Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.111764
Revised: August 28, 2025
Accepted: November 4, 2025
Published online: November 24, 2025
Processing time: 135 Days and 22.1 Hours
Pseudoachalasia mimics primary achalasia in symptoms and diagnostic findings, as observed in gastroscopy and barium swallow studies. However, pseudoachalasia, often associated with malignancies like metastatic breast cancer, requires prompt differentiation to avoid misdiagnosis and inappropriate treatment. This report highlights a rare case of pseudoachalasia secondary to metastatic breast cancer and highlights the diagnostic value of esophageal motility changes.
A 52-year-old woman presented with a one-year history of intermittent dysphagia following breast cancer surgery. Initial examinations suggested achalasia, but the patient’s high-resolution manometry (HRM) results showed a rapid shift from ineffective esophageal motility to type II achalasia within four months. Further investigations revealed metastatic adenocarcinoma of the cardia, originating from the breast.
In patients with a history of malignancy, rapidly evolving esophageal motility abnormalities should raise suspicion of pseudoachalasia. HRM plays a crucial role in differentiating between primary and secondary achalasia. Early diagnosis through advanced imaging and pathology is essential for proper management.
Core Tip: This case report presents a rare instance of pseudoachalasia secondary to metastatic breast cancer following surgery. Notably, high-resolution manometry (HRM) detected rapid changes in esophageal motility, progressing from normal motility to type II achalasia within a brief interval. This dynamic progression highlighted the diagnostic value of HRM in distinguishing pseudoachalasia from primary achalasia. The report emphasizes the importance of considering malignancy-related secondary achalasia in patients with a cancer history and unexplained dysphagia. It advocates for early HRM evaluation and pathological confirmation to guide timely treatment.
- Citation: Pan HY, Liu W, Ding W, Wang ZM, Feng YY, Yu AH, Cheng CS. Dynamic esophageal manometry reveals pseudoachalasia secondary to metastatic breast cancer: A case report. World J Clin Oncol 2025; 16(11): 111764
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/111764.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.111764
Achalasia is a condition characterized by symptoms such as dysphagia, vomiting, and reflux[1]. The classical ‘beak sign’ is always observed during a barium meal, and the cardia appears stenotic in gastroscopy. However, the gold standard for diagnosing achalasia is the characteristic esophageal motility changes detected through high-resolution manometry (HRM). Because pseudoachalasia often presents with the same symptoms and imaging features as achalasia, 2.4%-4.0% of patients initially diagnosed with achalasia may actually have pseudoachalasia[1-3]. This condition is most commonly caused by tumors around the gastroesophageal junction (EGJ)[3]. Therefore, clinicians must remain vigilant in distinguishing between the two.
This case report presents a unique instance of pseudoachalasia resulting from metastatic breast cancer, a rare occurrence with significant diagnostic challenges. The patient's condition evolved rapidly, showing marked changes in esophageal motility within just four months. Despite initial indications of primary achalasia, further investigations revealed metastatic involvement at the cardia. The rarity of gastrointestinal metastasis from breast cancer, combined with the unusual presentation of pseudoachalasia, makes this case particularly noteworthy.
A 52-year-old woman presented with a 1-year history of intermittent dysphagia, which had progressively worsened in the past 3 months with difficulty swallowing liquids, frequent vomiting, and an 11-kg weight loss.
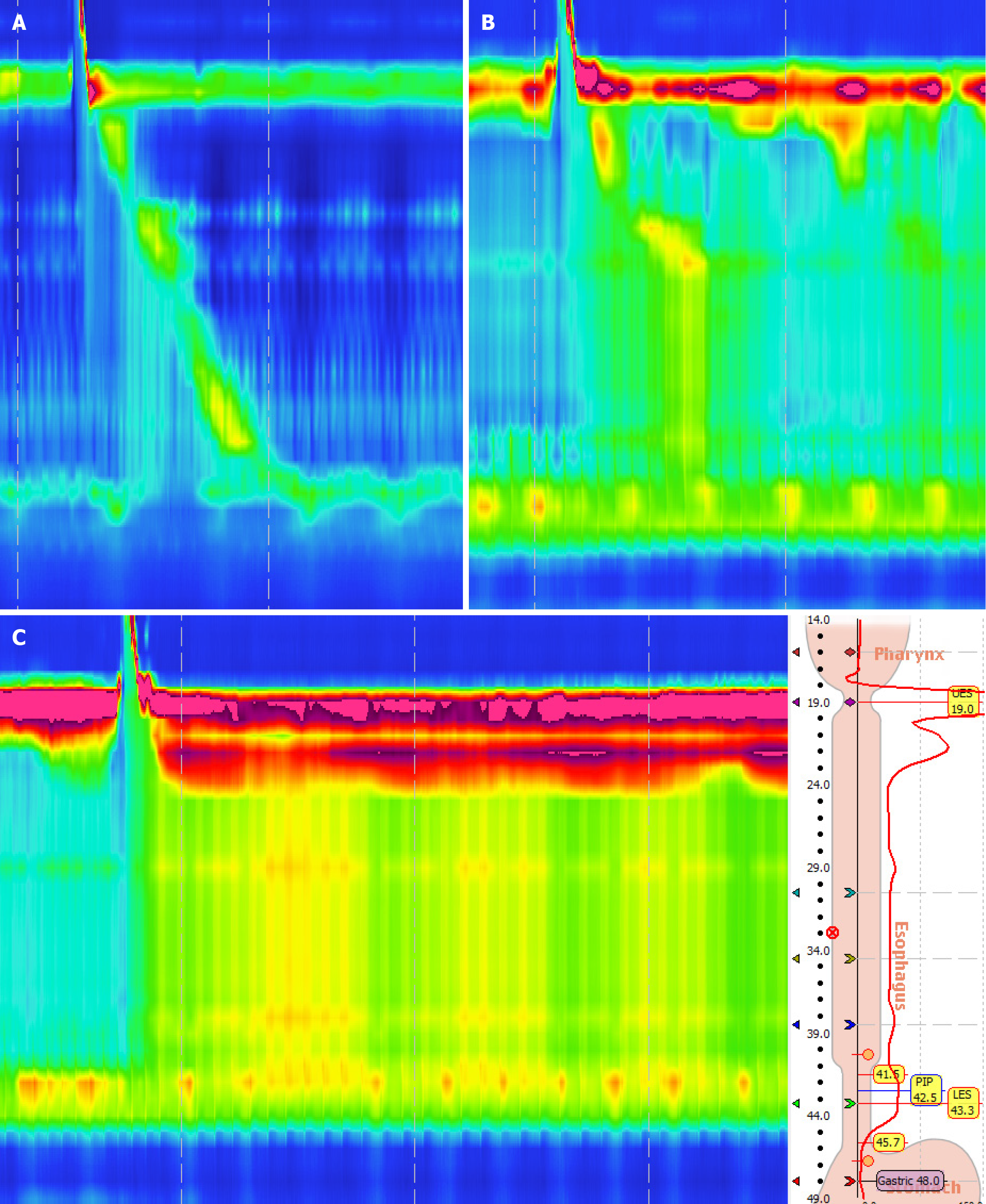
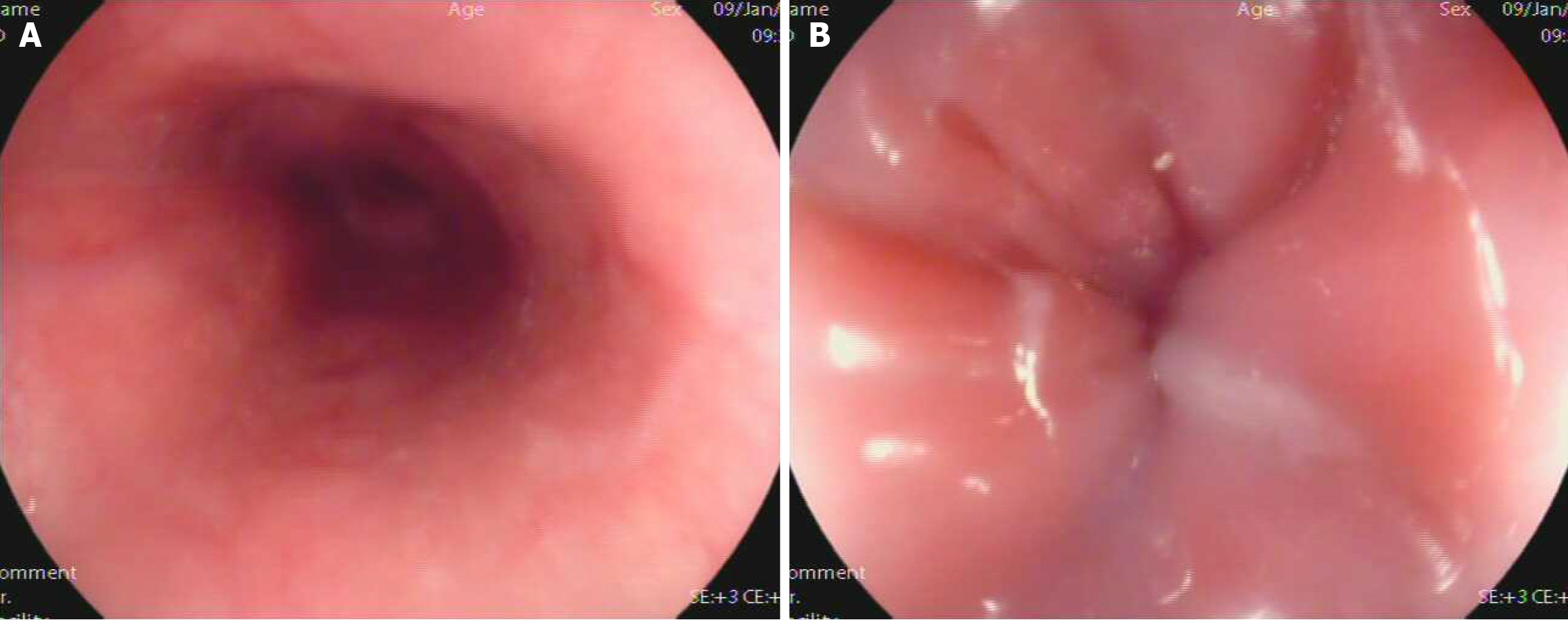
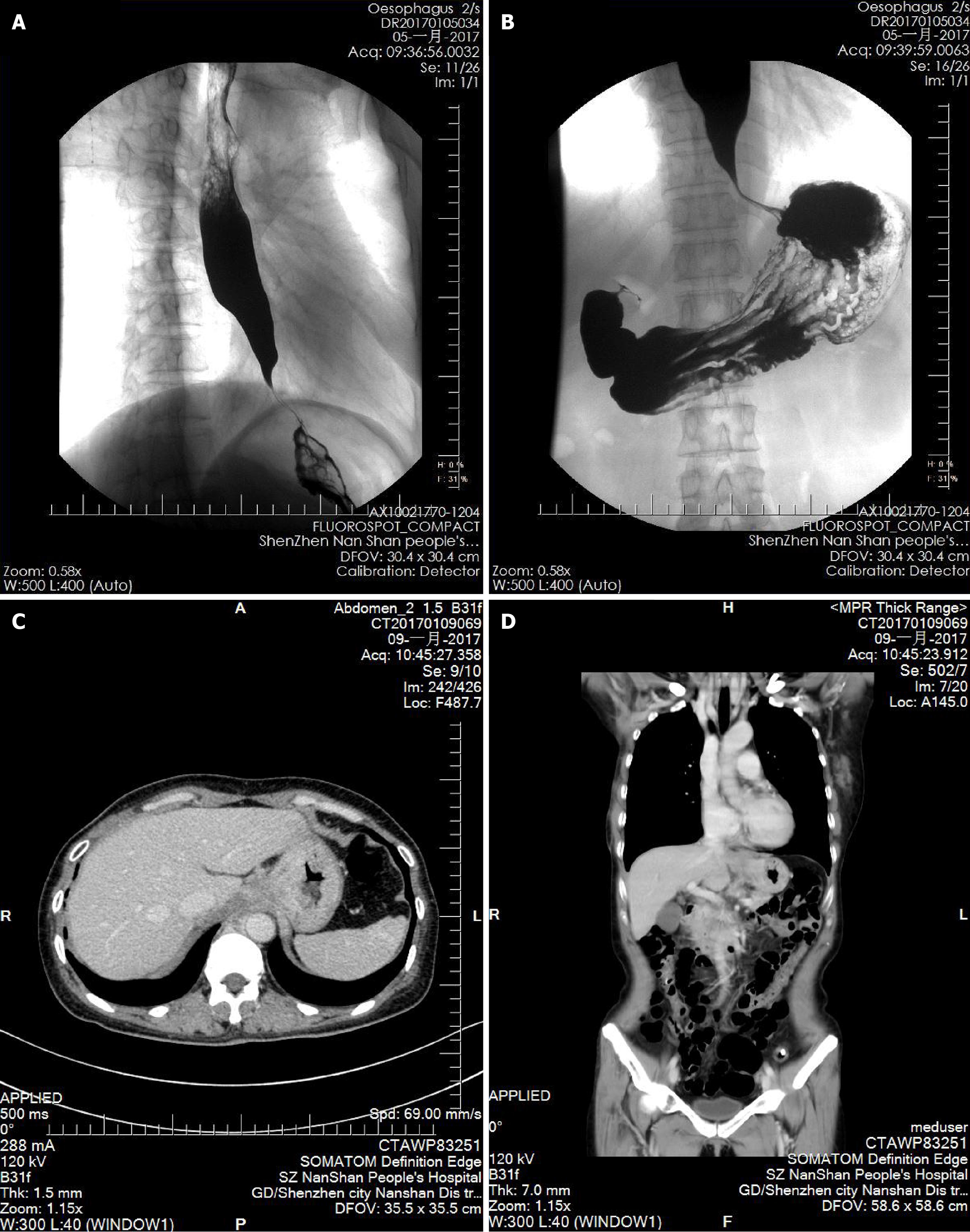
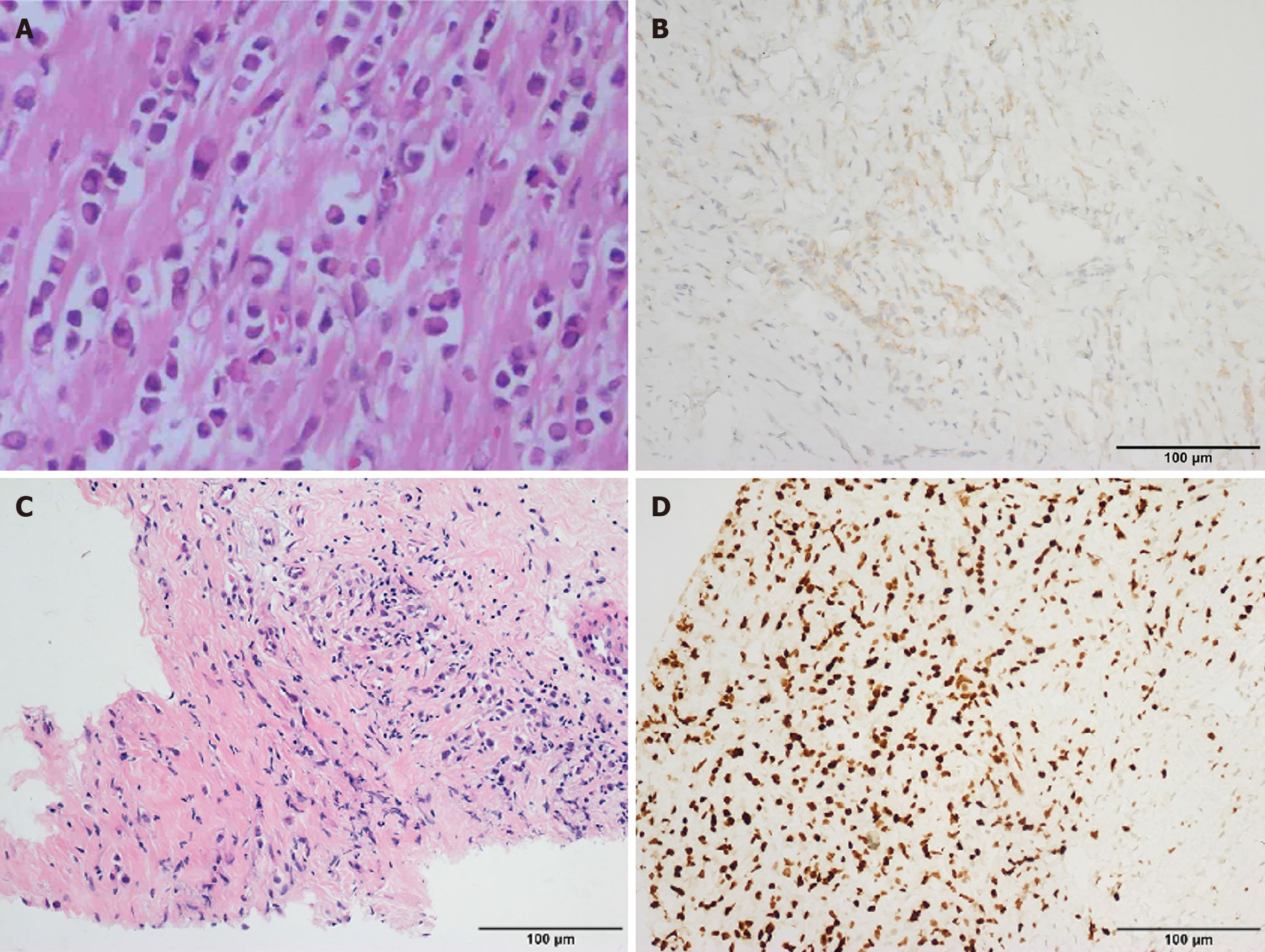
The patient first developed intermittent dysphagia in December 2015. Initial gastroscopy suggested chronic gastritis, and she was treated with omeprazole and mosapride for 1 week with limited relief. Symptoms persisted, and she gradually adapted to a liquid diet. In September 2016, a barium swallow was normal, but HRM revealed ineffective esophageal motility (IEM) (Figure 1A). Follow-up HRM in 2017 showed findings consistent with type II achalasia (Figure 1B-D). Despite mosapride, symptoms progressed to severe dysphagia with liquids and vomiting, leading to significant weight loss. Upon admission in December 2016, gastroscopy showed normal esophageal mucosa but severe stenosis of the cardia (Figure 2). A barium swallow demonstrated a typical “bird-beak sign”, suggestive of achalasia (Figure 3A and B). Given the rapid progression, pseudoachalasia was suspected. Thoracoabdominal computed tomography (CT) revealed a mass at the cardia and gastric fundus with metastatic retroperitoneal lymph nodes (Figure 3C and D). Endoscopic ultrasound-guided biopsy confirmed metastatic adenocarcinoma consistent with the patient’s primary breast carcinoma (Figure 4).
She underwent modified radical mastectomy for right breast invasive lobular carcinoma (ILC). Postoperative chemotherapy included liposomal adriamycin 32 mg + cyclophosphamide 800 mg (4 cycles), followed by paclitaxel liposomes 240 mg (4 cycles). Post-chemotherapy chest CT and breast ultrasound showed no recurrence or metastasis.
No relevant personal or family history of esophageal disease was reported. Family history of malignancy was not mentioned.
No specific abnormalities were reported in general physical examination on admission.
No remarkable abnormalities were mentioned in routine laboratory results.
Initial chest CT after breast surgery: Normal. Barium swallow (September 2016): Normal. HRM (2016): IEM. Gastroscopy (December 2016): Normal esophageal mucosa, severe cardia stenosis. Barium swallow (December 2016): Bird-beak sign suggestive of achalasia. HRM (2017): Pan-esophageal pressurization, integrated relaxation pressure (IRP) 49.6 mmHg, type II achalasia. Thoracoabdominal CT: Mass at cardia and gastric fundus with retroperitoneal lymph node metastases. Endoscopic ultrasound (EUS)-guided biopsy: Metastatic adenocarcinoma consistent with primary breast carcinoma.
The final diagnosis was pseudoachalasia secondary to metastatic invasive lobular breast carcinoma involving the EGJ and gastric cardia.
The patient had previously undergone modified radical mastectomy for breast cancer followed by adjuvant chemotherapy (liposomal adriamycin + cyclophosphamide × 4 cycles, paclitaxel liposomes × 4 cycles). After confirmation of pseudoachalasia, she was referred for further oncological management, including systemic therapy for metastatic breast carcinoma. Symptomatic management for dysphagia was also considered.
After endoscopic ultrasound-guided biopsy confirmed metastatic adenocarcinoma consistent with the primary breast carcinoma, the patient was referred to oncology for continuation of systemic treatment. At the time of reporting, long-term follow-up outcomes were not yet available.
This case report describes a rare instance of pseudoachalasia caused by metastatic breast cancer, presenting with rapidly progressive esophageal motility changes. Despite initial indications of primary achalasia, the patient's esophageal motility transitioned from IEM to type II achalasia within four months, ultimately revealing metastatic adenocarcinoma at the cardia. This case is unique due to the unusual gastrointestinal metastasis of breast cancer, which is rarely associated with pseudoachalasia. The rapid progression of symptoms and diagnostic findings underscores the importance of thorough investigation in patients with a history of malignancy.
Pseudoachalasia is commonly caused by primary or secondary neoplastic obstruction at the EGJ, paraneoplastic neurological syndromes, post-vagotomy, and post-gastric fundoplication changes[4]. Among these etiologies, the most difficult to identify are submucosal metastatic lesions at EGJ. The mechanisms of pseudoachalasia due to malignant tumors at EGJ include: (1) Tumor growth in the mediastinum or nearby cardia or gastric fundus, compressing the lower esophageal sphincter (LES); (2) Direct infiltration into the esophageal smooth muscle cells, causing impairment in smooth muscle relaxation; (3) Invasion of the myenteric plexus of the esophagus, resulting in the disappearance of smooth muscle peristalsis and LES relaxation disorder; and (4) Paraneoplastic effects, such as immune or hormonal influences, may also contribute. When tumors occur in the submucosa or outer layers, the characteristic manifestations of gastroscopy and barium swallow may mimic achalasia, often leading to misdiagnosis.
Both primary achalasia and pseudoachalasia present EGJ outflow obstruction on HRM. The 2021 updated Chicago Classification[5] of esophageal manometry also emphasizes once again that the key to distinguishing between achalasia and EGJ outlet obstruction is whether the motility of the esophageal body fully conforms to the characteristic motility manifestations of achalasia, such as aperistalsis, pan-esophageal pressurization, and premature swallowing. Therefore, whether there are normal peristaltic waves in the esophageal body is an important point of distinction between primary achalasia and pseudoachalasia. In our case, the second HRM showed a 4-s IRP median of 46.9 mmHg, with 90% of the esophageal body exhibiting pan-esophageal pressurization, which is highly consistent with manometric diagnostic criteria for achalasia. However, it is rare for HRM findings to show a transition from IEM to type II achalasia within only 4 months. Such a rapid change in esophageal motility patterns is atypical and may raise suspicion for underlying conditions such as pseudoachalasia.
Compared to patients with primary achalasia, those with pseudoachalasia tend to be older aged patients (≥ 55 years), have a shorter symptom duration (≤ 12 months), and experience greater weight loss (≥ 10 kg)[6,7]. These features are also helpful in identification.
Distant metastasis of breast cancer commonly involves local areas such as the chest wall, axillary lymph nodes, as well as the lungs and bones, with gastrointestinal metastasis being relatively rare[8]. A study by Borst et al[9], which followed a large number of breast cancer patients, found that only 0.4% had esophageal metastasis[9]. Scholars conducted an analysis of English-language case reports on esophageal metastasis from breast cancer since 1989 found that over 80% of metastases occur in the middle and lower segments of the esophagus. Moreover, the metastatic lesions tend to develop from outside the lumen inward, with mucosal metastasis in the esophagus is very rare[10]. Submucosal metastasis esophageal can present with a normal mucosal surface. It is noteworthy that although invasive ductal carcinoma is the most common histological subtype of breast cancer[11,12], ILC exhibits distinct biological characteristics and shows a higher propensity for metastasis to the gastrointestinal tract[13]. This feature may, to some extent, explain the unusual involvement of the cardia observed in our case, and it underscores the need for clinicians to maintain a high level of vigilance when ILC patients present with gastrointestinal symptoms. Therefore, conventional gastroscopy and barium swallow have significant limitations in detecting esophageal metastatic lesions from breast cancer, whereas EUS and CT provide better assessment.
We gained several practical insights: (1) Endoscopy may miss diagnoses of submucosal layer located tumor. If there is any suspicion, EUS or CT should be conducted as early as possible; (2) HRM plays a pivotal role in identifying the causes of dysphagia, especially for achalasia and the differentiation between pseudoachalasia. If the esophageal motility rapidly changes to achalasia within a short period, clinicians should consider the possibility of structural obstruction; and (3) Despite the rarity of gastrointestinal metastasis from breast cancer, it is still essential to carefully evaluate the possibility of esophageal or gastric involvement. In this case, the absence of continuous gastroscopic and imaging follow-up and assessment of the digestive tract post-surgery, which neglected the potential for cardia metastasis and resulted in a delayed diagnosis.
In conclusion, this case report highlights the diagnostic complexities of pseudoachalasia secondary to metastatic breast cancer, a rare but significant cause of esophageal motility disorders. The rapid progression from IEM to type II achalasia within four months, as demonstrated by HRM, provided a critical clue to its identification. Early differentiation between primary and secondary achalasia is essential for appropriate treatment and prognosis, particularly in patients with a history of malignancy. The case emphasizes the importance of utilizing advanced diagnostic tools, such as HRM, EUS, and CT, to detect submucosal or metastatic lesions that might be missed by conventional gastroscopy or barium swallow studies. Clinicians should remain alert for gastrointestinal metastasis breast cancer patients with rapidly evolving esophageal motility abnormalities, as early recognition can improve outcomes and guide appropriate therapeutic strategies.
| 1. | Campo SM, Zullo A, Scandavini CM, Frezza B, Cerro P, Balducci G. Pseudoachalasia: A peculiar case report and review of the literature. World J Gastrointest Endosc. 2013;5:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 2. | Liu W, Fackler W, Rice TW, Richter JE, Achkar E, Goldblum JR. The pathogenesis of pseudoachalasia: a clinicopathologic study of 13 cases of a rare entity. Am J Surg Pathol. 2002;26:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Haj Ali SN, Nguyen NQ, Abu Sneineh AT. Pseudoachalasia: a diagnostic challenge. When to consider and how to manage? Scand J Gastroenterol. 2021;56:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Zanini LYK, Herbella FAM, Velanovich V, Patti MG. Modern insights into the pathophysiology and treatment of pseudoachalasia. Langenbecks Arch Surg. 2024;409:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, Babaei A, Mittal RK, Rommel N, Savarino E, Sifrim D, Smout A, Vaezi MF, Zerbib F, Akiyama J, Bhatia S, Bor S, Carlson DA, Chen JW, Cisternas D, Cock C, Coss-Adame E, de Bortoli N, Defilippi C, Fass R, Ghoshal UC, Gonlachanvit S, Hani A, Hebbard GS, Wook Jung K, Katz P, Katzka DA, Khan A, Kohn GP, Lazarescu A, Lengliner J, Mittal SK, Omari T, Park MI, Penagini R, Pohl D, Richter JE, Serra J, Sweis R, Tack J, Tatum RP, Tutuian R, Vela MF, Wong RK, Wu JC, Xiao Y, Pandolfino JE. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0(©). Neurogastroenterol Motil. 2021;33:e14058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (1)] |
| 6. | Gergely M, Mello MD, Rengarajan A, Gyawali CP. Duration of symptoms and manometric parameters offer clues to diagnosis of pseudoachalasia. Neurogastroenterol Motil. 2021;33:e13965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Troskot Perić R, Bevanda D, Zgodić S, Paušak B, Madunić M, Hasanec M. How to Distinguish Idiopathic Achalasia from Pseudoachalasia? Psychiatr Danub. 2021;33:199-203. [PubMed] |
| 8. | Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and breast cancer risk: interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res Treat. 2015;150:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637-41; discussion 641. [PubMed] |
| 10. | Su H, Wu J, Liu H, Wei N, Lin W, Zhou Q, Wang M, Lv S, Yang Y. Review of esophageal metastasis from breast cancer. Gland Surg. 2020;9:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S, Ellis IO. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 12. | Pereira H, Pinder SE, Sibbering DM, Galea MH, Elston CW, Blamey RW, Robertson JF, Ellis IO. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology. 1995;27:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/