Published online Feb 24, 2025. doi: 10.5306/wjco.v16.i2.99635
Revised: September 24, 2024
Accepted: October 28, 2024
Published online: February 24, 2025
Processing time: 137 Days and 23.2 Hours
Colorectal cancer (CRC) ranks high among the most common types of malignant tumors. The primary cause of cancer-related mortality is metastasis, with lung metastases accounting for 32.9% of all cases of metastatic CRC (MCRC). However, cases of MCRC in the lungs, which present concurrently with primary peripheral lung adenocarcinoma, are exceptionally rare.
This report describes the case of a 52-year-old female patient who, following a colonoscopy, was diagnosed with moderately differentiated adenocarcinoma based on rectal mucosal biopsy findings. A preoperative chest computed tomography scan revealed a ground-glass nodule in the right lung and a small nodule (approximately 0.6 cm in diameter) in the extramural basal segment of the left lower lobe, which suggested multiple lung metastases from rectal cancer. Subsequent treatment and follow-up led to a diagnosis of rectal cancer with left lung metastasis and peripheral adenocarcinoma of the lower lobe of the right lung.
This case report describes the therapeutic journey of a patient with lung me
Core Tip: A 52-year-old female patient was found to have both lung metastases and primary lung adenocarcinoma after rectal cancer surgery. By combining pathology, immunohistochemistry and molecular genetics techniques, we were able to successfully differentiate between the two different tumor types and formulate a personalized treatment plan that included wedge resection of the left lung and partial resection of the right lung. Multidisciplinary collaboration plays an important role in the treatment process and provides a valuable reference for the clinical management of similar cases.
- Citation: Zhou FY, Song FH, Cheng ZH, Wu S. Discovery of primary lung cancer following resection of rectal cancer lung metastasis: A case report. World J Clin Oncol 2025; 16(2): 99635
- URL: https://www.wjgnet.com/2218-4333/full/v16/i2/99635.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i2.99635
Colorectal and lung cancers collectively contribute to one-third of all cancer-related fatalities throughout the world[1]. Colorectal cancer (CRC) is a predominant cause of cancer-related morbidity and mortality worldwide. Current evidence indicates that early-stage diagnosis of CRC can yield a 5-year survival rate as high as 90%. However, in more than 60% of cases, CRC has already metastasized at the time of detection. The lung is the most common site of extra-abdominal metastasis in CRC patients[2]. Conventional treatment modalities for CRC include chemotherapy, radiotherapy, and targeted therapy, with wedge resection of lung metastatic lesions remaining a vital surgical option for eligible patients[3]. Nonetheless, despite the high prevalence of lung metastases from CRC, instances of colon cancer lung metastasis coexisting with primary peripheral lung adenocarcinoma are exceptionally rare. Periportal adenocarcinoma, which is a rare type of lung adenocarcinoma, is typically characterized by an isolated nodule or mass and exhibits distinct clinical and pathological features. Moreover, the risk of metastasis and recurrence in primary peripheral lung adenocarcinoma is relatively positive compared with the risk of recurrence in other lung adenocarcinoma subtypes[4]. This case report delineates the diagnostic and therapeutic journey of a patient with lung metastasis from rectal cancer concurrent with primary peripheral lung adenocarcinoma. Multidisciplinary collaboration, tailored treatment strategies, and comprehensive patient rehabilitation guidance are crucial for improving overall treatment outcomes, as is emphasized in this report. The objective of this case report is to alleviate the diagnostic and therapeutic obstacles and risks associated with similar situations.
A 52-year-old woman presented to our hospital with an increased frequency of bowel movements and blood in the stool with no apparent cause.
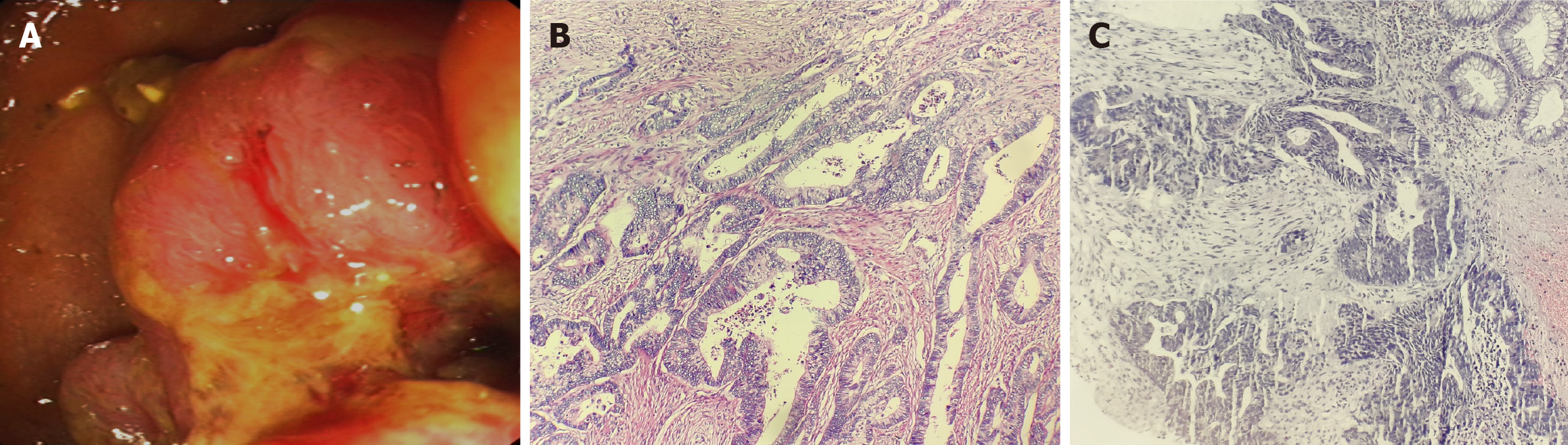
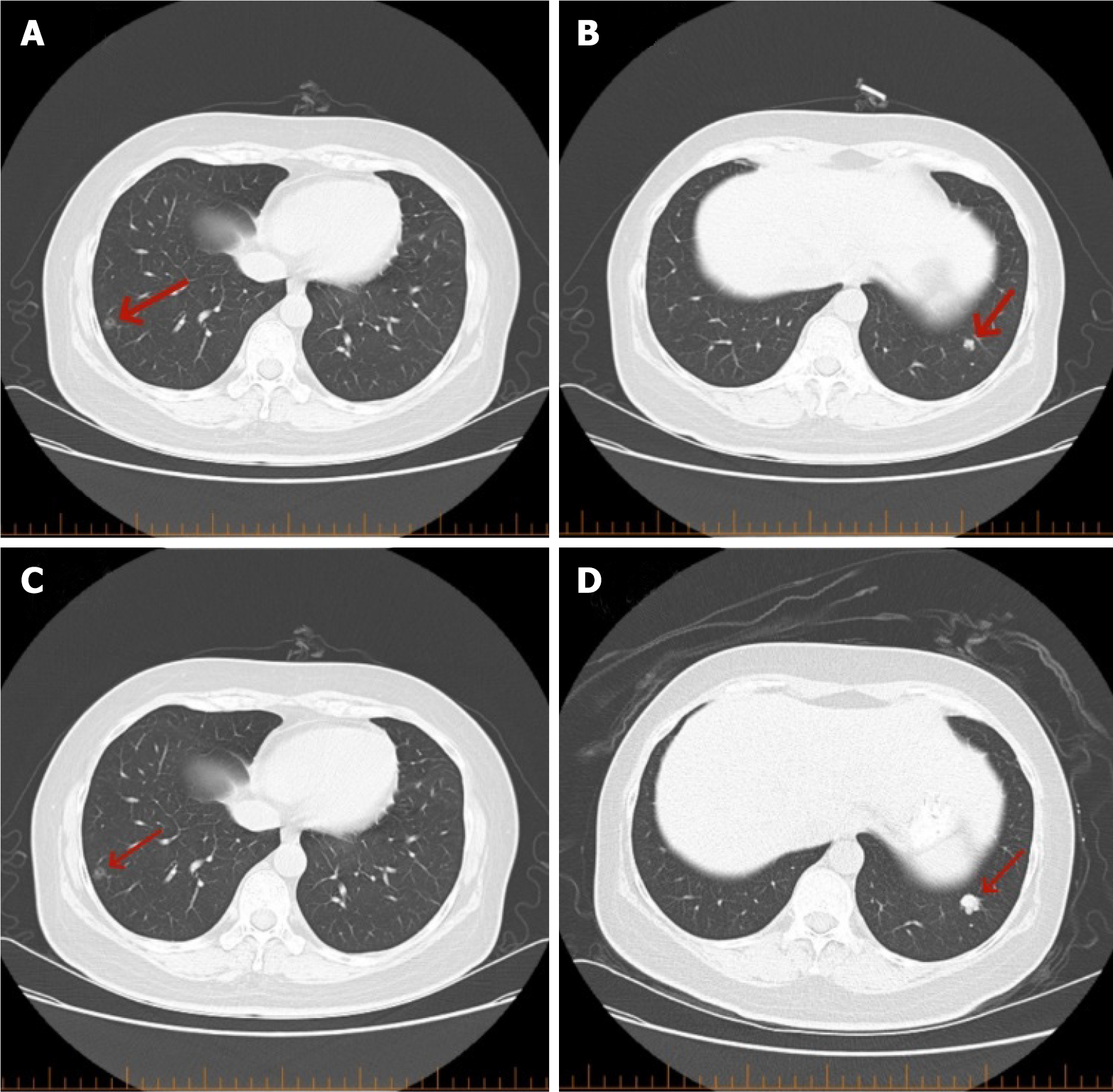
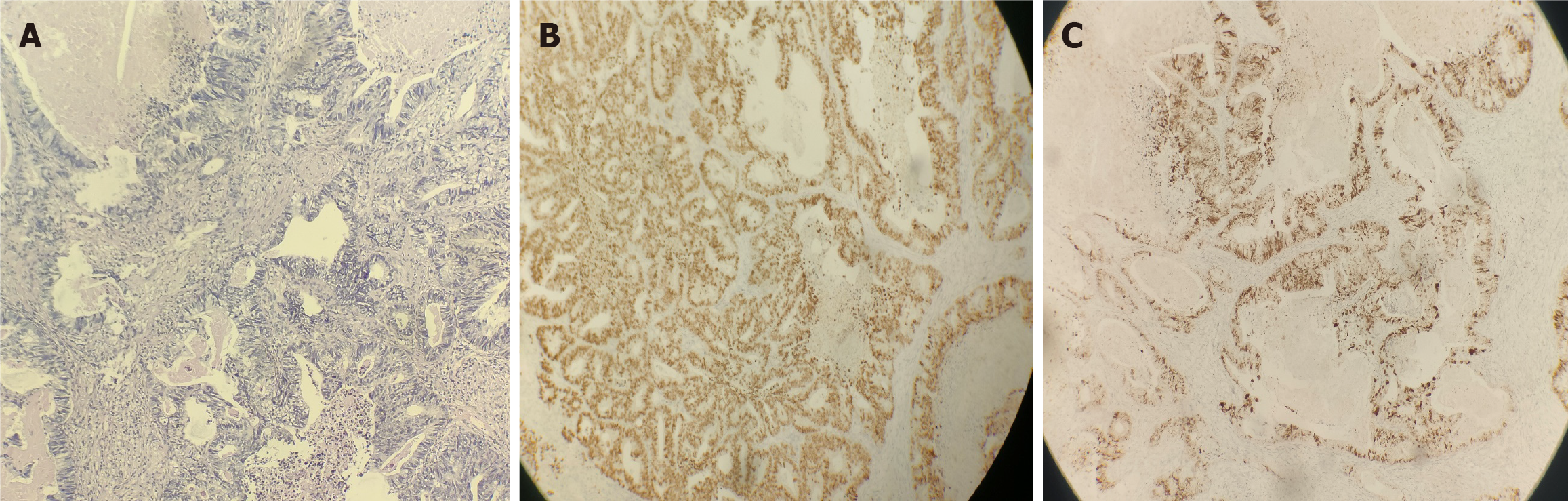
Patient data for rectal cancer: A 52-year-old female patient with no obvious cause of bowel changes and a positive fecal occult blood test was found to have a rectal tumor via colonoscopy (Figure 1A), and a biopsy revealed moderately differentiated adenocarcinoma of the rectal mucosa (Figure 1B). This patient underwent anterior resection of the rectum-rectosigmoid anastomosis on 07/12/2021, and postoperative pathology revealed an ulcerated type of moderately differentiated tubulointerstitial gland of the rectal mucosa (Figure 1C), with cancerous tissues invading the entire intestinal wall (pT3N2). Genetic testing demonstrated the KRAS G13D mutation, the APC R1273 mutation, and the APC R1399fs mutation. Before surgery, the patient underwent a chest computed tomography (CT) examination on July 10, 2021, which showed that the right lung had ground-glass nodular shadows. The larger shadow was located in the outer basal segment of the lower lobe of the right lung, with a diameter of approximately 0.8 cm (Figure 2A), and a nodular shadow with a diameter of approximately 0.6 cm was observed in the outer basal segment of the lower lobe of the left lung (Figure 2B). Postoperatively, the patient underwent a PET-CT scan, which revealed a soft tissue nodule in the lower lobe of the left lung with increased FDG metabolism. No FDG metabolism was observed in the remaining multiple ground-glass opacities in either lung. The clinician considered the nodule in the left lung as a metastatic focus of intestinal cancer and the ground-glass shadow on the right lung as a primary focus of lung cancer and advised the patient to follow up closely. The patient's lung metastasis was identified as advanced rectal cancer, and the clinician discussed that systemic treatment should be the mainstay of treatment. From August 2021 to January 2022, the patient received 7 cycles of adjuvant chemotherapy; after chemotherapy, a chest CT was performed on January 25, 2022, which revealed small nodules in the left lower lobe of the lungs and ground-glass nodules in the right lungs (the right lungs showed multiple ground-glass nodules, with the larger nodule located in the outer basal segment of the right lungs, with a diameter of approximately 0.8 cm). The larger segment was located in the exobasal segment of the lower lobe of the right lung, with a diameter of approximately 0.8 cm, and speckled low-density shadows were observed (Figure 2C). A nodular shadow with a diameter of approximately 1.2 cm was observed in the exobasal segment of the lower lobe of the left lung (Figure 2D), which was significantly larger than that in the CT image (from November 22, 2021), and was diagnosed as a metastasis in the left lung. The patient underwent thoracoscopic wedge resection of the left lung lobe with lymph node dissection of resection groups 7, 8, and 11 and was administered chemotherapy with an irinotecan + calcium folinic acid + 5-fluorouracil regimen for 4 cycles from March to May 2022 after the operation. Postoperative pathology revealed that adenocarcinoma was present in the lower lobe of the left lung (Figure 3A), and the presence of intestinal adenocarcinoma was considered in conjunction with the clinical history, as well as the following morphology and immunohistochemistry results: CK20 (3+) (Figure 3B) and CDX2 (3+) (Figure 3C).
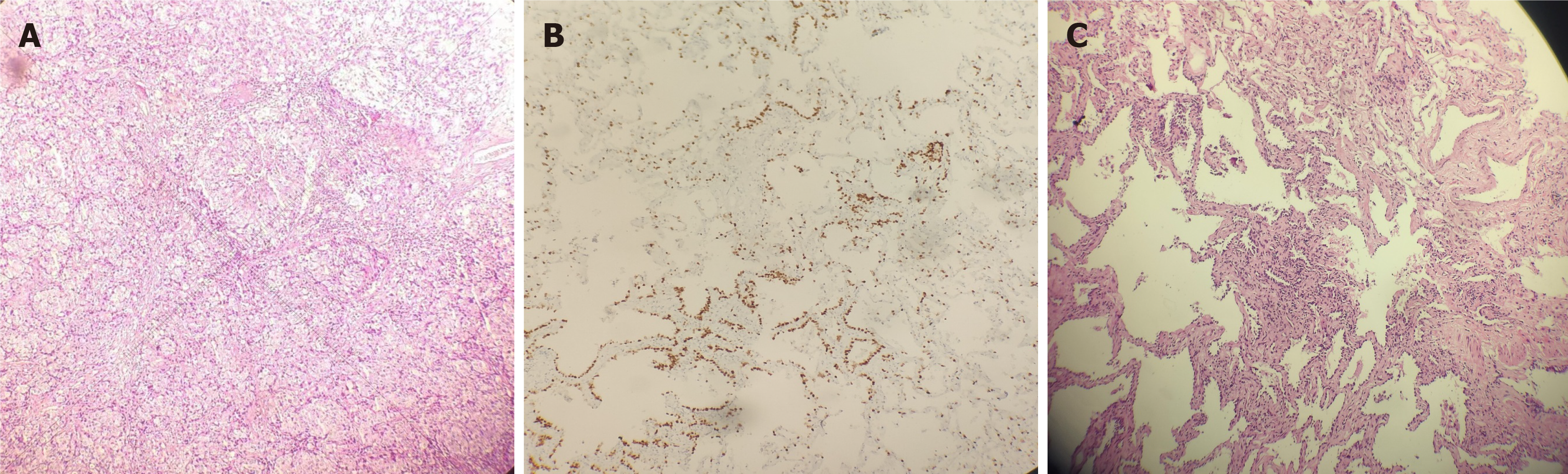
Patient data for peripheral lung adenocarcinoma: Two years after rectal cancer surgery, the patient was followed up and admitted to the hospital for review of the right lung-occupying lesion on chest CT, which exhibited no obvious change compared with the previous lesion. No new lesion had appeared, and the clinician considered it to be an early lung primary cancer. The patient strongly requested surgical treatment. The upper and lower lobes of the right lung were localized via CT-guided resection, followed by clearance of the lymph nodes from groups 2, 4, 7, 10, and 11. Intraoperative pathology revealed that the nodules in the upper and lower lobes of the right lung were microinvasive adenocarcinomas (Figure 4A and B), and immunohistochemical staining showed tumor cells with characteristics including thyroid transcription factor 1 (TTF-1): (3+) (Figure 4C); thus, the patient was diagnosed with peripheral adenocarcinoma of the right lung.
The patient was diagnosed with rectal cancer with lung metastases, and the patient was also diagnosed with peripheral lung adenocarcinoma.
Partial thyroidectomy for thyroid adenoma and thyroid nodules was performed in 2017.
The patient denied a family history of similar diseases or conditions.
On physical examination, all vital parameters were within the normal ranges, and the results of cardiopulmonary auscultation were normal.
The results of a fecal occult blood test were as follows: Carcinoembryonic antigen 2.12 ng/mL; glycoconjugate antigen 125 13.50 U/mL; and glycoconjugate antigen 19-9 8.35 U/mL, all of which were within normal limits. Immunohistochemical staining revealed the following tumor cells: TTF-1: (3+) (Figure 4C); therefore, the patient was diagnosed with peripheral adenocarcinoma of the right lung.
Imaging studies were performed. A ground-glass nodule (red arrow) was visible in the outer basal segment of the right lower lobe on the CT scan taken on July 10, 2021 (Figure 1A). A nodule (red arrow) was observed in the outer basal segment of the left lower lobe (Figure 1B). A ground-glass nodule (red arrow) was visible in the outer basal segment of the right lower lobe on the CT scan taken on January 25, 2022 (Figure 1C). A nodule (red arrow) was observed in the outer basal segment of the left lower lobe on the CT scan taken on January 25, 2022 (Figure 1D).
The patient was finally diagnosed with left lung metastasis from tubular adenocarcinoma of the rectal mucosal ulcer type (T3N2M1), which was a primary peripheral adenocarcinoma of the right lung.
The patient underwent anterior rectal resection-rectosigmoid anastomosis on December 7, 2021. The patient underwent thoracoscopic wedge resection of the left lung lobe with lymph node dissection in groups 7, 8, and 11 and was administered chemotherapy with an irinotecan + calcium folinic acid + 5-fluorouracil regimen for 4 cycles from March to May 2022 after the operation. The upper and lower lobes of the right lung were localized by CT-guided resection, followed by clearance of the lymph nodes from groups 2, 4, 7, 10, and 11.
The patient's condition remained stable after surgery, and the patient adhered to planned rehabilitation without signs of recurrence. Regular follow-up assessments revealed that all of the clinical indicators remained within normal limits, with no signs of tumor recurrence or metastasis and a good prognosis.
The increased use of chest CT scanning in recent years has led to an increase in the detection of lung metastases in CRC patients, with these cases now accounting for 32.9% of all metastatic CRC cases[5]. The timing of the appearance of lung metastases relative to the primary tumor is used to categorize synchronous and metachronous types of lung metastases. Synchronous lung metastases are found during the diagnostic examination of the primary colon tumor, whereas metachronous metastases are discovered at later times[3]. In particular, rectal cancer is associated with a greater risk of both synchronous [OR: 2.80 (1.65-4.76)] and metachronous [OR: 2.63 (1.69-4.08)] lung metastases than colon cancer[6]. Synchronized metastases are found in approximately 20% to 30% of newly diagnosed rectal cancer patients[7]. Accurate pathological diagnosis and therapeutic regimen development are challenging when rectal cancer lung metastases coexist with primary peripheral adenocarcinoma of the lung. This case report aims to provide a detailed analysis of the pathological features of this unique case, the complexities in diagnosis, and the selection of treatment strategies, thus offering a valuable reference for the clinical management of such complex cases.
In the lungs, there are often multiple nodules or masses of adenocarcinomas, with adenocarcinomas that are moderate- to well-differentiated and have relatively large glandular cavities and abundant epithelial cells[3]. The lung periphery is where the primary peripheral adenocarcinoma of the lung is typically located, and it typically consists of round or oval masses that have clear boundaries and distinct demarcations from adjacent lung tissues[8]. Their growth pattern is mainly acinar with smaller lumens, and they possess cells in the epithelium with the shape of hobnail-like epithelial cells[3]. In this case, the concurrent presence of both pathological types complicated the pathological diagnosis, thus necessitating a comprehensive approach using histology, immunohistochemistry, and molecular genetics to ensure diagnostic accuracy. In this case, positive immunohistochemical staining for TTF-1, which is a transcription factor predominantly expressed in thyroid and lung epithelial cells, was instrumental in diagnosing primary lung cancer[9]. TTF-1 expression in lung adenocarcinoma occurs in the nuclei of approximately 60%-75% of cases, in contrast with its rare expression in CRC[10]. This distinction facilitated the diagnosis of lung adenocarcinoma in this patient.
The patient's overall condition, tumor stage, and prognosis, which involved two distinct pathological types, must be taken into account when selecting a treatment in this case. Surgical intervention is not typically the first course of action for lung metastasis caused by rectal cancer, with chemotherapy, radiotherapy, and targeted therapy being more commonly used[11]. Conversely, for primary peripheral adenocarcinoma of the lung, surgery is usually the preferred option, with the specific surgical technique being tailored to the tumor's size and location and the patient's lung function[12]. In this case, we opted for laparoscopic wedge resection of the left lung lobe accompanied by postoperative adjuvant chemotherapy for metastatic cancer and CT-guided wedge resection for the primary adenocarcinoma localized in the upper and lower lobes of the right lung. The patient's unique circumstances were meticulously addressed via this approach, thus leading to favorable therapeutic outcomes.
Clinical practitioners will be provided with valuable insights and a reference point when confronting similar complex conditions when the dissemination of this case report is complete. Vigilant lung monitoring is crucial in the diagnosis and treatment of rectal cancer patients, thus allowing for early detection and intervention of lung lesions that can improve patient prognosis. In the future, we are committed to the ongoing monitoring of the patient's condition and adjusting treatment plans as necessary. Additionally, we aim to conduct in-depth research on the pathogenesis, diagnostic strategies, and treatment of rectal cancer lung metastasis, as well as primary adenocarcinoma of the lung. Our goal is to provide more effective treatments for patients.
| 1. | Shen M, Xie S, Rowicki M, Michel S, Wei Y, Hang X, Wan L, Lu X, Yuan M, Jin JF, Jaschinski F, Zhou T, Klar R, Kang Y. Therapeutic Targeting of Metadherin Suppresses Colorectal and Lung Cancer Progression and Metastasis. Cancer Res. 2021;81:1014-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2348] [Article Influence: 195.7] [Reference Citation Analysis (1)] |
| 3. | Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, Ba Y, Bai C, Bai L, Bai W, Bi F, Cai K, Cai M, Cai S, Chen G, Chen K, Chen L, Chen P, Chi P, Dai G, Deng Y, Ding K, Fan Q, Fang W, Fang X, Feng F, Fu C, Fu Q, Gu Y, He Y, Jia B, Jiang K, Lai M, Lan P, Li E, Li D, Li J, Li L, Li M, Li S, Li Y, Li Y, Li Z, Liang X, Liang Z, Lin F, Lin G, Liu H, Liu J, Liu T, Liu Y, Pan H, Pan Z, Pei H, Qiu M, Qu X, Ren L, Shen Z, Sheng W, Song C, Song L, Sun J, Sun L, Sun Y, Tang Y, Tao M, Wang C, Wang H, Wang J, Wang S, Wang X, Wang X, Wang Z, Wu A, Wu N, Xia L, Xiao Y, Xing B, Xiong B, Xu J, Xu J, Xu N, Xu R, Xu Z, Yang Y, Yao H, Ye Y, Yu Y, Yu Y, Yue J, Zhang J, Zhang J, Zhang S, Zhang W, Zhang Y, Zhang Z, Zhang Z, Zhao L, Zhao R, Zhou F, Zhou J, Jin J, Gu J, Shen L. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Jing L, Wang G. Risk factors for lymph node metastasis and surgical methods in patients with early-stage peripheral lung adenocarcinoma presenting as ground glass opacity. J Cardiothorac Surg. 2020;15:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Li Y, Zhou Z, Liu D, Zhou M, Tan F, Liu W, Zhu H. Predictive and Prognostic Factors of Synchronous Colorectal Lung-Limited Metastasis. Gastroenterol Res Pract. 2020;2020:6131485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 7. | Butte JM, Gonen M, Ding P, Goodman KA, Allen PJ, Nash GM, Guillem J, Paty PB, Saltz LB, Kemeny NE, Dematteo RP, Fong Y, Jarnagin WR, Weiser MR, D'Angelica MI. Patterns of failure in patients with early onset (synchronous) resectable liver metastases from rectal cancer. Cancer. 2012;118:5414-5423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Li Q, He XQ, Fan X, Luo TY, Huo JW, Huang XT. Computed Tomography Morphological Classification of Lung Adenocarcinoma and Its Correlation with Epidermal Growth Factor Receptor Mutation Status: A Report of 1075 Cases. Int J Gen Med. 2021;14:3687-3698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Guan L, Zhao X, Tang L, Chen J, Zhao J, Guo M, Chen C, Zhou Y, Xu L. Thyroid Transcription Factor-1: Structure, Expression, Function and Its Relationship with Disease. Biomed Res Int. 2021;2021:9957209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Schallenberg S, Dernbach G, Dragomir MP, Schlachtenberger G, Boschung K, Friedrich C, Standvoss K, Ruff L, Anders P, Grohé C, Randerath W, Merkelbach-Bruse S, Quaas A, Heldwein M, Keilholz U, Hekmat JK, Rückert C, Büttner R, Horst D, Klauschen F, Frost N. TTF-1 status in early-stage lung adenocarcinoma is an independent predictor of relapse and survival superior to tumor grading. Eur J Cancer. 2024;197:113474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Ganesh K, Wu C, O'Rourke KP, Szeglin BC, Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, Shady M, Sanchez-Vega F, Karthaus WR, Won HH, Choi SH, Pelossof R, Barlas A, Ntiamoah P, Pappou E, Elghouayel A, Strong JS, Chen CT, Harris JW, Weiser MR, Nash GM, Guillem JG, Wei IH, Kolesnick RN, Veeraraghavan H, Ortiz EJ, Petkovska I, Cercek A, Manova-Todorova KO, Saltz LB, Lavery JA, DeMatteo RP, Massagué J, Paty PB, Yaeger R, Chen X, Patil S, Clevers H, Berger MF, Lowe SW, Shia J, Romesser PB, Dow LE, Garcia-Aguilar J, Sawyers CL, Smith JJ. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 12. | Yin P, Yue B, Zhang J, Liu D, Bai D, Zhao G, Huang C, Geng G, Jiang J, Su Y, Yu X, Chen J. Optimal margins for early stage peripheral lung adenocarcinoma resection. BMC Cancer. 2021;21:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/