Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.108585
Revised: May 22, 2025
Accepted: August 4, 2025
Published online: September 24, 2025
Processing time: 158 Days and 15.9 Hours
Immuno-positron emission tomography (immuno-PET) is an innovative medical imaging technique that combines antibodies (Abs) or other immune-targeting molecules with positron-emitting radionuclides. By targeting antigens that are highly expressed in hematologic malignancies, immuno-PET has transformed diagnostic capabilities and enables precise monitoring of therapeutic responses through highly sensitive and specific tumor cell detection. Additionally, it plays a critical role in advancing therapeutic approaches by seamlessly linking diagnostic imaging with personalized treatment strategies. Its non-invasive nature and ability to provide whole-body imaging offer significant advantages over tradi
Core Tip: Fluorine-18 (18F)-fluorodeoxyglucose (FDG)-PET is commonly used to detect residual tumor cells and evaluate treatment responses in hematologic malignancies. However, as FDG uptake reflects glucose metabolism, it does not accurately indicate the presence of tumor cells expressing specific antigens. Recent studies have introduced immuno-PET, which labels antibodies or other immune-targeting molecules with positron-emitting radionuclides, as a promising alternative for visualizing tumor distribution and assessing therapeutic responses. This technique provides a specific imaging approach. In this minireview, we highlight recent progress in immuno-PET and discuss its future clinical applications in the management of hematologic malignancies.
- Citation: Goto H, Takano M, Shiraishi Y, Luanpitpong S. Immuno-positron emission tomography as a new frontier in imaging hematologic malignancies. World J Clin Oncol 2025; 16(9): 108585
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/108585.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.108585
Hematologic malignancies arise from blood-forming tissues, including the bone marrow and lymphatic system. Clinically, they are broadly categorized into myeloid and lymphoid malignancies depending on the origin of the malignant cells.
Myeloid malignancies include aggressive leukemias such as acute myeloid leukemia (AML), chronic forms like chronic myeloid leukemia, and other clonal disorders including myelodysplastic syndromes and myeloproliferative neoplasms, which exhibit varying degrees of ineffective hematopoiesis and abnormal cell proliferation. Lymphoid malignancies include a spectrum of diseases ranging from acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia to plasma cell disorders such as multiple myeloma (MM) and various forms of lymphoma.
Lymphomas arising from the malignant transformation of B-cells, T-cells, or Natural killer cells are broadly categorized as non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL). Over the past few decades, antibody (Ab)-based approaches have not only played a central role in the treatment of hematologic malignancies, but have also continued to evolve, with ongoing efforts to develop more effective and targeted therapies[1-4]. Cluster of differentiation 19 (CD19) and CD20 are the most important targets in B-cell malignancies. These markers are expressed across various stages of B-cell development and are commonly used for diagnostic and classification purposes. Notably, the landscape of B-cell NHL (B-NHL) has been dramatically transformed by the introduction of rituximab, an anti-CD20 mAb, leading to markedly improved patient outcomes[1]. More recently, CD19 has emerged as a promising therapeutic target, particularly in the context of chimeric antigen receptor T (CAR-T) cell therapy, reflecting the ongoing diversification of immunotherapeutic strategies beyond conventional Abs[5].
Immuno-positron emission tomography (immuno-PET) is a new medical imaging technique that combines Ab or immune-targeting molecules with positron-emitting radionuclides to enable the highly sensitive and specific identification of tumor cells. Traditional imaging techniques, such as computed tomography (CT) and magnetic resonance imaging, are limited by their inability to assess molecular targets in tumors[6]. Fluorine-18 (18F)-fluorodeoxyglucose (FDG)-PET has been widely used to detect residual tumor cells and assess treatment responses in patients with hematologic malignancies, especially lymphomas[7]. Nevertheless, 18F-FDG is highly dependent on glucose metabolism and cannot reveal the distribution of tumor cells expressing tumor antigens[8]. In immuno-PET with Abs, positron-emitting radionuclides require a half-life sufficient for Ab clearance. Recent studies have shown that long-lived positron-emitting radionuclides such as zirconium-89 (89Zr) have low positron energies and produce high sensitivity and resolution in cancer imaging[9,10]. Upon further investigation, immuno-PET can be utilized as a new imaging modality to evaluate tumor distribution and monitor therapeutic responses in the management of hematologic malignancies.
This minireview article summarizes the developments to date and future clinical uses of immuno-PET in the management of hematologic malignancies.
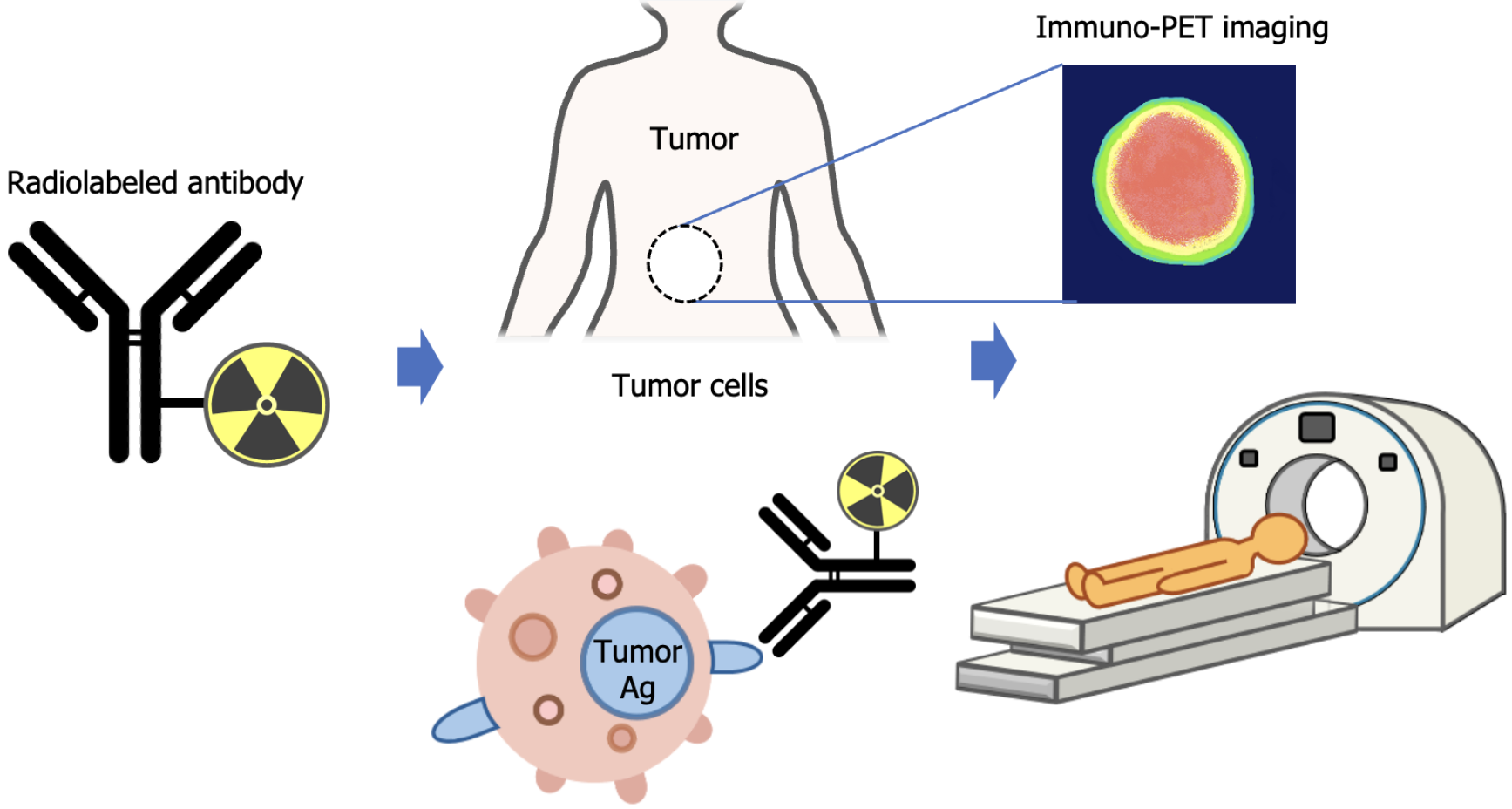
Immuno-PET is a radionuclide-based molecular imaging technique that combines the high sensitivity of PET with the specific targeting capabilities of biomolecules, such as Abs. Although Abs are commonly used, other immune-targeting molecules, including engineered proteins, can also be radiolabeled for immuno-PET applications[11]. The concept of immuno-PET involves detecting and imaging tumors using radiolabeled Abs, as illustrated in Figure 1.
This technique serves two main purposes: Visualizing the accumulation of radioimmunoconjugates in tumors or lesions prior to targeted therapy and evaluating tumor distribution and therapeutic response. Tumor antigens are often heterogeneously expressed, and information obtained from a single biopsy may not accurately reflect the overall tumor profile. Immuno-PET addresses this limitation by enabling non-invasive whole-body imaging, which allows for a comprehensive assessment of tumor heterogeneity and dynamic monitoring of treatment responses. Its non-invasive nature and ability to visualize diseases across the entire body provide significant advantages over traditional diagnostic approaches, particularly in detecting minimal residual disease and guiding adaptive therapeutic strategies.
Radioimmunoconjugates for immuno-PET consist of a targeting moiety (typically an Ab), a PET radionuclide, and a chemical linker—often a chelator—that ensures stable attachment of the radionuclide to the Ab.
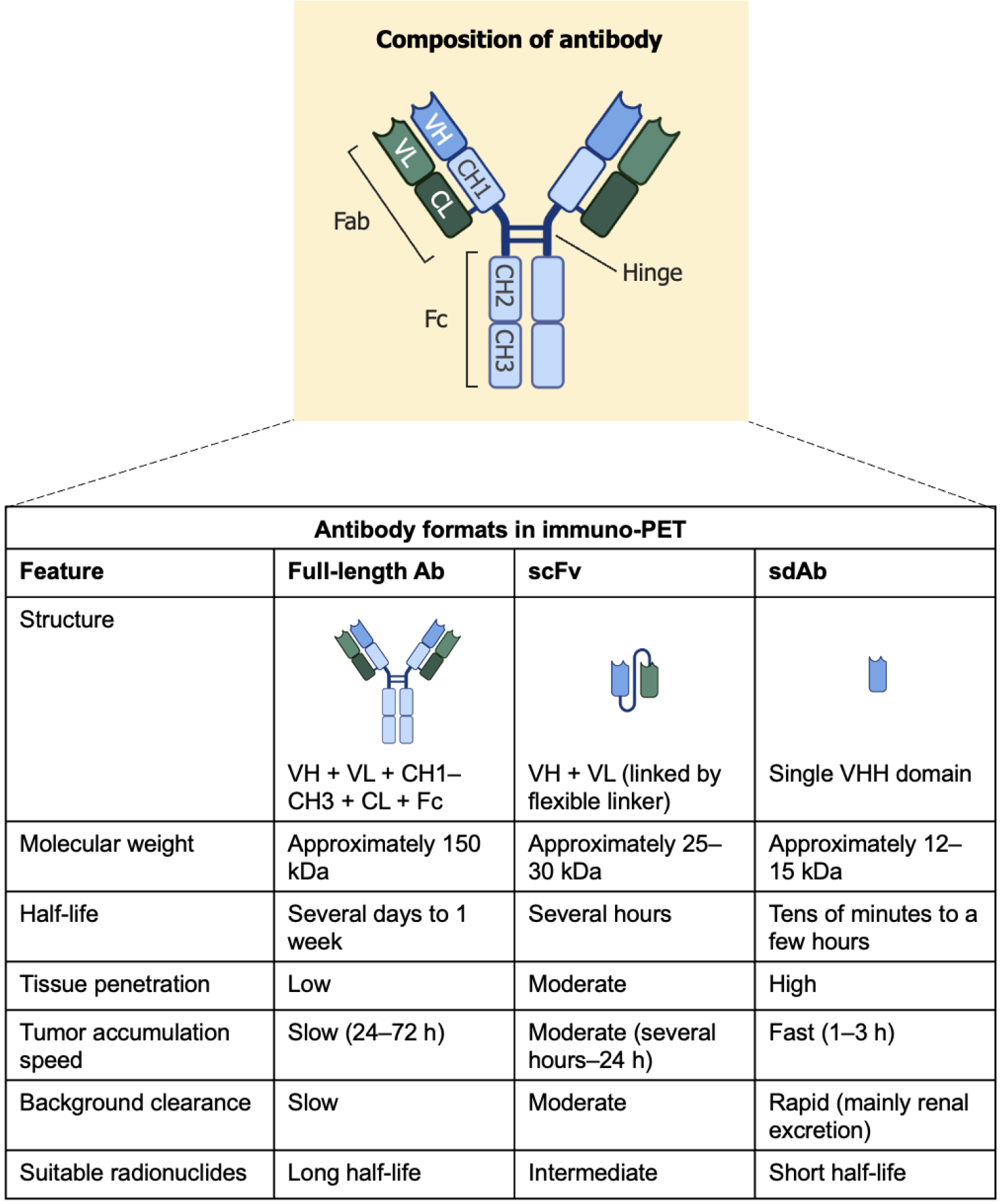
Various Ab formats are employed in immuno-PET to match the pharmacokinetics of the targeting agent to the physical half-life of the radionuclide employed[10]. The most commonly used Abs include full-length Abs, single-chain variable fragments (scFv), and single-domain Abs (sdAbs), also known as nanobodies. Each format exhibits distinct physicochemical and biological properties that influence imaging performance. Full-length Abs (approximately 150 kDa) contain both variable and constant domains, including the Fc region, and exhibit long serum half-lives, ranging from several days to weeks. Although this prolonged circulation allows for sustained tumor targeting, it often results in delayed tumor accumulation (24-72 h) and high background activity due to slow clearance. Therefore, Abs are typically labeled with long-lived radionuclides such as 89Zr. In contrast, scFv (approximately 25-30 kDa), composed of linked variable heavy and light chains, demonstrate faster tumor accumulation and moderate tissue penetration, with a serum half-life of several hours. These properties make them suitable for use with intermediate-half-life radionuclides such as Copper-64
Radionuclides provide the source of positron emission required for PET imaging. When a positron emitted from a radionuclide encounters an electron in the body, the two particles annihilate, producing a pair of 511 keV gamma photons that are emitted in opposite directions. These annihilation photons are detected using PET scanners to reconstruct detailed images of the tracer distribution in vivo. The selection of an appropriate radionuclide is crucial because its physical half-life should ideally match the biological half-life of the Ab or the targeting molecule to ensure optimal imaging contrast and minimal radiation burden. For example, 89Zr has a physical half-life of approximately 78.4 h, making it suitable for imaging with full-length Abs that require prolonged circulation times[9,10]. In contrast, 64Cu, which has a shorter half-life (12.7 hours), is often used with smaller Ab fragments or engineered targeting molecules because of its rapid pharmacokinetics[9,10]. 68Ga has a short half-life of approximately 68 min and is ideal for small molecules or peptides that are rapidly cleared from the bloodstream[10]. Its generator-based availability and favorable imaging characteristics have made it a widely used radionuclide for clinical PET imaging. Although other radionuclides such as 18F and iodine-124 (124I) have been explored, 18F (half-life: Approximately110 min) requires a cyclotron for production and typically involves complex organic synthesis techniques and elaborate precursor preparation for radiolabeling[10]. 124I, which has a suitable half-life (~100 h), emits high-energy positrons that lead to poor spatial resolution, and its in vivo deiodination can result in increased background signal and reduced tumor uptake[10]. The representative positron-emitting radionuclides discussed in this minireview for hematologic malignancies are listed in Table 1.
| Radionuclide | Emission | Half-life | β+ ray emitting rate |
| Copper-64 (64Cu) | β+, β-, EC | 12.7 h | 19% |
| Zirconium-89 (89Zr) | β+, EC | 78.4 h | 23% |
| Gallium-68 (68Ga) | β+, EC | 68 min | 89% |
In immuno-PET, chelators play a critical role in stably linking metal-based radionuclides to Abs. DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) is considered a ‘gold standard’ chelator for a wide range of radiometals, including 64Cu and 68Ga, due to its high thermodynamic stability[9]. However, efficient labeling with DOTA can require elevated temperatures and long reaction times, especially for 68Ga, which can be suboptimal for heat-sensitive biomolecules.
For 64Cu, alternative chelators such as TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid), CB-TE2A (a cross-bridged cyclam-based chelator), SarAr (sarcosine-based chelator), NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid), TE1PA (a phosphonate-containing NOTA derivative), and TE1A1P (a phosphinic acid-containing NOTA analog) have been developed to improve labeling efficiency and in vivo stability under milder conditions[9]. TE1PA, in particular, enables efficient complexation with 64Cu at lower temperatures and exhibits favorable pharmacokinetics[13], while TE1A1P offers enhanced complexation kinetics and exceptional in vivo stability, making it highly suitable for 64Cu-based immuno-PET applications[14].
Desferrioxamine (DFO) remains the most widely used chelator of Zr because of its high affinity for Zr ions[9]. However, its in vivo stability can be limited over extended periods, potentially affecting imaging accuracy. To address this, alternative chelators such as hydroxypyridinone (HOPO), 2,3-HOPO, and modified DFO analogs have been explored to enhance kinetic stability and reduce transchelation in vivo.
The selection of an appropriate chelator is critical for maintaining radiometal-chelator-Ab integrity, and the ongoing development of novel chelators aims to further improve the labeling efficiency, in vivo stability, and imaging performance across a range of immuno-PET applications.
Preclinical and clinical studies involving immuno-PET have been conducted for various hematologic malignancies, including lymphoma, MM, and leukemia, each with specific surface antigens that serve as imaging targets[15]. The surface antigens targeted by immuno-PET for hematologic malignancies discussed in this minireview are listed in Table 2.
| Types of hematologic malignancy | Surface antigens targeted in immuno-PET detection |
| Non-Hodgkin lymphoma | CD19, CD20, CXCR4 |
| Hodgkin lymphoma | CD30 |
| Multiple myeloma | CD38, CD138, VLA-4, BCMA, SLAMF7, CXCR4 |
| Acute myeloid leukemia | CD33, CXCR4 |
| Acute lymphoblastic leukemia | CXCR4 |
CD20 is a transmembrane phosphoprotein expressed on the surface of most B-cells, from the pre-B-cell stage to mature B-cells, but not on stem cells or plasma cells. Owing to its restricted expression pattern and stable presence in malignant B-cells, CD20 serves as an excellent therapeutic target in B-NHL. The introduction of rituximab, a chimeric mAb against CD20, has significantly improved the prognosis of patients with B-NHL by enhancing response rates and prolonging survival when combined with conventional chemotherapy[1,4]. Immuno-PET using 64Cu-DOTA-rituximab is feasible for identifying human CD20-expressing lesions in CD20 transgenic mice[16]. Raji cells, which are widely used as a representative model of Burkitt lymphoma—an aggressive B-cell lymphoma—have been xenografted into immunodeficient mice. Tumor-specific uptake has been demonstrated using immuno-PET with 64Cu-DOTA-rituximab in these tumor-bearing mice[17]. Lymphoma lesions in two patients with B-NHL were sensitively detected by immuno-PET using 64Cu-DOTA-rituximab[18]. Immuno-PET with 89Zr-labeled rituximab demonstrated distinct tumor accumulation in a murine preclinical model[19,20]. Owing to its greater physical half-life than 64Cu-labeled rituximab, 89Zr-labeled rituximab may enable extended monitoring of lesion targeting and therapeutic efficacy. A pilot study using 89Zr-rituximab in six patients with relapsed/refractory diffuse large B-cell lymphoma suggested that the tumor uptake of 89Zr-rituximab and CD20 expression in biopsies are concordant[21]. Tumor uptake in immuno-PET using 89Zr-rituximab is lower in patients previously treated with rituximab than in those not treated with rituximab, suggesting that preloading with non-radiolabeled rituximab affects the detection of radiolabeled rituximab[22]. Next-generation anti-CD20 Abs, obinutuzumab (humanized) and ofatumumab (fully human), are engineered with reduced murine content, potentially enabling improved tolerability and repeated dosing, in contrast to earlier agents, such as rituximab (chimeric) and tositumomab (murine), which incorporate a higher proportion of murine-derived sequences[23]. Obinutuzumab and ofatumumab recognize distinct epitopes on CD20 compared to rituximab and exhibit a lower internalization rate. Immuno-PET using radiolabeled obinutuzumab and ofatumumab has the potential to be more effective than immuno-PET with radiolabeled rituximab[20,24,25]. In preclinical mouse models expressing human CD20, immuno-PET using radiolabeled obinutuzumab demonstrated rapid and sustained tumor uptake with high contrast[25,26]. These results suggest that immuno-PET can effectively detect CD20-expressing lymphoma cells and aid in selecting patients who are likely to benefit from CD20-targeted therapies. Notably, this approach allows the use of a diagnostic Ab to identify tumor distribution by targeting one specific epitope, whereas a therapeutic Ab can simultaneously or subsequently target a different epitope, potentially enhancing treatment efficacy. However, a substantial portion of the data discussed is derived from murine models, which may limit its translatability to human clinical settings. Further studies, particularly in human clinical trials, are required to validate these findings and assess their clinical applicability.
Yttrium-90 (90Y)-labeled ibritumomab tiuxetan (Zevalin) was the first β-emitting radioimmunoconjugate approved by the US Food and Drug Administration in February 2002 for the treatment of relapsed or refractory, low-grade or follicular B-NHL[27,28]. This therapeutic agent combines the CD20-targeting capability of ibritumomab, a murine IgG1 mAb that recognizes the same antigen as rituximab, with the chelating compound tiuxetan, which stably binds 90Y through a covalent linkage to the Ab. 89Zr-labeled ibritumomab tiuxetan has been used in both preclinical and clinical studies to image tumors and evaluate therapeutic responses in patients with NHL[29,30]. Immuno-PET with 89Zr-labeled ibritumomab tiuxetan enables the clear visualization of known lesions and effectively predicts dose-limiting organs during therapy.
Immuno-PET imaging with sdAbs enhances tissue penetration and is a promising approach for tumor detection. Specifically, 68Ga-anti-CD20 sdAb exhibits targeted lymphoma uptake with minimal accumulation in non-target organs (except the kidneys) in human CD20+ lymphoma mouse models[31]. Although sdAbs theoretically offer a high potential for increased affinity, there is currently insufficient robust evidence supporting their superior specificity, indicating the need for further research to enable their clinical application. In future, when radioimmunotherapy with sdAbs is introduced for cancer treatment, immuno-PET using sdAbs will likely serve as a valuable tool to verify dosimetry.
CD19 is also expressed in B-NHL and has emerged as a key target for CAR-T cell therapy, and verification of CD19 expression prior to therapy is considered essential[5,32]. Immuno-PET imaging using a 64Cu-labeled mAb specific for human CD19 successfully achieved noninvasive and selective visualization of CD19-expressing lymphoma lesions in preclinical mouse models and in four human subjects with B-NHL[33].
C-X-C motif chemokine receptor 4 (CXCR4) is highly expressed on the surface of certain tumor cells in hematologic malignancies, including lymphoma[34]. Pentixather and pentixafor are CXCR4-targeting peptide-based ligands that are commonly used in molecular imaging and therapy. Immuno-PET using 64Cu-labeled pentixather targeting CXCR4 results in favorable resolution in mice bearing Burkitt lymphoma Daudi cells, which, like Raji cells, are established models of aggressive B-cell lymphoma[35]. The development and evaluation of immuno-PET targeting CXCR4 in patients with lymphoma are highly desirable.
CD30 is expressed in HL, particularly in Reed-Sternberg cells, and serves as a key diagnostic marker for the disease. In a CD30-positive human lymphoma mouse model, immuno-PET imaging with 89Zr-labeled anti-CD30 Ab (AC-10) demonstrated over threefold greater accumulation in CD30-positive tumors than in CD30-negative tumors[36]. Immuno-PET using 64Cu-labeled anti-CD30 (IMB16) enabled non-invasive assessment of CD30 expression, thereby allowing subsequent radioimmunotherapy in CD30-positive lymphoma[37].
These results highlight the potential of immuno-PET not only for visualizing lymphoma lesions but also for guiding and optimizing CD20- and CD19-targeted therapies, such as radioimmunotherapy and CAR-T cell therapy. Continued refinement of imaging agents and validation in clinical settings are essential to fully realize its role in personalized treatment strategies.
18F-FDG-PET may produce both false positives (due to inflammation) and false negatives (due to hyperglycemia)[38,39]. The incidence of false PET negativity was 11% in 227 newly diagnosed MM patients, and the expression of hexokinase-2, which catalyzes the initial step of glycolysis, was significantly reduced in PET false-negative cases (5.3-fold change)[40]. This underscores the need for alternative PET imaging techniques other than 18F-FDG-PET to assess treatment response in MM.
CD38 is a cell surface receptor that is highly expressed in plasma cells of MM patients, including malignant plasma cells. Daratumumab is a human mAb of (IgG1) that specifically targets CD38. Because CD38 is almost universally expressed by myeloma cells, it has demonstrated particularly strong clinical efficacy[41]. 89Zr-labeled daratumumab is safe and successfully identifies MM cells in a xenograft model using MM1.S and OPM-2 cells and in a first-in-human phase I clinical study that included 10 patients[42,43]. Immuno-PET using 64Cu-daratumumab also successfully detects MM cell dissemination in a xenograft model using MM1.S and enabled safe whole-body imaging of MM patients in a phase I trial[44,45]. To improve penetration into MM lesions, a 68Ga-labeled anti-CD38 sdAb (Nb1053) was developed, which successfully detected MM cell infiltration in human MM1.S xenograft mouse models, both in subcutaneous and disseminated disease forms[46].
Very late antigen-4 (VLA-4, also known as integrin α4β1), highly expressed on the surface of MM cells, is involved in cell trafficking and drug resistance via cell adhesion to bone marrow[47]. The KaLwRij mouse is an immunocompetent syngeneic model that allows for engraftment of 5TGM1 murine MM cells and closely resembles the clinical and biological features of human MM, including bone marrow tropism and monoclonal gammopathy[48]. Using this model, 64Cu-CB-TE1A1P-LLP2A efficiently detects VLA-4+ MM cells in subcutaneous tumors, spleen, and bone marrow[14].
B-cell maturation antigen (BCMA), also known as tumor necrosis factor receptor superfamily member 17, is related to the maturation and differentiation of plasma cells and is expressed as another MM antigen[49]. 68Ga-anti-BCMA sdAbs efficiently identify disease localization, demonstrating an excellent tumor-to-background ratio in an MM xenograft mouse model of disseminated disease[50].
CXCR4 is a suitable surface marker for tumor detection and the evaluation of treatment responses using immuno-PET, which is also applicable to MM[51]. 68Ga-pentixafor PET imaging enables the visualization of CXCR4 expression and demonstrates superior or comparable performance to 18F-FDG-PET in patients with MM[52-54].
CD138 (Syndecan-1), a cell surface proteoglycan highly expressed on MM cells, serves as an effective molecular target for immuno-PET imaging[55]. Using a 64Cu-labeled anti-CD138 Ab, immuno-PET enables sensitive detection of both subcutaneous MM tumors and bone marrow lesions. In the syngeneic 5T33 MM mouse model, immuno-PET imaging with 64Cu-CD138 Ab outperformed conventional 18F-FDG-PET in visualizing disease sites[13,56]. A comparison between the two anti-mouse CD138 Ab (9E7.4) conjugates demonstrated that 89Zr-DFO-9E7.4 exhibits higher nonspecific bone uptake than 64Cu-TE2A-9E7.4, suggesting that the 64Cu-labeled anti-CD138 Ab provides more favorable tumor-to-nontarget tissue ratios in the 5T33 MM syngeneic mouse model[57].
Signaling lymphocyte activation molecule F7 (SLAMF7), also known as CD2 subset 1 (CS1), is expressed in both normal and malignant plasma cells, and its expression has been associated with disease progression and relapse in MM patients[58]. Elotuzumab is a mAb used in the treatment of MM that targets SLAMF7, a surface protein highly expressed by myeloma cells. 89Zr-DFO-elotuzumab demonstrates significantly higher standardized uptake values in bone tissues affected by disseminated MM1.S tumors in mice[59]. This study highlights the potential of immuno-PET with 89Zr-DFO-elotuzumab as a predictive imaging approach to guide elotuzumab-based treatment strategies for MM patients.
These studies demonstrate how immuno-PET may be used to precisely detect MM-specific antigens, thereby circumventing the drawbacks of 18F-FDG imaging. This approach offers insights for personalized treatment planning and may play a critical role in evaluating therapeutic response and disease progression in MM.
In AML, CD33 is frequently expressed by leukemic blasts and serves as a target for therapeutic Abs including gemtuzumab ozogamicin, which have been approved for clinical use[60]. 64Cu-DOTA-anti-CD33-based immuno-PET imaging detected CD33+ AML cells in mice bearing MV4-11 or HL-60 with high sensitivity and specificity[61].
CXCR4 is frequently overexpressed in AML and plays a critical role in interactions between leukemic cells and the bone marrow microenvironment[62]. 68Ga-pentixafor immuno-PET imaging enables visualization of CXCR4 expression in patient-derived xenograft (PDX) mice with CXCR4+ AML[63].
Immuno-PET targeting AML surface antigens such as CD33 and CXCR4 provides a non-invasive means to visualize disease burden and microenvironmental interactions, highlighting its potential utility in both disease monitoring and treatment stratification.
Similar to AML, CXCR4 overexpression in ALL may contribute to leukemic cell-bone marrow interactions, and CXCR4-directed immuno-PET imaging could offer a non-invasive means of assessing its expression[62]. Notably, 68Ga-pentixafor immuno-PET also enables in vivo detection of CXCR4 expression in T-ALL PDX mice[63].
Even though CXCR4-targeted immuno-PET demonstrates potential for non-invasive imaging in ALL, there are still few studies on immuno-PET in this disease remains limited, emphasizing the need for further investigation.
Immuno-PET is a promising imaging modality that enables precise visualization of tumor-specific antigens in hematologic malignancies. Recent advances in radionuclide engineering, Ab engineering, and chelator chemistry have significantly improved the sensitivity, specificity, and translational potential of this technique. Further work is required to develop stable and biocompatible novel chelators, optimize the matching of Ab formats and radionuclide half-lives for suitable tumor antigens, and integrate artificial intelligence-based approaches for automated and quantitative image analysis in immuno-PET in order to fully realize its potential in hematologic oncology. Also, addressing challenges such as production costs, regulatory complexity, and limited accessibility will be essential for broader clinical adoption. Immuno-PET is poised to play a central role in precision medicine by enabling noninvasive, target-specific theranostics that support personalized treatment selection and real-time monitoring.
We thank Fukushima K for assistance with administrative tasks.
| 1. | Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med. 2008;359:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 2. | Goto H, Kojima Y, Matsuda K, Kariya R, Taura M, Kuwahara K, Nagai H, Katano H, Okada S. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer. 2014;50:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Goto H, Kudo E, Kariya R, Taura M, Katano H, Okada S. Targeting VEGF and interleukin-6 for controlling malignant effusion of primary effusion lymphoma. J Cancer Res Clin Oncol. 2015;141:465-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Han Y, Liu Z, Liu J, Yan W, Xia Y, Yue S, Yu J. Antibody-Based Immunotherapeutic Strategies for the Treatment of Hematological Malignancies. Biomed Res Int. 2020;2020:4956946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Globerson Levin A, Rivière I, Eshhar Z, Sadelain M. CAR T cells: Building on the CD19 paradigm. Eur J Immunol. 2021;51:2151-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep (Hoboken). 2023;6:e1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 7. | Hughes NM, Jacene HA. PET Imaging for Hematologic Malignancies. Radiol Clin North Am. 2021;59:705-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Hirata K, Tamaki N. Quantitative FDG PET Assessment for Oncology Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Badier L, Quelven I. Zirconium 89 and Copper 64 for ImmunoPET: From Antibody Bioconjugation and Radiolabeling to Molecular Imaging. Pharmaceutics. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Garaulet G, Báez BB, Medrano G, Rivas-Sánchez M, Sánchez-Alonso D, Martinez-Torrecuadrada JL, Mulero F. Radioimmunotheragnosis in Cancer Research. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Shabsigh M, Solomon LA. Peptide PET Imaging: A Review of Recent Developments and a Look at the Future of Radiometal-Labeled Peptides in Medicine. Chem Biomed Imaging. 2024;2:615-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 2402] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 13. | Navarro AS, Le Bihan T, Le Saëc P, Bris NL, Bailly C, Saï-Maurel C, Bourgeois M, Chérel M, Tripier R, Faivre-Chauvet A. TE1PA as Innovating Chelator for (64)Cu Immuno-TEP Imaging: A Comparative in Vivo Study with DOTA/NOTA by Conjugation on 9E7.4 mAb in a Syngeneic Multiple Myeloma Model. Bioconjug Chem. 2019;30:2393-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Soodgupta D, Hurchla MA, Jiang M, Zheleznyak A, Weilbaecher KN, Anderson CJ, Tomasson MH, Shokeen M. Very late antigen-4 (α(4)β(1) Integrin) targeted PET imaging of multiple myeloma. PLoS One. 2013;8:e55841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Caers J, Duray E, Vrancken L, Marcion G, Bocuzzi V, De Veirman K, Krasniqi A, Lejeune M, Withofs N, Devoogdt N, Dumoulin M, Karlström AE, D'Huyvetter M. Radiotheranostic Agents in Hematological Malignancies. Front Immunol. 2022;13:911080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Natarajan A, Gowrishankar G, Nielsen CH, Wang S, Iagaru A, Goris ML, Gambhir SS. Positron emission tomography of 64Cu-DOTA-Rituximab in a transgenic mouse model expressing human CD20 for clinical translation to image NHL. Mol Imaging Biol. 2012;14:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Lee CH, Lim I, Woo SK, Kim W, Kim KI, Lee KC, Song K, Lim SM. Targeted alpha immunotherapy of CD20-positive B-cell lymphoma model: dosimetry estimate of (225)Ac-DOTA-rituximab using (64)Cu-DOTA-rituximab. Ann Nucl Med. 2021;35:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Lee I, Lim I, Lee KC, Kang HJ, Lim SM. 64 Cu-DOTA-Rituximab PET/CT of B-Cell Non-Hodgkin Lymphoma for Imaging the CD20 Expression. Clin Nucl Med. 2023;48:e82-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Natarajan A, Habte F, Gambhir SS. Development of a novel long-lived immunoPET tracer for monitoring lymphoma therapy in a humanized transgenic mouse model. Bioconjug Chem. 2012;23:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Yoon JT, Longtine MS, Marquez-Nostra BV, Wahl RL. Evaluation of Next-Generation Anti-CD20 Antibodies Labeled with (89)Zr in Human Lymphoma Xenografts. J Nucl Med. 2018;59:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Jauw YW, Zijlstra JM, de Jong D, Vugts DJ, Zweegman S, Hoekstra OS, van Dongen GA, Huisman MC. Performance of 89Zr-Labeled-Rituximab-PET as an Imaging Biomarker to Assess CD20 Targeting: A Pilot Study in Patients with Relapsed/Refractory Diffuse Large B Cell Lymphoma. PLoS One. 2017;12:e0169828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Muylle K, Flamen P, Vugts DJ, Guiot T, Ghanem G, Meuleman N, Bourgeois P, Vanderlinden B, van Dongen GA, Everaert H, Vaes M, Bron D. Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging. 2015;42:1304-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Pierpont TM, Limper CB, Richards KL. Past, Present, and Future of Rituximab-The World's First Oncology Monoclonal Antibody Therapy. Front Oncol. 2018;8:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 24. | Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T, Muth G, Ziegler-Landesberger D, Van Puijenbroek E, Lang S, Duong MN, Reslan L, Gerdes CA, Friess T, Baer U, Burtscher H, Weidner M, Dumontet C, Umana P, Niederfellner G, Bacac M, Klein C. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 25. | Zettlitz KA, Tavaré R, Knowles SM, Steward KK, Timmerman JM, Wu AM. ImmunoPET of Malignant and Normal B Cells with (89)Zr- and (124)I-Labeled Obinutuzumab Antibody Fragments Reveals Differential CD20 Internalization In Vivo. Clin Cancer Res. 2017;23:7242-7252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Kang L, Li C, Rosenkrans ZT, Engle JW, Wang R, Jiang D, Xu X, Cai W. Noninvasive Evaluation of CD20 Expression Using (64)Cu-Labeled F(ab')(2) Fragments of Obinutuzumab in Lymphoma. J Nucl Med. 2021;62:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-López AJ, Multani P, White CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 803] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 28. | Goto H, Shiraishi Y, Okada S. Continuing progress in radioimmunotherapy for hematologic malignancies. Blood Rev. 2025;69:101250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Perk LR, Visser OJ, Stigter-van Walsum M, Vosjan MJ, Visser GW, Zijlstra JM, Huijgens PC, van Dongen GA. Preparation and evaluation of (89)Zr-Zevalin for monitoring of (90)Y-Zevalin biodistribution with positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:1337-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Rizvi SN, Visser OJ, Vosjan MJ, van Lingen A, Hoekstra OS, Zijlstra JM, Huijgens PC, van Dongen GA, Lubberink M. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin's lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging. 2012;39:512-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Krasniqi A, D'Huyvetter M, Xavier C, Van der Jeught K, Muyldermans S, Van Der Heyden J, Lahoutte T, Tavernier J, Devoogdt N. Theranostic Radiolabeled Anti-CD20 sdAb for Targeted Radionuclide Therapy of Non-Hodgkin Lymphoma. Mol Cancer Ther. 2017;16:2828-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Abramson JS. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus Med Rev. 2020;34:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Sonanini D, Schwenck J, Blaess S, Schmitt J, Maurer A, Ehrlichmann W, Ritter M, Skokowa J, Kneilling M, Jung G, Fend F, Krost S, Seitz CM, Lang P, Reischl G, Handgretinger R, Fougère C, Pichler BJ. CD19-immunoPET for noninvasive visualization of CD19 expression in B-cell lymphoma patients. Biomark Res. 2024;12:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Mehrpouri M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur J Pharmacol. 2022;920:174831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Poschenrieder A, Schottelius M, Osl T, Schwaiger M, Wester HJ. [(64)Cu]NOTA-pentixather enables high resolution PET imaging of CXCR4 expression in a preclinical lymphoma model. EJNMMI Radiopharm Chem. 2017;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Rylova SN, Del Pozzo L, Klingeberg C, Tönnesmann R, Illert AL, Meyer PT, Maecke HR, Holland JP. Immuno-PET Imaging of CD30-Positive Lymphoma Using 89Zr-Desferrioxamine-Labeled CD30-Specific AC-10 Antibody. J Nucl Med. 2016;57:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Yang X, Liu J, Li C, Zheng L, Lu X, Zhou Z, Zhu X, Gong J, Miao Q, Yang J. Preclinical evaluation of 64Cu/177Lu-labelled anti-CD30 monoclonal antibody for theranostics in CD30-positive lymphoma. Eur J Nucl Med Mol Imaging. 2025;52:1751-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Pijl JP, Nienhuis PH, Kwee TC, Glaudemans AWJM, Slart RHJA, Gormsen LC. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin Nucl Med. 2021;51:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 39. | Finessi M, Bisi G, Deandreis D. Hyperglycemia and 18F-FDG PET/CT, issues and problem solving: a literature review. Acta Diabetol. 2020;57:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Rasche L, Angtuaco E, McDonald JE, Buros A, Stein C, Pawlyn C, Thanendrarajan S, Schinke C, Samant R, Yaccoby S, Walker BA, Epstein J, Zangari M, van Rhee F, Meissner T, Goldschmidt H, Hemminki K, Houlston R, Barlogie B, Davies FE, Morgan GJ, Weinhold N. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood. 2017;130:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 41. | Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NW, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373:1207-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 936] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 42. | Ghai A, Maji D, Cho N, Chanswangphuwana C, Rettig M, Shen D, DiPersio J, Akers W, Dehdashti F, Achilefu S, Vij R, Shokeen M. Preclinical Development of CD38-Targeted [(89)Zr]Zr-DFO-Daratumumab for Imaging Multiple Myeloma. J Nucl Med. 2018;59:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Ulaner GA, Sobol NB, O'Donoghue JA, Kirov AS, Riedl CC, Min R, Smith E, Carter LM, Lyashchenko SK, Lewis JS, Landgren CO. CD38-targeted Immuno-PET of Multiple Myeloma: From Xenograft Models to First-in-Human Imaging. Radiology. 2020;295:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Caserta E, Chea J, Minnix M, Poku EK, Viola D, Vonderfecht S, Yazaki P, Crow D, Khalife J, Sanchez JF, Palmer JM, Hui S, Carlesso N, Keats J, Kim Y, Buettner R, Marcucci G, Rosen S, Shively J, Colcher D, Krishnan A, Pichiorri F. Copper 64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood. 2018;131:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Krishnan A, Adhikarla V, Poku EK, Palmer J, Chaudhry A, Biglang-Awa VE, Bowles N, Nathwani N, Rosenzweig M, Sahebi F, Karanes C, Simpson J, Sanchez JF, Yamauchi D, Parayno M, Chowdhury A, Caserta E, Marcucci G, Rockne R, Wu AM, Wong J, Forman SJ, Colcher D, Yazaki P, Shively J, Pichiorri F. Identifying CD38+ cells in patients with multiple myeloma: first-in-human imaging using copper-64-labeled daratumumab. Blood Adv. 2020;4:5194-5202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Wang C, Chen Y, Hou YN, Liu Q, Zhang D, Zhao H, Zhang Y, An S, Li L, Hou J, Huang G, Liu J, Zhao YJ, Wei W. ImmunoPET imaging of multiple myeloma with [(68)Ga]Ga-NOTA-Nb1053. Eur J Nucl Med Mol Imaging. 2021;48:2749-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 47. | Sanz-Rodríguez F, Teixidó J. VLA-4-dependent myeloma cell adhesion. Leuk Lymphoma. 2001;41:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Radl J, Hollander CF, van den Berg P, de Glopper E. Idiopathic paraproteinaemia. I. Studies in an animal model--the ageing C57BL/KaLwRij mouse. Clin Exp Immunol. 1978;33:395-402. [PubMed] |
| 49. | Cho SF, Xing L, Anderson KC, Tai YT. Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Wei W, Zhang Y, Zhang D, Liu Q, An S, Chen Y, Huang G, Liu J. Annotating BCMA Expression in Multiple Myelomas. Mol Pharm. 2022;19:3492-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341-4351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 340] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 52. | Philipp-Abbrederis K, Herrmann K, Knop S, Schottelius M, Eiber M, Lückerath K, Pietschmann E, Habringer S, Gerngroß C, Franke K, Rudelius M, Schirbel A, Lapa C, Schwamborn K, Steidle S, Hartmann E, Rosenwald A, Kropf S, Beer AJ, Peschel C, Einsele H, Buck AK, Schwaiger M, Götze K, Wester HJ, Keller U. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med. 2015;7:477-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 53. | Lapa C, Herrmann K, Schirbel A, Hänscheid H, Lückerath K, Schottelius M, Kircher M, Werner RA, Schreder M, Samnick S, Kropf S, Knop S, Buck AK, Einsele H, Wester HJ, Kortüm KM. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics. 2017;7:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Pan Q, Cao X, Luo Y, Li J, Feng J, Li F. Chemokine receptor-4 targeted PET/CT with (68)Ga-Pentixafor in assessment of newly diagnosed multiple myeloma: comparison to (18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2020;47:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Riccardi F, Tangredi C, Dal Bo M, Toffoli G. Targeted therapy for multiple myeloma: an overview on CD138-based strategies. Front Oncol. 2024;14:1370854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 56. | Bailly C, Gouard S, Lacombe M, Remaud-Le Saëc P, Chalopin B, Bourgeois M, Chouin N, Tripier R, Halime Z, Haddad F, Faivre-Chauvet A, Kraeber-Bodéré F, Chérel M, Bodet-Milin C. Comparison of Immuno-PET of CD138 and PET imaging with (64)CuCl(2) and (18)F-FDG in a preclinical syngeneic model of multiple myeloma. Oncotarget. 2018;9:9061-9072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Bailly C, Gouard S, Guérard F, Chalopin B, Carlier T, Faivre-Chauvet A, Remaud-Le Saëc P, Bourgeois M, Chouin N, Rbah-Vidal L, Tripier R, Haddad F, Kraeber-Bodéré F, Bodet-Milin C, Chérel M. What is the Best Radionuclide for Immuno-PET of Multiple Myeloma? A Comparison Study Between (89)Zr- and (64)Cu-Labeled Anti-CD138 in a Preclinical Syngeneic Model. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Malaer JD, Mathew PA. CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma. Am J Cancer Res. 2017;7:1637-1641. [PubMed] |
| 59. | Ghai A, Zheleznyak A, Mixdorf M, O'Neal J, Ritchey J, Rettig M, DiPersio J, Shokeen M, Achilefu S. Development of [(89)Zr]DFO-elotuzumab for immunoPET imaging of CS1 in multiple myeloma. Eur J Nucl Med Mol Imaging. 2021;48:1302-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130:2373-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 61. | Srideshikan SM, Brooks J, Zuro D, Kumar B, Sanchez J, Echavarria Parra L, Orellana M, Vishwasrao P, Nair I, Chea J, Poku K, Bowles N, Miller A, Ebner T, Molnar J, Rosenthal J, Vallera DA, Wong JYC, Stein AS, Colcher D, Shively JE, Yazaki PJ, Hui SK. ImmunoPET, [(64)Cu]Cu-DOTA-Anti-CD33 PET-CT, Imaging of an AML Xenograft Model. Clin Cancer Res. 2019;25:7463-7474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Cancilla D, Rettig MP, DiPersio JF. Targeting CXCR4 in AML and ALL. Front Oncol. 2020;10:1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 63. | Habringer S, Lapa C, Herhaus P, Schottelius M, Istvanffy R, Steiger K, Slotta-Huspenina J, Schirbel A, Hänscheid H, Kircher S, Buck AK, Götze K, Vick B, Jeremias I, Schwaiger M, Peschel C, Oostendorp R, Wester HJ, Grigoleit GU, Keller U. Dual Targeting of Acute Leukemia and Supporting Niche by CXCR4-Directed Theranostics. Theranostics. 2018;8:369-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/