Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.112392
Revised: August 4, 2025
Accepted: September 26, 2025
Published online: October 24, 2025
Processing time: 90 Days and 17.3 Hours
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by excessive blood cell production, which increases the risk of thrombosis. Ropeginterferon alfa-2b (RI) offers potential advantages over standard therapy (ST; including phlebotomy, hydroxyurea, and aspirin) by achieving hematologic and molecular responses. However, its comparative efficacy and safety remain understudied. We hypothesized that RI would improve hematologic and molecular outcomes but may differ in safety profiles compared to ST.
To evaluate the efficacy and safety of RI vs ST in patients with PV, focusing on hematologic response, molecular response, adverse events (AEs), and thrombotic risk.
This Preferred Reporting Items for Systematic Reviews and Meta-Analyses-compliant meta-analysis included randomized controlled trials comparing RI to ST in adult PV patients. PubMed, EMBASE, ClinicalTrials.gov, and ScienceDirect were searched from inception to July 2025. Outcomes included complete hematological response (CHR), molecular response, AEs leading to discontinuation, JAK2V617F allele burden, thrombotic events, and phlebotomy frequency. Pooled odds ratios (ORs) and MD with 95% confidence intervals (95%CIs) were calculated using random-effects models. Risk of bias was assessed with Cochrane RoB 2; evidence certainty was evaluated via GRADE.
Five studies involving 477 RI and 456 ST patients were included. RI significantly improved CHR (OR = 2.14, 95%CI: 1.18-3.88, P = 0.002) and molecular response (OR = 4.37, 95%CI: 0.99-19.38, P = 0.05), with substantial heterogeneity (I² = 76% and I² = 93%, respectively). AEs leading to discontinuation were higher with RI (OR = 3.89, 95%CI: 1.90-7.97, P = 0.0002; I² = 0%). No significant differences were observed in JAK2V617F allele burden (MD = -7.46, 95%CI: -21.12 to 6.20, P = 0.28; I² = 90%) or thrombotic events (OR = 0.93, 95%CI: 0.45-1.90, P = 0.83; I² = 0%). RI reduced phlebotomy frequency (MD = -1.52, 95%CI: -2.37 to -0.67, P = 0.0005; I² = 0%). Most studies had low to moderate risk of bias; evidence certainty was moderate for CHR and AEs, low for molecular response and thrombotic events, and very low for allele burden.
RI offers superior hematologic and molecular responses compared to ST in PV but is associated with higher discontinuation rates due to AEs. Comparable thrombotic risk and reduced phlebotomy needs highlight its potential, though tolerability requires careful management. The high heterogeneity in certain outcomes and potential for publication bias warrant cautious interpretation of these findings. Further long-term studies are needed to optimize dosing and patient selection.
Core Tip: Ropeginterferon alfa-2b significantly enhances complete hematologic and molecular responses in polycythemia vera compared to standard therapy, reducing phlebotomy needs without increasing thrombotic risk. However, higher adverse event-related discontinuations necessitate careful patient monitoring. Despite robust study designs, heterogeneity and potential publication bias warrant cautious interpretation. Long-term studies are essential to refine dosing and improve tolerability for optimal patient outcomes.
- Citation: Tom L, Mani S, Rawat A, Nan M, Mundada SM, Al Khatib B, Gopinath A, Taj O, Salahuddin N, Shaju F, Parsa S, Abdurrahman M, Das A, Khawar MMH, Sher A. Ropeginterferon alfa-2b vs standard therapy in polycythemia vera: A meta-analysis of efficacy and safety outcomes. World J Clin Oncol 2025; 16(10): 112392
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/112392.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.112392
Polycythemia vera (PV) is one of the classic BCR-ABL1-negative myeloproliferative neoplasms characterized by clonal hematopoietic stem cell dysfunction, leading to excessive production of blood cells[1]. This disorder affects men and women equally, with a median age at diagnosis of 61-65 years, though approximately 10% of patients are diagnosed below age 40[2]. The disease presents with a spectrum of clinical manifestations ranging from asymptomatic cases to complex symptomatic presentations.
Patients with PV may initially be asymptomatic but commonly develop various symptoms related to increased blood viscosity and hypercellularity. Microvascular disturbances frequently manifest as headaches, dizziness, visual disturbances, chest pain, and palpitations[3]. A hallmark symptom is erythromelalgia, characterized by severe burning pain, erythema, and warmth in the distal extremities, particularly the hands and feet[4]. This distinctive vasomotor symptom results from increased platelet count and aggregation, leading to microscopic vascular occlusion. Aquagenic pruritus, occurring after exposure to warm water, is another characteristic symptom present in 40%-65% of patients[5]. Additional manifestations may include splenomegaly, superficial thrombophlebitis, mucocutaneous bleeding, and overt thrombotic complications.
Laboratory findings at diagnosis typically include thrombocytosis in 53% of patients and leukocytosis in 49%. Splenomegaly is observed in over 30% of cases, while some patients may present with hepatomegaly and plethora.
The International Consensus Classification 2022 and recent World Health Organization criteria establish rigorous diagnostic standards for PV[6]. The major criteria include hemoglobin levels exceeding 16.5 g/dL in men and 16.0 g/dL in women, or hematocrit levels above 49% in men and 48% in women; bone marrow biopsy demonstrating age-adjusted hypercellularity with trilineage proliferation (panmyelosis), including prominent erythroid, granulocytic, and megakaryocytic elements with pleomorphic mature megakaryocytes; and presence of JAK2V617F or JAK2 exon 12 mutations[7]. The minor criterion consists of subnormal serum erythropoietin levels. Diagnosis requires either all three major criteria or the first two major criteria plus the minor criterion.
The main goal of treatment in PV is to prevent thrombotic and hemorrhagic complications by keeping hematocrit levels under 45%[2]. All patients require therapeutic phlebotomy as first-line treatment, typically combined with low-dose aspirin (81 mg once or twice daily) unless contraindicated. For high-risk patients (age ≥ 60 years or history of thrombosis), cytoreductive therapy is recommended[8].
Hydroxyurea represents the standard first-line cytoreductive agent, typically initiated at 500 mg twice daily in high-risk patients[9]. This agent effectively controls blood counts while reducing early thrombotic events and potentially decreasing the risk of acute myeloid leukemia transformation[10]. Alternative cytoreductive options include busulfan and pipobroman, though these carry distinct risk profiles. Busulfan demonstrates efficacy comparable to hydroxyurea with favorable long-term outcomes[11], while pipobroman, despite shorter survival and increased leukemic transformation risk compared to hydroxyurea, remains an option in specific clinical scenarios.
Interferon-alpha and its pegylated formulations have demonstrated significant therapeutic potential in PV management[12]. These agents achieve high rates of hematologic response while uniquely providing molecular responses with reductions in JAK2V617F allele burden[13]. The development of pegylated forms, such as interferon alfa-2a and alfa-2b, has enhanced their pharmacokinetics through increased stability, prolonged half-life, and reduced immunogenicity.
Ropeginterferon alfa-2b (RI) represents a novel monopegylated interferon formulation with promising clinical outcomes. Clinical trials have demonstrated superiority over hydroxyurea in achieving durable, complete hematologic and molecular responses. The drug is especially effective at lowering the JAK2V617F allele burden, and some patients have achieved undetectable levels. Studies utilizing higher starting doses (250-350-500 μg regimen) have shown enhanced efficacy, with complete hematological response (CHR) rates reaching 61.2% at 24 weeks[14].
Despite extensive clinical experience, several critical questions remain unanswered regarding optimal PV management. Current literature limitations include small sample sizes in comparative studies, inconsistent definitions of thrombotic events, variations in adverse event (AE) reporting, and limited long-term outcome data. The comparative efficacy and safety of ropeginterferon vs established therapies require systematic evaluation through comprehensive evidence synthesis[15].
Specifically, the relative efficacy of ropeginterferon compared to hydroxyurea or standard therapy (ST) in achieving CHRs, molecular responses, and long-term clinical outcomes remains incompletely characterized. Additionally, the comparative safety profiles, including treatment discontinuation rates due to AEs and the impact on thrombotic risk, warrant thorough investigation.
This meta-analysis comprehensively evaluates the safety and efficacy of ropeginterferon compared to hydroxyurea or ST in patients with PV. Primary objectives include assessing treatment response rates, thrombotic event incidence, discontinuation due to adverse effects, and changes in JAK2V617F allele burden. By synthesizing data from randomized controlled trials (RCTs) and long-term observational studies, this work aims to offer evidence-based guidance for therapeutic decision-making, support personalized patient management, and inform future clinical guidelines. As therapeutic options for PV advance, this analysis bridges key knowledge gaps and equips clinicians with robust insights for optimal disease management.
This systematic review and meta-analysis was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions[16,17]. The protocol has been prospectively registered with PROSPERO (ID: 1118732).
A comprehensive literature search was independently performed across major electronic databases, including PubMed/MEDLINE, EMBASE, ClinicalTrials.gov, and ScienceDirect, from database inception through July 2025. The search strategy incorporated a combination of Medical Subject Headings (MeSHs) terms and free-text keywords to maximize sensitivity, focusing on PV, ropeginterferon alfa, and ST. Key search strings included: ("Polycythemia Vera"[MeSH Terms] OR "Polycythemia Vera"[Title/Abstract]) AND ("Ropeginterferon"[Title/Abstract] OR "Interferon-alpha"[MeSH Terms] OR "Interferon-alpha"[Title/Abstract] OR "Pegylated Interferon"[Title/Abstract]) AND ("Phlebotomy"[MeSH Terms] OR "Phlebotomy"[Title/Abstract] OR "Hydroxyurea"[MeSH Terms] OR "Hydroxyurea"[Title/Abstract] OR "Aspirin"[MeSH Terms] OR "Aspirin"[Title/Abstract] OR "standard therapy"[Title/Abstract] OR "conventional therapy"[Title/Abstract]). The full search strategies tailored to each database are detailed in Supplementary Table 1. Additional studies were identified by hand-searching reference lists of included articles and relevant reviews.
Retrieved citations were imported into Zotero. Two reviewers (Rawat A and Mani S) independently screened titles and abstracts, followed by full-text evaluation for eligibility. Disagreements were resolved through discussion or arbitration by a third reviewer (Khawar MMH). Studies drawing from overlapping datasets or registries were cross-checked and prioritized to avoid data duplication based on sample size and recency.
Inclusion criteria encompassed: (1) RCTs and letters to editor involving adults (≥ 18 years) diagnosed with PV who underwent treatment with ropeginterferon alfa; (2) Comparisons between ropeginterferon alfa and ST (including phlebotomy, hydroxyurea, and aspirin); (3) Reporting of at least one outcome related to efficacy (e.g., CHR, partial molecular response, change in JAK2V617F allele burden, number of phlebotomies per year) or safety (e.g., AEs, incidence of thrombotic events); and (4) English-language publications.
Exclusion criteria included case reports, conference abstracts, editorials, clinical practice guidelines, consensus statements, animal studies, narrative reviews, systematic reviews, non-RCTs, and single-arm studies with no comparison group. After screening and eligibility assessment, four studies were deemed eligible and included in the final systematic review and meta-analysis[18-21].
Data extraction was performed independently by two reviewers (Tom L and Mani S) using a standardized form to gather information on study type, year of publication, number of participants, patient Characteristics [e.g., age, sex (male/female), hematocrit, platelet count, leukocyte count, spleen size and splenomegaly], intervention specifics [ropeginterferon alfa and ST (phlebotomy, hydroxyurea, and aspirin)], follow-up duration, and outcomes reported (including CHR, AEs leading to treatment discontinuation, thrombotic events, changes in the JAK2V617F gene allele burden, and the number of phlebotomies per year). Domains evaluated included the randomization process, deviations from intended interventions, missing outcomes data, measurement of the outcomes and selection of the reported result.
All analyses were performed using Review Manager (RevMan) Version 5.4 (Cochrane Collaboration). Because we expected heterogeneity in PV severity, types of interventions, and patient populations, we used a random-effects model. For outcomes with significant heterogeneity, we also calculated prediction intervals to better estimate the range of effects that could be expected in a future study. For dichotomous outcomes, pooled effect estimates were calculated as risk ratios with 95% confidence intervals (95%CIs) using the Mantel-Haenszel method. For continuous outcomes, MDs with 95%CIs were used. Heterogeneity was quantified with the Higgins I² statistic (low: 0%-25%; moderate: 25%-50%; substantial: 50%-75%; high: > 75%) and the Cochrane Q test (P < 0.10 indicating significance). Forest plots were generated for each clinical outcome and the publication bias was assessed through funnel plots. Sensitivity analyses, including leave-one-out approaches, were performed to test the robustness of the results. Subsequently, the quality of evidence for each outcome was appraised using the GRADE approach, which considers factors such as risk of bias, inconsistency, imprecision, indirectness, and publication bias to determine the overall certainty of the evidence. A two-sided P value ≤ 0.05 was considered statistically significant
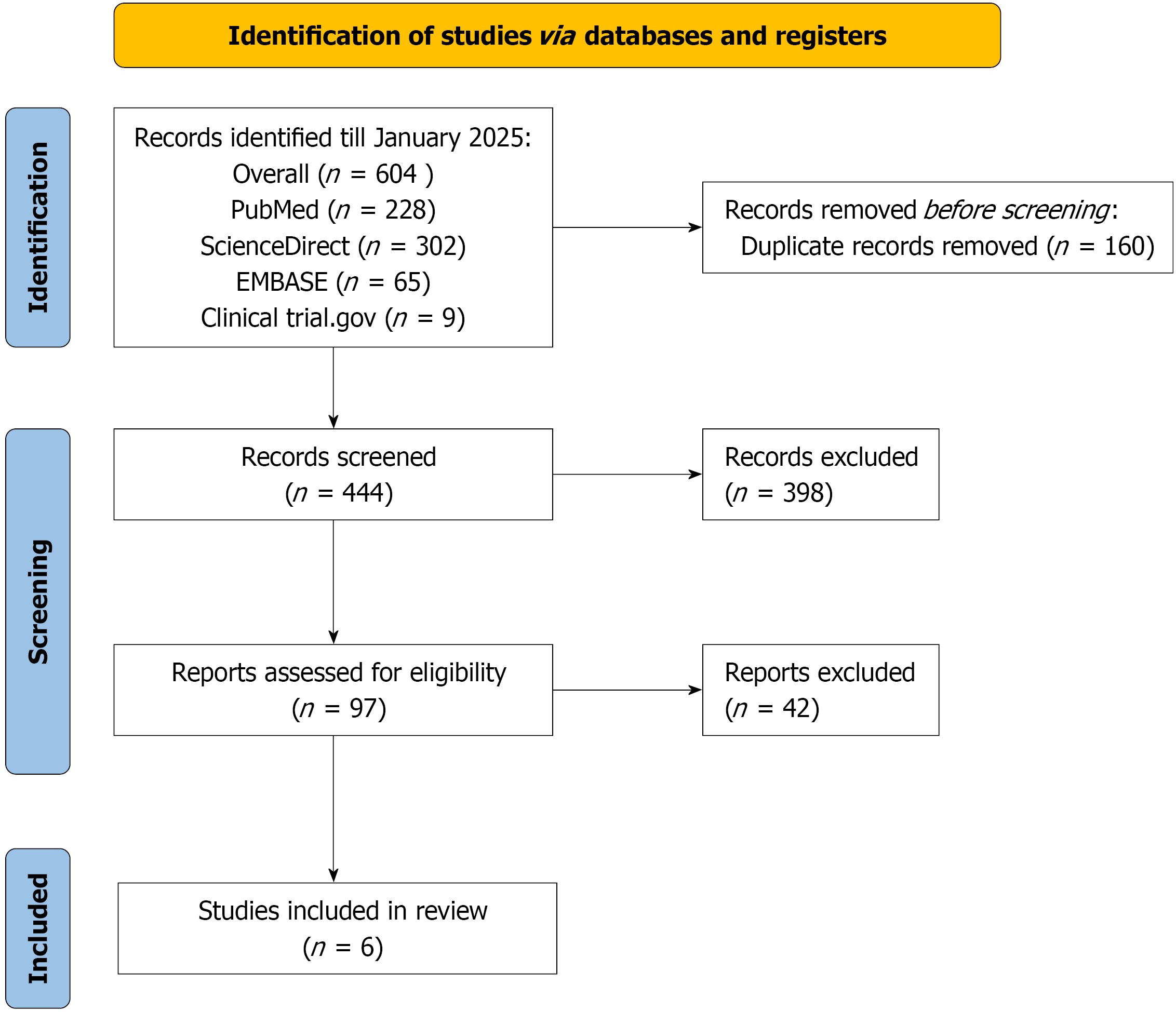
The literature search across multiple databases and registers up to January 2025 yielded a total of 604 records, comprising 228 from PubMed, 302 from ScienceDirect, 65 from EMBASE, and 9 from ClinicalTrials.gov. After removing 160 duplicate records, 444 unique records were screened based on titles and abstracts. Of these, 398 records were excluded due to irrelevance or failure to meet initial criteria. The remaining 46 reports were assessed for full-text eligibility, resulting in the exclusion of 42 reports for reasons such as inappropriate study design, insufficient data, or non-compliance with inclusion criteria. Ultimately, 4 studies[18-20,22] met all eligibility requirements and were included in the meta-analysis (PRISMA flow diagram in Figure 1).
A total of five reports from RCTs were included in the meta-analysis, derived from two primary trials: The Italian LOW-PV trial (reported at 2-year and 5-year follow-ups) and the European PROUD-PV/CONTINUATION-PV trial (reported across multiple publications and phases). The studies included 477 patients treated with RI and 456 patients receiving ST (primarily hydroxyurea or best available therapy), with some overlap in cohorts due to extended follow-up periods. All trials were conducted in Europe (Italy or multi-center European sites) and focused on patients with PV, with study durations ranging from 2 years to 5 years. Publications spanned 2020 to 2024. The proportion of male patients varied across studies, ranging from 46.5% to 73.4% in the ropeginterferon arms and 47.2% to 61.9% in the ST arms. All reports provided data on CHR, with four reports including molecular response, AEs leading to discontinuation, and thrombotic events. Three reports detailed changes in JAK2V617F allele burden, and two reports included the number of phlebotomies per patient-year. Detailed study characteristics and outcomes are summarized in Table 1.
| Ref. | Country | Study period (year) | Study design | Sample size (ropeginterferon; standard) | Male sex, % (ropeginterferon; standard) | Outcomes reported |
| Barbui et al[20], 2023 | Italy | 2 | RCT | 64; 63 | 73.4 (47/64); 61.9 (39/63) | CHR, molecular response, AEs leading to discontinuation, thrombotic events, change in JAK2V617F allele burden, number of phlebotomies per patient-year |
| Barbui et al[19], 2024 | Italy | 5 | RCT | 64; 63 | 73.4 (47/64); 61.9 (39/63) | CHR, AEs leading to discontinuation, thrombotic events, number of phlebotomies per patient-year |
| Gisslinger et al[22], 2020 (P) | Europe | 3.5 | RCT | 127; 127 | 46.5 (59/127); 47.2 (60/127) | CHR, molecular response, AEs leading to discontinuation, thrombotic events, change in JAK2V617F allele burden |
| Gisslinger et al[22], 2020 (C) | Europe | 5 | RCT | 95; 76 | 50.5 (48/95); 52.6 (40/76) | CHR, molecular response, thrombotic events, change in JAK2V617F allele burden |
| Kiladjian et al[18], 2022 (P) | Europe | 5 | RCT | 127; 127 | 46.5 (59/127); 47.2 (60/127) | CHR, molecular response, AEs leading to discontinuation, thrombotic events, change in JAK2V617F allele burden |
Baseline patient characteristics were well-balanced between the ropeginterferon and ST arms across the included reports. The mean age ranged from 50.8 years to 60.0 years in the ropeginterferon groups and 49.8 years to 60.0 years in the ST groups. The proportion of male patients varied from 48/95 (50.5%) to 59/127 (46.5%) in the ropeginterferon arms and 39/63 (61.9%) to 60/127 (47.2%) in the standard arms, with one report showing a higher male predominance in the ropeginterferon group (47/64, 73.4%). Hematocrit levels were comparable, with means of 43.9% to 48.0% (SD = 2.0% to 5.7%) for ropeginterferon and 44.0% to 49.7% (SD = 2.7% to 5.9%) for ST. Platelet counts ranged from 485 × 109/L to 620 × 109/L (SD = 188 × 109/L to 264 × 109/L) in ropeginterferon groups and 452 × 109/L to 640 × 109/L (SD = 250 × 109/L to 264 × 109/L) in standard groups. Leucocyte counts were between 10.6 × 109/L and 11.2 × 109/L (SD = 4.0 × 109/L to 5.0 × 109/L) for ropeginterferon and 10.4 × 109/L to 11.7 × 109/L (SD = 4.8 × 109/L to 5.0 × 109/L) for ST. Spleen size (measured below the costal margin) was reported in all studies, with means of 2.3 cm to 15.3 cm (SD = 0.8 cm to 6.7 cm) in ropeginterferon arms and 3.2 cm to 15.5 cm (SD = 2.3 cm to 10.7 cm) in standard arms. The prevalence of splenomegaly at baseline was low, ranging from 7% to 33.3% in ropeginterferon groups and 11% to 28.6% in standard groups. No data were available on prior cardiovascular interventions such as percutaneous coronary intervention or coronary artery bypass grafting. Detailed baseline characteristics are presented in Table 2.
| Ref. | Age (mean ± SD) ropeginterferon; standard | Sex (male/female) ropeginterferon; standard | Hematocrit (mean ± SD, %) ropeginterferon; standard | Platelet count (mean ± SD, × 109; L) ropeginterferon; standard | Leucocyte count (mean ± SD, × 109; L) ropeginterferon; standard | Spleen size (mean ± SD, cm) ropeginterferon; standard | Splenomegaly (%) ropeginterferon; standard |
| Barbui et al[20], 2023 | 50.8 ± 7.4; 49.8 ± 10.4 | 47/17; 39/24 | 43.9 ± 2.0; 44.0 ± 2.7 | 620 ± 188; 640 ± 260 | 10.9 ± 3.9; 10.4 ± 4.9 | 2.3 ± 0.8; 3.2 ± 2.3 | 33.3; 28.6 |
| Barbui et al[19], 2024 | 50.8 ± 7.4; 49.8 ± 10.4 | 47/17; 39/24 | 43.9 ± 2.0; 44.0 ± 2.7 | 620 ± 188; 640 ± 260 | 10.9 ± 3.9; 10.4 ± 4.9 | 2.3 ± 0.8; 3.2 ± 2.3 | 33.3; 28.6 |
| Gisslinger et al[22], 2020 (P) | 59.3 ± 10.5; 58.3 ± 14.3 | 59/68; 60/67 | 47.5 ± 5.3; 48.3 ± 5.3 | 502 ± 241; 482 ± 253 | 10.7 ± 4.1; 11.0 ± 5.0 | 13.0 ± 3.0; 13.2 ± 2.8 | 9.0; 12.0 |
| Gisslinger et al[22], 2020 (C) | 57.3 ± 10.5; 57.8 ± 12.5 | 48/47; 40/36 | 48.0 ± 5.7; 49.7 ± 5.2 | 513 ± 264; 486 ± 264 | 11.2 ± 5.0; 11.7 ± 4.8 | 13.3 ± 2.6; 13.2 ± 3.2 | 7.0; 11.0 |
| Kiladjian et al[18], 2022 (P) | 60.0 ± 10.4; 60.0 ± 14.1 | 59/68; 60/67 | 47.1 ± 5.3; 48.0 ± 5.9 | 485 ± 238; 452 ± 250 | 10.6 ± 4.0; 10.5 ± 4.9 | 15.3 ± 6.7; 15.5 ± 10.7 | 9.4; 11.8 |
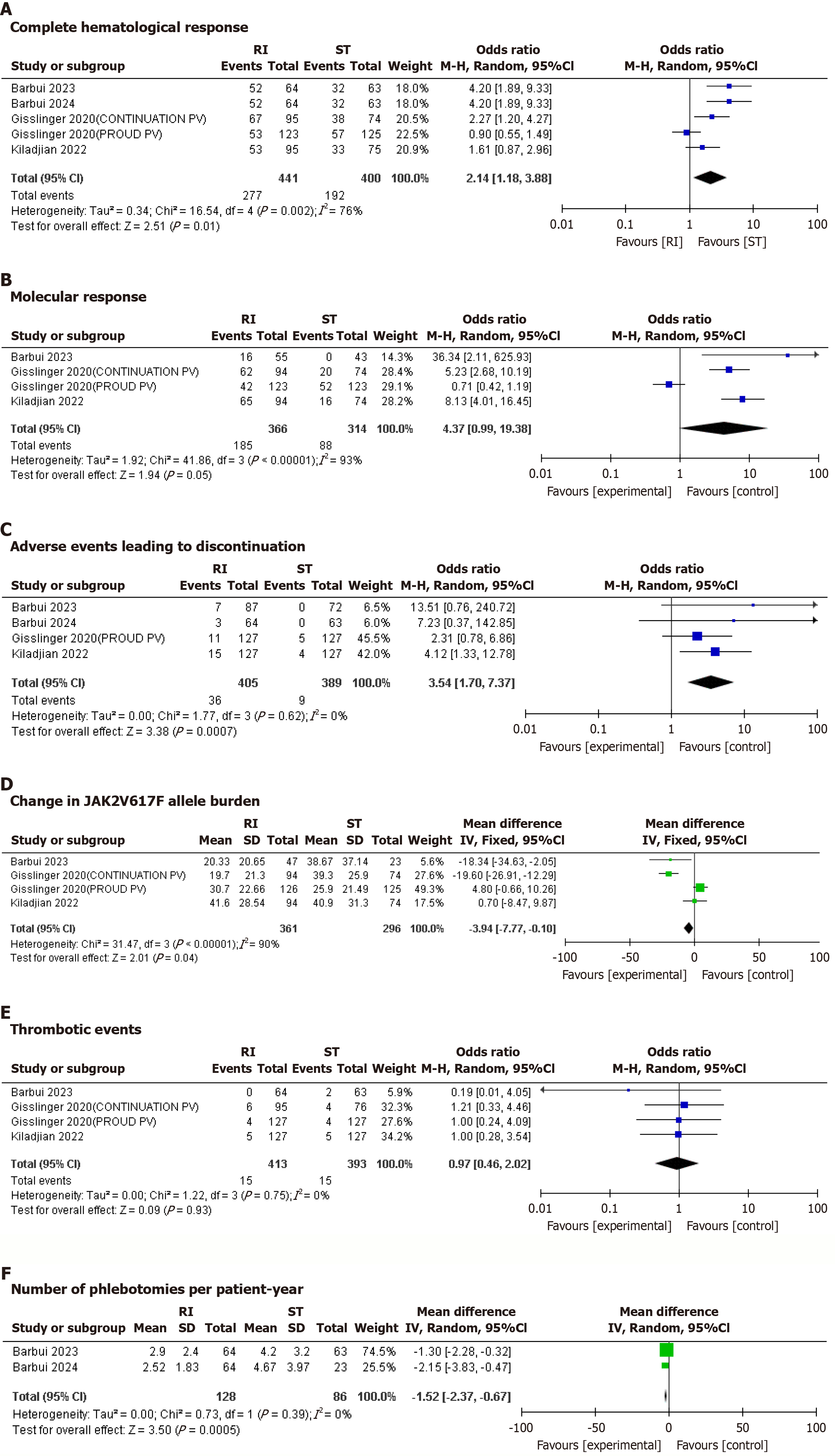
CHR: Five studies reported on CHR. RI was associated with a significantly higher CHR compared to ST. The pooled odds ratio (OR) was 2.14 (95%CI: 1.18-3.88; P = 0.002) with considerable heterogeneity (I² = 76%) that dropped considerably (I² = 48.3%) upon removal of Glissinger 2020(P).
Molecular response: Four studies reported molecular response to therapy. RI was associated with a significantly higher molecular response to therapy compared to ST. The pooled OR was 4.37 (95%CI: 0.99-19.38; P = 0.05) with substantial heterogeneity (I² = 93%) that dropped significantly to (I² = 12%) upon removal of Glissinger2020(P).
Adverse effects leading to discontinuation: Five studies reported adverse effects leading to discontinuation. RI was associated with significantly higher AEs leading to discontinuation compared to ST. The pooled OR was 3.89 (95%CI: 1.90-7.97; P = 0.0002) with unimportant heterogeneity I² = 0%.
JAK2V617F allele burden: Four studies reported change in JAK2V617F allele burden. No significant difference was observed between the JAK2V617F allele burden between the RI and ST groups. The pooled MD was -7.46 (95%CI: -21.12 to 6.20; P = 0.28) with considerable heterogeneity (I² = 90%) that dropped significantly (I² = 72%) upon removal of Gisslinger2020(P).
Thrombotic events: Four studies reported thrombotic events. No significant difference was observed in the thrombotic events between the RI and ST groups. The pooled OR was 0.93 (95%CI: 0.45-1.90; P = 0.83), with unimportant heterogeneity (I² = 0%).
Number of phlebotomies per patient per year: Two studies reported number of phlebotomies per patient per year. RI was associated with a significantly lower number of phlebotomies per patient per year compared to ST. The pooled MD was -1.52 (95%CI: -2.37 to -0.67; P = 0.0005), with unimportant heterogeneity (I² = 0%).
The forest plots showing all clinical outcomes are shown in Figure 2.
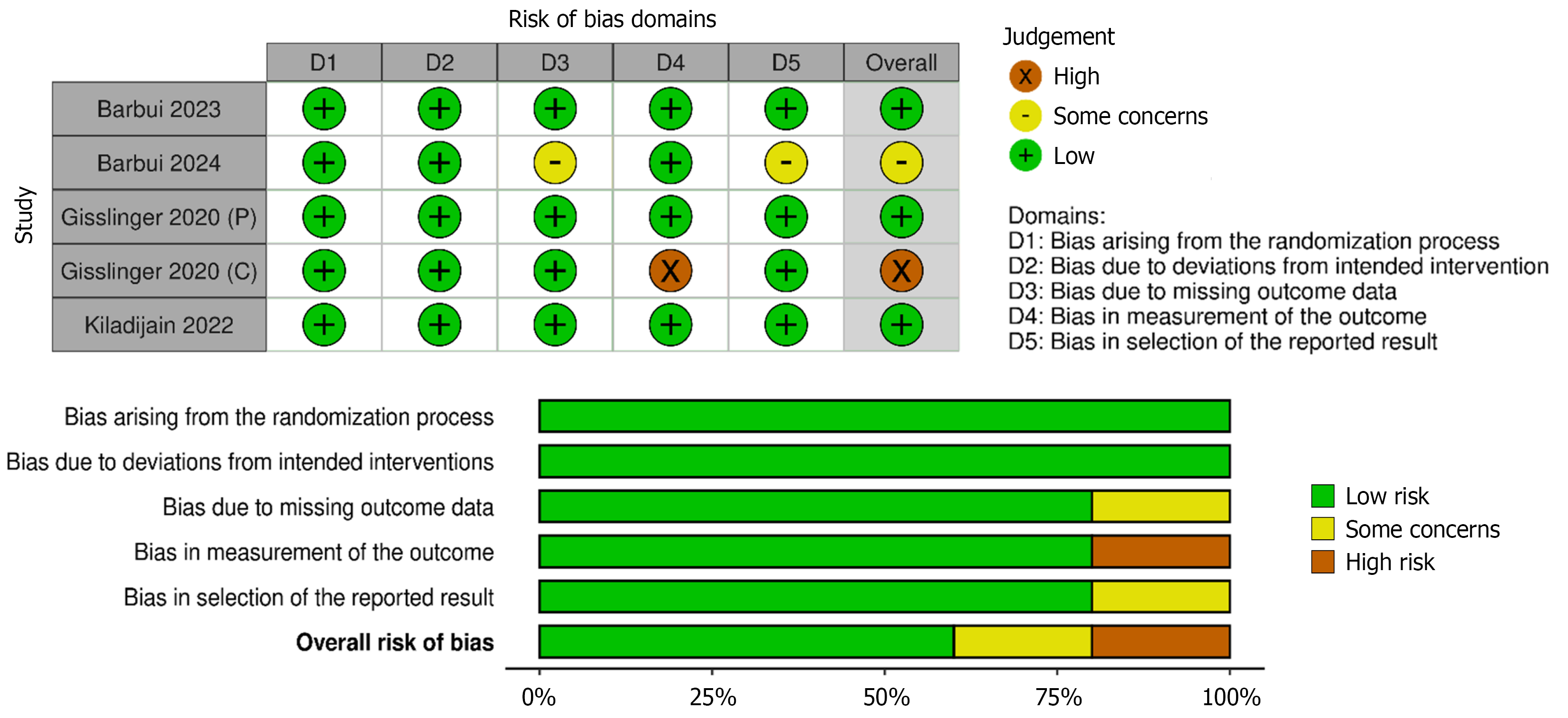
The Cochrane Risk of Bias (ROB 2.0) assessment revealed that majority of the included randomised controlled trials demonstrated a low risk of bias, indicating high methodological quality across domains and the internal validity of the meta-analysis.
Three studies were rated low risk overall, reflecting well-conducted trial methodology with minimal bias across domains. One study was assessed as having some concerns, mainly due to issues in outcome reporting and missing data domains. One trial, representing the continuation phase of a larger study, was rated as high risk of bias, particularly due to concerns in the outcome measurement.
These findings suggest that while most included studies are methodologically robust, the presence of one high-risk study necessitates cautious interpretation, mainly in pooled effect estimates. The detailed breakdown of domain-level judgement is presented in Figure 3.
The GRADE assessment of the meta-analysis revealed varying levels of evidence certainty across six outcomes comparing ropeginterferon (RI) to ST. CHR and AEs leading to discontinuation showed moderate to high certainty, with RI significantly improving response (OR = 2.14, 95%CI: 1.18-3.88) but also increasing discontinuation due toAEs (OR = 3.89, 95%CI: 1.90-7.97). Molecular response and JAK2V617F allele burden had low certainty due to substantial heterogeneity (I² = 93% and 90%, respectively) and imprecision, despite RI showing a higher molecular response (OR = 4.37, 95%CI: 0.99-19.38). Thrombotic events had moderate certainty with no significant difference (OR = 0.93, 95%CI: 0.45-1.90), while the number of phlebotomies per patient per year demonstrated high certainty, favoring RI with a significant reduction (MD = -1.52, 95%CI: -2.37 to -0.67). The presence of one high-risk of bias study and publication bias concerns for some outcomes necessitate cautious interpretation (Supplementary Table 1).
Publication bias was assessed using funnel plots for all outcomes, as each were based on fewer than 10 studies, precluding formal statistical tests for asymmetry like Egger's test in accordance with Cochrane recommendations; the funnel plot for CHR showed noticeable asymmetry with studies clustered on the right side favoring ropeginterferon and a potential gap in the left bottom quadrant, indicating possible underrepresentation of smaller studies with null or negative results, while the molecular response plot exhibited similar asymmetry with points predominantly on the right and apparent missing studies on the left; in contrast, the plot for AEs leading to discontinuation appeared relatively symmetrical, forming a typical inverted funnel shape without obvious gaps, and the JAK2V617F allele burden plot displayed scattered points without clear asymmetry, though limited studies hinder definitive assessment; the thrombotic events funnel plot was symmetrical with even distribution around the central line, and for number of phlebotomies per patient per year, based on only two studies, the plot showed no evident asymmetry, but interpretation remains limited due to the small number of trials (Supplementary Figure 1).
This meta-analysis demonstrated that RI greatly improves CHR and molecular response compared to ST, which includes phlebotomy, hydroxyurea, and aspirin. However, RI was also associated with a higher rate of AEs leading to discontinuation[22-24]. No significant differences were observed between RI and ST in terms of JAK2V617F allele burden reduction or thrombotic events[22,25,26]. The apparent contradiction between a statistically significant molecular response and a non-significant mean difference in JAK2V617F allele burden is likely due to the high heterogeneity (I² = 90%) and imprecision of the latter, which is a continuous measure. Heterogeneity was substantial for some outcomes but reduced following sensitivity analyses, particularly with the removal of Gisslinger 2020[22]. These findings suggest that RI provides superior efficacy in achieving hematologic and molecular control in PV, yet its tolerability remains a challenge[22,27].
The superior CHR and molecular response seen with RI indicate that it could be a top choice for treating PV, especially for patients requiring long-lasting hematologic control. The molecular response associated with RI suggests its role in targeting the JAK2V617F mutation, a key driver of PV pathogenesis[28,29]. These findings align with previous trials, such as the PROUD-PV and CONTINUATION-PV studies, which also demonstrated superior efficacy of RI in achieving hematologic remission and reducing allele burden compared to hydroxyurea[22,25]. The higher rate of AEs leading to discontinuation underscores the importance of careful patient selection and monitoring during RI therapy[21,23,27]. While our pooled analysis indicates a higher overall rate of discontinuation, a detailed examination of the specific types and severity of AEs is limited by the available data, which did not consistently report the incidence of specific AEs such as flu-like symptoms, cytopenias, or psychiatric effects.
The lack of significant differences in thrombotic events between RI and ST may reflect the effective thromboembolic prevention provided by both strategies, particularly through aspirin and phlebotomy[25,26]. However, the non-significant differences in the JAK2V617F allele burden reduction warrants further research, as this is an important marker of disease progression.
Our results are consistent with prior research, including Kiladjian et al[30] (2008) and Gisslinger et al[24] (2015), which reported improved hematologic and molecular responses with pegylated interferon. Nonetheless, discrepancies exist regarding the safety profile. Unlike our findings, some smaller trials reported tolerability comparable to hydroxyurea[24,30,31]. The larger pooled sample size in this meta-analysis, which provided greater power to detect AEs, may account for this difference. Additionally, variations in study populations, such as the inclusion of older patients or those with comorbidities, may explain the higher rate of treatment discontinuation observed in our analysis[21,23,27,32].
This meta-analysis has several limitations. First, the included studies varied in definitions of key outcomes, particularly thrombotic events and molecular response, contributing to heterogeneity[25,26]. Second, some studies had small sample sizes and limited long-term follow-up data[22,30]. Third, the funnel plots for CHR and molecular response showed noticeable asymmetry, indicating potential publication bias, where smaller studies with null or negative results may be underrepresented, which could lead to an overestimation of the beneficial effects of ropeginterferon. Fourth, the wide confidence intervals seen in some results, such as CHR and molecular response, indicate that variations in study design, patient characteristics, or the treatment protocols might have influenced the outcomes. Finally, data collection is ongoing, and newer trials with larger cohorts may alter these findings[32].
Our findings suggest that ropeginterferon represents a promising treatment option for managing hematologic and molecular aspects in PV. However, the higher rate of AEs indicates the need for personalized treatment approaches[21,22,26]. Future research should focus on identifying predictive biomarkers for RI tolerability and efficacy, along with conducting long-term studies to evaluate its impact on survival, risk of leukemia, and quality of life[26,32]. Additionally, trials comparing RI with newer agents or combination therapies could help refine treatment guidelines[31,32]. Finally, addressing the gaps in long-term data and standardizing outcome definitions will be critical to advancing the therapeutic landscape for PV[26,28,32].
RI offers superior hematologic and molecular responses compared to ST in PV, demonstrating sustained benefit over time and suggesting a disease-modifying effect. Nevertheless, higher discontinuation rates due to AEs highlight the need for vigilant patient selection and monitoring. Pending results from ongoing trials will help optimize dosing strategies to maximize efficacy while minimizing toxicity. Future investigations should establish long-term outcomes such as survival and leukemic progression and identify predictive markers to guide personalized therapy.
| 1. | Pizzi M, Croci GA, Ruggeri M, Tabano S, Dei Tos AP, Sabattini E, Gianelli U. The Classification of Myeloproliferative Neoplasms: Rationale, Historical Background and Future Perspectives with Focus on Unclassifiable Cases. Cancers (Basel). 2021;13:5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Podoltsev NA, Zhu M, Zeidan AM, Wang R, Wang X, Davidoff AJ, Huntington SF, Giri S, Gore SD, Ma X. The impact of phlebotomy and hydroxyurea on survival and risk of thrombosis among older patients with polycythemia vera. Blood Adv. 2018;2:2681-2690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Lanska DJ. Polycythemia vera and its neurologic manifestations. Jul 2, 2025. [cited 25 July 2025]. Available from: https://www.medlink.com/articles/polycythemia-vera-and-its-neurologic-manifestations. |
| 4. | Kurzrock R, Cohen PR. Erythromelalgia and myeloproliferative disorders. Arch Intern Med. 1989;149:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Michiels JJ. Erythromelalgia and vascular complications in polycythemia vera. Semin Thromb Hemost. 1997;23:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Thiele J, Kvasnicka HM, Orazi A, Gianelli U, Gangat N, Vannucchi AM, Barbui T, Arber DA, Tefferi A. The international consensus classification of myeloid neoplasms and acute Leukemias: myeloproliferative neoplasms. Am J Hematol. 2023;98:166-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Passamonti F. Classification of myeloproliferative neoplasms and prognostic factors. Am Soc Clin Oncol Educ Book. 2012;419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kuykendall AT. Treatment of hydroxyurea-resistant/intolerant polycythemia vera: a discussion of best practices. Ann Hematol. 2023;102:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | West WO. Hydroxyurea in the treatment of polycythemia vera: a prospective study of 100 patients over a 20-year period. South Med J. 1987;80:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Barbui T, De Stefano V, Ghirardi A, Masciulli A, Finazzi G, Vannucchi AM. Different effect of hydroxyurea and phlebotomy on prevention of arterial and venous thrombosis in Polycythemia Vera. Blood Cancer J. 2018;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Alvarez-Larrán A, Martínez-Avilés L, Hernández-Boluda JC, Ferrer-Marín F, Antelo ML, Burgaleta C, Mata MI, Xicoy B, Martínez-Trillos A, Gómez-Casares MT, Durán MA, Marcote B, Ancochea A, Senín A, Angona A, Gómez M, Vicente V, Cervantes F, Bellosillo B, Besses C. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol. 2014;93:2037-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Sun Y, Cai Y, Cen J, Zhu M, Pan J, Wang Q, Wu D, Chen S. Pegylated Interferon Alpha-2b in Patients With Polycythemia Vera and Essential Thrombocythemia in the Real World. Front Oncol. 2021;11:797825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Besses C, Alvarez-Larrán A, Martínez-Avilés L, Mojal S, Longarón R, Salar A, Florensa L, Serrano S, Bellosillo B. Modulation of JAK2 V617F allele burden dynamics by hydroxycarbamide in polycythaemia vera and essential thrombocythaemia patients. Br J Haematol. 2011;152:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Jin J, Zhang L, Qin A, Wu D, Shao Z, Bai J, Chen S, Duan M, Zhou H, Xu N, Zhang S, Zuo X, Du X, Wang L, Li P, Zhang X, Li Y, Zhang J, Wang W, Shen W, Zagrijtschuk O, Urbanski R, Sato T, Xiao Z. A new dosing regimen of ropeginterferon alfa-2b is highly effective and tolerable: findings from a phase 2 study in Chinese patients with polycythemia vera. Exp Hematol Oncol. 2023;12:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 15. | Ferrari A, Carobbio A, Masciulli A, Ghirardi A, Finazzi G, De Stefano V, Vannucchi AM, Barbui T. Clinical outcomes under hydroxyurea treatment in polycythemia vera: a systematic review and meta-analysis. Haematologica. 2019;104:2391-2399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5871] [Article Influence: 533.7] [Reference Citation Analysis (1)] |
| 17. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Aug 2024. [cited 25 July 2025]. Available from: https://www.cochrane.org/authors/handbooks-and-manuals/handbook. |
| 18. | Kiladjian JJ, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, Dulicek P, Illes A, Pylypenko H, Sivcheva L, Mayer J, Yablokova V, Krejcy K, Empson V, Hasselbalch HC, Kralovics R, Gisslinger H; PROUD-PV Study Group. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia. 2022;36:1408-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 19. | Barbui T, Carobbio A, De Stefano V, Alvarez-Larran A, Ghirardi A, Carioli G, Fenili F, Rossi E, Ciceri F, Bonifacio M, Iurlo A, Palandri F, Benevolo G, Pane F, Ricco A, Carli G, Caramella M, Rapezzi D, Musolino C, Siragusa S, Rumi E, Patriarca A, Cascavilla N, Mora B, Cacciola E, Calabresi L, Loscocco GG, Guglielmelli P, Gesullo F, Betti S, Ramundo F, Lunghi F, Scaffidi L, Bucelli C, Cattaneo D, Vianelli N, Bellini M, Finazzi MC, Tognoni G, Rambaldi A, Vannucchi AM. Ropeginterferon phase 2 randomized study in low-risk polycythemia vera: 5-year drug survival and efficacy outcomes. Ann Hematol. 2024;103:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Barbui T, Vannucchi AM, De Stefano V, Carobbio A, Ghirardi A, Carioli G, Masciulli A, Rossi E, Ciceri F, Bonifacio M, Iurlo A, Palandri F, Benevolo G, Pane F, Ricco A, Carli G, Caramella M, Rapezzi D, Musolino C, Siragusa S, Rumi E, Patriarca A, Cascavilla N, Mora B, Cacciola E, Mannarelli C, Loscocco GG, Guglielmelli P, Gesullo F, Betti S, Lunghi F, Scaffidi L, Bucelli C, Vianelli N, Bellini M, Finazzi MC, Tognoni G, Rambaldi A. Ropeginterferon versus Standard Therapy for Low-Risk Patients with Polycythemia Vera. NEJM Evid. 2023;2:EVIDoa2200335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Correction to Lancet Haematol 2021; 8: e175-84. Lancet Haematol. 2021;8:e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M, Rossiev V, Dulicek P, Illes A, Pylypenko H, Sivcheva L, Mayer J, Yablokova V, Krejcy K, Grohmann-Izay B, Hasselbalch HC, Kralovics R, Kiladjian JJ; PROUD-PV Study Group. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7:e196-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 23. | Tsai TH, Yu LH, Yu MS, Huang SH, Lin AJ, Lee KD, Chen MC. Real-world patient characteristics and treatment patterns of polycythemia vera in Taiwan between 2016 and 2017: a nationwide cross-sectional study. Ther Adv Hematol. 2023;14:20406207231179331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA, Wolf D, Kralovics R, Gisslinger B, Strecker K, Egle A, Melchardt T, Burgstaller S, Willenbacher E, Schalling M, Them NC, Kadlecova P, Klade C, Greil R. Ropeginterferon alfa-2b, a novel IFNα-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126:1762-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Gisslinger H, Klade C, Georgiev P, Skotnicki A, Gercheva-kyuchukova L, Egyed M, Rossiev V, Dulicek P, Illes A, Pylypenko H, Sivcheva L, Mayer J, Grohmann-Izay B, Hasselbalch H, Kralovics R, Kiladjian JJ. Final Results from PROUD-PV a Randomized Controlled Phase 3 Trial Comparing Ropeginterferon Alfa-2b to Hydroxyurea in Polycythemia Vera Patients. Blood. 2016;128:475. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Quintás-Cardama A, Kantarjian H, Manshouri T, Luthra R, Estrov Z, Pierce S, Richie MA, Borthakur G, Konopleva M, Cortes J, Verstovsek S. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27:5418-5424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Griesshammer M, Gisslinger H, Mesa R. Current and future treatment options for polycythemia vera. Ann Hematol. 2015;94:901-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95:1599-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 29. | Stein BL, Oh ST, Berenzon D, Hobbs GS, Kremyanskaya M, Rampal RK, Abboud CN, Adler K, Heaney ML, Jabbour EJ, Komrokji RS, Moliterno AR, Ritchie EK, Rice L, Mascarenhas J, Hoffman R. Polycythemia Vera: An Appraisal of the Biology and Management 10 Years After the Discovery of JAK2 V617F. J Clin Oncol. 2015;33:3953-3960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, Bellucci S, Grandchamp B, Chomienne C, Fenaux P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 31. | Mascarenhas J, Tashi T, El Chaer F, Priego V, Zagrijtschuk O, Qin A, Jessup J, Zimmerman C, Urbanski R, Masarova L. A Phase 3b, Randomized, Open-Label, Parallel Group, Multicenter Study to Assess Efficacy, Safety, and Tolerability of Two Dosing Regimens of Ropeginterferon Alfa-2b-Njft (P1101) in Adult Patients with Polycythemia Vera. Blood. 2023;142 Suppl 1:6444. [DOI] [Full Text] |
| 32. | Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol. 2013;6:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/