Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.112097
Revised: July 31, 2025
Accepted: September 2, 2025
Published online: October 24, 2025
Processing time: 99 Days and 3.6 Hours
Non-small cell lung cancer (NSCLC) is frequently characterized by poor response to cisplatin (DDP)-based chemotherapy, with increasing evidence suggesting that inflammatory cytokines in the tumor microenvironment contribute to chemoresistance.
To investigate the role of inflammatory cytokines in DDP resistance and to effect of IL-6 inhibition on chemosensitivity in NSCLC.
Twenty NSCLC patients were grouped into DDP-sensitive or DDP-resistant cohorts based on their clinical response. Cytokine levels in tumor tissues and NSCLC cell lines, including DDP-resistant A549/DDP and SK-MES-1/DDP, were quantified using enzyme-linked immunosorbent assay. To verify the effects of interleukin (IL)-6 on DDP resistance, NSCLC and resistant cells were treated with IL-6 inhibitors tocilizumab (TCZ), followed by DDP treatment. Cell viability, apoptosis, migration and invasion were detected via cell counting kit-8, flow cytometry, scratch assay, and transwell, respectively.
IL-6, IL-8, and tumor necrosis factor-α levels were significantly elevated in DDP-resistance tissues and cell models compared to sensitive controls (P < 0.05). TCZ treatment significantly reduced the half-maximal inhibitory concentration of DDP in resistant cells, induced apoptosis, and hindered migration and invasion (P < 0.05). IL-6 and IL-8 were identified as key cytokines associated with DDP resistance.
These findings demonstrated that IL-6 and related cytokines contribute to DDP resistance in NSCLC. IL-6 in
Core Tip: This study explores the association between inflammatory cytokines and cisplatin (DDP) resistance in non-small cell lung cancer (NSCLC). Elevated levels of interleukin (IL)-6, IL-8, and tumor necrosis factor-α were detected in DDP-resistant tissues and cells. Notably, inhibition of IL-6 using the receptor antagonist tocilizumab restored DDP sensitivity by reducing cell viability, migration, and invasion, while enhancing apoptosis. These findings underscore IL-6 as a promising therapeutic target to overcome DDP resistance in NSCLC.
- Citation: Dai Y, Liu YY, Cao N, Tian XW, Feng J, Hu ZZ, Xu JQ. Overcoming chemoresistance in non-small cell lung cancer: Insights into the influence of inflammatory factors on treatment response. World J Clin Oncol 2025; 16(10): 112097
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/112097.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.112097
Lung cancer remains one of the leading causes of cancer-related mortality worldwide. It ranks as the most prevalent malignancy among men and the third most common among women, accounting for 22% and 8.4% of all cancers respectively[1]. According to the International Agency for Research on Cancer, approximately 2.5 million new cases and 1.5 million deaths from lung cancer occur globally each year[2]. Among all histological types, non-small cell lung cancer (NSCLC)—primarily consisting of squamous cell carcinoma and adenocarcinoma—represents approximately 85% of all lung cancer cases[3]. Unfortunately, despite advancements in diagnosis and therapy, NSCLC continues to have a poor prognosis, with a 5-year survival rate of only around 15%[4].
Cisplatin (DDP) remains a first-line chemotherapeutic agent for NSCLC[5]. It is widely used in combination regimens or as neoadjuvant therapy due to its ability to interfere with DNA replication and repair in cancer cells[6,7]. However, a significant proportion of NSCLC patients exhibit intrinsic or acquired resistance to DDP, resulting in suboptimal responses, continued tumor progression, and reduced overall survival[8,9]. Overcoming this chemoresistance is essential to improving clinical outcomes, yet the underlying mechanisms remain incompletely understood.
Increasing evidence suggests that the tumor microenvironment (TME) plays a pivotal role in influencing drug response[10]. The TME is a complex and dynamic network composed of cancer cells, stromal cells, immune cells, extracellular matrix, and various soluble mediators. It is often characterized by chronic inflammation, which facilitates tumor proliferation, invasion, angiogenesis, and immune evasion[11]. In addition, inflammatory mediators within the TME can interfere with chemotherapy efficacy by altering drug metabolism, modifying cellular signaling, and promoting survival pathways[12-14].
Among the numerous cytokines present in the TME, interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) have been extensively documented for their functions in promoting tumor progression and chemoresistance. IL-6, secreted by both tumor and stromal cells, can activate autocrine and paracrine signaling loops that enhance cancer cell survival, proliferation, and resistance to therapy via pathways such as STAT3 and PI3K/Akt[15-18]. Similarly, IL-8 contributes to chemoresistance by promoting angiogenesis and enhancing metastatic potential[19]. While other cytokines such as IL-4 and IL-10 are typically considered to have anti-inflammatory effects, their roles in shaping the TME are more indirect and involve suppressing immune surveillance and promoting tumor tolerance[20,21]. These cytokines involved in modulating the efficacy of chemotherapy (albeit less direct) are crucial for understanding the full spectrum of immune interactions affecting drug response in NSCLC.
The present research concentrated on IL-4, IL-6, IL-8, IL-10, and TNF-α to further elucidate their roles in NSCLC resistance to DDP based on the significant impact of these cytokines on tumor biology and their potential as therapeutic targets. Our findings aim to shed light on the inflammatory mechanisms driving chemoresistance and provide a rationale for cytokine-targeted adjuvant therapies in NSCLC.
Following authorization from the Ethics Committee of Gezhouba Central Hospital of Sinopharm, this research was implemented in line with the approval guidelines. Each participant voluntarily provided written informed consent voluntarily before enrollment. Our investigation included 20 individuals confirmed to be suffering from NSCLC and underwent lung wedge resection from January 2016 to January 2018 in our hospital. The inclusion criteria were: (1) Age between 45 and 68 years; (2) No prior chemotherapy, radiotherapy, or immunotherapy before surgery; (3) Complete clinical and pathological data, including TNM staging (stage I-IV) and histological classification (squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma); and (4) Postoperative treatment with DDP (100 mg/m², intravenous drip). Patient characteristics were displayed in Table 1.
| Variable | Tumor (n = 20) |
| Sex | |
| Male | 11 (55.0) |
| Female | 9 (45.0) |
| Age (year) | 52.75 ± 6.52 |
| TNM stage | |
| Stage I | 3 (15.0) |
| Stage II | 5 (25.0) |
| Stage III | 8 (40.0) |
| Stage IV | 4 (20.0) |
| Histopathological type | |
| Squamous carcinoma | 12 (60.0) |
| Adenocarcinoma | 5 (25.0) |
| Adenosquamous carcinoma | 3 (15.0) |
During surgery, paired tumor tissues (tumor group) and paracancerous tissues (adjacent group) were collected and preserved under standard conditions. Following DDP-based chemotherapy, treatment responses were evaluated as per Response Evaluation Criteria in Solid Tumors criteria[22,23]. Patients who responded to DDP chemotherapy were defined as sensitive to DDP (sensitive group, n = 10), while those who did not respond to DDP chemotherapy were considered resistant to DDP (resistance group, n = 10).
A549 and SK-MES-1 cells, as human NSCLC cell lines, were acquired from the American Type Cell Culture Collection (VA, United States). DDP-resistant cells (including A549/DDP and SK-MES-1/DDP) were obtained from GuYan Industrial Co., Ltd (Shanghai, China). Dulbecco’s Modified Eagle’s Medium (DMEM) enriched with 10% FBS (Gibco, United States), 1% L-glutamine (Sigma-Aldrich, United States), and 2% penicillin/streptomycin (Servicebio, China) was exploited to culture the cells. The culture condition was set to 95% humidity and 5% CO2 in an incubator.
Subsequently, phosphate buffered saline (PBS; Beyotime, China) was exercised to dissolve DDP (Sigma, United States), which was then diluted to the appropriate concentrations with a complete medium. Next, diverse concentrations of DDP were applied to NSCLC cells for 48 hours of treatment. No treatment was administered to the cells assigned to the Control group. Moreover, the cells were incubated by IL-6 inhibitor tocilizumab (TCZ; 100 ng/mL) (HY-P9917, MedChemExpress, China) for 48 hours to verify the role of IL-6 in DDP-resistant cells[24].
Preliminary experiments were conducted to determine the optimal concentrations and incubation durations for DDP and TCZ treatments, ensuring effective drug response without excessive cytotoxicity.
Tissue samples were gently cut into small pieces using ophthalmic scissors, succeeded by supplementation with a suitable volume of PBS. Next, the samples were well homogenized using a homogenizer (Biospec, United States). After centrifugation (3000 rpm, 4 °C, 20 minutes) of the homogenate, the supernatant was harvested and preserved at -80 °C.
The culture supernatant was obtained by centrifuging the medium at 3000 rpm, 4 °C for 20 minutes to eliminate cellular residues, debris and other impurities. Upon centrifugation, the supernatant was carefully isolated and frozen at -80 °C.
The corresponding enzyme-linked immunosorbent assay (ELISA) kits were utilized for checking the IL-4 (Invitrogen, #BMS225-2, United States), IL-6 (Invitrogen, #BMS213-2, United States), IL-8 (Invitrogen, #BMS204-3, United States), IL-10 (Invitrogen, #BMS215-2, United States) and TNF-α (Invitrogen, #BMS223-4, United States) levels in tissue samples and cell supernatant. Briefly, the samples were diluted to appropriate concentrations and then placed into the ELISA plate. Based on the manufacturer's instructions, 50 μL of the prepared antibody dilution was supplemented to every plate well for 90 minutes of incubation at room temperature. Following rinsing, the samples in each well were supplemented with 100 μL of diluted horseradish peroxidase-conjugated antibody for incubation at room temperature for approximately 30 minutes. Upon discarding the antibody, each well was treated with the chromogenic substrate 3,3’,5,5’-tetramethylbenzidine (100 μL) and incubated for 30 minutes at room temperature in the absence of light. Ultimately, each well received stop solution (100 μL), after which readings at 450 nm were acquired via a microplate reader (Decca, China). The levels of cytokines in each sample were calculated based on the standard curve.
Log-phase cells were grown in 96-well plates (2 × 103 cells/well). Following 48 hours of independent incubation with TCZ or DDP, cell counting kit-8 solution (10 μL, Biofount, China) was applied to each well for another 2 hours of culture. Subsequently, absorbance readings at 450 nm were obtained employing a microplate reader (VL0000D2, Thermo Fisher Scientific, Waltham, MA, United States).
Flow cytometry worked to assess the NSCLC cell apoptosis rate. First, trypsin digestion was performed on the cells, after which they were rinsed utilizing pre-cooled PBS. Later, a 15-minute incubation of the samples was performed utilizing Annexin V-fluorescein isothiocyanate and propidium iodide at room temperature under dark conditions as directed by the Annexin V/Propidium Iodide Apoptosis Detection Kit procedure (Procell, China). Then, cell examination was conducted by Accuri C6 Plus Flow Cytometer (TOMY Digital Biology, United States) within 1 hour. Ultimately, apoptotic levels were researched by means of FlowJo v10.0 software (FlowJo, Canada).
Cell culture was executed under serum-free conditions in DMEM for 12 hours to avoid the impact of serum on the results. Next, the cells (1 × 103 cells/well) were introduced into the upper insert, and the DMEM embracing 10% fetal bovine serum was given to the lower insert. After that, the samples were maintained at 37 °C with 5% CO2 in a 90% humidity incubator. At 24 hours post-treatment, the culture medium was discarded, and the upper chamber was cleaned utilizing PBS. Afterward, a cotton swab was employed to gently remove the non-migrated cells from the upper chamber. The cells were first exposed to 4% paraformaldehyde for fixation and later stained with 0.1% crystal violet. Cells were then observed through an EVOS microscope (Thermo Fisher Scientific, United States). Cell counting was performed in five randomly selected fields of view.
Incubation of 1 × 104 NSCLC cells per well was completed in 6-well plates. Cells at 80% confluence were scratched with a pipette tip to form a vertical line at the well's bottom. Following PBS washing, the cells were incubated in serum-free DMEM. The EVOS microscope was used to analyze the cells before the beginning of the culture and after 24 hours of incubation. Besides, fields of view were randomly selected and photographed (five in total), and the interstitial region was measured with Image J software (National Institutes of Health, United States).
mean ± SD was adopted to present the data. To evaluate differences between the two groups, an independent t-test was performed. Group comparisons were carried out via one-way analysis of variance. Pairwise comparisons involved Tukey's test. In addition, univariate logistic regression served to analyze the correlation between DDP resistance and the levels of inflammatory cytokines (confidence interval = 95%). The effect of each factor on the risk for the DDP resistance was evaluated by calculating the odds ratio. Statistical analysis was accomplished via SPSS 24.0 software (IBM Corp., United States), and Graphpad Prism 9.0 (Graphpad, San Diego, CA, United States) was utilized to plot the data. P < 0.05 was considered statistical significance.
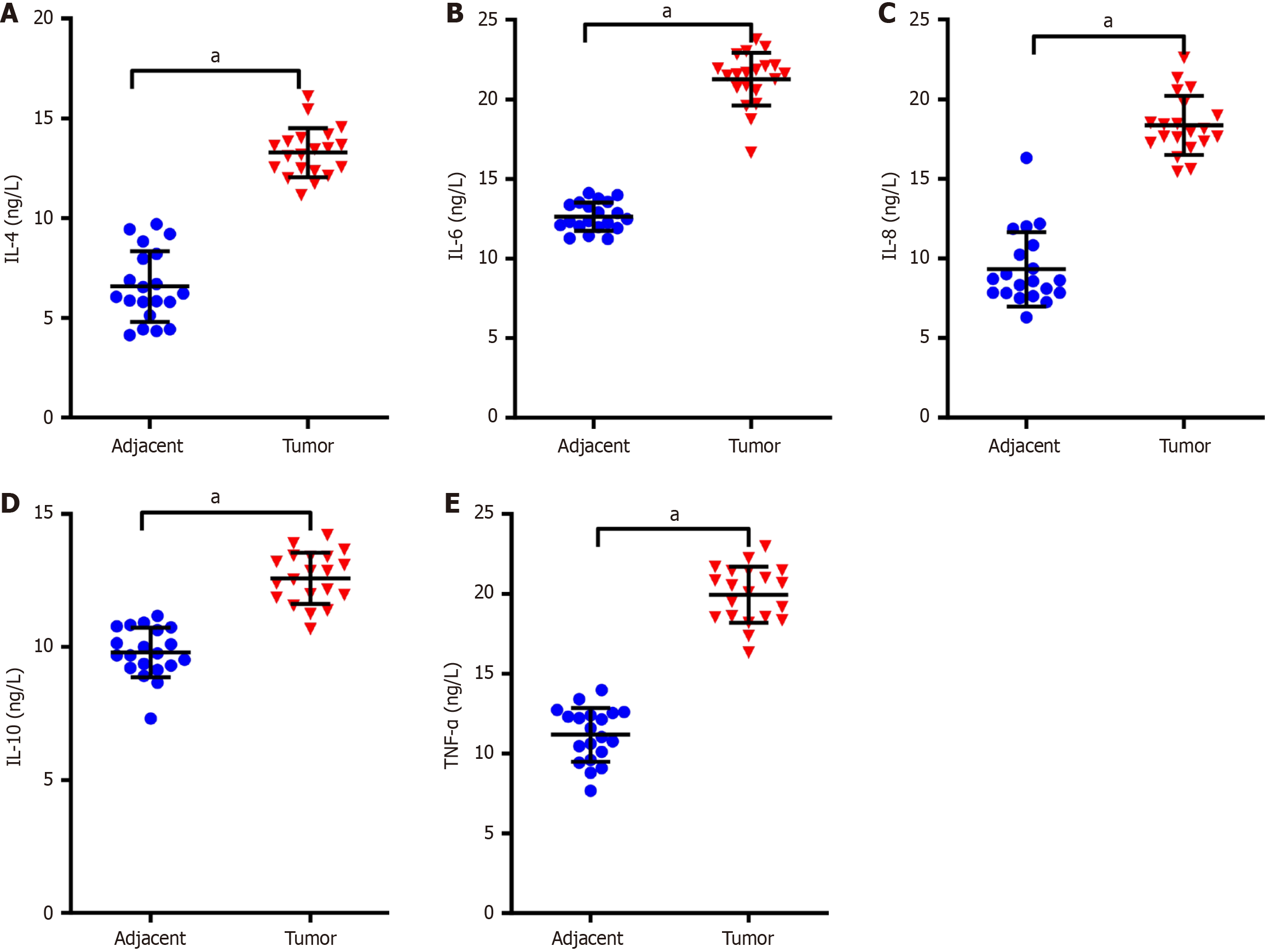
To determine whether inflammatory cytokines are up-regulated in NSCLC tumor tissues compared to paracancerous tissues, ELISA kits were utilized for testing the inflammatory factor levels in tumor and paracancerous samples of participants with NSCLC. The outcomes indicated that the IL-4, IL-6, IL-8, IL-10 and TNF-α levels were markedly higher in tumor tissues than those in paracancerous tissues (P < 0.01, Figure 1). These results suggest that NSCLC tumor tissues exhibit a pro-inflammatory environment noted for elevated IL-4, IL-6, IL-8, IL-10, and TNF-α levels, which could be tied to the pathogenesis and progression of NSCLC.
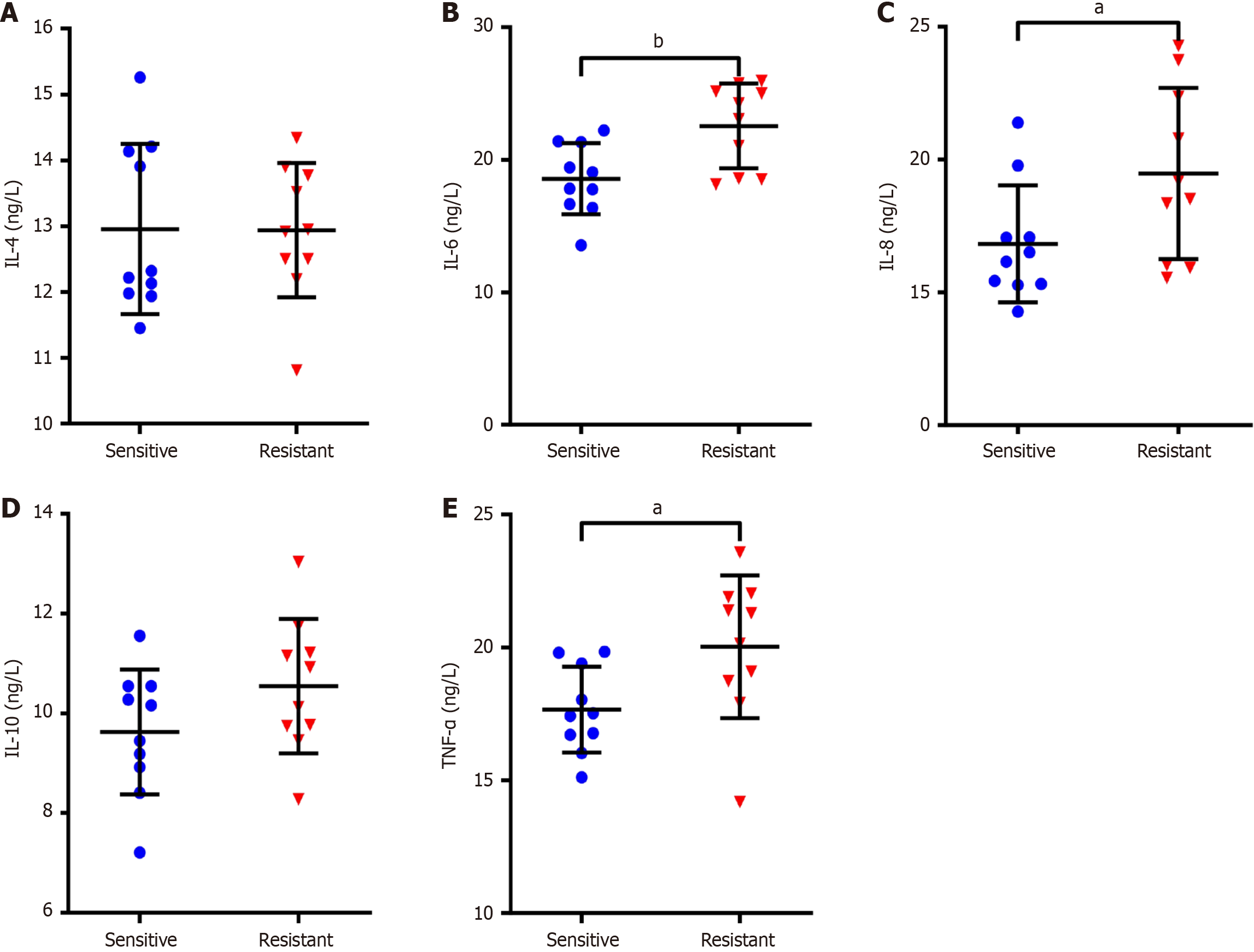
To investigate whether the elevated levels of inflammatory cytokines in tumor tissues are associated with resistance to DDP treatment in NSCLC patients, the changes in inflammatory cytokines were analyzed in tumor tissues of subjects suffering from NSCLC who were resistant to DDP. In relation to the sensitive group, IL-8, IL-6 and TNF-α expression of the tumor tissues was notably increased in the resistance group (P < 0.05), but both groups presented similar IL-10 and IL-4 Levels with no significant difference (P > 0.05, Figure 2). Subsequently, logistic regression analysis was employed to check the connection between the levels of inflammatory cytokines and DDP resistance. The findings suggested that the elevated IL-6 and IL-8 Levels in tumor tissues of sufferers with NSCLC were risk factors for DDP resistance (P < 0.05), while the IL-4, IL-10 and TNF-α levels were not markedly associated with DDP resistance (P > 0.05; Table 2).
| Indexes | OR (95%CI) | P value |
| IL-4 | 1.603 (0.380-6.773) | 0.521 |
| IL-6 | 1.968 (1.006-3.851) | 0.048 |
| IL-8 | 1.860 (1.014-3.414) | 0.045 |
| IL-10 | 1.833 (0.814-4.128) | 0.143 |
| TNF-α | 1.437 (0.692-2.988) | 0.331 |
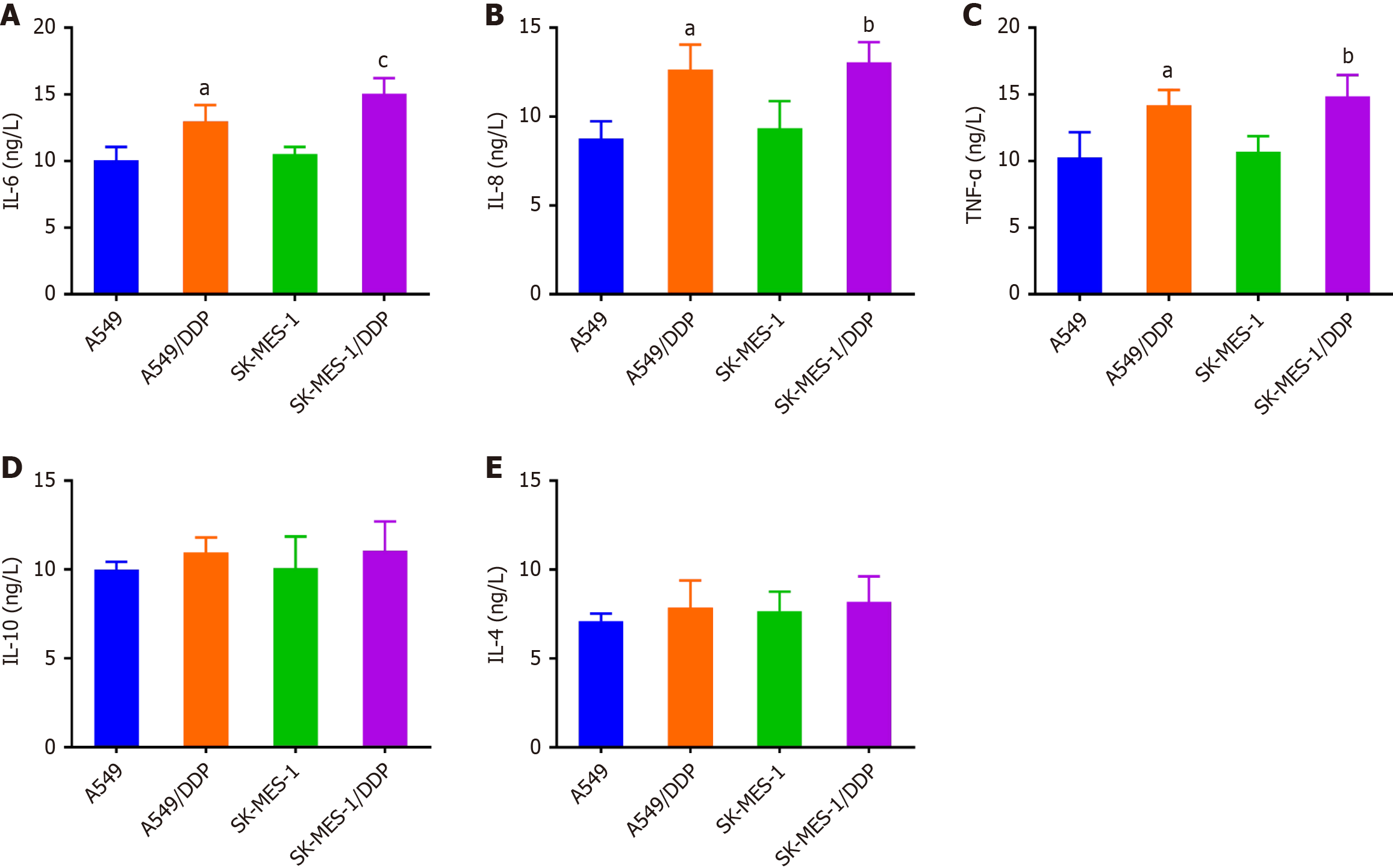
Further, we examined the inflammatory cytokine concentrations in the supernatant from NSCLC samples resistant to DDP. In comparison with A549 and SK-MES-1 cells, the IL-6, IL-8, and TNF-α levels went up in A549/DDP and SK-MES-1/DDP cells (P < 0.05), whereas no remarkable alterations was identified in IL-10 and IL-4 (Figure 3). The observed results pointed to a connection between inflammatory cytokine levels and the resistance of NSCLC cells to DDP.
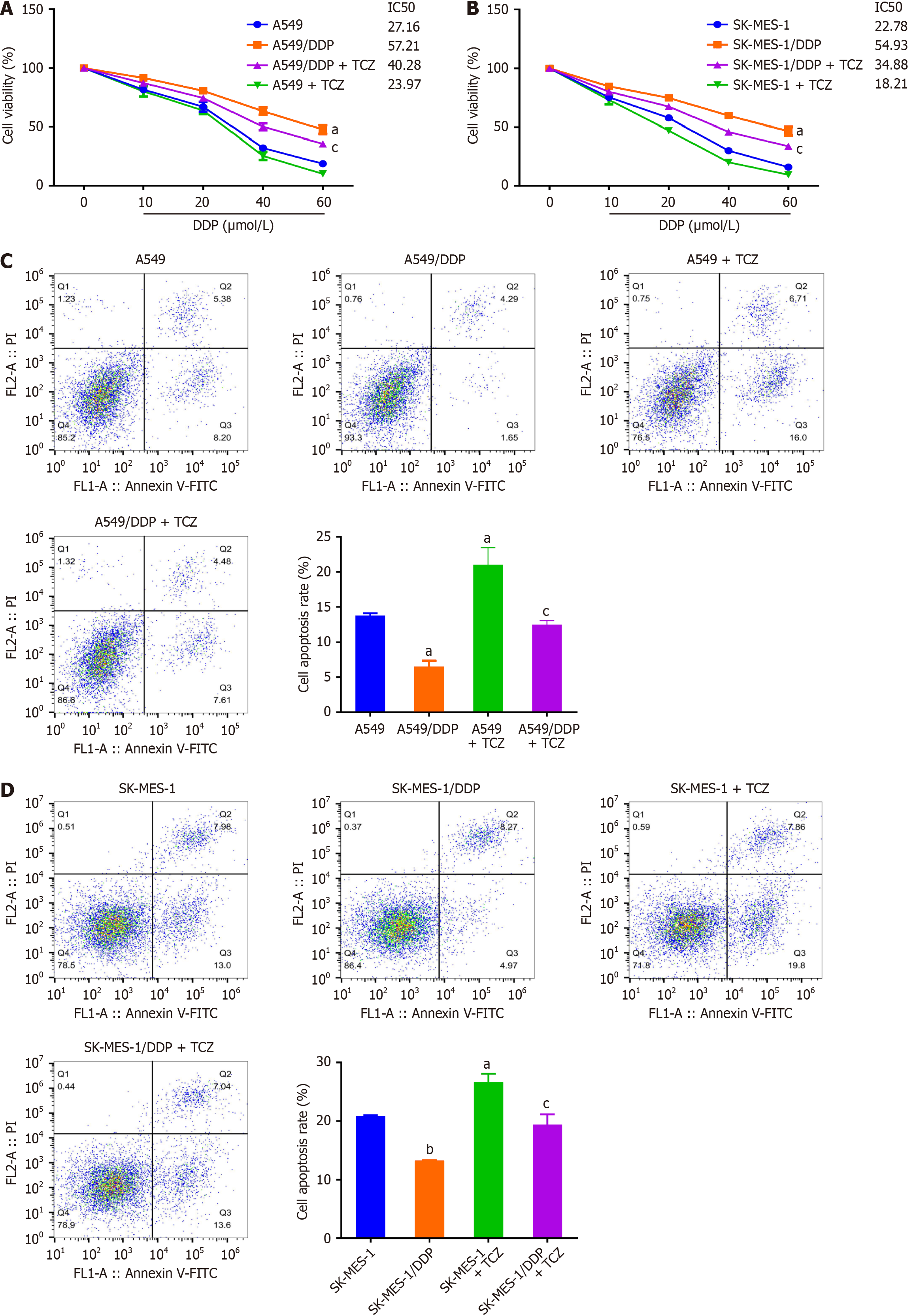
We hypothesized that IL-6 might participate in DDP resistance in NSCLC. For verifying this hypothesis, cells were treated with IL-6 inhibitors (TCZ), and the changes in cell viability were investigated after DDP treatment. The results (Figure 4A) indicated that the cell viability was higher in A549/DDP than that in A549 cells. Besides, the half-maximal inhibitory concentration (IC50) value was markedly elevated in SK-MES-1/DDP cells as opposed to SK-MES-1 cells (P < 0.05). Moreover, A549/DDP and SK-MES-1/DDP cells exhibited significantly higher resistance to DDP in contrast to A549 and SK-MES-1 cells. In addition, TCZ exposure reduced the IC50 value of DDP in resistant NSCLC cells (P < 0.05, Figure 4A). However, cell viability remained unchanged after TCZ treatment when compared to A549/SK-MES-1 cells. Then, TCZ’s effects on the NSCLC cell apoptosis were observed. Under DDP intervention, TCZ raised the apoptosis rate of A549/DDP and SK-MES-1/DDP cells (P < 0.05, Figure 4B). To sum up, inhibition of IL-6 Level increased the NSCLC cells’ sensitivity to DDP (Figure 4C and D).
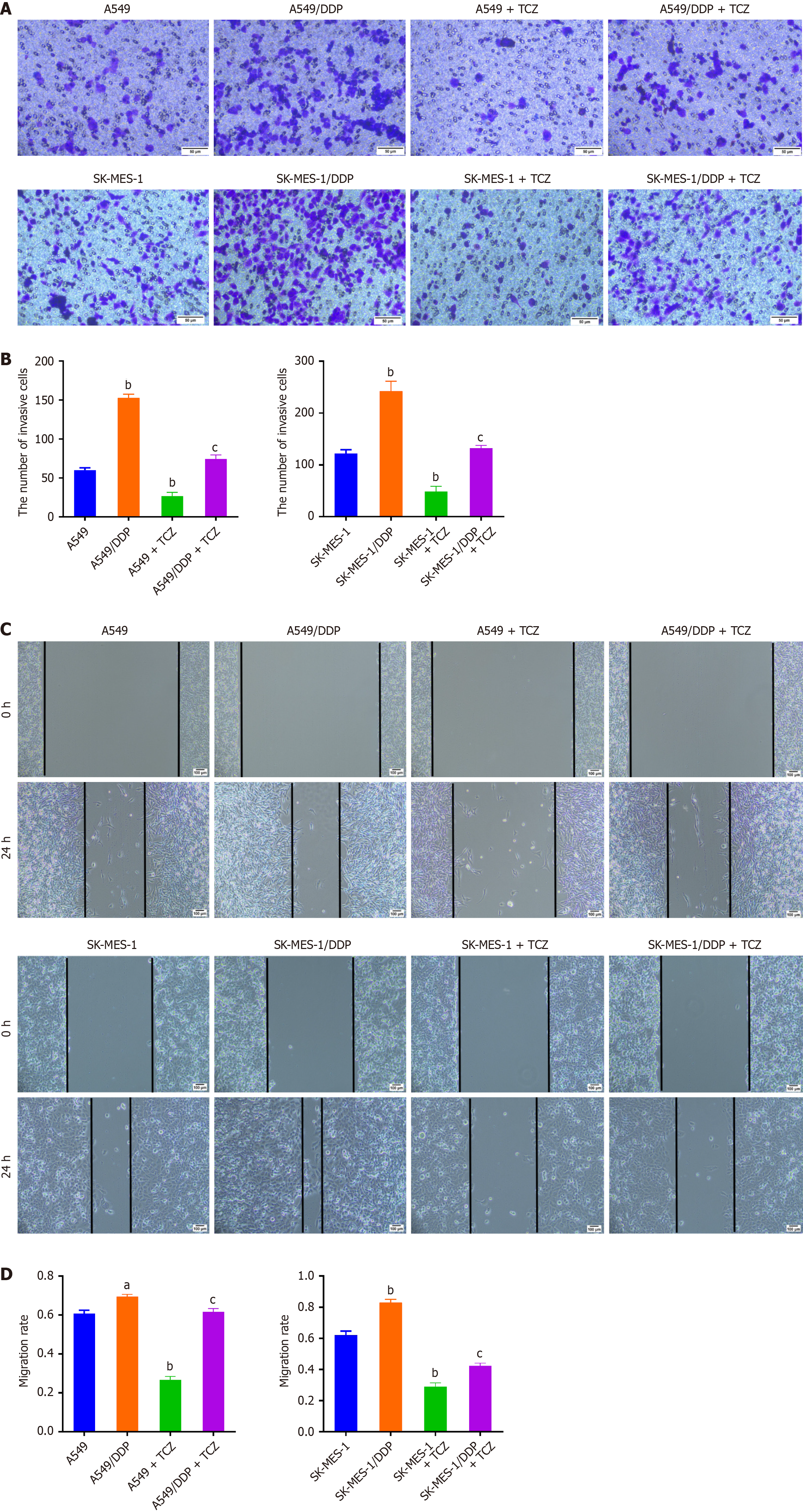
Further, we investigated the impact of TCZ on the invasion and migration of NSCLC cells. The results displayed that under DDP treatment, there was an evident enhancement in the migratory and invasive capabilities of A549/DDP and SK-MES-1/DDP cells relative to A549 and SK-MES-1 cells (P < 0.05, Figure 5A and B). Furthermore, TCZ weakened NSCLC cell invasiveness and motility (P < 0.05, Figure 5C and D). These data showed that inhibition of IL-6 significantly reduced DDP-resistant NSCLC cells’ migration and invasion capabilities.
NSCLC is a prevalent form of lung cancer known for its poor prognosis and low overall survival rate[3,4]. DDP is a first-line chemotherapy agent for NSCLC[25]. Unfortunately, DDP is completely ineffective in over 10% of NSCLC sufferers, and nearly 50% of subjects with NSCLC do not achieve the expected outcomes after DDP treatment[26]. Therefore, uncovering the causes and mechanisms of DDP resistance is key to optimizing its therapeutic impact in NSCLC.
Recent research highlights the critical role of the TME, especially its inflammatory component, in driving tumor progression and drug resistance[27]. In our study, we observed significantly elevated levels of IL-4, IL-6, IL-8, IL-10, and TNF-α in NSCLC tumor tissues compared to adjacent normal tissues, suggesting the presence of a chronically inflamed TME. These findings are consistent with prior studies demonstrating an enrichment of pro-inflammatory cytokines within NSCLC tissues[28,29]. Among them, IL-6 and IL-8 were found to be markedly elevated in both DDP-resistant NSCLC tissues and resistant cell models. Logistic regression analysis confirmed that elevated IL-6 and IL-8 Levels were significantly associated with poor DDP response.
These findings align with previous studies demonstrating the mechanistic involvement of IL-6 and IL-8 in NSCLC chemoresistance. IL-6 has been reported to promote resistance by inducing epithelial-mesenchymal transition, activating the NF-κB signaling pathway, and upregulating anti-apoptotic and DNA repair proteins such as Bcl-2, MCL-1, p53 and ERCC1[30-32]. Similarly, IL-8 can enhance drug efflux by promoting the expression of ATP-binding cassette transporters such as ABCA5, reducing intracellular drug concentrations and thereby limiting DDP efficacy[33]. Elevated serum levels of IL-8 and IL-6 have also been independently correlated with worse clinical prognosis in NSCLC patients[34,35].
While these previous studies primarily offered correlative insights, our study advances the field by providing functional validation. Using the clinically approved IL-6 receptor antagonist TCZ[24], we demonstrated that IL-6 blockade restores DDP sensitivity in resistant NSCLC cells. Specifically, TCZ treatment led to a significant reduction in IC50 values, enhanced apoptosis, and inhibited migration and invasion in DDP-resistant A549/DDP and SK-MES-1/DDP cells. This supports the hypothesis that IL-6 plays a central role not only in drug resistance but also in promoting ma
Our findings are consistent with the broader literature describing the protumorigenic effects of IL-6 in solid tumors. Under stress conditions such as hypoxia, acidosis, and high osmolarity, a wide array of cytokines, including IL-6 and IL-8, are secreted by tumor-associated macrophages, fibroblasts, and other immune cells, contributing to a pro-inflammatory TME[27-29]. While IL-4 and IL-10 are generally considered anti-inflammatory, their upregulation may reflect immune suppression and reduced anti-tumor surveillance[20,21,28]. However, their roles appear less directly related to chemoresistance, as no significant difference was observed between DDP-sensitive and resistant groups in our dataset.
Furthermore, the translational relevance of IL-6 inhibition is increasingly supported by emerging clinical evidence. TCZ has been explored in clinical trials for cancer-related inflammation and immune modulation. For instance, the ongoing trials NCT04191421 and NCT04940299 are investigating the safety and efficacy of TCZ in patients with advanced or metastatic NSCLC. In addition, a meta-analysis reported that elevated inflammatory indices, including IL-6, are associated with poor outcomes across various solid tumors, highlighting IL-6 as a potential therapeutic target[36]. Our findings support these translational trends, providing preclinical evidence for the efficacy of IL-6-targeted therapy in reversing chemoresistance.
Nevertheless, this study is not without limitations. First, we only assessed five inflammatory cytokines. Future studies should expand the cytokine panel to include other soluble mediators such as IL-1β, TGF-β, or CCL2. Second, while our clinical data provided key insights, the sample size was relatively small and lacked stratification by clinicopathological features such as TNM stage or smoking history. Third, although we demonstrated the functional relevance of IL-6 through in vitro models, further validation using in vivo animal models and patient-derived xenografts is necessary. Finally, downstream signaling pathways affected by IL-6/TCZ intervention remain to be elucidated and should be explored in future transcriptomic and proteomic studies.
This study demonstrated that DDP-resistant NSCLC samples exhibited significantly elevated levels of IL-8, IL-6 and TNF-α, with IL-6 and IL-8 identified as key contributors to chemoresistance. Functional assays confirmed that pharmacological inhibition of IL-6 using TCZ can effectively restore DDP sensitivity, reduce cell viability, and suppress malignant phenotypes such as migration and invasion in resistant NSCLC cell lines. These findings highlight the pivotal role of pro-inflammatory cytokines in the TME in mediating drug resistance. Clinically, targeting IL-6 signaling may serve as a promising adjunct strategy to overcome DDP resistance and improve therapeutic outcomes in NSCLC patients. Future research incorporating in vivo validation and clinical trials is warranted to further explore IL-6-targeted interventions as a viable component of precision oncology in lung cancer.
| 1. | Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health. 2019;85:8. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 947] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 3. | Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022;42:937-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 454] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 4. | Romaszko AM, Doboszyńska A. Multiple primary lung cancer: A literature review. Adv Clin Exp Med. 2018;27:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 5. | El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anticancer Agents Med Chem. 2021;21:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Vasconcellos VF, Marta GN, da Silva EM, Gois AF, de Castria TB, Riera R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2020;1:CD009256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Karam I, Jiang SY, Khaira M, Lee CW, Schellenberg D. Outcomes of small cell lung cancer patients treated with cisplatin-etoposide versus carboplatin-etoposide. Am J Clin Oncol. 2015;38:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015;1856:189-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 537] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 9. | Liu Z, Lin H, Gan Y, Cui C, Zhang B, Gu L, Zhou J, Zhu G, Deng D. P16 Methylation Leads to Paclitaxel Resistance of Advanced Non-Small Cell Lung Cancer. J Cancer. 2019;10:1726-1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Bożyk A, Wojas-Krawczyk K, Krawczyk P, Milanowski J. Tumor Microenvironment-A Short Review of Cellular and Interaction Diversity. Biology (Basel). 2022;11:929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 11. | Naser R, Fakhoury I, El-Fouani A, Abi-Habib R, El-Sibai M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy (Review). Int J Oncol. 2023;62:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 12. | Galizia D, Minei S, Maldi E, Chilà G, Polidori A, Merlano MC. How Risk Factors Affect Head and Neck Squamous Cell Carcinoma (HNSCC) Tumor Immune Microenvironment (TIME): Their Influence on Immune Escape Mechanisms and Immunotherapy Strategy. Biomedicines. 2022;10:2498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Guo X, Wang T, Huang G, Li R, Da Costa C, Li H, Lv S, Li N. Rediscovering Potential Molecular Targets for Glioma Therapy Through the Analysis of the Cell of Origin, Microenvironment and Metabolism. Curr Cancer Drug Targets. 2021;21:558-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nienhuis HH, Gaykema SB, Timmer-Bosscha H, Jalving M, Brouwers AH, Lub-de Hooge MN, van der Vegt B, Overmoyer B, de Vries EG, Schröder CP. Targeting breast cancer through its microenvironment: current status of preclinical and clinical research in finding relevant targets. Pharmacol Ther. 2015;147:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 16. | Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685-6693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 17. | Ene CV, Nicolae I, Geavlete B, Geavlete P, Ene CD. IL-6 Signaling Link between Inflammatory Tumor Microenvironment and Prostatic Tumorigenesis. Anal Cell Pathol (Amst). 2022;2022:5980387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Meng J, Zhang XT, Liu XL, Fan L, Li C, Sun Y, Liang XH, Wang JB, Mei QB, Zhang F, Zhang T. WSTF promotes proliferation and invasion of lung cancer cells by inducing EMT via PI3K/Akt and IL-6/STAT3 signaling pathways. Cell Signal. 2016;28:1673-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Zhai J, Shen J, Xie G, Wu J, He M, Gao L, Zhang Y, Yao X, Shen L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 20. | Bel'skaya LV, Loginova AI, Sarf EA. Pro-Inflammatory and Anti-Inflammatory Salivary Cytokines in Breast Cancer: Relationship with Clinicopathological Characteristics of the Tumor. Curr Issues Mol Biol. 2022;44:4676-4691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Shekari M, Kordi-Tamandani DM, MalekZadeh K, Sobti RC, Karimi S, Suri V. Effect of anti-inflammatory (IL-4, IL-10) cytokine genes in relation to risk of cervical carcinoma. Am J Clin Oncol. 2012;35:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Liu MY, Li XQ, Gao TH, Cui Y, Ma N, Zhou Y, Zhang GJ. Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients. J Thorac Dis. 2016;8:3314-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. Breast Cancer. 2005;12 Suppl 1:S16-S27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kim NH, Kim SK, Kim DS, Zhang D, Park JA, Yi H, Kim JS, Shin HC. Anti-proliferative action of IL-6R-targeted antibody tocilizumab for non-small cell lung cancer cells. Oncol Lett. 2015;9:2283-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Fennell DA, Summers Y, Cadranel J, Benepal T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C, Ferry D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat Rev. 2016;44:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 26. | Depierre A, Milleron B, Moro-Sibilot D, Chevret S, Quoix E, Lebeau B, Braun D, Breton JL, Lemarié E, Gouva S, Paillot N, Bréchot JM, Janicot H, Lebas FX, Terrioux P, Clavier J, Foucher P, Monchâtre M, Coëtmeur D, Level MC, Leclerc P, Blanchon F, Rodier JM, Thiberville L, Villeneuve A, Westeel V, Chastang C; French Thoracic Cooperative Group. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Farc O, Cristea V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp Ther Med. 2021;21:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Chen H, Zhang T, Zhang Y, Wu H, Fang Z, Liu Y, Chen Y, Wang Z, Jia S, Ji X, Shang L, Du F, Liu J, Lu M, Chong W. Deciphering the tumor microenvironment cell-infiltrating landscape reveals microenvironment subtypes and therapeutic potentials for nonsquamous NSCLC. JCI Insight. 2022;7:e152815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 29. | Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 30. | Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J Thorac Oncol. 2016;11:1482-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 31. | Duan S, Tsai Y, Keng P, Chen Y, Lee SO, Chen Y. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget. 2015;6:27651-27660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang YH, Wang YN, Li J, Gao FG. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Sci. 2014;105:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Li L, Chen C, Xiang Q, Fan S, Xiao T, Chen Y, Zheng D. Transient Receptor Potential Cation Channel Subfamily V Member 1 Expression Promotes Chemoresistance in Non-Small-Cell Lung Cancer. Front Oncol. 2022;12:773654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Liao C, Yu Z, Guo W, Liu Q, Wu Y, Li Y, Bai L. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: a systematic review and meta-analysis. Cancer Biomark. 2014;14:469-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Cury SS, de Moraes D, Freire PP, de Oliveira G, Marques DVP, Fernandez GJ, Dal-Pai-Silva M, Hasimoto ÉN, Dos Reis PP, Rogatto SR, Carvalho RF. Tumor Transcriptome Reveals High Expression of IL-8 in Non-Small Cell Lung Cancer Patients with Low Pectoralis Muscle Area and Reduced Survival. Cancers (Basel). 2019;11:1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Li S, Feng X, Cao G, Wang Q, Wang L. Prognostic significance of inflammatory indices in hepatocellular carcinoma treated with transarterial chemoembolization: A systematic review and meta-analysis. PLoS One. 2020;15:e0230879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/