NIR light into visible or UV light, which then initiates the release of chemotherapeutic agents like doxorubicin from the MSN matrix. When tested in vitro, these unique MSNs effectively induced apoptosis in cancer cells by releasing doxorubicin precisely through NIR stimulation. They penetrate deep into the brain tissue and activate localized areas, suggesting they can be used as targeted brain tumor therapies with minimal effect on healthy tissue. Later in 2022, Ge et al[163] developed transferrin-decorated MSNs co-loaded with 5-aminolevulinic acid and paclitaxel to provide synergistic glioma therapy by combining chemotherapy with photodynamic treatment. Incorporating transferrin enhanced the selective uptake of drugs by tumor cells through transferrin receptor-mediated endocytosis, while NIR-responsive delivery facilitated the precise, localized delivery of drugs into tumor cells. Based on this dual strategy, PTX was enhanced for its anti-tumor activity while simultaneously activating 5-aminolevulinic acid’s photodynamic effects, offering a promising platform for targeted and image-guided glioma treatment.
Magnetic fields: A promising technique for targeting drugs to GBM sites is the use of external magnetic fields and MSNs enhanced with iron oxide nanoparticles. Using this design, not only can the target be pinpointed more accurately, but systemic toxicity can also be minimized, allowing a more concentrated therapeutic effect to be achieved[164].
The MCM-41 containing zero-valent iron core exhibits a unique magnetic responsiveness, which enables them to be used in targeted GBM therapy, as well as providing them with magnetic resonance imaging capabilities that allow non-invasive monitoring of their biodistribution and tracking[110]. As negative contrast agents, these magnetic nanoparticles facilitate the visual assessment of their accumulation in brain tumors, enhancing the effectiveness of magnetic resonance imaging for GBM and therapeutic response. They also enhance cytotoxicity by producing ROS within tumor cells. In biodistribution studies, magnetic MSNs were found to be retained dose-dependently in glioma tissue, confirming their ability to cross the BBB.
pH: pH-responsive nanocarriers can selectively release their therapeutic payloads in the acidic TME, reducing off-target effects and enhancing drug accumulation at the tumor site[165]. This approach also leverages the acidic conditions within endosomes and lysosomes, further improving the efficiency of drug release during cellular uptake.
Knežević et al[166] developed novel pH-responsive MSNs for more efficient and targeted GBM therapy. These MSNs were surface decorated with an iridium (III)-based complex via a pH-sensitive hydrazone linker, thereby demonstrating enhanced drug release at weakly acidic pH levels (5 and 6), compared to physiological conditions. Nanoparticles were found to exhibit low toxicity to healthy MRC-5 cells, while being cytotoxic to U251 cells. Liu et al[167] fabricated plasma complex component-functionalized manganese-doped MSNs, loaded with paclitaxel, for glioma treatment. These MSNs were able to cross the BBB, as evidenced by the in vivo imaging. Additionally, this nanoplatform demonstrated pH-responsiveness, and oxidative stress studies revealed its sensitivity to redox reactions. After 10 hours, 84.1% of paclitaxel was released at pH 5.0, compared to 53.7% at pH 7.4 after 24 hours, indicating that the drug's release is pH-dependent and controlled.
The study by Fei et al[168] introduced aptamer-siRNA chimera-capped MSNs for GBM therapy. Gint4.T aptamers were employed to specifically target platelet-derived growth factor receptors, which are abundantly expressed in GBM cells. They were also capped with siRNA that targets hepatoma-derived growth factor (HDGF), effectively inhibiting tumor growth. As soon as the MSNs arrive at the tumor site, they release temozolomide into the GBM microenvironment. The chimera effectively controlled drug release, with pH-sensitive bond cleavage under acidic conditions. The sequential release of siHDGF followed by temozolomide enhanced GBM cell apoptosis, reduced viability, and inhibited migration and invasion, suggesting that this construct holds promise for synergistic anti-GBM therapy.
Furthermore, Mundžić et al[169] prepared PTX-loaded MSNs that were capped with β-cyclodextrin via a hydrazone linkage, cleavable in weakly acidic environments, and functionalized with chlorotoxin for glioma targeting. In vitro, U87 GBM cells showed a slower uptake of CHX-modified nanoparticles and higher toxicity, likely as a result of the higher PTX release kinetics in acidic environments. There was a significant pH-responsive behavior of these cap-modified MSNs, with PTX being released more rapidly at pH 5.0 than at pH 7.4.
Redox: Redox-responsive drug delivery systems have received increasing attention in cancer therapy due to the ability of these systems to interact with the tumor cells' distinct redox environment. These systems typically demonstrate stability in the bloodstream but release active cargo in the presence of intracellular reducing agents, like glutathione, which are predominantly found in tumors.
In an attempt to enhance chemotherapeutic efficacy, Dong et al[170] developed a biodegradable glioma-targeting delivery system utilizing periodic mesoporous organosilica nanoparticles (PMO) modified with lactoferrin. These PMOs, functionalized with thioether groups, were designed to degrade at increased glutathione concentrations within the TME of the tumor. PMO-lactoferrin nanoparticles significantly improved the uptake of doxorubicin by C6 glioma cells with reduced apoptosis, demonstrating enhanced therapeutic efficacy. Biocompatibility studies revealed minimal hemolysis at high concentrations, with no injury to vital organs. More recently, Zhu et al[116] incorporated glutathione-sensitive components, such as copper sulfide or thiol groups, to selectively release therapeutic agents or activate reactions that are specifically enhanced in tumor tissues, minimizing off-target effects. This approach enabled precise targeting and enhanced therapeutic efficacy, particularly in therapies like chemodynamic therapy and ferroptosis-based treatments, where redox-responsive reactions augmented tumor cell death.
Enzymes: The enzyme-mediated mechanism of drug release offers a viable avenue for drug release, thanks to the overexpression of specific enzymes at the intended biological target. Drugs delivered in this way possess several benefits, including rapid and selective delivery to cancer cells, thus enhancing therapeutic efficacy[171]. Despite the widespread application of enzyme-responsive MSNs in cancer therapy, their use in glioblastoma remains mostly unexplored. While studies in other tumors show strong potential for precise, localized drug release[172,173], the complex enzymatic profile of glioma offers an untapped opportunity. Tailoring enzyme-responsive MSNs to this microenvironment could overcome key therapeutic barriers, but dedicated research is still needed to realize their full potential in glioma treatment.
Hypoxia: Hypoxia typically occurs in deeper portions of tumors due to disrupted blood supply, resulting in insufficient oxygen reaching the cells. Several methods have been developed to trigger the release of drugs from nanoparticles under hypoxia, involving reducible functional groups within the particles. Hypoxia causes electron transfer between these groups, leading to changes in material properties and causing drug release[174].
Tang et al[175] developed a hypoxia-responsive selenium (Se)-engineered mesoporous silica nanocapsule designed to treat radiotherapy-resistant GBM (rrGBM). These Se-MSNs were functionalized with an Angiopep-2 peptide on the nanoparticle's shell to enhance BBB penetration, and an endosome escape cationic PLL, effectively delivering siRNA to the cytosol, effectively inhibiting rrGBM invasion under low-dose X-ray exposure. When rrGBM cells were irradiated with X-rays, siCFL1-loaded SeMSN significantly inhibited their migration and proliferation by 84% and 80%, respectively. The X-ray also triggered the dissociation of SeMSN and released the loaded cofilin-1 siRNA, leading to significant inhibition of rrGBM migration and invasion. Moreover, the incorporation of hypoxia-sensitive metronidazole polymers further improved tumor suppression by strengthening radiosensitization and stabilizing ROS-induced DNA damage, thereby promoting tumor cell apoptosis. Consequently, hypoxia-induced metronidazole conversion further enhanced radiosensitization by preventing DNA damage repair and inducing apoptosis, which led to 90% cell death (Table 2)[137,138,148,165,176].
Since the early development of silica-based nanocarriers, particularly MSNs, they have undergone extensive preclinical evaluation as oral drug delivery systems that significantly enhance the solubility and bioavailability of poorly water-soluble drugs[177-180]. In a randomized study conducted in 2014, lipid-nanoporous silica hybrid (Lipoceramic) formula
Despite the robust preclinical evidence supporting the efficacy of MSNs in crossing the BBB and selectively targeting glioma cells, to the best of our knowledge, no clinical trials to date directly evaluate their use in glioblastoma therapy. This underscores the significant translational gap between laboratory innovation and clinical application. There are precedents for silica-based systems that have been investigated in human trials, which are encouraging for future applications in brain delivery. One notable example is the evaluation of hybrid silica nanoparticles like Cornell dots in the imaging of brain tumors (NCT01266096, NCT03465618), which demonstrated their safety, renal clearance, and effective tumor targeting.
Finally, the translation of MSN-based glioma therapies into clinical practice faces several regulatory and logistical challenges. These include meeting stringent FDA or EMA requirements for biocompatibility, pharmacokinetics, and toxicity under Good Manufacturing Practice conditions. It is also imperative to scale up MSNs' synthesis with acceptable batch consistency and ensure sterility and stability to proceed with clinical trials.
MSNs offer a versatile and effective platform for overcoming key challenges in glioma treatment, including the BBB, BTB, and complex TME. With their high drug-loading capacity, tunable porosity, and ability to respond to internal and external stimuli, MSNs enable precise, targeted, and controlled drug delivery. Functionalization with tumor-specific ligands further enhances selectivity and reduces off-target effects. Ongoing advancements in mesoporous silica design, especially multifunctional, stimuli-responsive systems and integration with personalized medicine, hold great potential to improve therapeutic outcomes of glioma treatment.
| 1. | Codrici E, Popescu ID, Tanase C, Enciu AM. Friends with Benefits: Chemokines, Glioblastoma-Associated Microglia/Macrophages, and Tumor Microenvironment. Int J Mol Sci. 2022;23:2509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018;4:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 450] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 3. | Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19:v1-v88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1204] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 4. | Zhao Y, Xu Z, Zhang Y, Liu Y, Ye M, Chen R, Cao Z, Zhou H, Zhou Y. Emerging Trends in Glioma Incidence and Prognostic Factors: A Comprehensive Analysis of the United States (2000-2018). [DOI] [Full Text] |
| 5. | Liang J, Lv X, Lu C, Ye X, Chen X, Fu J, Luo C, Zhao Y. Prognostic factors of patients with Gliomas - an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. 2020;20:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, Baranowska-Bosiacka I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers (Basel). 2022;14:2412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 368] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 7. | Davis ME. Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs. 2016;20:S2-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 832] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 8. | Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20:iv1-iv86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1634] [Article Influence: 233.4] [Reference Citation Analysis (0)] |
| 9. | Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494-503; quiz 1 p following 516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 649] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | Alizadeh M, Broomand Lomer N, Azami M, Khalafi M, Shobeiri P, Arab Bafrani M, Sotoudeh H. Radiomics: The New Promise for Differentiating Progression, Recurrence, Pseudoprogression, and Radionecrosis in Glioma and Glioblastoma Multiforme. Cancers (Basel). 2023;15:4429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 11. | Barthel L, Hadamitzky M, Dammann P, Schedlowski M, Sure U, Thakur BK, Hetze S. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022;41:53-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Alexandru O, Horescu C, Sevastre AS, Cioc CE, Baloi C, Oprita A, Dricu A. Receptor tyrosine kinase targeting in glioblastoma: performance, limitations and future approaches. Contemp Oncol (Pozn). 2020;24:55-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Patel R, Leung HY. Targeting the EGFR-family for therapy: biological challenges and clinical perspective. Curr Pharm Des. 2012;18:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neuro Oncol. 2002;4:196-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Barzegar Behrooz A, Talaie Z, Jusheghani F, Łos MJ, Klonisch T, Ghavami S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int J Mol Sci. 2022;23:1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 16. | Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 1306] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 17. | Abdel-Hamid NM, Abass SA. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol Biol Rep. 2021;48:6525-6538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Ajibare AJ, Odetayo AF, Akintoye OO, Oladotun AJ, Hamed MA. Zinc abates sodium benzoate -induced testicular dysfunction via upregulation of Nrf2/ HO-1/ Nf-κB signaling and androgen receptor gene. J Trace Elem Med Biol. 2024;86:127505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Seyfrid M, Maich WT, Shaikh VM, Tatari N, Upreti D, Piyasena D, Subapanditha M, Savage N, McKenna D, Mikolajewicz N, Han H, Chokshi C, Kuhlmann L, Khoo A, Salim SK, Archibong-Bassey B, Gwynne W, Brown K, Murtaza N, Bakhshinyan D, Vora P, Venugopal C, Moffat J, Kislinger T, Singh S. CD70 as an actionable immunotherapeutic target in recurrent glioblastoma and its microenvironment. J Immunother Cancer. 2022;10:e003289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Nabors LB. Management of Gliomas: Individualized Treatment Options. J Nati Compr Cancer Netw. 2020;18:985-988. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Ma R, Taphoorn MJB, Plaha P. Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry. 2021;92:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70:299-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 1319] [Article Influence: 219.8] [Reference Citation Analysis (6)] |
| 23. | Liu Z, Ji X, He D, Zhang R, Liu Q, Xin T. Nanoscale Drug Delivery Systems in Glioblastoma. Nanoscale Res Lett. 2022;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Rončević A, Koruga N, Soldo Koruga A, Rončević R, Rotim T, Šimundić T, Kretić D, Perić M, Turk T, Štimac D. Personalized Treatment of Glioblastoma: Current State and Future Perspective. Biomedicines. 2023;11:1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 25. | Barbato MI, Nashed J, Bradford D, Ren Y, Khasar S, Miller CP, Zolnik BS, Zhao H, Li Y, Bi Y, Shord SS, Amatya AK, Mishra-Kalyani PS, Scepura B, Al-Matari RA, Pazdur R, Kluetz PG, Donoghue M, Singh H, Drezner N. FDA Approval Summary: Dabrafenib in Combination with Trametinib for BRAFV600E Mutation-Positive Low-Grade Glioma. Clin Cancer Res. 2024;30:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 26. | Rudà R, Horbinski C, van den Bent M, Preusser M, Soffietti R. IDH inhibition in gliomas: from preclinical models to clinical trials. Nat Rev Neurol. 2024;20:395-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 27. | Ballo MT, Conlon P, Lavy-Shahaf G, Kinzel A, Vymazal J, Rulseh AM. Association of Tumor Treating Fields (TTFields) therapy with survival in newly diagnosed glioblastoma: a systematic review and meta-analysis. J Neurooncol. 2023;164:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Li X, Jia Z, Yan Y. Efficacy and safety of tumor-treating fields in recurrent glioblastoma: a systematic review and meta-analysis. Acta Neurochir (Wien). 2022;164:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1915] [Article Influence: 212.8] [Reference Citation Analysis (0)] |
| 30. | Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 343] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 31. | Banerjee K, Núñez FJ, Haase S, McClellan BL, Faisal SM, Carney SV, Yu J, Alghamri MS, Asad AS, Candia AJN, Varela ML, Candolfi M, Lowenstein PR, Castro MG. Current Approaches for Glioma Gene Therapy and Virotherapy. Front Mol Neurosci. 2021;14:621831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Argyo C, Weiss V, Bräuchle C, Bein T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem Mater. 2014;26:435-451. [DOI] [Full Text] |
| 33. | Manzano M, Vallet‐regí M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv Funct Materials. 2020;30. [DOI] [Full Text] |
| 34. | Wu SH, Hung Y, Mou CY. Mesoporous silica nanoparticles as nanocarriers. Chem Commun (Camb). 2011;47:9972-9985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 279] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 35. | Wu SH, Mou CY, Lin HP. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42:3862-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 883] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 36. | Liu D, Lin B, Shao W, Zhu Z, Ji T, Yang C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl Mater Interfaces. 2014;6:2131-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Song Y, Du D, Li L, Xu J, Dutta P, Lin Y. In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier. ACS Appl Mater Interfaces. 2017;9:20410-20416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Mo J, He L, Ma B, Chen T. Tailoring Particle Size of Mesoporous Silica Nanosystem To Antagonize Glioblastoma and Overcome Blood-Brain Barrier. ACS Appl Mater Interfaces. 2016;8:6811-6825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Zhu J, Zhang Y, Chen X, Zhang Y, Zhang K, Zheng H, Wei Y, Zheng H, Zhu J, Wu F, Piao JG, Zhu Z, Li F. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem Biophys Res Commun. 2021;534:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Hsu TI, Chen YP, Zhang RL, Chen ZA, Wu CH, Chang WC, Mou CY, Chan HW, Wu SH. Overcoming the Blood-Brain Tumor Barrier with Docetaxel-Loaded Mesoporous Silica Nanoparticles for Treatment of Temozolomide-Resistant Glioblastoma. ACS Appl Mater Interfaces. 2024;16:21722-21735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 41. | Kan LK, Drummond K, Hunn M, Williams D, O'Brien TJ, Monif M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol Open. 2020;2:e000069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Jones J, Nguyen H, Drummond K, Morokoff A. Circulating Biomarkers for Glioma: A Review. Neurosurgery. 2021;88:E221-E230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 43. | Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol Rev. 2018;70:412-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 44. | Thenuwara G, Curtin J, Tian F. Advances in Diagnostic Tools and Therapeutic Approaches for Gliomas: A Comprehensive Review. Sensors (Basel). 2023;23:9842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 45. | Chen C, Ou X, Wang J, Guo W, Ma X. Radiomics-Based Machine Learning in Differentiation Between Glioblastoma and Metastatic Brain Tumors. Front Oncol. 2019;9:806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Neska-Matuszewska M, Bladowska J, Sąsiadek M, Zimny A. Differentiation of glioblastoma multiforme, metastases and primary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone-Searching for a practical approach. PLoS One. 2018;13:e0191341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 47. | Castillo M. History and evolution of brain tumor imaging: insights through radiology. Radiology. 2014;273:S111-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Sharma P, Aaroe A, Liang J, Puduvalli VK. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol Adv. 2023;5:vdad009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 49. | Cha GD, Jung S, Choi SH, Kim DH. Local Drug Delivery Strategies for Glioblastoma Treatment. Brain Tumor Res Treat. 2022;10:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 50. | Barzegar Behrooz A, Talaie Z, Syahir A. Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable. Pharmaceutics. 2022;14:1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 51. | Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah SAA, Akhter N, Hassan SSU. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed Pharmacother. 2017;92:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 52. | Tomaszewski W, Sanchez-Perez L, Gajewski TF, Sampson JH. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin Cancer Res. 2019;25:4202-4210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 53. | Liu Y, Zhou F, Ali H, Lathia JD, Chen P. Immunotherapy for glioblastoma: current state, challenges, and future perspectives. Cell Mol Immunol. 2024;21:1354-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 54. | Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, Park DM. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017;19:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 55. | Feldman L. Hypoxia within the glioblastoma tumor microenvironment: a master saboteur of novel treatments. Front Immunol. 2024;15:1384249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 56. | Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, Adam V. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 311] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 57. | Mi Y, Guo N, Luan J, Cheng J, Hu Z, Jiang P, Jin W, Gao X. The Emerging Role of Myeloid-Derived Suppressor Cells in the Glioma Immune Suppressive Microenvironment. Front Immunol. 2020;11:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 58. | Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 850] [Reference Citation Analysis (0)] |
| 59. | Himes BT, Geiger PA, Ayasoufi K, Bhargav AG, Brown DA, Parney IF. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front Oncol. 2021;11:770561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 60. | Huang J, Zhao Y, Zhao K, Yin K, Wang S. Function of reactive oxygen species in myeloid-derived suppressor cells. Front Immunol. 2023;14:1226443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 61. | Ren J, Xu B, Ren J, Liu Z, Cai L, Zhang X, Wang W, Li S, Jin L, Ding L. The Importance of M1-and M2-Polarized Macrophages in Glioma and as Potential Treatment Targets. Brain Sci. 2023;13:1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 62. | Zhang L, Jiang Y, Zhang G, Wei S. The diversity and dynamics of tumor-associated macrophages in recurrent glioblastoma. Front Immunol. 2023;14:1238233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 63. | Miao Y, Deng Y, Liu J, Wang J, Hu B, Hao S, Wang H, Zhang Z, Jin Z, Zhang Y, Li C, Zhang P, Wan H, Zhang S, Feng J, Ji N. Anti-cancer effect of targeting fibroblast activation protein alpha in glioblastoma through remodeling macrophage phenotype and suppressing tumor progression. CNS Neurosci Ther. 2023;29:878-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 64. | Zarodniuk M, Steele A, Lu X, Li J, Datta M. CNS tumor stroma transcriptomics identify perivascular fibroblasts as predictors of immunotherapy resistance in glioblastoma patients. NPJ Genom Med. 2023;8:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 65. | Lootens T, Roman BI, Stevens CV, De Wever O, Raedt R. Glioblastoma-Associated Mesenchymal Stem/Stromal Cells and Cancer-Associated Fibroblasts: Partners in Crime? Int J Mol Sci. 2024;25:2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Ajarrag S, St-Pierre Y. Galectins in Glioma: Current Roles in Cancer Progression and Future Directions for Improving Treatment. Cancers (Basel). 2021;13:5533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 67. | Zheng Y, Ma X, Feng S, Zhu H, Chen X, Yu X, Shu K, Zhang S. Dendritic cell vaccine of gliomas: challenges from bench to bed. Front Immunol. 2023;14:1259562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 68. | Zha C, Meng X, Li L, Mi S, Qian D, Li Z, Wu P, Hu S, Zhao S, Cai J, Liu Y. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17:154-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 200] [Reference Citation Analysis (0)] |
| 69. | Aili Y, Maimaitiming N, Mahemuti Y, Qin H, Wang Y, Wang Z. The Role of Exosomal miRNAs in Glioma: Biological Function and Clinical Application. Front Oncol. 2021;11:686369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3452] [Cited by in RCA: 4006] [Article Influence: 200.3] [Reference Citation Analysis (0)] |
| 71. | Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 1237] [Article Influence: 206.2] [Reference Citation Analysis (0)] |
| 72. | Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 1171] [Article Influence: 195.2] [Reference Citation Analysis (0)] |
| 73. | Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 74. | Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2891] [Cited by in RCA: 3645] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 75. | Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 902] [Article Influence: 300.7] [Reference Citation Analysis (0)] |
| 76. | Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 77. | Lim SH, Yee GT, Khang D. Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier. Int J Nanomedicine. 2024;19:2529-2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 78. | Cui J, Xu Y, Tu H, Zhao H, Wang H, Di L, Wang R. Gather wisdom to overcome barriers: Well-designed nano-drug delivery systems for treating gliomas. Acta Pharm Sin B. 2022;12:1100-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 16393] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 80. | Rathi S, Griffith JI, Zhang W, Zhang W, Oh JH, Talele S, Sarkaria JN, Elmquist WF. The influence of the blood-brain barrier in the treatment of brain tumours. J Intern Med. 2022;292:3-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 81. | Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 552] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 82. | Wen L, Tan Y, Dai S, Zhu Y, Meng T, Yang X, Liu Y, Liu X, Yuan H, Hu F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017;24:1843-1855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 84. | An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37:1561-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 484] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 85. | Agosti E, Zeppieri M, Ghidoni M, Ius T, Tel A, Fontanella MM, Panciani PP. Role of glioma stem cells in promoting tumor chemo- and radioresistance: A systematic review of potential targeted treatments. World J Stem Cells. 2024;16:604-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 86. | Auffinger B, Spencer D, Pytel P, Ahmed AU, Lesniak MS. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother. 2015;15:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 87. | Chen B, Zhou X, Yang L, Zhou H, Meng M, Wu H, Liu Z, Zhang L, Li C. Glioma stem cell signature predicts the prognosis and the response to tumor treating fields treatment. CNS Neurosci Ther. 2022;28:2148-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Mattei V, Santilli F, Martellucci S, Delle Monache S, Fabrizi J, Colapietro A, Angelucci A, Festuccia C. The Importance of Tumor Stem Cells in Glioblastoma Resistance to Therapy. Int J Mol Sci. 2021;22:3863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Hsieh CH, Wu CP, Lee HT, Liang JA, Yu CY, Lin YJ. NADPH oxidase subunit 4 mediates cycling hypoxia-promoted radiation resistance in glioblastoma multiforme. Free Radic Biol Med. 2012;53:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Huang TY, Piunti A, Lulla RR, Qi J, Horbinski CM, Tomita T, James CD, Shilatifard A, Saratsis AM. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 91. | Eckerdt F, Platanias LC. Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance. Cancers (Basel). 2023;15:3458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 92. | Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 93. | Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1059] [Article Influence: 62.3] [Reference Citation Analysis (10)] |
| 94. | Fei X, Wu J, Tian H, Jiang D, Chen H, Yan K, Wang Y, Zhao Y, Chen H, Xie X, Wang Z, Zhu W, Huang Q. Glioma stem cells remodel immunotolerant microenvironment in GBM and are associated with therapeutic advancements. Cancer Biomark. 2024;41:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 532] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 96. | Dewdney B, Jenkins MR, Best SA, Freytag S, Prasad K, Holst J, Endersby R, Johns TG. From signalling pathways to targeted therapies: unravelling glioblastoma's secrets and harnessing two decades of progress. Signal Transduct Target Ther. 2023;8:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 97. | Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983-10985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 710] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 98. | Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2161] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 99. | Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 983] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 100. | Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3089] [Cited by in RCA: 3891] [Article Influence: 299.3] [Reference Citation Analysis (1)] |
| 101. | Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280:5350-5370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 102. | Ullah R, Yin Q, Snell AH, Wan L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin Cancer Biol. 2022;85:123-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 339] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 103. | Tsai CY, Ko HJ, Chiou SJ, Lai YL, Hou CC, Javaria T, Huang ZY, Cheng TS, Hsu TI, Chuang JY, Kwan AL, Chuang TH, Huang CF, Loh JK, Hong YR. NBM-BMX, an HDAC8 Inhibitor, Overcomes Temozolomide Resistance in Glioblastoma Multiforme by Downregulating the β-Catenin/c-Myc/SOX2 Pathway and Upregulating p53-Mediated MGMT Inhibition. Int J Mol Sci. 2021;22:5907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 104. | Li C, Yang J, Lei S, Wang W. SKA3 promotes glioblastoma proliferation and invasion by enhancing the activation of Wnt/β-catenin signaling via modulation of the Akt/GSK-3β axis. Brain Res. 2021;1765:147500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Guo T, Wu C, Zhang J, Yu J, Li G, Jiang H, Zhang X, Yu R, Liu X. Dual blockade of EGFR and PI3K signaling pathways offers a therapeutic strategy for glioblastoma. Cell Commun Signal. 2023;21:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 106. | Edeler D, Kaluđerović MR, Dojčinović B, Schmidt H, Kaluđerović GN. SBA-15 mesoporous silica particles loaded with cisplatin induce senescence in B16F10 cells. RSC Adv. 2016;6:111031-111040. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Pevná V, Zauška Ľ, Almáši M, Hovan A, Bánó G, Máčajová M, Bilčík B, Zeleňák V, Huntošová V. Redistribution of hydrophobic hypericin from nanoporous particles of SBA-15 silica in vitro, in cells and in vivo. Int J Pharm. 2023;643:123288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 108. | Drača D, Edeler D, Saoud M, Dojčinović B, Dunđerović D, Đmura G, Maksimović-Ivanić D, Mijatović S, Kaluđerović GN. Antitumor potential of cisplatin loaded into SBA-15 mesoporous silica nanoparticles against B16F1 melanoma cells: in vitro and in vivo studies. J Inorg Biochem. 2021;217:111383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Ortiz-islas E, Sosa-arróniz A, Manríquez-ramírez ME, Rodríguez-pérez CE, Tzompantzi F, Padilla JM. Mesoporous silica nanoparticles functionalized with folic acid for targeted release Cis-Pt to glioblastoma cells. Rev Adv Mater Sci. 2021;60:25-37. [DOI] [Full Text] |
| 110. | Shevtsov MA, Parr MA, Ryzhov VA, Zemtsova EG, Arbenin AY, Ponomareva AN, Smirnov VM, Multhoff G. Zero-valent Fe confined mesoporous silica nanocarriers (Fe(0) @ MCM-41) for targeting experimental orthotopic glioma in rats. Sci Rep. 2016;6:29247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Lewandowski D, Ruszkowski P, Pińska A, Schroeder G, Kurczewska J. SBA-15 Mesoporous Silica Modified with Gallic Acid and Evaluation of Its Cytotoxic Activity. PLoS One. 2015;10:e0132541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Almáši M, Beňová E, Zeleňák V, Madaj B, Huntošová V, Brus J, Urbanová M, Bednarčík J, Hornebecq V. Cytotoxicity study and influence of SBA-15 surface polarity and pH on adsorption and release properties of anticancer agent pemetrexed. Mater Sci Eng C Mater Biol Appl. 2020;109:110552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Turan O, Bielecki P, Perera V, Lorkowski M, Covarrubias G, Tong K, Yun A, Rahmy A, Ouyang T, Raghunathan S, Gopalakrishnan R, Griswold MA, Ghaghada KB, Peiris PM, Karathanasis E. Delivery of drugs into brain tumors using multicomponent silica nanoparticles. Nanoscale. 2019;11:11910-11921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 114. | Attia MS, Kijanka G, Nguyen NT, Zhang J, An H. Advances and prospects of RNA delivery nanoplatforms for cancer therapy. Acta Pharm Sin B. 2025;15:52-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 115. | Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics. 2018;10:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 495] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 116. | Zhu H, Ni X, Su J, Qin Y, He X, Liu B, Ding S, Wang H, Zhang X, Huang J, Hu Q, Ma R, Cai J. Multifunctional Mesoporous Silicon Nanoparticles for MRI-Based Diagnostic Imaging and Glioma Therapy. ACS Appl Mater Interfaces. 2025;17:26416-26430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 117. | Liu T, Li L, Teng X, Huang X, Liu H, Chen D, Ren J, He J, Tang F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials. 2011;32:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 118. | Gao YM, Chiu SH, Busa P, Liu CL, Kankala RK, Lee CH. Engineered Mesoporous Silica-Based Core-Shell Nanoarchitectures for Synergistic Chemo-Photodynamic Therapies. Int J Mol Sci. 2022;23:11604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 119. | Ravindran Girija A, Balasubramanian S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics. 2019;3:1-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 120. | Chen YP, Chou CM, Chang TY, Ting H, Dembélé J, Chu YT, Liu TP, Changou CA, Liu CW, Chen CT. Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood-Brain Barrier. Front Chem. 2022;10:931584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 121. | Bouchoucha M, Béliveau É, Kleitz F, Calon F, Fortin MA. Antibody-conjugated mesoporous silica nanoparticles for brain microvessel endothelial cell targeting. J Mater Chem B. 2017;5:7721-7735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Baghirov H, Karaman D, Viitala T, Duchanoy A, Lou YR, Mamaeva V, Pryazhnikov E, Khiroug L, de Lange Davies C, Sahlgren C, Rosenholm JM. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS One. 2016;11:e0160705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 123. | Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A. 2015;112:12486-12491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 124. | Ye Y, Sun Y, Zhao H, Lan M, Gao F, Song C, Lou K, Li H, Wang W. A novel lactoferrin-modified β-cyclodextrin nanocarrier for brain-targeting drug delivery. Int J Pharm. 2013;458:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Shadmani N, Makvandi P, Parsa M, Azadi A, Nedaei K, Mozafari N, Poursina N, Mattoli V, Tay FR, Maleki A, Hamidi M. Enhancing Methotrexate Delivery in the Brain by Mesoporous Silica Nanoparticles Functionalized with Cell-Penetrating Peptide using in Vivo and ex Vivo Monitoring. Mol Pharm. 2023;20:1531-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 126. | Heggannavar GB, Vijeth S, Kariduraganavar MY. Development of dual drug loaded PLGA based mesoporous silica nanoparticles and their conjugation with Angiopep-2 to treat glioma. J Drug Deliv Sci Technol. 2019;53:101157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Giri K, Kuschnerus I, Ruan J, Garcia‐bennett AE. Influence of a Protein Corona on the Oral Pharmacokinetics of Testosterone Released from Mesoporous Silica. Adv Ther. 2020;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Clemments AM, Muniesa C, Landry CC, Botella P. Effect of surface properties in protein corona development on mesoporous silica nanoparticles. RSC Adv. 2014;4:29134-29138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Kuo Y, Hsu C. Anti-melanotransferrin and apolipoprotein E on doxorubicin-loaded cationic solid lipid nanoparticles for pharmacotherapy of glioblastoma multiforme. J Taiwan Inst Chem Eng. 2017;77:10-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Athalye M, Chorawala M, Sharma A, Patel S, Patel R, Patel M. Apolipoprotein E3 functionalized mesoporous silica nanoparticles for targeted and enhanced therapeutic efficacy of Levetiracetam in brain tumor-associated epilepsy: Insights into brain uptake, biodistribution and pharmacokinetic behavior. Int J Biol Macromol. 2025;310:143174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 131. | Chen ZA, Wu CH, Wu SH, Huang CY, Mou CY, Wei KC, Yen Y, Chien IT, Runa S, Chen YP, Chen P. Receptor Ligand-Free Mesoporous Silica Nanoparticles: A Streamlined Strategy for Targeted Drug Delivery across the Blood-Brain Barrier. ACS Nano. 2024;18:12716-12736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 132. | Li L, Liu T, Fu C, Tan L, Meng X, Liu H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomedicine. 2015;11:1915-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 133. | Li J, Sun R, Xu H, Wang G. Integrative Metabolomics, Proteomics and Transcriptomics Analysis Reveals Liver Toxicity of Mesoporous Silica Nanoparticles. Front Pharmacol. 2022;13:835359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 134. | Hozayen WG, Mahmoud AM, Desouky EM, El-Nahass ES, Soliman HA, Farghali AA. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed Pharmacother. 2019;109:2527-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 135. | Yu Y, Duan J, Li Y, Li Y, Jing L, Yang M, Wang J, Sun Z. Silica nanoparticles induce liver fibrosis via TGF-β(1)/Smad3 pathway in ICR mice. Int J Nanomedicine. 2017;12:6045-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 136. | Kim IY, Joachim E, Choi H, Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine. 2015;11:1407-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 137. | Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5:5717-5728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 138. | Paula AJ, Martinez DST, Araujo Júnior RT, Souza Filho AG, Alves OL. Suppression of the hemolytic effect of mesoporous silica nanoparticles after protein corona interaction: independence of the surface microchemical environment. J Braz Chem Soc. 2012;23:1807-1814. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 139. | Zhang RL, Pratiwi FW, Chen BC, Chen P, Wu SH, Mou CY. Simultaneous Single-Particle Tracking and Dynamic pH Sensing Reveal Lysosome-Targetable Mesoporous Silica Nanoparticle Pathways. ACS Appl Mater Interfaces. 2020;12:42472-42484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 642] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 141. | He Q, Shi J, Zhu M, Chen Y, Chen F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 2010;131:314-320. [DOI] [Full Text] |
| 142. | Choi Y, Lee JE, Lee JH, Jeong JH, Kim J. A Biodegradation Study of SBA-15 Microparticles in Simulated Body Fluid and in Vivo. Langmuir. 2015;31:6457-6462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 143. | Zhang Y, Lin X, Chen X, Fang W, Yu K, Gu W, Wei Y, Zheng H, Piao J, Li F. Strategies to Regulate the Degradation and Clearance of Mesoporous Silica Nanoparticles: A Review. Int J Nanomedicine. 2024;19:5859-5878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 144. | Mahmoud AM, Desouky EM, Hozayen WG, Bin-Jumah M, El-Nahass ES, Soliman HA, Farghali AA. Mesoporous Silica Nanoparticles Trigger Liver and Kidney Injury and Fibrosis Via Altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 Signaling in Rats. Biomolecules. 2019;9:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 145. | Barguilla I, Candela-Noguera V, Oliver P, Annangi B, Díez P, Aznar E, Martínez-Máñez R, Marcos R, Hernández A, Marcos MD. Toxicological Profiling and Long-Term Effects of Bare, PEGylated- and Galacto-Oligosaccharide-Functionalized Mesoporous Silica Nanoparticles. Int J Mol Sci. 2023;24:16158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 146. | Breznan D, Das DD, MacKinnon-Roy C, Bernatchez S, Sayari A, Hill M, Vincent R, Kumarathasan P. Physicochemical Properties Can Be Key Determinants of Mesoporous Silica Nanoparticle Potency in Vitro. ACS Nano. 2018;12:12062-12079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VS. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano. 2011;5:1366-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 387] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 148. | Joglekar M, Roggers RA, Zhao Y, Trewyn BG. Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells. RSC Adv. 2013;3:2454. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Huang X, Li L, Liu T, Hao N, Liu H, Chen D, Tang F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390-5399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 635] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 150. | Sun A, Qian D, Wang Z, Xu Y, Ye H, Fang C, Yan C. Protective effect of lipoic acid modification on brain dysfunctions of mice induced by mesoporous silica nanoparticles. Chem Eng J. 2021;415:128957. [DOI] [Full Text] |
| 151. | Lin YS, Haynes CL. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc. 2010;132:4834-4842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 582] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 152. | Roggers RA, Joglekar M, Valenstein JS, Trewyn BG. Mimicking red blood cell lipid membrane to enhance the hemocompatibility of large-pore mesoporous silica. ACS Appl Mater Interfaces. 2014;6:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 153. | Orlando A, Cazzaniga E, Tringali M, Gullo F, Becchetti A, Minniti S, Taraballi F, Tasciotti E, Re F. Mesoporous silica nanoparticles trigger mitophagy in endothelial cells and perturb neuronal network activity in a size- and time-dependent manner. Int J Nanomedicine. 2017;12:3547-3559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 154. | Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25:2265-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 155. | Cabellos J, Gimeno-Benito I, Catalán J, Lindberg HK, Vales G, Fernandez-Rosas E, Ghemis R, Jensen KA, Atluri R, Vázquez-Campos S, Janer G. Short-term oral administration of non-porous and mesoporous silica did not induce local or systemic toxicity in mice. Nanotoxicology. 2020;14:1324-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 156. | Fu C, Liu T, Li L, Liu H, Chen D, Tang F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials. 2013;34:2565-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 157. | Attia MS, Hassaballah MY, Abdelqawy MA, Emad-Eldin M, Farag AK, Negida A, Ghaith H, Emam SE. An updated review of mesoporous carbon as a novel drug delivery system. Drug Dev Ind Pharm. 2021;47:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 158. | Castillo RR, Lozano D, González B, Manzano M, Izquierdo-Barba I, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert Opin Drug Deliv. 2019;16:415-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 159. | Zhang Y, Hou Z, Ge Y, Deng K, Liu B, Li X, Li Q, Cheng Z, Ma P, Li C, Lin J. DNA-Hybrid-Gated Photothermal Mesoporous Silica Nanoparticles for NIR-Responsive and Aptamer-Targeted Drug Delivery. ACS Appl Mater Interfaces. 2015;7:20696-20706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 160. | Wang Y, Wang K, Zhao J, Liu X, Bu J, Yan X, Huang R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J Am Chem Soc. 2013;135:4799-4804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 161. | Vivero-Escoto JL, Elnagheeb M. Mesoporous Silica Nanoparticles Loaded with Cisplatin and Phthalocyanine for Combination Chemotherapy and Photodynamic Therapy in vitro. Nanomaterials (Basel). 2015;5:2302-2316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 162. | Reddy KL, Sharma PK, Singh A, Kumar A, Shankar KR, Singh Y, Garg N, Krishnan V. Amine-functionalized, porous silica-coated NaYF4: Yb/Er upconversion nanophosphors for efficient delivery of doxorubicin and curcumin. Materials Science and Engineering: C. 2019;96:8695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 163. | Ge P, Liu Y, Chen Q, Su Z, Du Y, Luo S, Zhao X, Cao X, Song H, Zhu X. Transferrin receptors/magnetic resonance dual-targeted nanoplatform for precise chemo-photodynamic synergistic cancer therapy. Nanomedicine. 2022;39:102467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Dhar D, Ghosh S, Das S, Chatterjee J. A review of recent advances in magnetic nanoparticle-based theranostics of glioblastoma. Nanomedicine (Lond). 2022;17:107-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 165. | Martínez-Carmona M, Lozano D, Colilla M, Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 166. | Knežević NŽ, Ilić N, Kaluđerović GN. Mesoporous Silica Nanoparticles for pH-Responsive Delivery of Iridium Metallotherapeutics and Treatment of Glioblastoma Multiforme. Inorg. 2022;10:250. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 167. | Liu Y, Yu S, Jiang X, Wu Q, Shen W, Zou Z, Wei W, Wu C, Gao Y. Functional paclitaxel-manganese-doped mesoporous silica nanoparticles for orthotopic brain glioma targeted therapy. Mater Des. 2024;238:112715. [DOI] [Full Text] |
| 168. | Fei H, Jin Y, Jiang N, Zhou Y, Wei N, Liu Y, Miao J, Zhang L, Li R, Zhang A, Du S. Gint4.T-siHDGF chimera-capped mesoporous silica nanoparticles encapsulating temozolomide for synergistic glioblastoma therapy. Biomaterials. 2024;306:122479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 169. | Mundžić M, Ultimo A, Mladenović M, Pavlović A, Gobbo OL, Ruiz-Hernandez E, Santos-Martinez MJ, Knežević NŽ. Chlorotoxin-functionalized mesoporous silica nanoparticles for pH-responsive paclitaxel delivery to Glioblastoma multiforme. Heliyon. 2025;11:e41151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 170. | Dong M, Liu Y, Liu B, Peng J, Tang Y, Lu G, Shi H, Zhu F. Enhanced anti-glioma efficacy of biodegradable periodic mesoporous organosilica nanoparticles through target delivery of chemotherapeutics. J Mater Sci Mater Med. 2023;34:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Zhang L, Bei HP, Piao Y, Wang Y, Yang M, Zhao X. Polymer-Brush-Grafted Mesoporous Silica Nanoparticles for Triggered Drug Delivery. Chemphyschem. 2018;19:1956-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 172. | Cheng YJ, Luo GF, Zhu JY, Xu XD, Zeng X, Cheng DB, Li YM, Wu Y, Zhang XZ, Zhuo RX, He F. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2015;7:9078-9087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (8)] |
| 173. | Liu J, Zhang B, Luo Z, Ding X, Li J, Dai L, Zhou J, Zhao X, Ye J, Cai K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale. 2015;7:3614-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 174. | Thambi T, Son S, Lee DS, Park JH. Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater. 2016;29:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 175. | Tang X, Wang Z, Xie Y, Liu Y, Yang K, Li T, Shen H, Zhao M, Jin J, Xiao H, Liu H, Gu N. Radiation-Triggered Selenium-Engineered Mesoporous Silica Nanocapsules for RNAi Therapy in Radiotherapy-Resistant Glioblastoma. ACS Nano. 2023;17:4062-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 176. | Yildirim A, Ozgur E, Bayindir M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J Mater Chem B. 2013;1:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 177. | Attia MS, Ghazy FES. Ameliorating the Poor Dissolution Rate of Selexipag in Aqueous Acidic Conditions Following Confinement into Mesoporous Silica. Ind J Pharm Edu Res. 2023;57:1002-1011. [DOI] [Full Text] |
| 178. | Attia MS, Yahya A, Monaem NA, Sabry SA. Mesoporous silica nanoparticles: Their potential as drug delivery carriers and nanoscavengers in Alzheimer's and Parkinson's diseases. Saudi Pharm J. 2023;31:417-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 179. | El-Gendy ZA, Ammar NM, Kassem AM, Attia MS, Afifi SM, Ibrahim AH, Emam SE, Ms Korany R, El-Nasser G El-Gendy A, Elshamy AI. Myricetin-loaded SBA-15 silica nanoparticles for enhanced management of pyrexia, pain, and inflammation through modulation of MAPK/NF-κB and COX-2/PGE-2 pathways: Evidence from the biochemical, histological, and metabolomic analysis. Int J Pharm. 2024;666:124775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 180. | Attia MS, Hasan AA, Ghazy FS, Gomaa E. Mesoporous silica nanoparticles-embedded hydrogel: A potential approach for transdermal delivery of carvedilol to pediatric population. Int J Pharm. 2025;676:125605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 181. | Tan A, Eskandar NG, Rao S, Prestidge CA. First in man bioavailability and tolerability studies of a silica-lipid hybrid (Lipoceramic) formulation: a Phase I study with ibuprofen. Drug Deliv Transl Res. 2014;4:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Mirzaali F, Dizaj SM, Shahi S, Memar MY, Parnia F. The Antibacterial Effect of Tetracycline-loaded Mesoporous Silica Nanoparticles in the Gingival Fluid at Implant-abutment Junction: A Randomized Clinical Trial Study. Pharm Nanotechnol. 2023;11:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 183. | Baek J, Robert-Nicoud G, Herrera Hidalgo C, Borg ML, Iqbal MN, Berlin R, Lindgren M, Waara E, Uddén A, Pietiläinen K, Bengtsson T. Engineered mesoporous silica reduces long-term blood glucose, HbA1c, and improves metabolic parameters in prediabetics. Nanomedicine (Lond). 2022;17:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/