Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.110047
Revised: July 15, 2025
Accepted: September 25, 2025
Published online: October 24, 2025
Processing time: 140 Days and 19.6 Hours
Human epidermal growth factor receptor 2 (HER2) plays pivotal roles in cellular proliferation, survival, and differentiation of several malignancies. Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy. The clinical and molecular significance of HER2 expression level in UTUC remains poorly characterized vs bladder cancer.
To comprehensively evaluate HER2 expression patterns and their association with UTUC patients’ clinicopathological features.
Data were retrospectively collected from patients diagnosed with UTUC at The First Affiliated Hospital of Guangxi Medical University between January 2023 and December 2024. HER2 status was evaluated by immunohistochemistry in 145 UTUC patients who met the inclusion criteria. Its associations with tumor grade, tumor stage, and other clinicopathological parameters were assessed. The χ2 test or Fisher’s exact test, along with univariate and multivariate logistic regression analyses, were performed to determine the influences of clinicopathological factors on HER2 expression.
HER2 positivity was significantly associated with high tumor grade (P = 0.003), while other variables, including sex, anatomical tumor location, pathological T stage, Ki-67 proliferation index, nodal metastasis status, lymphovascular invasion, and tumor laterality failed to demonstrate statistically significant correlations. These findings were further substantiated through univariate logistic regression modeling, yielding an odds ratio of 3.56 [95% confidence interval (CI): 1.30-9.75; P = 0.013] for the association between high tumor grade and HER2 positivity. Importantly, this relationship remained robust (hazard ratio = 3.42, 95%CI: 1.22-9.60; P = 0.019) even after implementing multivariate logistic regression analysis. With a median follow-up time of 8 months (interquartile range, 4-14) months, 14 patients experienced intravesical recurrence after radical nephroureterectomy. Certain patient characteristics, such as HER2-negative, male sex, high-grade tumors, and luminal phenotype, were associated with a higher risk of intravesical recurrence.
In UTUC, HER2 overexpression is closely associated with tumor dedifferentiation (high grade), while it does not correlate with conventional indicators of disease progression, indicating that HER2 may serve a distinct biological function in this cancer type.
Core Tip: This is a retrospective single-center observational study to evaluate human epidermal growth factor receptor 2 (HER2) expression patterns by immunohistochemistry in 145 upper tract urothelial carcinoma (UTUC) specimens and assessed its correlation with tumor grade, stage, molecular subtype, and other clinicopathological parameters. HER2 overexpression in UTUC is strongly linked to tumor dedifferentiation (high grade), while other variables including sex, anatomical tumor location, pathological T stage, Ki-67 proliferation index, nodal metastasis status, lymphovascular invasion, and tumor laterality all failed to demonstrate statistically meaningful correlations. This distinct pattern suggests HER2 may play a unique biological role in UTUC compared to other epithelial malignancies.
- Citation: Huang L, He J. Human epidermal growth factor receptor 2 overexpression is associated with high-grade tumors in upper tract urothelial carcinoma. World J Clin Oncol 2025; 16(10): 110047
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/110047.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.110047
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy, accounting for only 5%-10% of all urothelial carcinomas (UC) and having an estimated annual incidence of 1-2 cases per 100000 individuals in Western countries[1]. Peak incidence of UTUC occurs predominantly in patients aged 70-90 years, demonstrating a 2:1 male predominance. At initial diagnosis, muscle-invasive disease is present in approximately two-thirds of UTUC cases-significantly higher than the 15%-25% observed in de novo bladder cancer (BC). Metastatic disease is detected in approximately 9% of patients at presentation. Concurrent BC occurs in 17% of UTUC cases, while prior BC history is reported in 41% of American male UTUC patients vs just 4% among Chinese males[1]. Despite its lower prevalence compared with BC, UTUC exhibits distinct biological characteristics, clinical manifestations, and therapeutic challenges. UTUC, involving the renal pelvis and ureter, is characterised by aggressive biological behaviours, a high recurrence rate, and limited therapeutic options[2]. While significant progress has been made in understanding the molecular mechanism of BC, UTUC remains comparatively understudied, particularly in terms of potential therapeutic targets, such as human epidermal growth factor receptor 2 (HER2).
HER2 is a well-characterised oncogenic driver in several malignancies, particularly breast and gastric malignancies, where its amplification or overexpression is linked to aggressive tumor progression, and it functions as an essential biomarker for targeted treatment[3,4]. HER2 belongs to the epidermal growth factor receptor family of receptor tyrosine kinases, playing pivotal roles in cellular proliferation, survival, and differentiation[5]. Upon dimerization, HER2 triggers downstream signaling pathways, notably the PI3K/AKT and MAPK signaling pathways, thereby promoting tumor proliferation and resistance to apoptosis[6]. In breast cancer, HER2-targeted therapies, such as trastuzumab, pertuzumab, and antibody-drug conjugates (ADC) (e.g., trastuzumab emtansine and trastuzumab deruxtecan), have transformed treatment strategies, significantly improving clinical outcomes for HER2-positive patients[7]. Given the aggressive nature of high-grade disease and the limited efficacy of conventional chemotherapy for patients with UTUC, identifying actionable molecular targets, such as HER2 may have significant therapeutic implications. A clearer understanding of HER2 prevalence and its clinicopathological correlations in UTUC is essential before such therapies can be systematically evaluated.
Similar to breast cancer and gastric cancer, HER2 overexpression in UC is associated with higher tumor grades and reduced survival[8]. Recent trials have shown promising clinical efficacy of anti-HER2 ADCs in HER2-positive [immunohistochemistry (IHC) 3+] UC[9]. Notably, Ye et al[10] demonstrated that approximately one-third of UTUC patients in Southwest China exhibited HER2 overexpression, suggesting that anti-HER2 therapy could be a promising treatment option for this region. Furthermore, HER2 positivity in this cohort could be correlated with high tumor grade and luminal phenotype. However, the role of HER2 in UTUC remains poorly defined, and only limited and often contradictory studies are available[10-13]. Among them, the associations between HER2 status and pathological features or prognostic value in UTUC patients remain inconclusive and highly variable. Whether HER2 expression level correlates with clinicopathological features such as tumor grade, stage, or survival remains elusive. Therefore, the present study aimed to evaluate HER2 expression level in a well-characterized cohort of patients with UTUC using IHC, and to assess its relationship with clinicopathological characteristics, including tumor grade, tumor stage, lymphovascular invasion, and clinical outcomes.
This retrospective study enrolled patients who were diagnosed with UTUC at The First Affiliated Hospital of Guangxi Medical University (Nanning, China) between January 2023 and December 2024. The study protocol was approved by the Institutional Ethics Committee of The First Affiliated Hospital of Guangxi Medical University. Clinicopathological data, including age, sex, tumor location, tumor grade, pT stage, Ki-67 expression level, pN stage, lymphovascular invasion, laterality, and molecular subtypes, were obtained from the hospital’s electronic patient record system. Inclusion criteria were summarized as follows: (1) Histopathological confirmation of UC after surgery; (2) Availability of well-preserved postoperative paraffin-embedded tissue specimens; and (3) Complete clinical and follow-up information. Exclusion criteria were summarized as follows: (1) Presence of non-UC malignancies; (2) Diagnosis of synchronous malignancies at other anatomical sites; (3) Incomplete clinicopathological information; and (4) Any other conditions regarded inappropriate for inclusion at the discretion of the attending physician. All participants provided written informed consent for the use of their samples.
Ki-67 expression level was categorized as low or high, using a cut-off value of 20%, as documented previously[10]. According to the expression levels of CK20 and CK5/6 proteins[10,14,15], UTUC was classified into four molecular subtypes: Luminal type (CK20+, CK5/6-), basal type (CK20-, CK5/6+), double-positive type (CK20+, CK5/6+), and double-negative type (CK20-, CK5/6-). HER2 expression was assessed using IHC, with scores of 0 or 1+ defined as negative, and scores of 2+ or 3+ regarded as positive.
Four-micrometer paraffin-embedded UTUC tissue sections were dewaxed and then washed with phosphate-buffered saline. Antigen retrieval was performed using EDTA antigen repair buffer (ZLI-9069; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Blocking was carried out using hydrogen peroxide (H44024859; Guangdong Nanguo Pharmaceutical Co., Ltd., Guangzhou, China) for 10 minutes at room temperature. The sections were then incubated overnight at 4 °C with the anti-HER2 antibody (mouse anti-human, #Kit-0043; Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China), the anti-CK5/6 antibody (mouse anti-human, #2301050744a; Fuzhou Maixin Biotechnology Development Co., Ltd.), and the anti-CK20 antibody (rabbit anti-human, #24100517; Zhongshan Golden Bridge Biotechnology Co., Ltd.). This was followed by incubation with a secondary antibody (PV-6000; Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 1 hour at room temperature. Peroxidase activity was visualized using the DAB reagent kit (× 20) (DAB-1031; Fuzhou Maixin Biotechnology Development Co., Ltd.). Nuclei were counterstained with hematoxylin (Lot: 180301; Shanghai Biological Technology Development Co., Ltd., Shanghai, China).
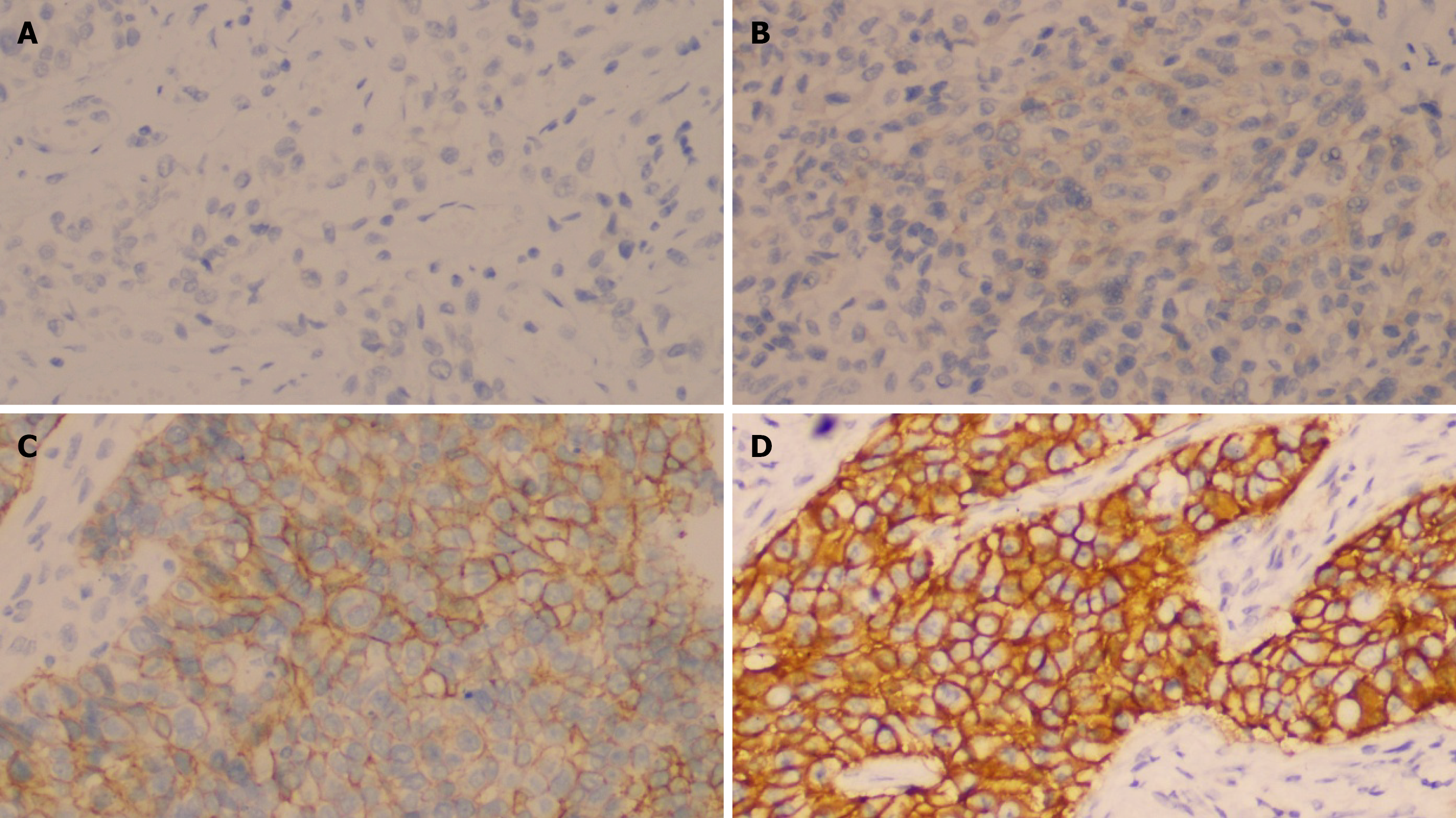
HER2 staining protocols applied in the Department of Pathology were evaluated according to the Chinese Expert Consensus on HER2 Testing in UC (2021 edition)[16]. HER2 expression was scored as follows: A score of 0 was assigned when there was no membrane staining or when < 10% of invasive tumor cells exhibited incomplete and faint/barely perceptible membrane staining; a score of 1+ was given when ≥ 10% of invasive tumor cells showed incomplete and faint/barely perceptible membrane staining; a score of 2+ was assigned when either ≥ 10% of invasive tumor cells displayed complete, weak-to-moderate circumferential membrane staining or < 10% of cells exhibited complete circumferential staining of any intensity; and a score of 3+ was given when ≥ 10% of invasive tumor cells showed complete, strong circumferential membrane staining. HER2 positivity was defined as IHC 2+ (equivocal) or IHC 3+ (positive/overexpression). All slides were independently reviewed and scored by two senior pathologists who were blinded to clinical outcomes, and any discrepancies were resolved through consensus discussion.
Clinicopathological variables were analyzed to assess their associations with HER2 expression level using SPSS 19.0 software (IBM Corp., Armonk, NY, United States). Categorical variables (e.g., age, sex, tumor location, tumor grade, pT stage, Ki-67 expression level, pN stage, lymphovascular invasion, laterality, and molecular subtypes) were compared using the χ2 or Fisher’s exact test. Univariate and multivariate logistic regression analyses were conducted to evaluate the influences of clinicopathological parameters on HER2 expression level. All statistical analyses were two-sided, and P-value < 0.05 was considered statistically significant.
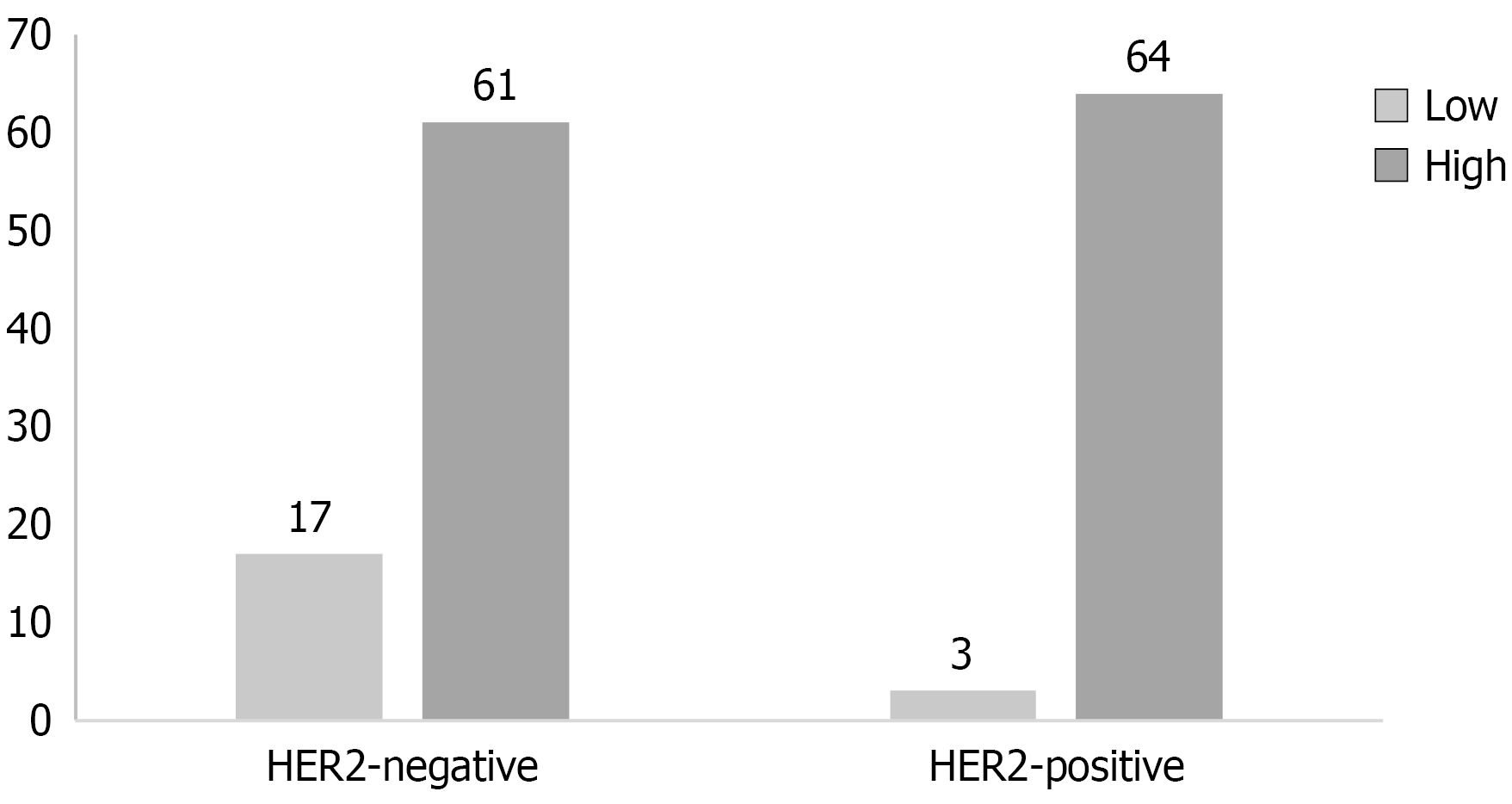
In total, 145 patients with UTUC, who were admitted to The First Affiliated Hospital of Guangxi Medical University between January 2023 and December 2024, were enrolled in this study. Among them, the male gender was predominant, accounting for 64% of the cohort. Patients’ median age was 66 (range, 32-88) years. Final pathological scores of 0, 1+, 2+, and 3+ were recorded in 29, 49, 50, and 17 patients, respectively. Immunohistochemical analysis revealed that HER2 overexpression (defined as 2+/3+ staining) was present in 46.2% of cases (67/145), indicating that nearly half of UTUC tumors demonstrated potentially targetable HER2 expression levels. When stratified by tumor grade, a significant difference was found: HER2 positivity was identified in only 15% (3/20) of low-grade tumors, whereas the prevalence rose markedly to 51.2% (64/125) in high-grade carcinomas (Figure 1), suggesting a strong biological relationship between tumor dedifferentiation and HER2 activation. The distribution across T stages indicated a moderately higher HER2 positivity in non-muscle-invasive (≤ T1) tumors (55.6%) than in muscle-invasive tumors (T2: 41.9%, ≥ T3: 43.6%), although these differences did not reach statistical significance. The primary tumor sites were the renal pelvis in 78 cases (53.8%), the ureter in 64 (44.1%), and both locations in 3 (2.1%). According to CK5/6 and CK20 immunohistochemical staining, 22 (15.2%) tumors were categorized as the basal phenotype, while 102 (70.3%) tumors were identified as the luminal phenotype. Detailed clinicopathological characteristics are summarized in Table 1.
| Variable | Category | Total | HER2-negative | HER2-positive | P value |
| Age | 66 (32-88) | 68 (32-84) | 65 (35-88) | ||
| Gender | 0.736 | ||||
| Male | 93 | 51 | 42 | ||
| Female | 52 | 27 | 25 | ||
| Location | 0.156 | ||||
| Renal pelvis | 78 | 44 | 34 | ||
| Ureter | 64 | 34 | 30 | ||
| Both | 3 | 0 | 3 | ||
| pT stage | 0.426 | ||||
| ≤ T1 | 36 | 16 | 20 | ||
| T2 | 31 | 18 | 13 | ||
| ≥ T3 | 78 | 44 | 34 | ||
| Grade | 0.003 | ||||
| Low | 20 | 17 | 3 | ||
| High | 125 | 61 | 64 | ||
| Ki-67 | 0.479 | ||||
| Low | 30 | 14 | 16 | ||
| High | 115 | 62 | 53 | ||
| pN stage | 0.646 | ||||
| Yes | 8 | 4 | 4 | ||
| No | 137 | 74 | 63 | ||
| Lymphovascular invasion | 0.135 | ||||
| pN1 | 39 | 17 | 22 | ||
| pN0 | 106 | 61 | 45 | ||
| Laterality | 0.639 | ||||
| Left | 68 | 30 | 38 | ||
| Right | 77 | 31 | 46 | ||
| Molecular subtypes | 0.788 | ||||
| Luminal | 102 | 53 | 49 | ||
| Basal | 22 | 14 | 8 | ||
| Double-positive | 10 | 5 | 5 | ||
| Double-negative | 11 | 6 | 5 |
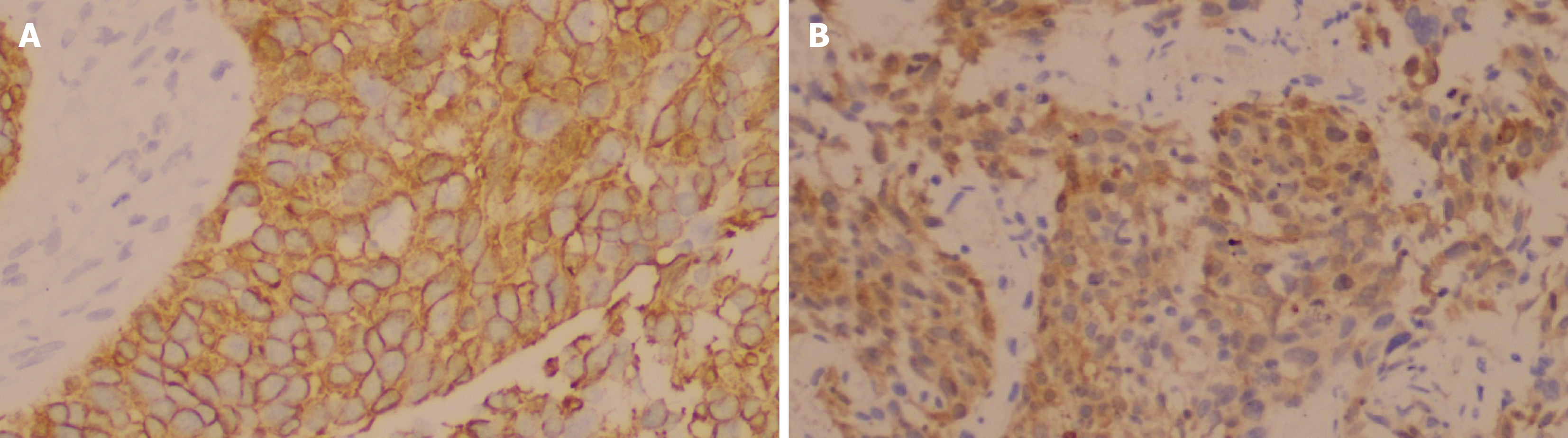
All 145 patients’ specimens were immunohistochemically stained for HER2, CK5/6, and CK20. Representative immunohistochemical staining patterns for the four different intensity grades of HER2 are illustrated in Figure 2. Positive HER2 staining was detected in the epithelial cell membranes. Representative staining patterns for CK5/6 and CK20 are depicted in Figure 3, with positive staining for both markers detected in the cytoplasm of epithelial cells.
As presented in Table 1, the χ2 test confirmed that tumor grade was the only clinicopathological parameter significantly associated with HER2 status (P = 0.003). However, other variables, including sex, anatomical tumor location, pathological T stage, Ki-67 proliferation index, nodal metastasis status, lymphovascular invasion, tumor laterality, and molecular subtypes, did not demonstrate significant correlations. These findings were further supported by univariate logistic regression modelling (Table 2), which yielded an odds ratio (OR) of 3.56 [95% confidence interval (CI): 1.30-9.75, P = 0.013] for the association between high tumor grade and HER2 positivity. This indicates that high-grade UTUC tumors demonstrated a significantly higher frequency of HER2 overexpression than low-grade tumors. Importantly, this relationship remained robust (hazard ratio = 3.42, 95%CI: 1.22-9.60, P = 0.019) even after implementing multivariate logistic regression analysis (Table 2), confirming tumor grade as an independent predictor of HER2 status in UTUC.
| Variable | Univariate HR (95%CI) | P value | Multivariate HR (95%CI) | P value |
| Age (per year) | 1.01 (0.98-1.04) | 0.62 | ||
| Gender (female vs male) | 1.12 (0.58-2.17) | 0.74 | ||
| Tumor location (ureter vs renal pelvis) | 1.13 (0.60-2.13) | 0.71 | ||
| pT stage (T2 vs ≤ T1) | 0.72 (0.30-1.74) | 0.47 | ||
| pT stage (≥ T3 vs ≤ T1) | 0.77 (0.36-1.65) | 0.5 | ||
| Grade (high vs low) | 3.56 (1.30-9.75) | 0.013a | 3.42 (1.22-9.60) | 0.019a |
| Ki-67 (high vs low) | 0.76 (0.34-1.70) | 0.5 | ||
| Lymph node metastasis (yes vs no) | 1.17 (0.30-4.58) | 0.82 | ||
| Lymphovascular invasion (yes vs no) | 1.56 (0.76-3.20) | 0.23 | ||
| Laterality (right vs left) | 1.17 (0.60-2.28) | 0.647 | ||
| Molecular subtype (basal vs luminal) | 0.62 (0.24-1.60) | 0.32 | ||
| Molecular subtype (double-positive vs luminal) | 1.08 (0.30-3.94) | 0.9 | ||
| Molecular subtype (double-negative vs luminal) | 0.90 (0.26-3.14) | 0.87 |
With a median follow-up time of 8 months (interquartile range, 4-14 months), 14 patients experienced intravesical recurrence following radical nephroureterectomy (Table 3). Certain patient characteristics, such as HER2 negativity, male sex, high-grade tumors, and luminal phenotype, were associated with a higher risk of intravesical recurrence. During subsequent follow-up, 8 patients underwent transurethral resection of bladder tumors. Pathological analysis revealed that 25% (2/8) of the recurrent bladder tumors had transitioned from HER2-positive status in the primary tumor to HER2-negative status.
| Variable | n (%) |
| All | 14 |
| Gender | |
| Male | 7 (50) |
| Female | 7 (50) |
| Grade | |
| Low | 4 (29) |
| High | 10 (71) |
| Tumor location | |
| Ureter | 8 (57) |
| Renal pelvis | 6 (43) |
| HER2 | |
| Negative | 9 (64) |
| Positive | 5 (36) |
| Stage | |
| ≤ T1 | 4 (29) |
| T2 | 1 (7) |
| ≥ T3 | 9 (64) |
| Molecular subtype | |
| Luminal | 10 (71) |
| Basal | 4 (29) |
The molecular mechanism of UTUC has remained relatively understudied compared with its bladder counterpart, particularly regarding potential therapeutic targets, such as HER2. The present study provided a comprehensive analysis of HER2 expression patterns and their clinicopathological correlations in UTUC, revealing several important findings with both biological and clinical implications.
The HER2 positivity rate of 47.6% indicates that UTUC demonstrates a relatively high frequency of HER2 expression compared with other solid tumors, significantly exceeding rates found in colorectal cancer (3%-5%) and ovarian cancer (10%-30%), and surpassing those typically identified in gastric cancer (20%-30%) and breast cancer (15%-20%)[17-20]. This prevalence is particularly notable given that no targeted therapies have yet been approved for HER2-positive UTUC. The striking grade-dependent expression pattern (15.0% in low-grade vs 51.2% in high-grade tumors) suggests that HER2 may play a role in tumor dedifferentiation, aligning with findings of a previous study on BC where HER2 amplification correlates with higher tumor grade and stage[21]. In the present study, the results of the χ2 test confirmed tumor grade as an independent predictor of HER2 status (P = 0.003), further confirming this biological relationship.
The robust association between HER2 positivity and high tumor grade remains the most significant finding of multivariate logistic regression analysis (OR = 3.42, P = 0.019). This outcome confirms the established role of HER2 in cellular differentiation pathways, as demonstrated by Fleischmann et al[22] who concentrated on UC molecular profiling. The biological etiology of this association may involve HER2-mediated activation of critical signaling pathways, particularly the PI3K-AKT-mTOR axis, influencing tumor differentiation in multiple epithelial malignancies[5,6].
Contrary to patterns identified in breast and gastric adenocarcinomas, where HER2 overexpression typically correlates with advanced disease stage[23], results of the present study indicated no significant association between HER2 status and either T stage (P = 0.426) or nodal metastasis (P = 0.646). This finding is consistent with the results reported by Ye et al[10], who also found no association between HER2 amplification and muscle invasion in UTUC. The apparent dissociation between HER2 status and disease progression in UTUC points to significant differences in the biological role of HER2 compared with other epithelial malignancies, potentially reflecting tissue-specific signaling network configurations.
The relationship between HER2 status and molecular subtypes presents a notable divergence from BC biology. While luminal-papillary bladder tumors frequently harbor HER2 alterations[15], the present study revealed no subtype-specific enrichment of HER2 positivity in UTUC (P = 0.788). This finding supports the emerging concept that upper and lower tract UC are molecularly distinct entities, as first proposed in the comprehensive genomic characterization by Robertson et al[24]. The biological etiology of this discrepancy may involve differential developmental origins or microenvironmental influences along the urinary tract.
The therapeutic implications of our findings are multifaceted. Although the absence of an association between HER2 expression and advanced stage may indicate limited utility for HER2-targeted therapies in metastatic UTUC, the strong correlation with high-grade disease suggests potential therapeutic relevance in this subset. Recent advances in ADC technology, particularly trastuzumab deruxtecan, have shown significant efficacy in HER2-low breast cancer[7], enhancing the possibility of analogous applications in UTUC. Moreover, emerging evidence indicates that HER2 signaling pathway may exert immunomodulatory effects that could enhance the efficacy of immune checkpoint inhibitors[25], highlighting a promising direction for combined treatment strategies.
The dissociation between HER2 status and Ki-67 proliferative index (P = 0.479) warrants further investigation. This finding diverges from certain reports in the breast cancer literature, while is consistent with urothelial-specific research by Sathe et al[26], indicating that HER2 may impact differentiation pathways in UTUC independently of its effects on cellular proliferation. This outcome carries potential implications for therapeutic sequencing because the differentiation state can significantly impact treatment response.
Clinically, intravesical recurrence following radical nephroureterectomy remains a significant challenge, with published data reporting an incidence rate of 31.7% (71/224 patients) over a median follow-up of 33.3 months[27]. In the present study, 14 cases of intravesical tumor recurrence were identified, accompanied by alterations in the HER2 status. Similar findings have been documented in UC research, particularly in BC. Barth et al[28] conducted a comprehensive analysis of 48 matched pairs of bladder carcinoma in situ and their corresponding invasive tumors, revealing a notable luminal phenotype in carcinoma in situ lesions, followed by downregulation of luminal markers and upregulation of basal markers during progression. This molecular shift may reflect underlying biological alterations in the tumor microenvironment, carrying substantial therapeutic implications.
Several limitations of the present study should be acknowledged. The retrospective design might introduce potential selection biases, and the single-institution nature might limit the generalizability of the findings. While the sample size is substantial for a rare cancer, such as UTUC, it may still have been underpowered to detect more subtle associations between HER2 expression and clinical parameters. Additionally, the follow-up time was relatively short, limiting our ability to draw conclusions regarding the influence of HER2 on UTUC patients’ prognosis. Future research should overcome these limitations by employing prospective, multi-center designs that incorporate standardized testing protocols and longer follow-up periods.
Several important research directions arise from these findings. Firstly, preclinical studies employing UTUC models should investigate the functional effects of HER2 overexpression and assess potential therapeutic approaches. Secondly, clinical trials are warranted to evaluate the efficacy of HER2-targeted treatments in high-grade, HER2-positive UTUC patients, with close consideration of possible differences in drug sensitivity relative to BC. Finally, comprehensive molecular profiling studies should investigate the correlation between HER2 alterations and other genomic and microenvironmental factors in UTUC.
In conclusion, this study biologically delineated a distinct HER2 pattern in UTUC, which was characterized by a strong association with tumor grade. These findings highlight the importance of tissue-specific molecular characterization and provide potential therapeutic directions for high-grade disease.
| 1. | Masson-Lecomte A, Birtle A, Pradere B, Capoun O, Compérat E, Domínguez-Escrig JL, Liedberg F, Makaroff L, Mariappan P, Moschini M, Rai BP, van Rhijn BWG, Shariat SF, Smith EJ, Teoh JYC, Soukup V, Wood R, Xylinas EN, Soria F, Seisen T, Gontero P. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: Summary of the 2025 Update. Eur Urol. 2025;87:697-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Jaime-Casas S, Tripathi A, Pal SK, Yip W. Clinical Implications of the Molecular and Genomic Landscape of Upper Tract Urothelial Carcinoma. Curr Urol Rep. 2024;26:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Check DK, Jackson BE, Reeder-Hayes KE, Dinan MA, Faherty E, Kwong J, Mehta S, Spees L, Wheeler SB, Wilson LE, Lam C. Characteristics, healthcare utilization, and outcomes of patients with HER2-low breast cancer. Breast Cancer Res Treat. 2024;203:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Quaquarini E, Grillo F, Gervaso L, Arpa G, Fazio N, Vanoli A, Parente P. Prognostic and Predictive Roles of HER2 Status in Non-Breast and Non-Gastroesophageal Carcinomas. Cancers (Basel). 2024;16:3145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 751] [Article Influence: 53.6] [Reference Citation Analysis (6)] |
| 6. | Cheng X. A Comprehensive Review of HER2 in Cancer Biology and Therapeutics. Genes (Basel). 2024;15:903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 7. | Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA; DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1806] [Article Influence: 451.5] [Reference Citation Analysis (0)] |
| 8. | Bai X, He W, Yin H, Li X, Zhou X, Wei Z, Yu H, Gou X. Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: A retrospective single‑center experience. Exp Ther Med. 2022;24:704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Xu Z, Ma J, Chen T, Yang Y. Case report: The remarkable response of pembrolizumab combined with RC48 in the third-line treatment of metastatic urothelial carcinoma. Front Immunol. 2022;13:978266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ye J, Chen Z, Liao X, Wang X, Zhang C, Han P, Wei Q, Bao Y. HER2 expression in upper tract urothelial carcinoma and the relationship with clinicopathological characteristics-an analysis of 155 patients in Southwest China. World J Urol. 2024;42:521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Chou LP, Hsu CW, Yang SF, Lee CT, Ou YC, Lin KC, Hu CY, Jou YC, Tsai YS, Chow NH. Clinicopathologic Analysis of Micropapillary Urothelial Carcinoma of the Upper Urinary Tract: Implications for HER2-Targeted Therapy. Clin Genitourin Cancer. 2023;21:508.e1-508.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Yang M, Yao Y, Wang K, Qi L, Yang B, Khudadad M, Guo Y, Wang Y, Liu Y, Li L, Cao L, Huang Q, Guo Q, Li Q, Yao X, Wang C, Cao W. Clinicopathological characteristics and prognostic significance of HER2 status evaluation in patients with urothelial carcinoma: a retrospective single-center experience in China. Virchows Arch. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Ye J, Liao X, Qiu Y, Wei Q, Bao Y. A systematic review and meta-analysis for human epidermal growth factor receptor 2 on upper tract urothelial carcinoma patients. Tumori. 2024;110:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Sikic D, Keck B, Wach S, Taubert H, Wullich B, Goebell PJ, Kahlmeyer A, Olbert P, Isfort P, Nimphius W, Hartmann A, Giedl J; Bridge Consortium. Immunohistochemiocal subtyping using CK20 and CK5 can identify urothelial carcinomas of the upper urinary tract with a poor prognosis. PLoS One. 2017;12:e0179602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, Melquist J, Bondaruk J, Majewski T, Zhang S, Pretzsch S, Baggerly K, Siefker-Radtke A, Czerniak B, Dinney CP, McConkey DJ. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1360] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 16. | Tumor Pathology Committee of Chinese Anti-Cancer Association; Expert Committee on Urothelial Carcinoma of Chinese Society of Clinical Oncology. [Clinical pathological expert consensus on HER-2 testing in urothelial carcinoma in China]. Zhonghua Zhong Liu Za Zhi. 2021;43:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5527] [Article Influence: 345.4] [Reference Citation Analysis (3)] |
| 18. | Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 432] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 862] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 20. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8264] [Article Influence: 330.6] [Reference Citation Analysis (11)] |
| 21. | Cost NG, Delacroix SE Jr, Sleeper JP, Smith PJ, Youssef RF, Chapin BF, Karam JA, Culp S, Abel EJ, Brugarolas J, Raj GV, Sagalowsky AI, Wood CG, Margulis V. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1250] [Cited by in RCA: 1701] [Article Influence: 212.6] [Reference Citation Analysis (0)] |
| 24. | Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, Castro MAA, Gibb EA, Kanchi RS, Gordenin DA, Shukla SA, Sanchez-Vega F, Hansel DE, Czerniak BA, Reuter VE, Su X, de Sa Carvalho B, Chagas VS, Mungall KL, Sadeghi S, Pedamallu CS, Lu Y, Klimczak LJ, Zhang J, Choo C, Ojesina AI, Bullman S, Leraas KM, Lichtenberg TM, Wu CJ, Schultz N, Getz G, Meyerson M, Mills GB, McConkey DJ; TCGA Research Network, Weinstein JN, Kwiatkowski DJ, Lerner SP. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171:540-556.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1684] [Article Influence: 187.1] [Reference Citation Analysis (7)] |
| 25. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 521] [Article Influence: 104.2] [Reference Citation Analysis (1)] |
| 26. | Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol Biol. 2018;1655:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Somiya S, Kobori G, Ito K, Nakagawa H, Takahashi T, Koterazawa S, Takaoka N, Haitani T, Nagahama K, Ito M, Megumi Y, Higashi Y, Moroi S, Akao T, Yamada H, Kanno T. Preoperative risk classification for intravesical recurrence after laparoscopic radical nephroureterectomy for upper tract urothelial carcinoma in a multi-institutional cohort. Int J Urol. 2023;30:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Barth I, Schneider U, Grimm T, Karl A, Horst D, Gaisa NT, Knüchel R, Garczyk S. Progression of urothelial carcinoma in situ of the urinary bladder: a switch from luminal to basal phenotype and related therapeutic implications. Virchows Arch. 2018;472:749-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/