Published online Dec 24, 2025. doi: 10.5306/wjco.v16.i12.112639
Revised: September 4, 2025
Accepted: November 14, 2025
Published online: December 24, 2025
Processing time: 144 Days and 4.3 Hours
Metabolic syndrome (MetS), characterized by central obesity, insulin resistance, dyslipidemia, and hypertension, has been increasingly recognized as a significant contributor to the development and progression of colorectal cancer (CRC). This review comprehensively summarizes current evidence linking MetS to CRC risk and outcomes from mechanistic, epidemiological, and clinical perspectives. Mechanistic studies suggest that hyperinsulinemia, activation of the insulin-like growth factor axis, chronic systemic inflammation, and adipokine dysregulation create a tumor-promoting environment. Epidemiological data from large-scale cohort studies and meta-analyses consistently demonstrate a positive association between MetS and CRC incidence, with abdominal obesity and hyperglycemia identified as key components. Mendelian randomization studies further support a causal relationship between visceral adiposity and CRC risk. Clinically, MetS is associated with increased risk of recurrence and reduced overall and disease-free survival in CRC patients. Emerging evidence also indicates that persistent meta
Core Tip: This comprehensive review highlights the emerging role of metabolic syndrome (MetS) as a critical modifiable risk factor in colorectal cancer (CRC) initiation, progression, and prognosis. It synthesizes mechanistic evidence involving insulin resistance, chronic inflammation, and adipokine dysregulation, and consolidates global epidemiological data linking MetS - especially abdominal obesity and hyperglycemia - to increased CRC incidence and mortality. The review also discusses the adverse impact of MetS on CRC outcomes, including recurrence and survival, and evaluates the potential of lifestyle and surgical interventions in mitigating these risks. This article underscores the importance of integrating metabolic health assessment into personalized CRC prevention and management strategies.
- Citation: Gao F, Jiao Y, Wang HL. Metabolic syndrome and colorectal cancer: Mechanisms, epidemiological evidence, and clinical implications. World J Clin Oncol 2025; 16(12): 112639
- URL: https://www.wjgnet.com/2218-4333/full/v16/i12/112639.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i12.112639
Colorectal cancer (CRC) remains one of the leading causes of cancer-related morbidity and mortality worldwide. The increasing prevalence of metabolic syndrome (MetS) has drawn significant attention due to its potential contribution to colorectal carcinogenesis. MetS is characterized by a combination of risk factors, including abdominal obesity, insulin resistance, hyperglycemia, hypertension, and dyslipidemia, which collectively lead to systemic inflammation and metabolic disturbances[1,2]. According to widely accepted criteria, the diagnosis of MetS is established when three or more of these five components are present. The growing global burden of both CRC and MetS highlights the urgent need to explore the intersection of these two conditions, especially given that MetS affects 25%-40% of adults in developed countries[1,2].
Epidemiological studies have increasingly pointed to MetS as a significant risk factor for CRC, with its components, especially abdominal obesity and hyperglycemia, being closely linked to CRC initiation and progression[3]. This review aims to examine the molecular mechanisms, epidemiological associations, and clinical implications of MetS in CRC, while addressing existing controversies and knowledge gaps in the current literature[1,4].
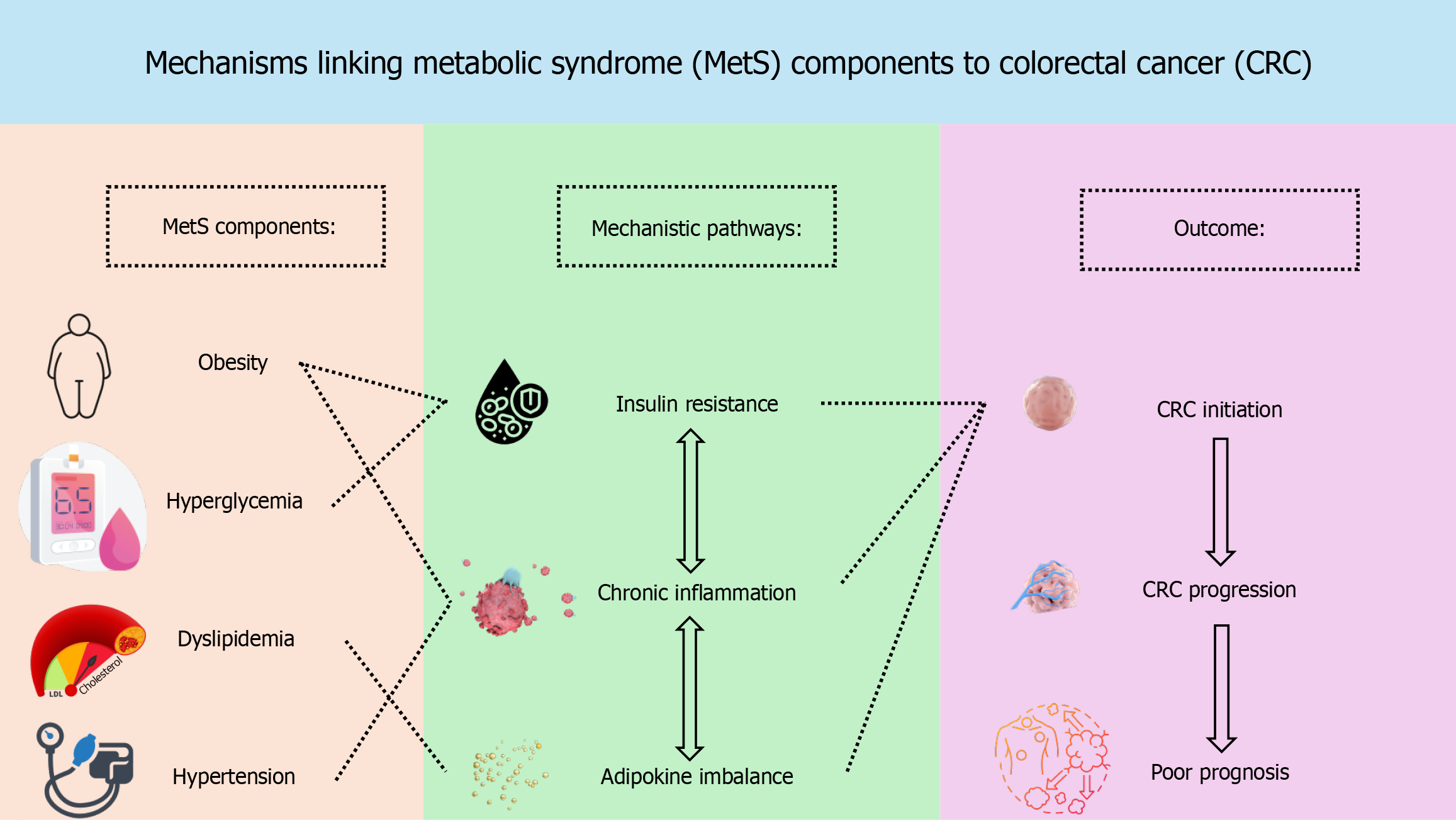
The mechanisms by which MetS contributes to CRC development are multifactorial, with insulin resistance, chronic inflammation, and altered lipid metabolism representing key mechanistic pathways, whereas abdominal obesity and hyperglycemia are the major clinical phenotypes most consistently linked to CRC risk. Insulin resistance, commonly seen in MetS, leads to elevated levels of insulin and activation of the insulin-like growth factor pathway, promoting cellular proliferation and inhibiting apoptosis[1,5]. Additionally, chronic low-grade inflammation is a hallmark of MetS and contributes to CRC through increased secretion of pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and C-reactive protein, which enhance tumor growth and survival[1,5].
Furthermore, adipokine dysregulation, particularly the imbalance between elevated leptin and reduced adiponectin levels, plays a significant role in CRC development[1,6]. Elevated leptin can stimulate cellular proliferation, angiogenesis, and pro-inflammatory signaling, while reduced adiponectin diminishes anti-inflammatory and pro-apoptotic pathways, thereby synergistically fostering a pro-tumorigenic environment. Dysregulated lipid metabolism, commonly seen in MetS, has also been shown to promote CRC by altering cell membrane dynamics and generating oxidative stress[1,6]. These mechanisms collectively create a tumor-promoting environment that enhances the risk of CRC in individuals with MetS (Figure 1).
Epidemiological data consistently show that MetS is a major risk factor for CRC (Table 1). Meta-analyses and large cohort studies have demonstrated that individuals with MetS have a 25%-70% increased risk of developing CRC compared to those without MetS, with risk rising proportionally to the number of MetS components present[7,8]. Among the components of MetS, abdominal obesity and hyperglycemia have emerged as the most influential factors in CRC risk, with several studies identifying visceral fat as a key contributor[3].
| Ref. | Year | Study design/population | MetS definition/components analyzed | Main findings on CRC risk |
| Han et al[2] | 2021 | Systematic review and meta-analysis (17 studies) | NCEP ATP III/multiple components | MetS associated with approximately 30% increased CRC incidence; abdominal obesity and hyperglycemia strongest drivers |
| Shen et al[4] | 2021 | Systematic review and meta-analysis (25 cohorts) | Mixed definitions | MetS increased CRC risk (RR approximately of 1.37); stronger effect in men; heterogeneity by region |
| Zhan et al[7] | 2024 | Meta-analysis (31 cohorts + MR) | Standardized criteria | MetS and abdominal obesity causally linked to CRC; hyperglycemia consistently associated |
| Wang et al[8] | 2024 | Prospective cohort, China (number approximately 100000) | Chinese criteria | MetS significantly increased CRC incidence; abdominal obesity main contributor |
| Yuan et al[9] | 2024 | Mendelian randomization | Genetic instruments for MetS | Causal effect of abdominal obesity on CRC risk confirmed |
| Stocks et al[13] | 2008 | Prospective cohort, Europe (number approximately 560000; men and women) | MetS components separately analyzed | Abdominal obesity and hyperglycemia increased CRC risk in both sexes; stronger in men |
| Bhome et a[14] | 2021 | Prospective cohort, United Kingdom (n = 1006 CRC patients) | Clinical diagnosis of MetS | MetS predicted higher recurrence risk, especially liver-specific recurrence |
Despite methodological variations across studies, abdominal obesity and hyperglycemia consistently emerge as the strongest predictors of CRC risk in diverse populations, including Asian, European, and United States cohorts. For example, large cohort studies from Europe and the United States have reported similar associations between abdominal obesity and CRC risk, even though the definitions of MetS components and measurement techniques varied[2]. This consistency across different populations and study designs highlights the robustness of these risk factors and underscores the need for targeted prevention strategies.
Mendelian randomization studies have provided causal evidence supporting the role of abdominal obesity in increasing CRC risk, reinforcing the need to target abdominal fat in CRC prevention strategies[9,10]. Furthermore, early-onset CRC, which is becoming increasingly prevalent, is also associated with MetS, highlighting the importance of addressing metabolic health in younger populations[11,12].
In addition to increasing CRC risk, MetS has a significant impact on CRC prognosis. Studies have shown that MetS is associated with higher recurrence rates and poorer overall survival in CRC patients, particularly those with liver-specific recurrence after surgical resection[13]. The adverse effect of MetS on prognosis is independent of traditional oncological factors such as tumor stage and grade, underscoring the importance of managing metabolic dysfunction in CRC care[3].
For instance, several studies have shown that metformin, a commonly used medication for managing hyperglycemia in diabetic patients, improves survival outcomes in CRC patients with diabetes[14]. Specifically, metformin has been associated with reduced CRC recurrence and improved overall survival in patients with coexisting diabetes, suggesting its potential as an adjunctive treatment in MetS-related CRC management. Furthermore, weight reduction through bariatric surgery has been shown to significantly reduce the incidence of CRC in patients with severe obesity. Bariatric surgery, by improving metabolic health and reducing visceral fat, has led to favorable long-term outcomes in terms of both metabolic control and cancer prevention.
Lifestyle factors, such as obesity, smoking, and sedentary lifestyle and poor dietary patterns affecting glucose metabolism, are significant contributors to survival outcomes in MetS patients with CRC, suggesting that interventions targeting MetS components could improve long-term survival[3,14]. Furthermore, the integration of inflammatory markers, such as CRP, into MetS assessment may help identify high-risk individuals for CRC recurrence and poor prognosis[15].
Among the individual components of MetS, abdominal obesity and hyperglycemia consistently show the strongest associations with CRC risk. Numerous studies have found that abdominal obesity, measured by waist circumference or waist-to-hip ratio, is a robust predictor of CRC risk, with combined obesity and hyperglycemia marking the highest risk for CRC[16-18]. In addition, evidence from European cohorts has demonstrated that these associations are not limited to Asian male populations; for example, Stocks et al[16] reported that abdominal obesity and hyperglycemia significantly increased CRC risk in both men and women, although the effect sizes varied between sexes. This indicates that while men may experience higher absolute risks, abdominal obesity and hyperglycemia are also important determinants of CRC risk in women.
Other components of MetS, such as hypertension and dyslipidemia, have also been implicated in CRC risk, although their associations are less consistent across studies[6,8]. Studies suggest that the combination of obesity and hyperglycemia with hypertension and dyslipidemia significantly amplifies CRC risk, even though the individual effects of hypertension and dyslipidemia may be weaker. These interactions highlight the complex nature of MetS and its contribution to CRC risk, suggesting that multi-component MetS profiles, rather than individual components, are more strongly associated with poor prognosis and higher CRC incidence[7,17].
The strong association between MetS and CRC highlights the need for incorporating metabolic health into CRC prevention strategies. Given the modifiable nature of MetS, interventions aimed at improving metabolic function, such as weight loss, increased physical activity, and dietary changes, are crucial in reducing CRC risk[14,19]. Pharmacological treatments, including metformin for glucose control and statins for dyslipidemia, may also contribute to reducing CRC risk and improving outcomes in MetS patients[14].
Bariatric surgery has also emerged as a promising intervention for MetS-related CRC prevention, particularly in individuals with severe obesity[20]. Given the rising incidence of both MetS and CRC, integrating metabolic health assessments into CRC screening protocols could significantly improve early detection and personalized treatment strategies[3].
Although growing evidence supports the association between MetS and CRC, important controversies and gaps remain. The lack of uniform diagnostic criteria for MetS leads to heterogeneity in prevalence estimates and complicates cross-study comparisons. Moreover, the potential influence of sex-specific and age-specific factors is insufficiently understood, as some studies suggest stronger associations in men and younger adults, yet results remain inconsistent. In addition, prospective interventional studies directly addressing whether control of MetS components reduces CRC risk or improves prognosis are scarce. Finally, the integration of metabolic and inflammatory profiling into CRC risk stratification frameworks is still in its early stages, requiring further validation.
The association between MetS and CRC underscores the need for personalized approaches to prevention and treatment. Future research should focus on metabolic profiling and biomarker discovery to better understand the unique metabolic signatures that contribute to CRC development in MetS patients. These efforts could enable the identification of novel biomarkers for early detection, risk stratification, and treatment selection in CRC patients with metabolic abnormalities.
In addition, precision prevention approaches that tailor interventions based on an individual’s metabolic profile, genetic background, and environmental factors could hold great promise in reducing CRC risk. Personalized treatment strategies, such as the use of pharmacological agents (e.g., metformin) or lifestyle interventions (e.g., bariatric surgery), could be optimized based on metabolic and genetic assessments, leading to more effective outcomes in patients with MetS.
Moreover, addressing sex-specific and age-specific mechanisms in MetS-related CRC is critical. Subgroup analyses, including those focused on early-onset CRC, could offer valuable insights into the differential impact of MetS components across different populations. Integrating multi-omics technologies, including genomics, proteomics, and metabolomics, could further refine personalized prevention and treatment strategies.
MetS is a significant and modifiable risk factor for CRC, with compelling evidence linking its components, particularly abdominal obesity and hyperglycemia, to increased CRC risk and poorer clinical outcomes. Mechanistically, MetS promotes CRC development through insulin resistance, chronic inflammation, and adipokine imbalance. Epidemiological studies consistently show a higher risk of CRC in individuals with MetS, especially those with multiple metabolic disturbances. Clinically, MetS is associated with higher CRC recurrence rates and reduced survival, underscoring the importance of managing metabolic health in CRC prevention and treatment. Lifestyle interventions and pharmacological treatments targeting MetS components may help mitigate CRC risk and improve outcomes. Future research should focus on refining MetS definitions, identifying metabolic biomarkers for CRC prediction, and evaluating the effectiveness of interventions to reduce CRC risk in MetS patients.
| 1. | Mendonça FM, de Sousa FR, Barbosa AL, Martins SC, Araújo RL, Soares R, Abreu C. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Han F, Wu G, Zhang S, Zhang J, Zhao Y, Xu J. The association of Metabolic Syndrome and its Components with the Incidence and Survival of Colorectal Cancer: A Systematic Review and Meta-analysis. Int J Biol Sci. 2021;17:487-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Zhu C, Mao C, Cai W, Zheng J, Yang H, You T, Chen J, Yu Y, Shen X, Li L. The effect of metabolic syndrome on postoperative complications and long-term survival of patients with colorectal cancer. Front Oncol. 2023;13:1036458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Shen X, Wang Y, Zhao R, Wan Q, Wu Y, Zhao L, Wu X. Metabolic syndrome and the risk of colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2215-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Colloca A, Donisi I, Anastasio C, Balestrieri ML, D'Onofrio N. Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer. Cells. 2024;13:663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Takayama T, Sato Y, Muguruma N. Role of Alcohol and Metabolic Diseases in Colorectal Carcinogenesis. In: Yoshiji H, Kaji K, editors. Alcoholic/Non-Alcoholic Digestive Diseases. Singapore: Springer, 2019: 43-52. [DOI] [Full Text] |
| 7. | Zhan ZQ, Chen YZ, Huang ZM, Luo YH, Zeng JJ, Wang Y, Tan J, Chen YX, Fang JY. Metabolic syndrome, its components, and gastrointestinal cancer risk: a meta-analysis of 31 prospective cohorts and Mendelian randomization study. J Gastroenterol Hepatol. 2024;39:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Chen R, Zhang L, Chen Y, Li J, Li S, Xu L, Hu Y, Bai Y. Association between metabolic syndrome and the risk of colorectal cancer: a prospective study in China. Eur J Cancer Prev. 2024;33:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yuan C, Shu X, Hu Z, Jie Z. Genetic prediction of the relationship between metabolic syndrome and colorectal cancer risk: a Mendelian randomization study. Diabetol Metab Syndr. 2024;16:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Kong W, Liu M, Li Q, Wang Y, Zheng Y, Zhou Y. Metabolic syndrome and risk of colorectal cancer: A Mendelian randomization study. Heliyon. 2024;10:e23872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Seki H, Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, Fujiu K, Michihata N, Jo T, Takeda N, Morita H, Yasunaga H, Komuro I. Association between metabolic syndrome and incident colorectal cancer in young adults: analysis of a nationwide epidemiological database. Eur Heart J. 2021;42:ehab724.3130. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Jin EH. Alteration of metabolic syndrome and the risk of colorectal cancer in individuals younger than 50 years. J Clin Oncol. 2024;42:81. [DOI] [Full Text] |
| 13. | Bhome R, Peppa N, Karar S, McDonnell D, Mirnezami A, Hamady Z. Metabolic syndrome is a predictor of all site and liver-specific recurrence following primary resection of colorectal cancer: Prospective cohort study of 1006 patients. Eur J Surg Oncol. 2021;47:1623-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jeon JY, Meyerhardt JA. Can we change the past for colorectal cancer patients and how do we move forward? Cancer. 2014;120:1450-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Liu T, Fan Y, Zhang Q, Wang Y, Yao N, Song M, Zhang Q, Cao L, Song C, Shi H. The combination of metabolic syndrome and inflammation increased the risk of colorectal cancer. Inflamm Res. 2022;71:899-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 16. | Stocks T, Lukanova A, Johansson M, Rinaldi S, Palmqvist R, Hallmans G, Kaaks R, Stattin P. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond). 2008;32:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Li X, Chen H, Gang W, Feng X, Lyu Z, Wei L, Wen Y, Chen S, Wu S, Dai M, Li N, He J. IDDF2019-ABS-0179 The association between components of metabolic syndrome and colorectal cancer risk in chinese males. Gut. 2019;68:A17-A18. [DOI] [Full Text] |
| 18. | Li X, Chen H, Wang G, Feng X, Lyu Z, Wei L, Wen Y, Chen S, Wu S, Hang D, Dai M, Li N, He J. Metabolic Syndrome Components and the Risk of Colorectal Cancer: A Population-Based Prospective Study in Chinese Men. Front Oncol. 2019;9:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Deng L, Liu T, Liu CA, Zhang Q, Song MM, Lin SQ, Wang YM, Zhang QS, Shi HP. The association of metabolic syndrome score trajectory patterns with risk of all cancer types. Cancer. 2024;130:2150-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Wu PN, Liu JL, Fang MJ, Fu XS, Wei JL, Wang Y, Qian HH, Zhang D. Global trends in colorectal cancer and metabolic syndrome research: a bibliometric and visualization analysis. Int J Surg. 2024;110:3723-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/