INTRODUCTION
The incidence of pancreatic cancer (PC) has more than doubled worldwide, and in terms of cancer-related mortality, it is currently ranked fourth in the United States[1]. The PC related death rate is expected to increase, and pancreatic ductal adenocarcinoma (PDAC) is projected to become the second leading cause of cancer-related death by 2030 in the United States[2], while according to the prediction by Ferlay et al[3], PC will cause 111500 deaths in Europe by 2025, which is approximately 50% more than the number of recorded deaths attributed to the same disease in 2010. Moreover, with a 5-year survival rate of roughly 7.1% and 10% in the United States and other Western countries, PC has the poorest prognosis[1,3]. Early diagnosis and the consequent resectability are crucial for improving the dismal prognosis of PDAC, as the majority of cases have an extremely poor prognosis[4]. However, early detection of PC is very challenging to accomplish[5]. The last few years have seen a significant increase in our understanding of its precursor lesions; familial PC (FPC) registries, especially in Western countries, revealed that a large number of individuals had several, microscopic pancreatic intraepithelial neoplasms (PanINs) surrounding the tumor with different KRAS mutations, resembling the colorectal polyposis observed in familial adenomatous polyposis (FAP) patients[6,7]. Additionally, "anticipation" - as well as other inherited disorders - has been documented in FPC families which indicate a tendency for younger age and a worse prognosis if diagnosed later[6].
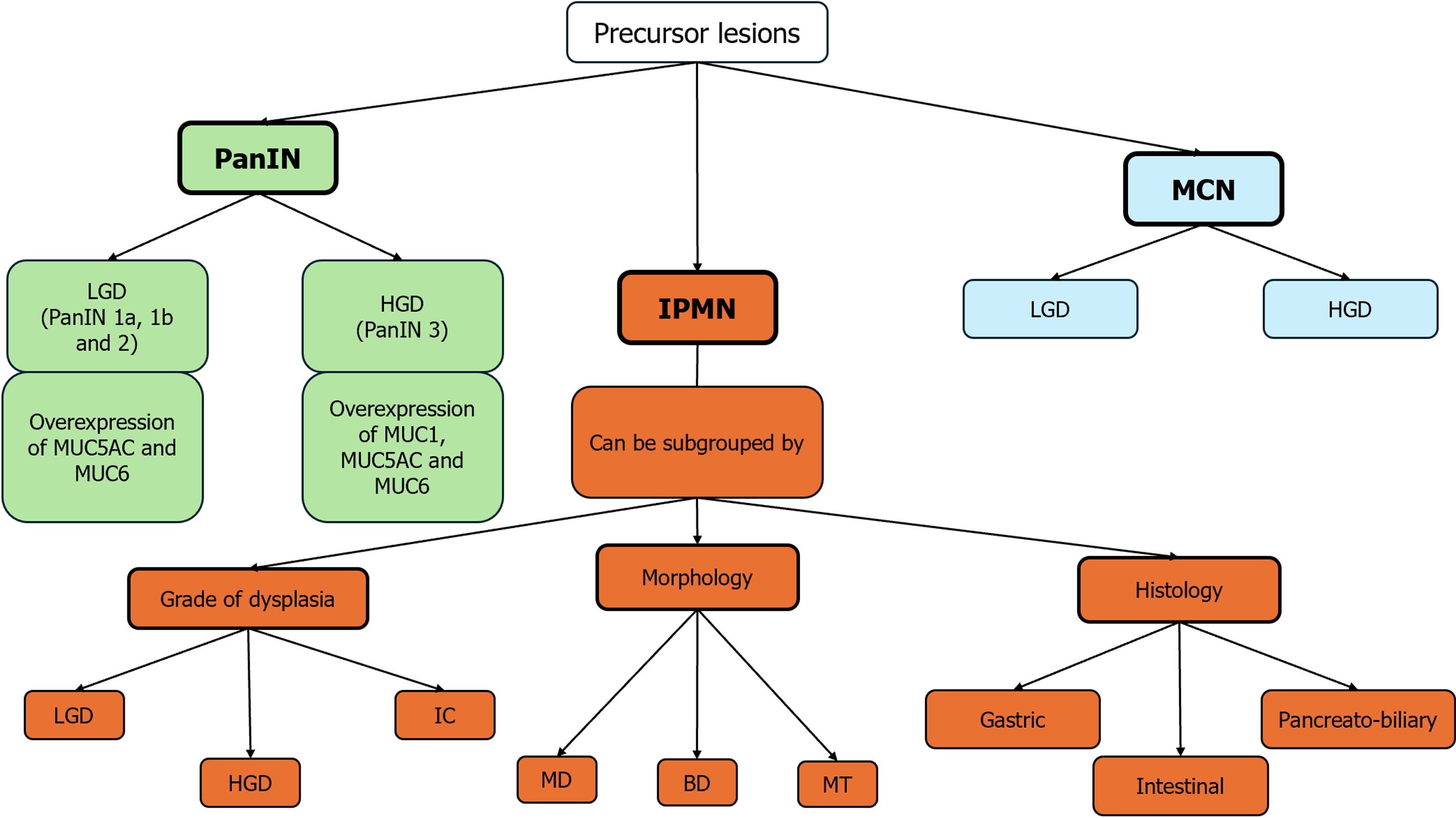
The other identified cancer precursor are mucinous pancreatic cysts that are nevertheless frequently encountered, but only few pancreatic cysts have the potential to become cancerous. The most common pancreatic cystic neoplasms include mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs) which are commonly recognized as PC precursor lesions[8,9] (Figure 1).
Figure 1 Precursor lesions of pancreatic cancer: A schematic overlook.
BD: Branch duct; HGD: High grade dysplasia; IC: Invasive cancer; IPMN: Intraductal papillary mucinous neoplasm; LGD: Low grade dysplasia; MCN: Mucinous cystic neoplasm; MD: Main duct; MT: Mixed type; PanIN: Pancreatic intraepithelial neoplasia.
According to the conventional model of pancreatic tumor carcinogenesis, a cell or group of cells presents early genetic changes, subsequently followed by clonal expansion leading to complete transformation. An alternative scenario is that early dissemination of PC cells is followed by independent transformation[10]. The most significant and well-known precursor of pancreatic adenocarcinoma is PanIN, a microscopic flat epithelial lesion, typically asymptomatic, that develops in the pancreatic ducts. Measuring less than 5 mm in size, PanIN accounts for 75%-80% of pancreatic adenocarcinomas[6,11]. PanINs are classified, according to the degree of structural and cytological atypia, into low-grade PanINs (LG-PanINs, previously PanIN 1A, PanIN 1B, and PanIN 2), which present minimal to moderate structural and cytological atypia, and high-grade PanINs (HG-PanINs), previously known as PanIN 3 or “carcinoma in situ”, which present severe structural and cytological atypia. The degree of dysplasia affects the immunohistochemical properties of PanINs. Specifically, apomucins are often overexpressed in gastrointestinal tract epithelial malignancies. MUC1, which is normally expressed by the pancreatic ducts and centroacinar cells, is responsible for the monitoring of lumen formation. It appears to be overexpressed in HG-PanINs and is frequently associated with invasive pancreatic adenocarcinoma[7]. While MUC5AC and MUC6 are not expressed by normal pancreatic ducts, they are present in LG-PanIN lesions and, like MUC1, in the majority of invasive ductal carcinomas[7,9].
Telomere shortening and gene mutations, such as KRAS, cyclin-dependent kinase inhibitor 2A, and p21WAF/CIP1 mutations, are the unique genetic alterations induced by PanIN-1s. Furthermore, a variety of downstream molecular abnormalities, including cyclin D1, COX-2, TP53, and SMAD4 mutations, are found in the intermediate- and late-stage PanINs, similar to gene mutations in the early-stage PanINs, such as KRAS mutations[12].
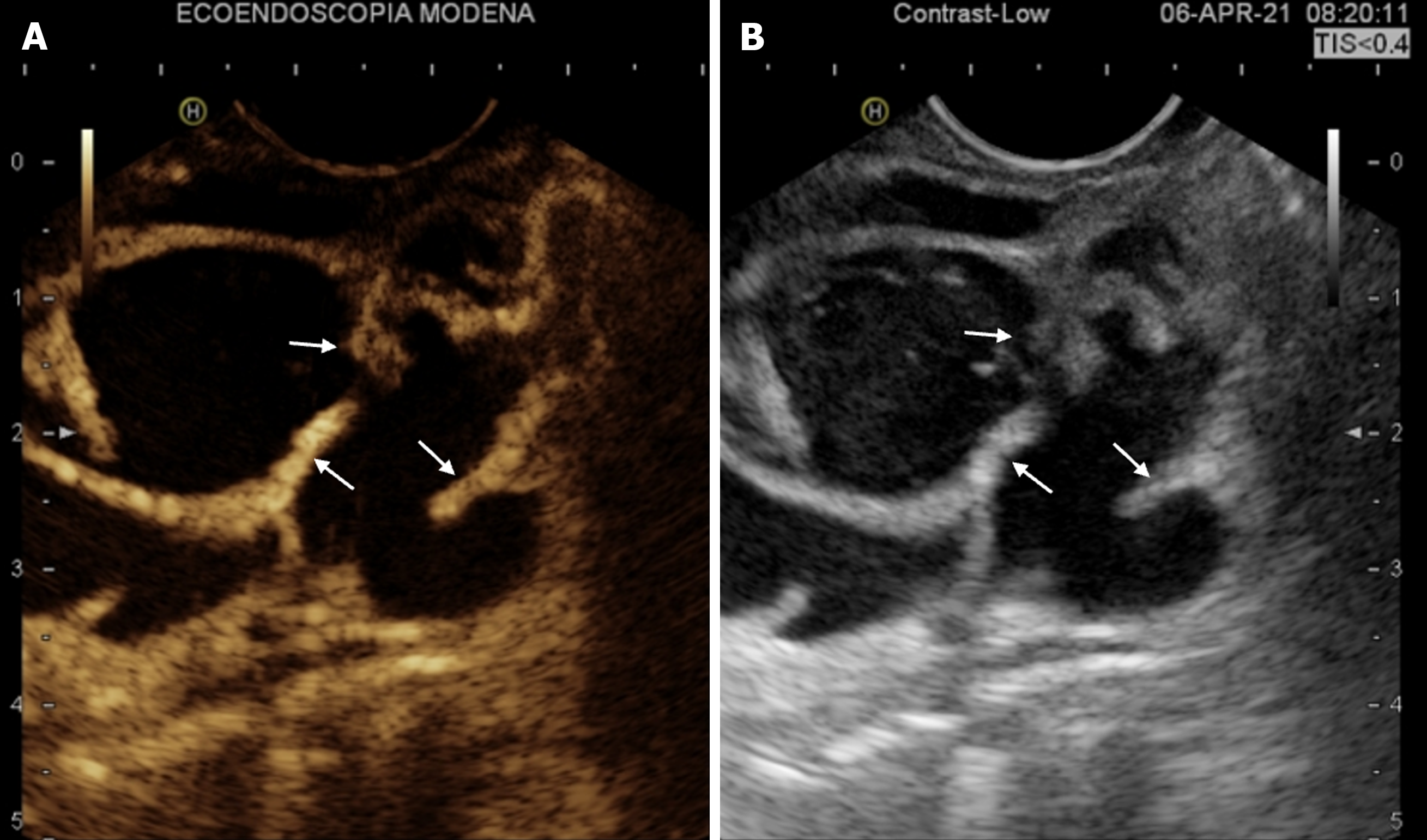
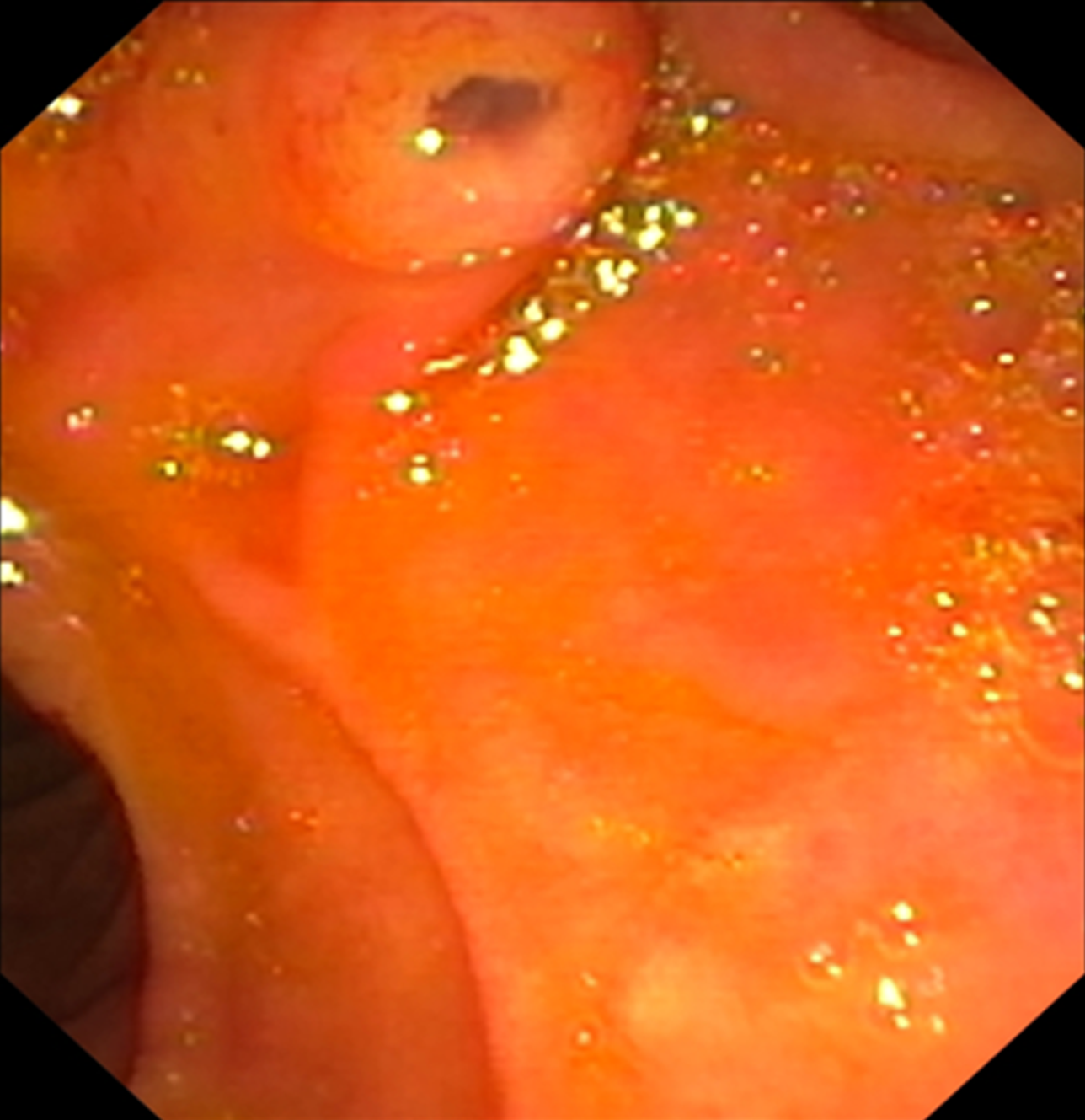
IPMNs are a type of cystic epithelial neoplasm that mostly has papillary projections and mucin production. IPMNs show different types of growth and latent cancer and are most frequently located in the head of the pancreas, either as single or multiple lesions. IPMNs are typically classified according to their morphology, which includes main duct (MD), branch duct (BD), and mixed type (MT); MD-IPMN (Figure 2) is usually defined by segmental or diffuse main pancreatic duct (MPD) dilatation greater than 5 mm in the absence of additional MPD obstruction. The “fish-eye sign” is known to be an endoscopic pathognomonic sign for IPMN diagnosis, and is characterized by a bulging, prominent ampulla extruding mucin (Figure 3)[7]; Unfortunately, this is a very rare finding, therefore not very helpful for diagnosis[8]. IPMNs can also be stratified according their histology, which includes gastric, intestinal, and pancreatobiliary. However, recently, several histologic patterns have been found in the same single cyst, supporting the theory that epithelial alterations are linked to the early development of cancer. MPD IPMN, is associated with intestinal and pancreatobiliary phenotypes and has a higher rate of malignant progression[8,13].
Figure 2 Contrast-enhanced endoscopic ultrasound study of intraductal papillary mucinous neoplasms-branch duct.
A: Contrast-enhanced-endoscopic ultrasound (EUS) of a large multilocular cyst with contrast-enhanced thickened septa (arrows); B: EUS B-mode image of the same lesion.
Figure 3 Fish-eye sign.
Endoscopic view of a bulging ampulla actively extruding mucus.
BD-IPMN originates from the smaller branches of the MD, rarely causes MPD dilation, and is frequently associated with gastric phenotype. MT-IPMN has characteristics that include both MD and BD-IPMN. IPMNs can be classified pathologically by their dysplasia grade: Invasive carcinoma (IC), high-grade dysplasia, and low-grade dysplasia[7,9]. IPMN and MCN (40%-67%) are two examples of mucinous pancreatic cystic tumors that commonly exhibit a KRAS mutation at codon 12[8,14].
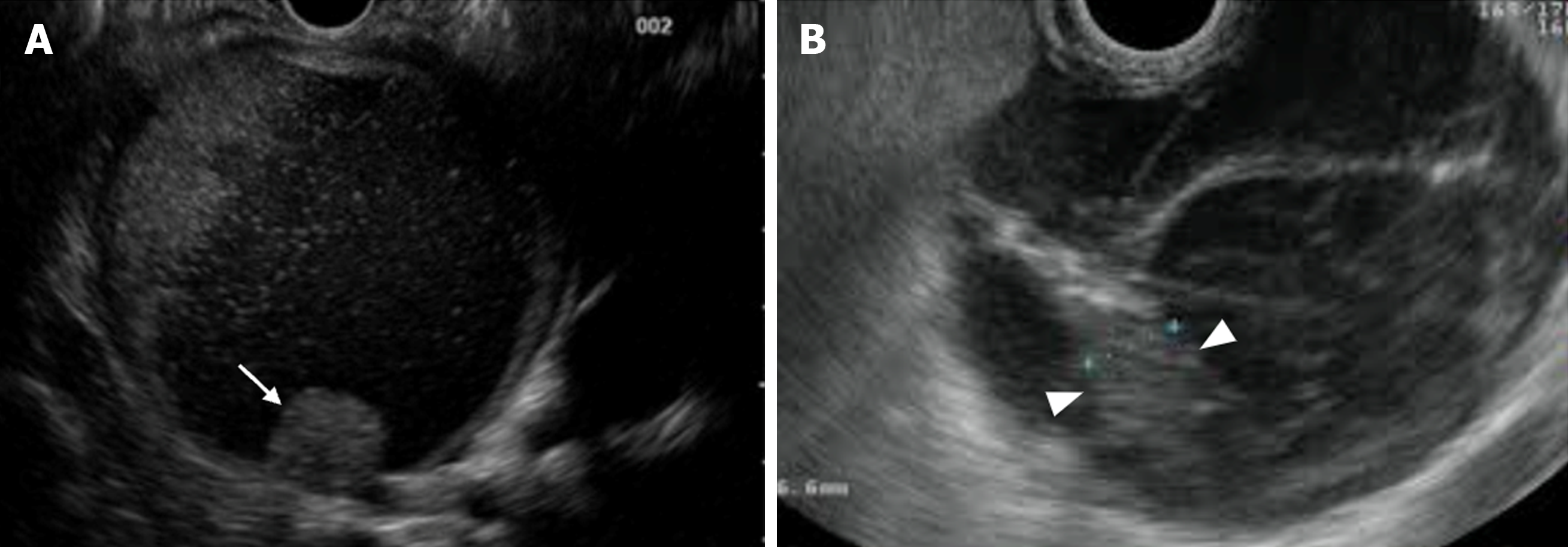
MCNs are another important precursor to PC. These are cystic neoplasms with a lining of mucin-producing columnar cells with varying degrees of dysplasia and an ovarian-type stroma beneath the epithelial layer. These lesions are more common in women in contrast to IPMNs. They usually appear as single unilocular cysts in the pancreas body or tail, and only sometimes connect to the pancreatic duct. About 25% of MCNs may have peripheral wall or septal calcifications. MCNs have a higher chance of developing cancer and approximately 15% of resected MCNs contain invasive cancer, and risk factors for malignancy include: Size > 6 cm, enhancing nodules, thick irregular walls, and peripheral calcifications (Figure 4)[7,13,15].
Figure 4 Mucinous cystic neoplasms with “worrisome” features.
A: Mural nodule (arrow); B: Thickened septum (arrow heads).
PC SCREENING: HOW AND WHEN?
Traditional cross-sectional imaging modalities are unable to diagnose many cases of early PC, especially HG-PanIN/carcinoma in situ, and these findings currently serve only as pathological diagnoses. It is possible to use “indirect findings” to detect early-stage pancreatic lesions such as pancreatic duct dilatation and/or irregular stenosis, pancreatic cysts, and focal atrophy of the pancreas[5].
In this setting, it has been reported that magnetic resonance cholangiopancreatography (MRCP) has a greater ability than computed tomography (CT) to detect pancreatic lesions[16]; furthermore, MRCP and endoscopic ultrasound (EUS) have a major role in detecting pancreatic abnormalities such as focal irregular MPD stenosis, changes in its caliber, small cystic lesions, or BD dilatation[13,15]. A recent multicenter prospective study showed that EUS and MRCP detected any pancreatic lesions (solid or cystic) in 42.6% of 216 asymptomatic subjects with high risk of PC, with an agreement between the two methods of approximately 91%. Additionally, no subjects in this study developed invasive PC during the mean follow-up period of 28.8 months. However, in three of the five asymptomatic patients, high grade neoplasia in an IPMN and/or multiple PanINs was detected and treated, in line with previous screening studies[17,18].
However, in contrast to EUS, MRCP demonstrated high sensitivity in identifying cystic lesions of any size, but had some important limitations regarding the timely detection of solid lesions[19]. As shown by Kitano et al[20], in expert hands EUS is considered the technique of choice for the study of pancreatic lesions, with a median sensitivity of 94%, even for smaller lesions[19,20].
Additionally, EUS can help to identify hypoechoic lesions surrounding MPD stenosis, which are common in some patients with HG-PanINs[21]; these lesions have been interpreted as a result of localized inflammation, fibrosis, and fatty changes due to PC in situ[22]. EUS findings (lobularity with/without honeycombing; hyperechoic foci without shadowing; dilated side branches; hyperechoic MPD margin) on the non-cancerous area could suggest chronic pancreatitis, which is a well-known risk factor for PC[23].
Moreover, the utility of EUS in the detection of early stage PC is sustained by the potential to carry out fine-needle aspiration (FNA) and fine needle biopsy, which allow for confirmation of the diagnosis, with an accuracy of 96% and a sensitivity of 90% for lesions smaller than 10 mm[22,24]. However, EUS cannot provide reliable results in cases of PC without sufficient mass lesions, including PanIn and PC in situ[11,25,26]. In these cases (stage 0 and I, without a clear mass detected by other imaging modalities), endoscopic retrograde cholangiopancreatography (ERCP) with ERCP-based pancreatic juice cytology may be helpful for pathological diagnosis. However, similar to the unsatisfactory sensitivity of brush cytology, the positive rate of single pancreatic juice cytology was also rather low[21]. It has been observed that the single-cytology sensitivity for PC can range from 33% to 76%. This sensitivity increases when secretin is administered to collect pancreatic juice[27]. According to a recent study by Ikemoto et al[28], the sensitivity of single pancreatic juice cytology was 38%, but the sensitivity of serial pancreatic juice aspiration cytology (SPACE) showed a notable improvement, rising to 75% when limited to cases with stage 0 PC[28]. Iiboshi et al[25] first introduced the endoscopic placement of a nasopancreatic drainage tube during ERCP to collect multiple cytologic samples 1-3 days after ERCP. In their study, comparing the endoscopic nasopancreatic drainage placement (ENPD) group with the single-cytology group, Iiboshi et al[25] found that SPACE had better sensitivity and accuracy, respectively, improving the former from 50% to 100% and the latter from 45.5% to 95% by placing the ENPD[25]. According to previous studies, SPACE has a sensitivity range of 30.8% to 100% and may accurately diagnose tiny PCs[29]. When the tip was positioned upstream as opposed to downstream from the stricture (87% vs 74%) and when brush cytology was carried out prior to drainage (86% vs 74%), the ENPD approach showed higher sensitivity for cancer in the pancreatic head (90%) than in the body and tail (68%)[30].
According to current Japanese guidelines for PC, in cases of MPD localized stenosis or its caliber change, together with dilatation of the BD, ERCP followed by SPACE after ENPD placement may be highly accurate in diagnosing HG-PanIN[31]. Based on variations in MRCP over time, Furuya et al[32] used continuous cytology of pancreatic juice to diagnose HG-PanIN. The authors reviewed all previous relevant reports on the same subject and found 27 cases of high-grade PanIN, which showed some recurrent features that should be considered as possibly early markers: MPD stenosis, caudal MPD dilatation, and, based on histopathological findings, fibrosis around the MPD was discovered to be the cause of the stenosis. When considering an early PC diagnosis system, this case is thought to be highly suggestive[32].
Several molecular targets, such as microRNA, methylated DNA markers, and telomerase activity, have been considered as biomarkers in pancreatic juice that can help identify PC early. Some studies have reported that abnormal expression of p53 or SMAD4 may indicate the intraductal spread of invasive PC[33,34]. However, further studies are needed to allow their possible use in clinical practice.
Per-oral pancreatoscopy (POPS) is gaining more and more consensus in the setting of indeterminate pancreatic duct strictures for the study of IPMNs and for therapeutic purposes (for example, POPS-guided lithotripsy)[8,34-36]. There are 5 types of protruding lesions in the pancreatic MD, based on how they look during pancreatoscopy: Granular mucosa (type 1), fish-egg-like protrusions without vascular images (type 2), fish-egg-like protrusions with vascular images (type 3), villous protrusions (type 4), and vegetative protrusions (type 5). Types 1 and 2 are associated with benign lesions, while types 3, 4 and 5 are associated with malignant lesions[8,13,37]. POPS also allows tissue and pancreatic juice sample collection, raising the diagnostic accuracy of the procedure[38].
In order to identify the differential application of EUS-FNA, ERCP, including SPACE, and POPS for the diagnosis of early-stage PC, we suggest that more prospective studies are necessary.
BIOMARKERS, GENE MUTATIONS AND NEW PROMISING TOOLS
Currently, there are no defined screening tests for PC in clinical settings, and there is still uncertainty over the best surveillance approach, including the best modalities to use, the frequency of surveillance, and the subgroups of high risk individuals (HRIs) to monitor. The first suggested step is the identification of HRIs, such as those with hereditary syndromes (Peutz-Jeghers syndrome, Lynch syndrome, FAP, cystic fibrosis, hereditary pancreatitis, hereditary breast and ovarian cancer), familiar PC, chronic pancreatitis, pancreatic cysts, new-onset diabetes, etc. Another step is the definition of the appropriate age to start screening HRIs: In patients with family risk, the American College of Gastroenterology guidelines[39] recommend beginning at age 50 or 10 years younger than the youngest relative with PC; at the age of 40 years in PRSS1 mutation carriers with hereditary pancreatitis and in CKDN2A mutation carriers; at the age of 35 years in patients with Peutz-Jeghers syndrome; and at 45 years in patients with ataxia telangiectasia mutated, Lynch syndrome, BRCA1, and BRCA2. As already explained, the current preferred modalities for screening are magnetic resonance imaging (MRI) and EUS, the former especially for cystic lesions and the latter for solid lesions[40]. In a recent multicentric Italian study from 2015 to 2022, Paiella et al[41] observed 156 high-risk patients for PC in a 3-year follow-up period with MRCP or EUS, finding 8 PCs (3 in stage I) and 1 PanIN3, with a resectability rate over 60%, confirming that PC surveillance is feasible and safe.
Another large cohort study at a tertiary care center conducted by Canto et al[42] showed consistent results: Over a surveillance period exceeding 16 years (from 1998 to 2014) the author followed and analyzed 354 individuals with high risk PC (for genetic factors and family history) with a median follow-up time of 5.6 years; all patients were evaluated at baseline by endoscopic endosonography and underwent surveillance with endoscopic ultrasonography, MRI and/or CT scan. Seven percent of 354 patients had neoplastic progression, and it was found that most PCs detected during surveillance were resectable (9/10) with 85% of these patients surviving for 3 years[42].
According to a recent analysis of the literature, either EUS, MRI, or both may be more cost-effective than no surveillance when compared to previously published articles evaluating the PC surveillance cost-effectiveness across hereditary or familial HRIs. There was no dominant surveillance strategy when directly comparing EUS, MRI, and other surveillance modalities. Instead, the imaging strategy of choice depended on the cost of the surveillance approach and PC risk, which differed globally[43].
The major difficulty in PC screening, as previously mentioned, is developing the ideal test for early detection and prevention, which should include sensitive and reliable markers to detect asymptomatic tumors that are otherwise clinically and radiographically undetectable. The most promising biomarkers were found in pancreatic juice (mutant TP53/SMAD4) and in blood from liquid biopsies (CA19-9, HbA1C, circulating tumor cells or DNA, exosomes) or cell-free DNA[13], but they still remain under investigation for clinical utility in specific settings, and further data are needed before implementation in routine screening. Additionally, a number of molecular genetic changes, including KRAS mutations, are present in PanIN, the most prevalent PC precursor. PC therapeutic intervention studies and reasonable early detection tactics are made possible by the availability of noninvasive molecular biomarkers for PanIN. Use of these markers can resolve the diagnostic conundrum for early detection of PanIN progression to invasive cancer and serves as a guideline for the design of novel therapeutic intervention strategies. Chemical entities and therapeutic genes such as small interfering RNA and microRNA may be employed to regulate signal pathways, including KRAS, tyrosine kinase receptors, Notch, and BRCA2, for PC therapy. In addition, targeting inhibition of cancer stem cells (CSCs) and regulation of tumor stroma are emerging promising routes[12]. Other studies regarding stool biomarkers and the potential role of microbiota are ongoing, but the knowledge in this field is too premature[44]. Furthermore, radiomics, Artificial Intelligence, and machine learning applications represent promising tools in the detection of early PC, but other studies and research in this field are mandatory[45].
CONCLUSION
The case report by Furuya et al[32] on the challenging diagnosis of early pancreatic adenocarcinoma focusing on new tools for an earlier diagnosis, prompted us to carry out a literature review on the subject. As already mentioned, most PCs are diagnosed at an advanced or even metastatic stage due to the highly aggressive characteristics and lack of typical early symptoms of PC. Thus, early diagnosis is still challenging. Many studies have shown that “indirect findings” such as a slight dilatation of the MPD, caudal PD dilation and small cystic lesions should be considered as possibly early markers. Detecting these abnormal findings in HRIs through an effective screening system including diagnostic imaging (MRI, EUS, ERCP), analysis of pancreatic juice, and application of new knowledge regarding artificial intelligence and the microbiota. Additional comprehension of the complex downstream cascades of molecular genetic changes is required, as is the creation of appropriate targeting medications and treatment plans to prevent the progression of PanINs. In conclusion, given the worldwide increasing incidence and mortality of HG-PanIN, in light of the relatively good prognosis of early stage disease, we believe further prospective studies are needed to explore new methods for the detection and diagnosis of early-stage PC. Further research should aim to define new diagnostic and therapeutic algorithms based on well-defined criteria for EUS, MRI, SPACE and POPS application as well as patient selection for screening programs and early diagnosis and application of genetic and molecular biomarkers.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade A, Grade D
Novelty: Grade A, Grade C
Creativity or Innovation: Grade A, Grade C
Scientific Significance: Grade A, Grade C
P-Reviewer: Adnyana IMDM; Gugulothu D S-Editor: Li L L-Editor: Webster JR P-Editor: Cai YX