Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.109385
Revised: May 24, 2025
Accepted: September 2, 2025
Published online: October 24, 2025
Processing time: 168 Days and 17.7 Hours
Pancreatic cancer (PC) remains a highly lethal malignancy worldwide. Increasing evidence indicates that inflammation is a critical factor in the pathogenesis of pancreatic ductal adenocarcinoma (PDAC). Inflammatory cell infiltration within the PDAC tumor microenvironment promotes tumor growth and metastasis, while both local and systemic chronic inflammation is associated with an elevated risk of developing the disease. Given that the same immune cell populations are involved in mediating both inflammatory and immune responses, there exists a close interrelationship between inflammation and immunity in the context of PDAC. Various studies have reported the relationship between inflammation and PC. This review article provided a comprehensive summary of the roles of er
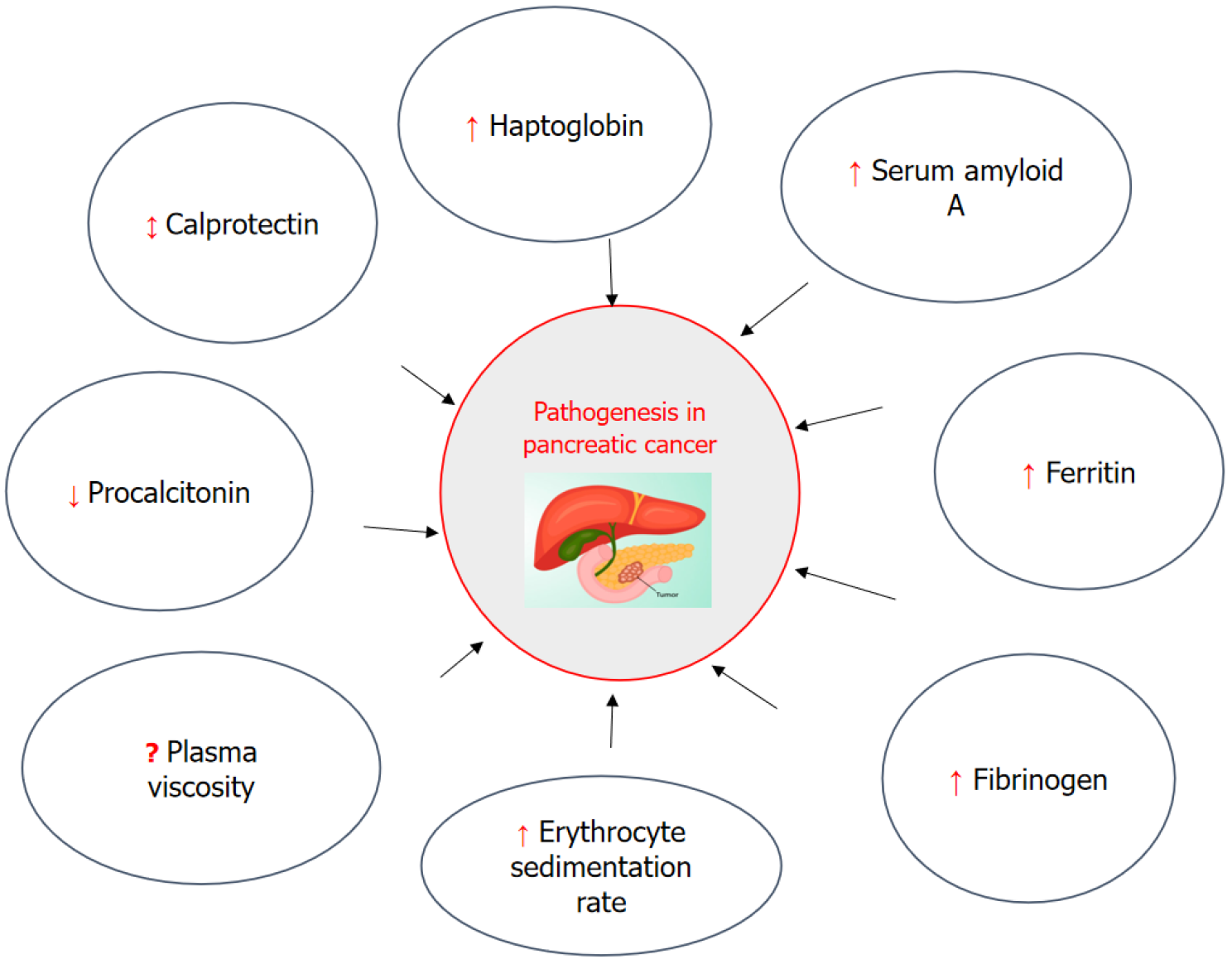
Core Tip: Inflammation is a key contributor to the initiation, progression, and metastatic dissemination of pancreatic cancer. In pancreatic ductal adenocarcinoma, inflammatory cell infiltration within the tumor microenvironment significantly promotes tumor proliferation and metastatic potential. Moreover, both systemic and localized chronic inflammatory conditions have been identified as important risk factors in the development of pancreatic ductal adenocarcinoma. This article provided a comprehensive summary of the roles of erythrocyte sedimentation rate, plasma viscosity, procalcitonin, calprotectin, haptoglobin, serum amyloid A, ferritin, and fibrinogen in the pathophysiological mechanisms underlying pancreatic cancer.
- Citation: Aslam A, Hamid H, Fatima E, Pervaiz S, Arif M, Irshad H, Shafqat A, Khan S, Khurshid H, Rafaqat S. Inflammatory biomarkers in the pathogenesis of pancreatic cancer: A literature review. World J Clin Oncol 2025; 16(10): 109385
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/109385.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.109385
Pancreatic cancer (PC) is one of the most lethal malignancies with a poor 5-year survival rate of around 5%-9% that has remained almost still since the 1960s[1,2]. The vast majority of cases over 85% are attributed to pancreatic ductal adenocarcinoma (PDAC), which arises from the ductal epithelial cells, predominantly located in the head region of the pancreas[2]. PC is a general term that encompasses all malignant tumors originating in the pancreas, including both exocrine and endocrine types. Among these, PDAC is the most prevalent, accounting for approximately 90%-95% of all PC cases. PDAC specifically arises from the epithelial cells lining the pancreatic ducts, making it a subtype of exocrine PC. In contrast, other forms of PC include acinar cell carcinoma, mucinous cystic neoplasms, and pancreatic neuroendocrine tumors, each differing in cellular origin, biological behavior, and clinical course. PDAC is particularly aggressive, characterized by rapid progression, late diagnosis, and a poor prognosis, with a five-year survival rate of less than 10%. While PC as a whole includes tumors with varying degrees of malignancy and outcomes, PDAC is generally the most lethal and commonly referred to in clinical discussions and research due to its overwhelming predominance and clinical significance.
Malignant pancreatic tumors can have a variety of histological features, including: (1) Ductal adenocarcinoma; (2) Cystadenocarcinoma; and (3) Other malignant tumors (sarcomas, metastatic, etc.)[3]. PDAC is preceded by cystic lesions such as intraductal papillary mucinous neoplasm (IPMN), intraductal tubulopapillary neoplasm and mucinous cystic neoplasm, while pancreatic intraepithelial neoplasia is a non-cystic lesion. Radiological techniques are used to identify cystic lesions clinically, whereas pancreatic intraepithelial neoplasia are found under a microscope in tissues following pancreatic resection or biopsy[4]. There are several different types of IPMN, ranging from benign to malignant, and it is a precursor lesion for PC. Several cancers have been connected to increased systemic inflammation. Cancer-related inflammation is thought to contribute to the development of malignancy by providing growth factors, promoting angiogenesis and invasion, and inhibiting antitumor activity by altering the immune cell population[5].
The global incidence of PC is projected to increase; however, the rate of this rise varies across countries, with some nations exhibiting a decelerating or declining trend in incidence[6]. PDAC accounts for 90%-95% of all PC cases reported worldwide, making it the fourth most lethal kind of cancer[7]. PC currently ranks as the third leading cause of cancer-related mortality in the United States, with approximately 62200 new diagnoses and 48800 deaths reported annually. Among pancreatic malignancies, PDACs represent nearly 90% of cases[8]. In several Asia-Pacific countries, including Armenia, Japan, Kazakhstan, New Zealand, Australia, and South Korea, PC is associated with notably high mortality rates. Conversely, in other nations such as China, mortality due to PC is on an upward trajectory, suggesting that peak death rates may be observed in the coming years[9].
Countries with a high human development index consistently report higher incidence, prevalence, and mortality rates associated with PC. This pattern is similarly observed across the Asia-Pacific region, where nations with elevated human development index levels demonstrate a greater burden of disease. The rising incidence in the Asia-Pacific population is attributed in part to increased life expectancy and ongoing socioeconomic development. However, in many low- and middle-income countries within the region, limited cancer registry infrastructure contributes to significant underreporting of PC cases[10].
By 2040, the global prevalence of PC is expected to rise, with projections indicating a higher susceptibility among women. When accounting for percentage changes, regions characterized by lower socioeconomic status are predicted to bear a disproportionately greater burden of PC compared to more affluent areas[11].
PC incidence and death rates were expected to increase dramatically in the next years, according to GLOBOCAN 2020 statistics[12]. The estimates, encompassing 36 cancer types by sex and age across 185 countries and territories, are derived from the most reliable local data sources available - primarily population-based cancer registries for incidence data and national vital statistics for mortality. As reported in the most recent GLOBOCAN estimates, PC is associated with one of the lowest survival rates among all malignancies and currently ranks as the sixth leading cause of cancer-related mortality worldwide[13,14]. Less than 5% of PC patients, of both sexes, survive for five years[15]. Between 2009 and 2018, the incidence of PC has risen across all ethnic groups and in both sexes. The disease disproportionately affects minority populations, males, and individuals residing in rural areas. Furthermore, the incidence is notably higher among older adults, indicating an elevated risk within this demographic group[16]. A marked disparity in PC survival rates exists between rural and urban areas, which may be attributed to variations in healthcare infrastructure, differences in stage at diagnosis, and underlying socioeconomic and lifestyle-related factors[17]. Notwithstanding significant progress in the biology of cancer, PC incidence and mortality rates are still rising yearly[18].
Cytokines, which are low-molecular-weight proteins involved in regulating various biological functions such as immune response, inflammation, metabolism, cell proliferation, and differentiation, play a dual role in cancer biology. In addition to exerting direct effects on cancer cells, they actively modulate the tumor microenvironment (TME). Several cytokines have a critical impact on both cancer progression and the processes underlying cancer immunoediting[19].
PC, particularly PDAC, is a highly lethal malignancy. Its development and progression are driven by a combination of genetic mutations, epigenetic alterations, TME changes, and inflammatory signaling. In addition to innate and adaptive immune cells, interleukins (ILs) and related cytokines serve as a conduit for communication between non-immune cells and tissues. As a result, ILs are crucial for the development, progression, and control of cancer. These ILs properties can be utilized to improve immunotherapies to boost their effectiveness and lessen their side effects[20,21]. For many years, the link between inflammation and cancer has been recognized due to strong connections between carcinogenesis and chronic inflammatory illnesses[22].
In PC, inflammation is characterized by complex interactions among immune cells, inflammatory cells, cytokines, and chemokines. Both cancer cells and the surrounding stromal and inflammatory components contribute to the development of an inflammatory TME. Inflammation is intricately linked to immune function, with immune cells playing dual roles in mediating both inflammatory processes and immune responses. TME is defined by two predominant features: Tumor-promoting inflammation and tumor-suppressive immunity in PC[23].
The TME in PC comprises a diverse array of cell types and intricate signaling pathways that collectively facilitate tumor progression. A central component of this unique microenvironment is the symbiotic interaction between PC cells and pancreatic stellate cells (PSCs), which plays a pivotal role in shaping the TME. Pancreatic stellate cells contribute to several malignancy-associated processes, including resistance to apoptosis, enhanced invasion and metastasis, angio
PDAC represents the most aggressive subtype of PC, characterized by extensive inflammation and desmoplasia. These features contribute to a hypoxic microenvironment, metabolic reprogramming, and immune suppression, all of which facilitate tumor progression and metastasis. A major barrier to effective treatment is the limited ability of current the
Various studies have reported the relationship between inflammation and PC. This review article provided a comprehensive summary of the roles of erythrocyte sedimentation rate (ESR), plasma viscosity, procalcitonin (PCT), calprotectin (CP), haptoglobin (Hp or Hpt), serum amyloid A (SAA), ferritin, and fibrinogen (Fib) in the pathophysiological me
Inflammation plays a pivotal role in the pathogenesis of various cancer types. It is believed to influence tumor initiation and progression through multiple mechanistic pathways, including the increased formation of DNA adducts, promotion of angiogenesis, and disruption of apoptotic signaling pathways[26].
Inflammation plays a critical role in the initiation, progression, and metastasis of PC. Several established risk factors, including alcohol consumption, tobacco use, diabetes, and chronic pancreatitis, are strongly associated with the de
Inflammatory infiltration within the TME of PDAC plays a significant role in tumor growth and metastasis. Both systemic and localized chronic inflammation have been implicated as risk factors for PDAC development. Given the overlapping roles of immune cell populations in inflammation and immune responses, these processes are closely interconnected. The PDAC microenvironment is characterized by an immunosuppressive profile, with an increased presence of myeloid-derived suppressor cells (MDSCs), M2-polarized macrophages, and regulatory T cells (Tregs), while M1 macrophages, dendritic cells (DCs), and effector CD4+ and CD8+ T lymphocytes are less prevalent. This immunosuppressive environment is primarily driven by soluble and exosome-associated cytokines, although direct, contact-dependent interactions between inflammatory and cancer cells also contribute. Among these factors, tumor necrosis factor-alpha (TNF-α) plays a pivotal role in promoting cancer risk, tumor progression, and cancer-associated cachexia[28].
Inflammation has been identified as a critical factor in the development and progression of various solid tumors, including PC. Both spontaneous and hereditary forms of chronic pancreatitis are associated with an increased risk of pancreatic malignancy. The inflammatory response contributes to increased cellular proliferation and genomic instability, thereby promoting the malignant transformation of pancreatic cells. Reactive oxygen species (ROS), cytokines, and inflammatory signaling mediators such as nuclear factor kappa B (NF-κB) and cyclooxygenase-2 (COX-2) play pivotal roles by enhancing cell cycle progression, suppressing tumor suppressor functions, and driving oncogene activation. Therapeutic strategies targeting inflammation, including anti-cytokine vaccines, thiazolidinediones, antioxidants, and inhibitors of NF-κB and COX-2, may hold promise for the prevention or treatment of PC. Advancing research on the molecular pathways linking pancreatic inflammation and carcinogenesis may yield novel anti-inflammatory agents or alternative approaches for early cancer detection and prevention. Pancreatic inflammation, driven by cytokines, ROS, and pro-inflammatory pathways, is considered a key contributor to the early onset of PC[29].
Tumor cells and associated stromal cells (e.g., CAFs, macrophages) secrete pro-inflammatory cytokines, including IL-6, which stimulate hepatic synthesis of acute-phase proteins like C-reactive protein (CRP). Tumor-associated macrophages and MDSCs within the TME produce IL-6. IL-6, in turn, drives the systemic acute-phase response, elevating CRP levels and promoting tumor-associated inflammation. Inflammation is recognized as a key contributor to carcinogenesis, mediated in part by pro-inflammatory markers such as IL-6 and CRP[30].
Additionally, the K-ras mutation represents a critical genetic event in the development of PC. Another study aimed to evaluate and compare serum levels of IL-6 and CRP among patients with PDAC, chronic pancreatitis, and healthy controls (HCs), and to investigate potential associations between these inflammatory markers and disease status, overall survival (OS), and K-ras mutation status in PDAC patients[30]. The study cohort comprised 135 PDAC patients, 25 individuals with chronic pancreatitis, and 25 HCs. Serum IL-6 and CRP levels were quantified using enzyme-linked immunosorbent assay, while K-ras mutations were detected through polymerase chain reaction-restriction fragment length polymorphism analysis. Serum levels of both IL-6 and CRP were significantly elevated in PDAC patients compared to HCs[30].
Elevated IL-6 and CRP levels were associated with more advanced disease features, including locally advanced tumors, lymphatic invasion, distant metastasis, and higher tumor stage. Among patients with unresectable PDAC, increased IL-6 Levels correlated with the presence of K-ras mutations. These findings suggest that IL-6 and CRP may play key roles in PDAC progression, with elevated levels reflecting more aggressive tumor behavior. Moreover, the observed association between IL-6 and K-ras mutations implies potential molecular cross-talk contributing to the pathogenesis and advancement of PDAC[30].
In PC, elevated CRP and IL-6 Levels are driven by tumor-derived and immune cell-mediated inflammation, activation of oncogenic signaling pathways, tissue necrosis, and systemic metabolic alterations. These biomarkers not only reflect disease burden but may also actively contribute to cancer progression and poor clinical outcomes. The primary limitation of study is that it focused on a limited subset of cytokines, whereas multiple cytokines are known to play roles in inflammation and tumorigenesis. A broader cytokine profile analysis is necessary to achieve a more comprehensive understanding of their involvement in disease progression. Nevertheless, the findings indicate that elevated serum IL-6 Levels are associated with more aggressive disease phenotypes and the presence of K-ras mutations in patients with unresectable PDAC[30].
Smoking, excessive body fat, chronic pancreatitis, and long-term diabetes are known risk factors for PC and are all characterized by elements of inflammatory processes. Prospective research on the association between inflammatory indicators and the risk of PC is, however, lacking. The risk of PC was not substantially correlated with any of the inflammatory markers, however, there was a marginally significant correlation between increased circulating soluble tumor necrosis factor-receptor 2 (sTNF-R2). However, in women, there was a significant correlation between elevated levels of sTNF-R1 and the risk of PC[31].
A greater body mass index and diabetes appeared to be associated with an increased risk for sTNF-R2. CRP and IL-6 do not appear to play a part in prospective analysis of PC risk, whereas sTNF-R1 appeared to be a risk factor in women and sTNF-R2 may operate as a mediator in the association between PC risk and overweight and diabetes. Proinflammatory proteins and cytokines’ significance in the pathophysiology of exocrine PC need more extensive prospective research[31].
Another study examined correlations between PC risk and common pre-diagnostic blood indicators of chronic inflammation (CRP, albumin, Hp, and leukocytes) in the Swedish Apolipoprotein-related MORtality RISk prospective cohort research. Serum levels of Hp, CRP, and leukocytes before diagnosis were linked to an increased risk of PC. Findings underscore the need to further explore this link and point to a potential involvement of chronic inflammation in the etiology of PC[32].
Inflammatory characteristics are believed to accelerate the growth of tumors, resulting in a worse median OS. Numerous minor investigations have concentrated on a broad spectrum of prognostic markers based on inflammation. The neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), and CRP to albumin ratio were among the inflammatory markers that were assessed using adequate data from 1294 out of 2323 patients who were diagnosed with PC at cancer centre between 2009 and 2021. The inflammatory benchmark index (IBI), a new composite score, was discovered. Advanced age, gender, tumor stage, cancer antigen 19-9 (CA19-9), NLR, LMR, PLR, CRP to albumin ratio, and IBI were shown to be prognostic indicators in univariate statistical analyses. Independent prognostic indicators in multivariate analyses included advanced age, gender, tumor stage, CA19-9, NLR, LMR, CRP to albumin ratio, and IBI. In addition to offering clinicians readily accessible predictive indicators and highlighting the role of inflammation in PC, these findings might also serve as helpful stratification criteria for studies that target patient inflammation or immune response[33].
PC is very difficult to diagnose early due to the absence of sensitive liquid biopsy techniques and reliable biomarkers. The authors tried to see if circulating inflammatory markers might also be used to diagnose PC in its early stages in addition to CA19-9. PC patients had considerably greater levels of circulating Fib, neutrophils, and monocytes, whereas their circulating albumin, prealbumin, lymphocytes, and platelets were significantly lower than those of HCs and other pancreatic tumors (OPT) patients. Patients with PC had significantly higher ratios of Fib to albumin (FAR), Fib to prealbumin (FPR), NLR, PLR, monocyte to lymphocyte (MLR), and Fib to lymphocyte (FLR) than patients with HC and OPT, and their prognostic nutrition index values (PNI) were lower[34].
Using the FAR, FPR, and FLR in conjunction with CA19-9 demonstrated the greatest diagnostic value in the training sets for separating individuals with early-stage PC from HC [area under the curve (AUC)] of 0.964 and early-stage PC from OPT (AUC of 0.924). When comparing patients with pancreatic head cancer and other pancreatic head tumors, the AUC was 0.915 for the combination of CA19-9, FAR, FPR, and FLR. When comparing patients with pancreatic body and tail cancer and other pancreatic body and tail tumors, the AUC was 0.894. To distinguish early-stage PC from HC and OPT, particularly early-stage pancreatic head cancer, a combination of FAR, FPR, FLR, and CA19-9 may be a useful non-invasive biomarker[34].
In addition to causing invasion and metastatic spread, inflammation accelerates tumor growth. CRP, CA19-9, and standard laboratory values were investigated in this retrospective analysis as preoperative predictive variables in patients with PC. Patients with CRP and CA19-9 Levels below the cut-off values at 5 years were 45% disease-free and 49% alive. The equivalent survival rates for patients with neoadjuvant therapy were 52% and 45%, and for those having surgery upfront, they were 45% and 40%, respectively. This new prognostic score, which combines CA19-9 and CRP, is a helpful preoperative tool for predicting survival[35].
Before receiving first- and second-line palliative chemotherapy for patients with unresectable PC, there are few unequivocal indicators available for predicting prognosis. To determine the most effective prognostic inflammatory marker, it examined the correlations between six inflammatory markers and OS: Glasgow prognostic score, PNI, platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, prognostic index, and CRP/albumin ratio. Higher CRP/albumin ratio patients had a worse OS than first-line patients with lower CRP/albumin ratio. Moreover, OS was better for patients with lower CRP/albumin ratio before second-line treatment than for those with greater CRP/albumin ratio. Consequently, CRP/albumin ratio may be a valuable biomarker for unresectable PC patient prognosis prediction in both first- and second-line treatment[36].
In summary, prediagnostic levels of circulating TNF-αR2, IL-6, and CRP were not linked to the risk of PC, indicating that systemic inflammation as indicated by circulating inflammatory components is unlikely to be a significant contri
Investigating the predictive importance of NLR, PLR, CRP, and CA19-9 in patients with PDAC was the goal of another study to better validate pre-operative risk classification and therapy. Individuals with radiographic lymph node metastases had statistically significantly higher levels of both CRP and CA19-9 than individuals without disease. In metastatic disease, NLR and CA19-9 Levels were likewise elevated. It discovered a negative link between the survival time and the CRP and neutrophil count in every patient. Simple, reproducible, affordable, and widely accessible markers, preoperative CRP, CA19-9, and NLR can provide data on the survival and metastasis of solid organs and lymph nodes, provide prognostic hints, and aid in the clinical staging of PDAC patients[38].
Shadhu and Xi[39], in 2019, explain inflammation markers such as NF-κB, IL-6, toll-like receptors, transforming growth factor β (TGF-β), TNF-α, IL-1-α, IL-4, IL-8, IL-1β, COX-2, serine protease inhibitor Kazal type-1 gene, ROS, C-X-C motif chemokine ligand 12. This article provided a comprehensive summary of the roles of ESR, plasma viscosity, PCT, CP, Hp, SAA, ferritin, and Fib in the pathophysiological mechanisms underlying PC as explained in Figures 1, 2, 3, and 4. The Circulating levels of inflammatory biomarkers in the pathogenesis of PC are explained in Figure 1.
The ESR, also known as the sed rate, is a commonly utilized hematological test that provides a non-specific indicator of systemic inflammation. It measures the rate at which erythrocytes settle in a column of anticoagulated whole blood under standardized conditions over one hour. Changes in blood composition, especially the presence of acute-phase reactants, are reflected in the ESR, which is an indirect indicator of systemic inflammation. Cytokine signaling, particularly that of IL-6, TNF-α, and IL-1, is the main cause of its rise. Hepatocytes are stimulated to create acute-phase proteins such as Fib, CRP, and SAA[40].
By encouraging the production of rouleaux and changing the surface charge and aggregation characteristics of erythrocytes, these proteins hasten the sedimentation of red blood cells. Immunomodulatory processes also affect ESR because persistent infection or inflammation increases immune cell activation and inflammatory mediator production, maintaining the acute-phase response. It is a clinical biomarker used to detect and monitor systemic inflammatory activity associated with various pathological conditions, including autoimmune disorders, infections, and malignancies. In a reported case, a 63-year-old male presented with a two-month history of fatigue, nocturnal sweating, and persistent daily fevers. Laboratory findings demonstrated elevated serum CRP levels, increased ESR, and leukocytosis. Imaging via computed tomography revealed the presence of a tumor situated between the duodenum and the pancreatic head[41]. ESR is a helpful, although generic, indicator of continuing inflammatory or immunological processes because it captures the physiologic interaction between immune activation and hepatic protein synthesis, despite its lack of specificity.
Systemic inflammation causes an increase in plasma viscosity, a measure of the fluid’s resistance to flow that reflects changes in the concentration and make-up of plasma proteins, especially acute-phase reactants. IL-6, IL-1, and TNF-α are examples of inflammatory cytokines that cause the liver to create more Fib, immunoglobulins, and other acute-phase proteins, which thicken and make plasma stickier. In addition to directly influencing viscosity, these proteins also have an impact on immunological and vascular reactions. Elevated plasma viscosity is therefore a result of cytokine-driven hepatic protein synthesis and reflects the body’s immune-modulatory response to inflammation, infection, or malignancy. While less commonly used than other inflammatory markers like CRP or ESR, plasma viscosity provides a sensitive and stable indicator of chronic inflammatory states[42].
Plasma viscosity is a hematological parameter used to assess blood thickness, which can provide insights into inflammatory processes. Inflammatory responses increase plasma protein levels, leading to elevated blood viscosity. PDAC remains one of the most lethal malignancies, largely due to the presence of a dense fibrotic stroma generated by tumor-associated stromal (TAS) cells, which supports tumor cell survival and impedes therapeutic efficacy. In this study, the authors developed a three-dimensional in vitro model that replicates the mechanical characteristics of the PDAC TME, offering a valuable platform for investigating disease pathophysiology. Using mesoscale indentation techniques, viscoelastic properties - specifically Steady-State Modulus and viscosity were quantified across resected PDAC tumors, chronically inflamed chronic pancreatitis regions, and histologically normal pancreatic tissue. Both tumors and pancreatitis exhibit elevated Steady-State Modulus values compared to normal tissue, indicating increased stiffness[43].
Notably, pancreatitis samples demonstrated significantly lower viscosity relative to both normal and tumor tissues. To recapitulate these biomechanical alterations in vitro, PDAC and TAS cells were isolated from patient tumors, and TAS cells were cultured within collagen hydrogels supplemented with PDAC cell-conditioned medium. After seven days, TAS-laden hydrogels cultured in a control medium exhibited mechanical properties similar to those of normal pancreatic tissue, whereas those exposed to a conditioned medium developed increased stiffness, mirroring tumor-like mechanical behaviour. Collectively, these findings establish a physiologically relevant in vitro model that emulates the in vivo stiffening observed in PDAC and highlights the potential of incorporating viscosity parameters into elastography techniques to improve the diagnostic distinction between between pancreatic cancer and pancreatitis[43].
PCT is a 14.5 kDa peptide consisting of 116 amino acids. It is composed of three parts: Calcitonin carboxyl-terminus peptide 1, sometimes referred to as katacalcin (21 amino acids), immature calcitonin (33 amino acids), and the amino terminus (57 amino acids). It reported the first instance in which closely monitored the shift in the serum PCT level and lesion size in a patient with PCT-secreting metastatic PanNET (G2). Following therapy, the patient’s pancreatic bulk and many liver lesions decreased, and their serum PCT level significantly dropped as well. However, more research is required to determine if PCT alterations and the clinical course of PanNET patients are related[44]. Similar processes create acute phase proteins such as CRP and PCT. One of the main sources of PCT production seems to be the liver. PCT might therefore be regarded as an acute phase protein. PCT’s apparent diagnostic capacity to distinguish bacterial infection from other sources of inflammation may be explained by its distinct kinetics rather than a radically different afferent route[45].
Approximately 60% of the soluble cytosol proteins in human neutrophil granulocytes are composed of CP, a 36-kDa protein that binds calcium and zinc. As dietary practices became more Westernized globally, the prevalence of inflammatory bowel disorders increased. Both Crohn’s disease and ulcerative colitis are long-term, crippling illnesses that affect people with significant morbidity and pose problems for healthcare systems worldwide. Since CP was discovered and described in the 1980s, fecal CP has become a very reliable, non-invasive biomarker for assessing intestinal inflammation. Faecal CP depicts the progression of human inflammatory bowel disorders and distinguishes between inflammatory and non-inflammatory gut disorders. The biological roles of the CP subunits S100A8 and S100A9 during the coordination of an inflammatory response at mucosal surfaces throughout organ systems have been clarified by recent research[46].
Inflammation and disease management in PDAC are closely related. Recent findings have identified damaged-associated molecular patterns in PDAC tissue, which are known to trigger inflammation in the innate immune system. One damaged-associated molecular pattern known to be a possible mediator of the disease control process in a variety of cancers is CP. The effects of circulating CP on disease control in patients receiving first-line treatment for advanced PDAC are examined. During first-line treatment for advanced PDAC, the disease control rate (DCR) was linked to a drop in the amount of circulating CP. In advanced PDAC, the innate immune system contributes to the effectiveness of chemo
There are data available for individuals with pancreatic illnesses, however fecal CP determination is helpful in the diagnosis of certain inflammatory gastrointestinal disorders. Assessing fecal CP determined whether intestinal inflammation was present in pancreatic disease patients. Low fecal elastase-1 concentration was the only variable independently linked to a high fecal CP concentration, according to the multivariate analysis, while the presence of diarrhoea and low fecal elastase-1 concentration were significantly associated with abnormally high fecal CP concentration in univariate analyses. The intestinal ecology may be altered by pancreatic insufficiency, which might lead to intestinal inflammation[48].
Current biomarkers for PCs have low sensitivity and specificity, and the surgical excision of these tumors is linked to substantial morbidity and death. Since serum levels of CP and calgranulin C are potential biomarkers of some cancers and major surgery complications, the current study examined the levels of both proteins in patients who had surgically removed benign and malignant pancreatic tumors. In comparison to the HCs, patients with malignant and benign tumors had substantially greater baseline blood levels of CP and calgranulin C. Benign tumor patients had considerably greater blood levels of both proteins than individuals with malignant tumors[49].
Following surgery, CP and calgranulin C blood levels were considerably higher than baseline, and this increase lasted for the duration of the seven-day follow-up period. Interestingly, the blood levels of both proteins were considerably greater in the 37 patients who developed postoperative pancreatic fistulas (POPFs) than in the patients who recovered without any problems from the first day of the postoperative period. Furthermore, the serum levels of CP and calgranulin C showed a strong predictive value for the onset of POPF; these two proteins’ predictive values outperformed those of the white blood cell count and the serum level of CRP. The findings imply that serum levels of CP and calgranulin C may be biomarkers for pancreatic tumors, surgical damage to the pancreatic tissue, and the emergence of POPFs[49].
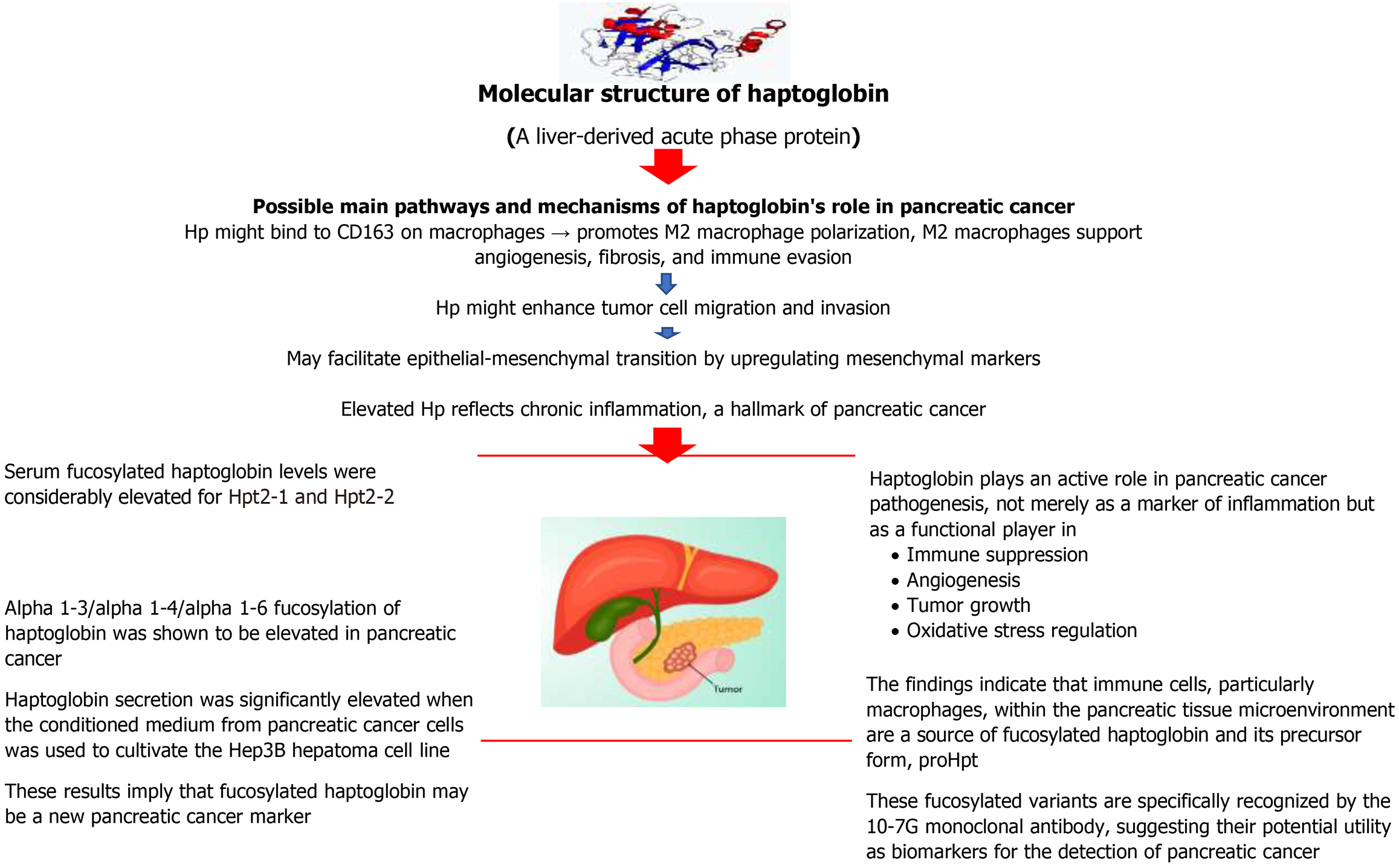
To eliminate the hemoglobin that is outside of your red blood cells, your liver produces a protein called Hp or Hpt. Oxygen is transported from your lungs to the rest of your body by the protein hemoglobin, which is found in red blood cells. IL-6-type cytokines and other inflammatory mediators greatly stimulate the hepatic production of Hp, a member of the evolutionarily conserved group of acute phase plasma proteins (APPs). Whether in connection with infections or a disease state like cancer, the function of inflammatory mediators in guiding host immune activity is increasingly being researched. It is unclear how these mediators’ downstream targets, the APPs, contribute to this tightly controlled immune response. The idea that Hp may have a regulatory role in immune function stems from the close relationship between inflammation and the cytokine-induced increase of Hp expression, which is known to be essential for immune system regulation. Consistent with plasma Hp’s suggested anti-inflammatory properties during an acute phase reaction[50].
Effective circulating biomarkers for pancreatic adenocarcinoma (PA) are lacking, which hinders timely and accurate diagnosis. The widely used biomarker, CA19-9, cannot be improved by a single test to successfully distinguish PA from benign diseases. To ascertain if the combination of Hp, SAA and CA19-9 would enhance the diagnosis of PA more than CA19-9 alone, the study set out to validate two acute phase proteins, Hp and SAA, as biomarkers for PA. Serum levels of Hp and SAA were considerably higher in PA patients than in HC participants and CP patients[51].
In contrast to individuals with other benign conditions, PA patients’ sera had substantially higher levels of Hp, whereas SAA did not reach significance in the same comparison. AUC = 0.792 for Hp and AUC = 0.691 for SAA were shown to be superior diagnostic markers across a range of threshold cutoffs, according to receiver operating characteristic analysis. When using certain cutoffs to reduce overall misclassification, Hp produced a sensitivity of 82.7% and a specificity of 71.1%, whereas SAA produced a sensitivity of 34.7% and a specificity of 90.2% when separating PA patients from all non-PA controls. The sensitivity and specificity of CA19-9 in the same sample collection were 77.3% and 91.1%, respectively. With a sensitivity of 81.3% and a specificity of 95.5%, the panel diagnostic screen that included data from Hp, SAA, and CA19-9 outperformed CA19-9 alone in terms of overall accuracy. In a panel diagnostic screen, these findings show that Hp and SAA help differentiate PA from benign diseases and HCs. For a more accurate diagnosis of PA, this study advocates using a combination of biomarkers[51].
Certain malignant transformations have been shown to alter oligosaccharide structures; as a result, these changes may serve as tumor markers for specific cancer types. The conditioned medium of the PC cell lines includes several fu
PC was discovered to have a highly fucosylated protein of about 40 kD, which an N-terminal analysis identified as the beta chain of Hp. Although other illnesses such as hepatocellular carcinoma, liver cirrhosis, gastric cancer, and colon cancer have been shown to have fucosylated-Hp (Fuc-Hpt), the prevalence of PC was noticeably greater. When PC reached an advanced stage, Fuc-Hpt was more commonly seen; following surgery, it vanished. Alpha 1-3/alpha 1-4/alpha 1-6 fucosylation of Hp was shown to be elevated in PC, according to a mass spectrometry study of Hp that was separated from the blood of individuals with the disease and the medium from a PC cell line, PSN-1[52].
Hp secretion was significantly elevated when the conditioned medium from PC cells was used to cultivate the Hep3B hepatoma cell line. These results imply that Fuc-Hpt may be a new PC marker. There were two potential outcomes for the fucosylation of Hp. Two theories exist: First, PC cells themselves create Fuc-Hpt; second, PC produces a factor that causes the liver to make Fuc-Hpt[52].
Fucosylation is a crucial glycosylation implicated in inflammation and cancer. Numerous investigations have shown that a range of cancer patients had markedly elevated blood levels of Fuc-Hpt. Three distinct phenotypes of Hp have been identified based on its genetic background: Hpt1-1, Hpt2-1, and Hpt2-2. The occurrence of several human illnesses is linked to the normal levels of serum Hpt, which vary depending on the Hpt phenotype. Here, it used two types of enzyme-linked immunosorbent assay (ELISA) to examine the impact of Hpt phenotype on measured Fuc-Hpt. Re
The glyco-biomarker of PC, Fuc-Hpt is well-established. A new anti-glycan antibody [10-7G monoclonal antibody (mAb)] that selectively detects Fuc-Hpts, such as proHpt, was recently developed by us. Patients with PC had higher serum concentrations of the 10-7G value, as determined by ELISA, than did HCs. Which particular tissue or cell type creates proHpt, or Fuc-Hpts, is yet unclear, nevertheless. It used 10-7G mAb to perform immunohistochemistry (IHC) and ELISA studies on tissue samples from PC in this investigation[54].
One pancreatic tissue segment out of twenty-one had direct 10-7G mAb staining of pancreatic cells. On the other hand, 12 out of 21 sections showed positive immune cell staining. Serum 10-7G median value was somewhat higher in IHC-positive patients, but there was no significant difference in 10-7G expression between the positive and negative staining IHC groups. The greatest amounts of proHpt were detected by Western blot using 10-7G mAb in differentiated THP-1 cells (a human acute monocytic leukaemia cell line), a human acute monocytic leukaemia cell line, out of all the leukemic cell lines that were tested. Remarkably, either hypoxic circumstances or IL-6 therapy resulted in a significant increase in proHpt synthesis in vitro. The findings indicate that immune cells, particularly macrophages, within the pancreatic tissue microenvironment are a source of Fuc-Hpt and its precursor form, proHpt. These fucosylated variants are specifically recognized by the 10-7G mAb, suggesting their potential utility as biomarkers for the detection of PC[54].
A mass spectrometric technique was created to use fucosylated glycans as possible indicators for PC and to clarify the N-glycan structures of serum glycoproteins. To compare the N-glycans from the blood of 16 patients with PC with those from 15 people with benign conditions - five normals, five with CP, and five with type II diabetes - this technique was used to measure Hp in human serum. A mAb was utilized to extract Hp from just 10 μL of serum, and desialylated N-glycans were subjected to quantitative permethylation before MALDI-QIT-TOF MS analysis[55].
Eight desialylated N-glycan structures of Hp were found, and PC samples were the first to describe a bifucosylated triantennary structure. Samples from PC had higher levels of core and antennary fucosylation than samples from benign circumstances. A substantial difference was found between the benign conditions examined and patients with PC at all stages, according to the estimated fucosylation degree indexes. According to this study, a blood test based on mass spectrometric analysis of Hp fucosylation patterns might be a novel way to diagnose PC[55]. The overall summary of Hp causes in pathogenesis in PC is shown in Figure 2.
SAA, an acute-phase reactant, plays a significant role in both acute and chronic inflammatory processes and is widely utilized in clinical laboratories as a biomarker of inflammation. Although both SAA and CRP are commonly used acute-phase proteins, SAA demonstrates superior diagnostic sensitivity in specific clinical scenarios, including viral infections, severe acute pancreatitis, and renal transplant rejection. Its utility is particularly notable in immunocompromised patients, individuals with cystic fibrosis receiving corticosteroid therapy, and preterm neonates with late-onset sepsis. However, for broader inflammatory and infectious conditions, particularly bacterial infections, the combined use of SAA with other APPs such as CRP and PCT enhances diagnostic sensitivity and provides a more comprehensive assessment of the inflammatory status. This integrative approach can better inform clinical decisions, particularly regarding antibiotic management. Despite its promise, the diagnostic implications of various SAA isotypes remain incompletely understood due to the limited availability of clinically validated data, as current knowledge is largely based on experimental studies. Moreover, existing A-SAA assays, which utilize polyclonal antibodies, lack isotype specificity and may cross-react with multiple inflammatory conditions. Consequently, in clinical practice, SAA measurement is often interpreted alongside other biomarkers to improve diagnostic accuracy and clinical utility[56].
SAA is a major acute-phase protein produced predominantly by the liver in response to pro-inflammatory cytokines, particularly IL-6, IL-1β, and TNF-α. Persistently elevated SAA levels, especially in chronic inflammatory diseases, can lead to secondary amyloidosis, where misfolded SAA fragments deposit as amyloid fibrils in organs, disrupting normal function. Thus, SAA is both a marker and mediator of inflammation, linking cytokine signaling, acute-phase protein synthesis, and immune system activation[57]. SAA is an excellent indicator of inflammation in the body and has been shown to rise quickly by up to 1000 times in response to acute inflammation[58]. Several cytokines, such as IL-1, IL-6, and TNF-α, promote the development of SAA, an acute-phase hepatic protein released during acute infections and tissue damage[59]. A higher level of SAA protein is seen in cancer patients at an early stage in a variety of common cancers, including melanoma, lung, ovarian, renal, uterine, and nasopharyngeal cancer[60].
SAA might be a valuable cancer biomarker. Finding a predictive biomarker is crucial to assisting patients with advanced PC (PCa) in selecting the best chemotherapy treatments. The purpose of another study was to ascertain if baseline SAA levels were related to treatment response, progression-free survival (PFS), and OS in patients with PCa who were receiving chemotherapy. The results of the multivariate analysis demonstrated that SAA was an independent predictor of both OS and PFS. Extended OS and PFS were linked to low SAA. In contrast to patients who received nab-paclitaxel plus gemcitabine (AG) or SOXIRI, those with a low SAA who received modified FOLFIRINOX had longer OS and PFS. There was no significant difference between the three chemotherapy regimens in patients with a high SAA. Because baseline SAA can be easily and quickly analyzed from peripheral blood, it may be a valuable clinical biomarker for patients with PCa, both as a prognostic biomarker and as a guide for choosing chemotherapy treatments[61].
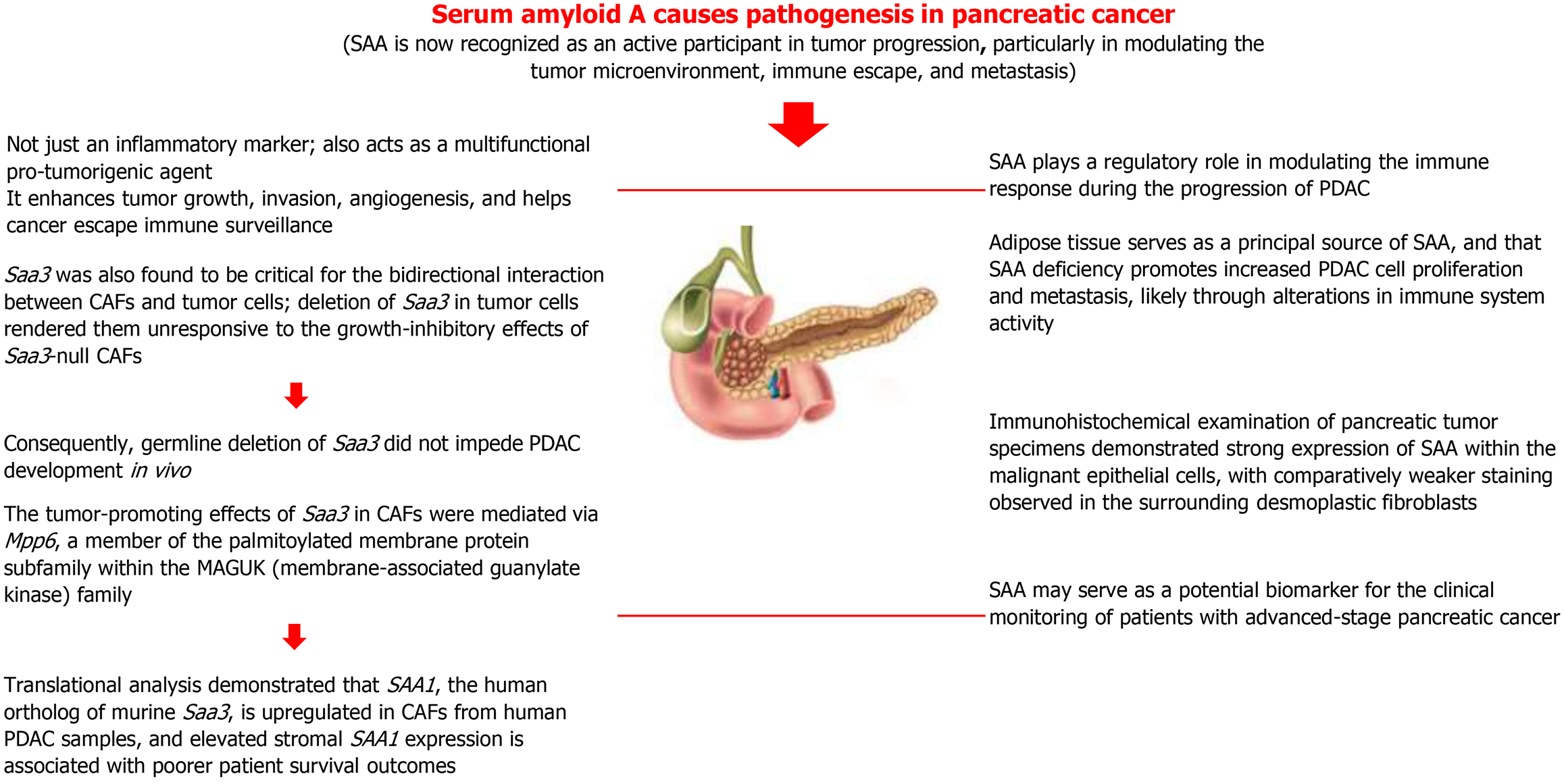
The detection and tracking of human PC depend on new biomarkers. Serums obtained from naked mice that had human PC cells transplanted in their pancreas were examined using Surface Enhanced Laser Desorption Ionization mass spectrometry in our earlier work. The amount of SAA was strongly connected with the tumors’ gradual development. Yokoi et al[62] investigated the potential correlation between patient plasma levels of SAA and the stage of PC. SAA levels in the plasma of patients with PC were substantially greater than those of normal volunteers, and they tended to rise as the disease progressed. In particular, the SAA level in stage IV B patients was substantially greater than that of normal volunteers and stage I patients. An immunohistochemical examination of a pancreatic tumor sample showed more SAA expression in the carcinomas’ malignant epithelium and less desmoplastic fibroblast staining. Patients with APC may benefit from SAA as a biomarker[62].
PDAC cells interfere with the immune response and interact with the host system. The host cells produce mediators as a result of this communication, which fosters the expansion of PDAC cells and the development of premetastatic niches. Despite advancements, little is known about the precise processes and contributing elements of immune regulation in the development of PDAC. To create PDAC mouse models (WT-KPCL and KOSAA-KPCL), male C57BL/6 mice aged eight weeks were given intraperitoneal injections of luciferase-expressing KPC cells (KPCL). Phosphate-buffered saline was given to the control groups, KOSAA and WT. As the tumor grew, KPCL mice lost a lot of weight and developed skeletal muscle and adipose tissue loss, which is a marker of PC[63].
According to a comparative analysis, tumor mice had 86 upregulated and 35 downregulated circulating proteins out of 734 total. It was shown that the most highly expressed proteins in tumor mice’s plasma were SAA 1 and 2, which were remarkably 1024 times more plentiful than in controls. It assessed SAA1, 2, and 3 expressions in adipose tissue, liver, muscle, and tumor to identify the primary source of SAA in tumor animals. It is noteworthy that in WT-KPCL, adipose tissue contributed significantly to SAA levels. However, it could not find SAA expression in the KOSAA-KPCL tumor tissue, suggesting that KPCL cells are a very small source of SAA. It investigated the function of SAA in PDAC cell proliferation in vivo since we were intrigued by these results. SAA-KO mice (KOSAA-KPCL) and controls (WT-KPCL) were given injections of luciferase-expressing KPCL. Interestingly, bioluminescence imaging showed that the pancreas of rats lacking SAA had a sharp increase in KPCL. A considerably greater risk of liver metastases - 62.5% higher in KOSAA-KPCL than in WT-KPCL - supported this[63].
The protein SAA binds retinol, and retinoic acid is essential for the immune system. In the tumor tissue separated from KOSAA-KPCL, it observed a substantial drop in CD8 expression, together with a lower quantity of retinoic acid in the plasma of KOSAA-KPCL animals. These findings imply that during PDAC cell development, SAA regulates the immune response. Through immunological regulation, adipose tissue is probably a key source of SAA, and its absence promotes the proliferation and metastasis of PDAC cells. These results imply that SAA may be used therapeutically to treat PC[63].
PDAC is characterized by a dense desmoplastic stroma, predominantly composed of CAFs, which are known to contribute to tumor progression, therapeutic resistance, and immunosuppressive mechanisms. In another study, the tran
The analysis revealed Saa3, a member of the SAA apolipoprotein family, as the most differentially expressed gene and a central mediator of the tumor-supportive activity of platelet-derived growth factor receptor alpha-positive CAFs. Functionally, Saa3-proficient CAFs enhanced tumor cell growth in orthotopic models, whereas Saa3-deficient CAFs suppressed tumor progression. Saa3 was also found to be critical for the bidirectional interaction between CAFs and tumor cells; deletion of Saa3 in tumor cells rendered them unresponsive to the growth-inhibitory effects of Saa3-null CAFs. Consequently, germline deletion of Saa3 did not impede PDAC development in vivo. The tumor-promoting effects of Saa3 in CAFs were mediated via Mpp6, a member of the palmitoylated membrane protein subfamily within the membrane-associated guanylate kinase family. The translational analysis demonstrated that SAA1, the human ortholog of murine Saa3, is upregulated in CAFs from human PDAC samples and elevated stromal SAA1 expression is associated with poorer patient survival outcomes. These findings suggest that targeted inhibition of SAA1 in CAFs may represent a novel therapeutic strategy for PDAC[64]. The overall summary of SAA causes in pathogenesis in PC is shown in Figure 3.
Ferritin is primarily known as an intracellular iron storage protein, but it also functions as an acute-phase reactant that is upregulated during inflammation. Its expression is strongly induced by pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α as part of the cytokine-mediated acute-phase response[65].
During systemic inflammation or infection, ferritin synthesis increases in hepatocytes and macrophages, leading to elevated serum levels. This response serves a dual purpose: It sequesters iron away from pathogens (limiting microbial growth) and reflects immune-modulatory activity, as ferritin itself can influence immune responses by modulating T-cell activity and suppressing pro-inflammatory cytokine production in some contexts. Elevated ferritin levels are commonly observed in inflammatory conditions, infections, malignancies, and autoimmune diseases, and in severe cases such as macrophage activation syndrome or cytokine storms, it can signal hyperinflammation. Therefore, ferritin serves both as a marker of inflammation and an active participant in immune regulation and iron homeostasis[66].
Although serum ferritin (SF) is another well-known inflammatory measure, it is not clear if it either causes or reflects inflammation or if it is a part of an inflammatory cycle. SF is a sign of cellular damage because it is produced by damaged cells. Although SF is thought to be a harmless protein, it has lost (dumped) the majority of its usual iron complement, which is very hazardous when unliganded[67].
SF is elevated in patients with PC. PC was associated with a significantly higher SF level than controls (P < 0.01), calcifying (P < 0.05), non-calcifying (P < 0.05), and recurrent (P < 0.01) CP. 10 out of 36 patients with CP and 10 out of 26 patients with non-pancreatic illnesses had elevated SF levels, however, whereas 6 out of 22 patients with PC had ferritin levels within the normal range. Despite being often elevated in PC, the findings imply that SF is not a reliable indicator of PC[68].
Ferritin and carcinoembryonic antigen (CEA) were highlighted as clinically beneficial in the detection of PC. CEA was shown to be elevated in 51% of PC patients, as well as aberrant in 31% of extra-pancreatic illnesses and 22% of CP patients. It was discovered that the CEA was higher in people with metastatic PC than in those with localized tumors. With IgG for liver cirrhosis and alkaline phosphatase for extra-pancreatic gastrointestinal cancers, CEA was associated with the participants’ ages in all of the materials. In addition to being elevated in 73% of patients with PC, ferritin was also abnormal in 38% of patients with extra-pancreatic illnesses and 40% of patients with CP[69].
All of the patients with CP who were examined after a relapse had high blood ferritin levels. It may be inferred that because of their low sensitivity and specificity, ferritin and CEA are not reliable indicators of PC. Several variables impact both of them: Ferritin is determined by the presence of acute inflammation and cell necrosis, while CEA is mostly determined by age and liver dysfunction[69].
To examine the various hepatic alterations that impact SF in chronic pancreatic and other digestive disorders, SF, prealbumin, pscudocholinesterase, a-I-antitrypsin, and caeruloplasmin were measured in control subjects and patients with PC, CP, or extra-pancreatic disease primarily of gastrointestinal origin. Comparing extra-pancreatic illness and PC to controls, elevated levels of circulating ferritin were observed. Ferritin was shown to be correlated with the other proteins under investigation as well as with alkaline phosphatase, alanine aminotransferase, and total bilirubin. It concluded that liver alterations, with cholestasis most likely being the main cause, are substantially responsible for the rise in SF in chronic pancreatic and other gastrointestinal disorders[70]. SF may be a useful indicator for predicting the outcome of hypofractionated radiation treatment for PC[71].
Some malignancies, such as breast and pancreatic tumors, have higher blood levels of ferritin, an iron-storing protein. Through iron transport, immunological control, and angiogenesis, ferritin has been demonstrated to contribute to carcinogenesis as well as the proliferation and survival of cancer cells. Ferritin might be a useful biomarker for assessing PC patients’ chances of survival. Patients with increased SF had a worse OS rate when examined continuously. Upon categorical analysis, individuals with greater ferritin levels had a lower OS rate[72].
Ferritin was moving toward significance in multivariate analysis with pretreatment SF, tissue inhibitor of metalloproteinase 1, urokinase-type plasminogen activator (uPA), and vascular endothelial growth factor, whereas tissue inhibitor of metalloproteinase 1 was the sole significant predictive predictor. In a phase III study of metastatic PC, pretreatment SF was associated with a noticeably worse OS. For PC, the pretreatment SF level is a useful prognostic biomarker that should be assessed as a predictor of response to new treatment approaches[72].
Detecting malignant IPMNs is facilitated by elevated SF. One potential diagnostic for detecting cancer in IPMNs is SF[73]. One of the worst conditions without an early warning sign is PC. SF and possible genes that may contribute to ferritin and PC risk were examined to see if they were indicators of PC risk. A set of genes that showed high correlations between ferritin and PC was investigated using an Oncomine database as a platform. Here, it demonstrates that elevated SF levels can signal a risk for PC, pointing to SF as a novel tumor marker that could aid in the detection of PC. Fur
The growth and spread of pancreatic tumors are influenced by inflammation linked to cancer. Additionally, current research has linked the inflammatory TME to resistance-inducing and therapeutic response modulation. Another study examined the predictive and prognostic significance of the inflammatory biomarkers SF and CRP in patients with APC. In a categorical analysis, OS was worse for individuals with greater ferritin (> median) or CRP (> 25th percentile). Fur
However, only individuals with elevated levels of both inflammatory biomarkers showed a substantial decline in OS when patients were assessed based on their ferritin and CRP levels. For individuals with inoperable pancreatic tumors, SF and CRP are independent predictors of a reduced survival time. Furthermore, the assessment of patients using both biomarkers revealed that, while being independent, their prognostic significance represented the overall level of inflammation linked to cancer. Therefore, more research should be done on SF and CRP as clinical indicators[75].
Fib is a soluble plasma glycoprotein produced by the liver and functions as a key acute-phase protein. Blood clotting, fibrinolysis, cellular and matrix interactions, inflammation, wound healing, and neoplasia are all significant processes in which fibrin and Fib have overlapping functions. Fibrin synthesis itself and the complimentary interactions between certain binding sites on Fib and extrinsic molecules such as proenzymes, clotting factors, enzyme inhibitors, and cell receptors play a major role in regulating these activities. Within the N-terminal E domain, disulfide bridging connects two sets of three polypeptide chains, A alpha, B beta, and gamma, to form Fib[76].
Fib synthesis markedly increased in response to pro-inflammatory cytokines, especially IL-6, as well as IL-1β and TNF-α. As part of the cytokine-driven acute-phase response, Fib plays a dual role in hemostasis and immune modulation. In the coagulation cascade, it is converted to fibrin by thrombin to form blood clots, aiding in tissue repair and limiting pathogen spread. Elevated Fib levels during inflammation increase plasma viscosity and promote erythrocyte agg
Although there is no unique biomarker for the early detection of PDAC, early detection is crucial since PDAC can be fatal. It previously used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to identify Fib α chain as a viable biomarker for distinguishing between individuals with and without PDAC. Chung et al[78] confirmed serum Fib’s clinical utility as a PDAC biomarker. There was a substantial difference in mean Fib levels between the PDAC and control groups. When comparing blood Fib levels to distinguish PDAC patients from control patients using receiver operating characteristic analysis, the overall sensitivity and specificity were 67.4% and 83.6%, respectively, using a threshold value of 427 ng/mL. PDAC patients with distant metastases had substantially higher serum Fib levels than those without. Serum Fib levels and carbohydrate antigen 19-9 Levels did not correlate well, but when combined, these two indicators improved survival prediction. A potential biomarker for PDAC diagnosis and prognosis prediction is serum Fib levels[78].
Although the FPR, a new immune-nutritional biomarker, has been linked to prognosis in many cancer types, its function in predicting the prognosis of resectable PC remains unclear. The FPR cutoff value of 0.29 was ideal. According to multivariate analysis, the following factors independently predicted OS: Tumor node metastasis stage, CEA, CA19-9, FPR, and managing nutritional status. The five previously mentioned parameters were used to create the nomogram. The nomogram’s predictive and discriminative capabilities were superior to those of the traditional tumor node metastasis stage, according to decision curve analysis and time-dependent AUC. For individuals with PC that is treatable, FPR is a viable biomarker for prognostic prediction. When developing personalized treatment plans and predicting survival, practitioners can benefit from the nomogram based on FPR[79].
An association between plasma Fib and PC has not been demonstrated in a large-scale clinical investigation, even though variations of plasma Fib have been reported in a small number of pancreatic malignant tumors. Patients with PC had considerably greater levels of plasma Fib than those with benign pancreatic tumors. In PC, 41.1% of patients had hyperfibrinogenemia (> 4.20 g/L), while in benign pancreatic illness, no favourable outcomes were found[80].
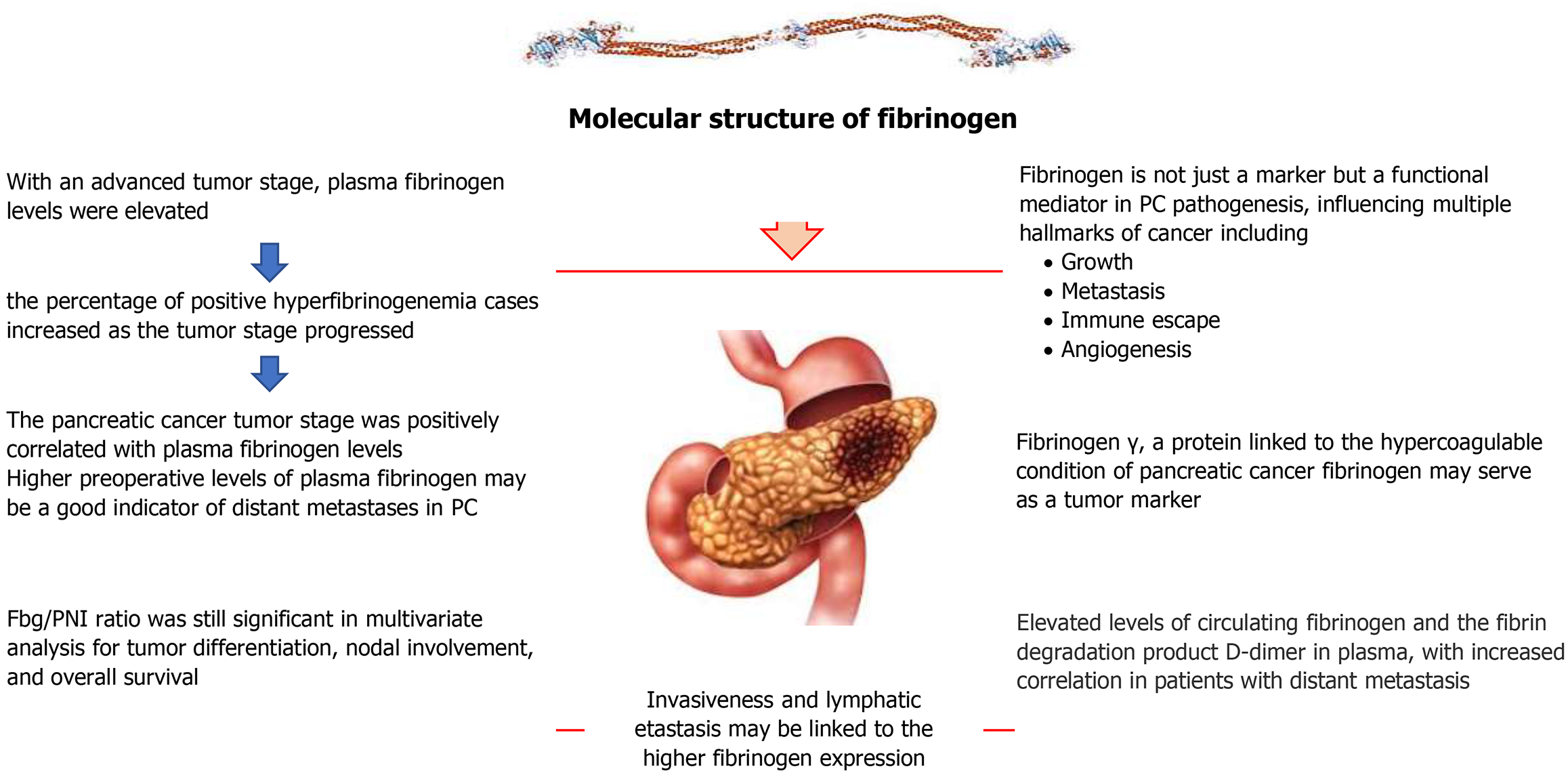
In PC with an advanced tumor stage, plasma Fib levels were elevated. In PC, the percentage of positive hyperfibrinogenemia cases increased as the tumor stage progressed. Compared to the group that did not have distant metastases, the distant-metastasis group had significantly higher levels of plasma Fib. Both univariate and multivariate analysis showed that distant metastases of PC were strongly correlated with high plasma Fib levels. The PC tumor stage was positively correlated with plasma Fib levels. Higher preoperative levels of plasma Fib may be a good indicator of distant metastases in PC in humans[80].
One of the worst prognoses of any solid cancer is that of PDAC, which has an extremely low overall and PFS rate. Clinically, tumors from PDAC patients exhibit elevated expression levels of many coagulation systems (such as tissue factor) and fibrinolytic system components, such as urokinase plasminogen activator (uPA) and uPA receptor (uPAR). Furthermore, individuals with distant metastases of PC exhibit higher levels of circulating Fib and the fibrin breakdown product D-dimer in their plasma[81].
Another study hypothesis was that the growth of PDAC tumors would be disrupted by targeting the components of the plasminogen activation pathway. Individual components of the fibrinolytic system (uPA and uPAR) were removed utilizing clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) technology on PC cell lines from the KPC animal model (K-rasLSL.G12D/+; p53LSL.R172H/+; Elas-CreER). Compared to Cas9 control tumors, KPCL with uPA and uPAR knocked out had noticeably smaller tumors. KPC murine PC cells injected orthotopically in Plg-/-mice showed significantly reduced tumor growth when compared to WT mice. This suggests that the pro-tumor effect of tumor cell-derived uPA/uPAR expression was linked, at least in part, to plasmin(ogen) activation to enhance PDAC tumor progression[81].
It employed Plg or Fib-specific antisense oligonucleotide (ASO) therapy to precisely reduce plasminogen or Fib levels in mice with human PDAC tumors to further examine the role of Plg or Fib in the microenvironment in PDAC de
It has been difficult to find serum indicators for PC. CA19-9 is the most widely used, yet its sensitivity and specificity are not very high. It looked for possible serum indicators for PC using large-scale proteomics. All pancreatic tumors had a similar overexpression of 154 proteins. Among them, Fib γ stood out as being overexpressed in PC tissue by IHC (67% vs 29%, P < 0.05) and serum by enzymatic analysis (54.1 ± 64.1 compared to 0.0 ± 0.0 mg/dL, P < 0.05) in comparison to normal pancreas. Mass spectrometry and two-dimensional gel electrophoresis were used in proteomic research to successfully identify 154 putative serum indicators for PC. Fib γ, a protein linked to the hypercoagulable condition of PC, was able to distinguish between cancer and normal sera among individuals. In PC, Fib may serve as a tumor marker[82].
Chang et al[83] aimed to clarify the role of the preoperative Fib to FPR, which has not been well investigated in the past, in forecasting the prognosis of PDAC. The ideal FPR threshold of 14.77 was established, which made it easier to stratify patients into groups with low and high FPR values. The high FPR cohort’s patients showed notably worse OS and recurrence-free survival (RFS) rates than their low FPR counterparts. For both RFS and OS, multivariate Cox regression analysis highlighted FPR as an independent predictive factor. Clinical utility and predictive accuracy were better with FPR than with the NLR. RFS and OS in PDAC patients having radical resection may be independently predicted by the preoperative Fib to FPR. FPR could be a useful supplement to the existing prognostic models, possibly assisting in the formulation of treatment choices and patient care plans for PDAC[83].
Patients with PC had higher Fib levels. Invasiveness and lymphatic metastasis may be linked to higher Fib expression. Blood loss during surgery was not decreased by using vitamin K in perioperative care[84].
Examining the predictive significance of the serum Fib to prognostic nutritional index (Fbg/PNI) ratio in patients having resection for pancreatic ductal adenocarcinom was assessed. The univariate study of OS and disease-free survival (DFS) revealed that Fbg/PNI was a significant predictive predictor. Fbg/PNI was still significant in multivariate analysis for tumor differentiation, nodal involvement, and OS. Using multivariate analysis, Fbg/PNI represented a significant independent prognostic predictor of poor DFS. Intending to cure PC, preoperative Fbg/PNI represents a new substantial independent prognostic predictor for OS and DFS[85].
Surgery is a crucial therapy for PDAC; the prognostic indicators currently in use are insufficient. The usefulness of the FA score (Fib/albumin ratio) in forecasting PDAC postoperative outcomes was assessed. All patients with extended survival times had FA < 130, whereas none of the patients with FA ≥ 130 Lived for more than 3 years. All other indices were not as good in predicting postoperative outcomes as the FA score. Recurrence-free and OS were considerably worse in patients with FA ≥ 130 than in those with FA < 130. For predicting the postoperative results of PDAC, the FA score is helpful. By identifying patients who are expected to have poor postoperative prognoses before surgery, adjuvant or neoadjuvant treatment may improve results[86]. Fib, CA19-9, and D-dimer can all be employed as markers for tracking disease recurrence in patients with resectable PC after surgery, but only preoperative D-dimer can predict survival[87].
One of the main drivers of the development and spread of cancers, including PC, is the systemic inflammatory response. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and FAR were the prognostic factors determined and compared in this study between resectable PC and locally progressed or metastatic PC. The OS of patients with resectable PC who had low preoperative MLR and PLR was considerably higher. Low FAR, MLR, NLR, and PLR were substantially linked to a lower chance of dying for individuals with locally advanced or metastatic PC. MLR and FAR were independent predictors of whether PC was metastatic or locally progressed. In resectable PC and locally progressed or metastatic PC, the prognostic functions of FAR, MLR, NLR, and PLR were distinct. The predictive value of FAR was highest in cases with locally progressed or metastatic PC. In locally progressed or metastatic PC, low FAR was positively connected with OS, which might be utilized to forecast the prognosis[88].
Coagulation and fibrinolysis abnormalities are commonly seen in cancer patients. In several cancer forms, new information revealed a correlation between tumor stage and prognosis with plasma D-dimer and Fib levels. The predictive significance of Fib and plasma D-dimer in digestive malignancies is investigated[89]. In intestinal cancer, elevated plasma D-dimer levels were higher than Fib and were associated with poorer OS. The most detrimental effects of increased Fib and plasma D-dimer on OS were found in colorectal cancer. High plasma D-dimer was more predictive of non-metastatic patients, but it had a more unfavorable prognostic effect on metastatic patients as compared to non-metastatic patients with digestive cancer. Pretreatment Fib and plasma D-dimer were both strong indicators of poor survival in individuals with digestive cancer who had various characteristics. To confirm their effects on cancer prognosis, more research is necessary[89]. The overall summary of Fib causes in pathogenesis in PC is shown in Figure 4.
PC remains a highly lethal malignancy, largely due to challenges in early detection and the limited effectiveness of conventional therapeutic modalities. While surgical resection combined with chemotherapy offers potential curative outcomes when the disease is diagnosed at an early stage, a significant proportion of patients exhibit resistance or limited response to standard treatments. Notably, advancements in targeted therapies designed to interfere with specific molecular pathways critical for tumor growth and invasion have demonstrated encouraging potential in improving treatment efficacy. These therapies represent a novel and evolving class of cancer treatment that selectively targets oncogenic drivers within pancreatic tumor cells. Continued progress in diagnostic techniques, surgical innovations, and therapeutic strategies is anticipated to significantly improve patient survival[90].
In such cases, a multidisciplinary treatment approach is essential to optimize clinical outcomes and extend survival. Surgical resection remains the cornerstone of treatment for localized tumors, and recent advancements in minimally invasive surgical techniques have significantly enhanced both the safety and efficacy of these procedures. Systemic chemotherapy has also demonstrated a substantial impact on OS, and the incorporation of neoadjuvant therapy - often in combination with radiation has further improved outcomes by increasing resectability and targeting micrometastatic disease. In the context of metastatic PC, molecular profiling has facilitated the introduction of targeted therapies tailored to specific genetic alterations, yielding promising clinical responses. Additionally, novel immunotherapeutic strategies, including immune checkpoint inhibitors, PARP inhibitors, and cancer vaccines, have emerged as valuable treatment options, contributing to improved survival metrics. Despite the historically poor prognosis associated with PC, recent advances in systemic therapy and personalized medicine are reshaping the therapeutic landscape and enhancing patient outcomes[91].
Cytokines within the TME serve as key effectors and signaling molecules that mediate interactions between inflammatory and stromal components in PC. Targeting cytokine signaling pathways has emerged as a promising therapeutic strategy for inhibiting tumor progression[23].
The critical role of cytokines in PC is supported by clinical trial data. Bruton tyrosine kinase (BTK), a non-receptor tyrosine kinase of the Tec family, is predominantly expressed in mast cells and myeloid cells within the peritumoral inflammatory stroma. BTK signaling plays a crucial role in sustaining TME. Inhibition of BTK has been shown to induce phenotypic reprogramming of M2-like macrophages into a pro-inflammatory M1-like phenotype, thereby enhancing CD8+ T cell-mediated cytotoxic responses. Overman et al[92] conducted a randomized Phase II clinical trial to assess the therapeutic potential of BTK inhibition using acalabrutinib - administered either as monotherapy or in combination with the anti-programmed cell death 1 (PD-1) antibody pembrolizumab - in patients with APC. The study confirmed the safety of both treatment regimens and observed a reduction in granulocytic MDSCs (CD15+ MDSCs). However, clinical efficacy remained limited, with an overall response rate and DCR of 0% and 14.3%, respectively, for acalabrutinib monotherapy, and 7.9% and 21.1%, respectively, for the combination therapy[92].
In a separate phase IIa trial, the CXCR4 antagonist BL-8040 (motixafortide) was evaluated in combination with pembrolizumab and chemotherapy in patients with metastatic PC. This regimen led to enhanced infiltration of CD8+ effector T cells into tumors, as well as reductions in both MDSCs and circulating Tregs. The combination demonstrated improved therapeutic benefit, with an overall response rate of 32%, a DCR of 77%, and a median duration of response of 7.8 months, indicating a promising immunotherapeutic approach in the management of APC[93].
In recent years, the United States Food and Drug Administration (FDA) has authorized several innovative immunotherapies. Among these are: (1) Immunomodulatory antibodies that block checkpoints in patients with a variety of cancers, such as solid tumors with high microsatellite instability, non-small cell lung cancer, melanoma, Hodgkin’s disease, etc.; and (2) T cell immunotherapy for B cell malignancies that is altered by chimeric antigen receptor (CAR) T cell immunotherapy[94,95].
Approved immune checkpoint inhibitors include antibodies that block PD-1, programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein 4. The use of PC immunotherapy in combination with anti-PD-1 (pembrolizumab and nivolumab) immunotherapy for all solid tumors with high microsatellite instability or mismatch repair deficiency was recently authorized by the FDA[96].
Single-agent Treg inhibitors have demonstrated limited efficacy in the treatment of PC. To investigate the immunomodulatory effects within the TME, Lutz et al[97] evaluated an irradiated, granulocyte macrophage-colony-stimulating factor-secreting allogeneic PC vaccine, administered either alone or in combination with low-dose cyclophosphamide aimed at Treg depletion. The study revealed that the addition of cyclophosphamide to granulocyte macrophage-colony-stimulating factor-secreting allogeneic PC vaccine shifted the intratumoral immune balance toward a more favorable effector T-cell response by reducing Treg-mediated immunosuppression[97].
Arshad et al[98] reported that in patients with APC undergoing treatment with gemcitabine and intravenous omega-3 fish oil, a reduction in pro-angiogenic and pro-inflammatory factors was linked to improved clinical outcomes. This therapeutic effect was associated with decreased levels of CAFs, suggesting a potential mechanism underlying the enhanced prognosis[98]. A phase I/II clinical trial was conducted to evaluate the safety and efficacy of the toll-like receptor 2/6 agonist macrophage-activating lipopeptide-2 in combination with gemcitabine in patients with pancreatic carcinoma. The findings demonstrated a significant improvement in mean survival, attributed to macrophage-activating lipopeptide-2-mediated activation of monocytes, macrophages, and previously anergic DCs[99].
Mayanagi et al[100] conducted a phase I pilot study to evaluate the feasibility and immunogenicity of DC vaccination using Wilms tumor gene 1 peptide-pulsed DCs in combination with gemcitabine in patients with APC. The study demonstrated that this therapeutic approach is both feasible and capable of eliciting robust anti-tumor T-cell responses[100].
Conventional therapeutic strategies have shown limited efficacy in patients with PDAC, prompting increased interest in immunotherapeutic approaches, which have yielded promising outcomes in preclinical models. However, despite these encouraging findings, significant biological and clinical barriers continue to constrain the translation of immunotherapy into effective treatment for PDAC. It is essential to recognize that each immunotherapeutic modality presents distinct advantages and limitations. In a comprehensive review, Farhangnia et al[101] examined a broad spectrum of immunotherapeutic strategies, including oncolytic virus therapy and various forms of adoptive cell transfer, such as T-cell receptor-engineered T cells, CAR T-cell therapy, CAR natural killer cell therapy, and cytokine-induced killer cell therapy. The review also addressed the potential of immune checkpoint blockade, immunomodulatory agents, cancer vaccines, and interventions targeting the tumor-associated myeloid cell compartment. Additionally, innovative technologies such as CRISPR/Cas9 gene editing and modulation of the gut microbiome were discussed in the context of enhancing immunotherapeutic efficacy in PDAC[101].
DNA methyltransferase inhibitors represent a pivotal advancement in targeting aberrant epigenetic regulation in cancer. Agents such as decitabine can reverse hypermethylation-mediated silencing of tumor suppressor genes, thereby reinstating their expression and contributing to anti-tumor effects. In this study, decitabine, a DNA methyltransferase 1 inhibitor, was encapsulated within polylactic-co-glycolic acid (PLGA) nanoparticles (NPs) to form Dec@PLGA. These NPs were subsequently coated with a hybrid vesicle composed of an anti-PD-L1 (aPD-L1) antibody and macrophage mem
Although gemcitabine is the standard chemotherapeutic agent used in its management, its clinical utility is hindered by considerable systemic toxicity. Previous clinical trials investigating the combination of gemcitabine with erlotinib have yielded limited therapeutic benefits and raised concerns regarding adverse effects. To address these limitations, the encapsulation of chemotherapeutic agents within PLGA NPs has emerged as a promising strategy, offering controlled drug release and targeted delivery to minimize off-target toxicity. Moreover, surface modification of these NPs with macrophage-derived membranes enhances immune evasion and tumor-targeting capabilities. In this context, a novel nanoplatform was developed comprising gemcitabine-loaded PLGA NPs cloaked with macrophage membranes (MPGNPs), aiming to enhance tumor-specific accumulation and reduce systemic toxicity. The co-administration of MPGNPs with erlotinib demonstrated a synergistic inhibitory effect on PC cell proliferation both in vitro and in vivo. Mechanistically, this combinatorial approach exerted its anti-tumor effects through simultaneous modulation of the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin and rat sarcoma virus/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase /extracellular-signal-regulated kinase signaling cascades. Additionally, the macrophage membrane coating facilitated immune evasion and passive tumor targeting by circumventing phagocytic clearance. The findings by Cai et al[104] provide compelling preclinical evidence supporting the therapeutic potential of combining MPGNPs with erlotinib for the treatment of PC.
Another study reported the design and synthesis of hollow-structured copper sulfide (CuS) NPs loaded with an ataxia telangiectasia mutated (ATM) kinase inhibitor and functionalized with an anti-TGF-β antibody on their surface (CuS-ATMi@TGF-β NPs). These engineered NPs exhibited excellent photostability, efficient drug release, and the capacity to elevate local temperature under near-infrared irradiation, enabling controlled, near-infrared-responsive activation. The CuS-ATMi@TGF-β NPs demonstrated selective tumor targeting, resulting in marked inhibition of tumor growth through a synergistic therapeutic approach combining low-temperature photothermal therapy and chemotherapy. This na
PC, despite accounting for only approximately 3% of all new cancer diagnoses, is projected to become the second leading cause of cancer-related mortality shortly. Advancements in patient survival are anticipated to result from improved identification of risk factors, earlier detection, enhanced integration of locoregional and systemic treatment modalities, and the development of more effective pharmacological agents driven by a refined understanding of the tumor’s molecular biology[106].
For patients with unresectable, non-metastatic disease, combined modality approaches - including primary chemo
Inflammatory biomarkers play a crucial role in the clinical management of PC, particularly PDAC, due to their in
The ESR, a non-specific marker of systemic inflammation, has limited diagnostic specificity in PC but may offer clinical utility as a prognostic and potentially predictive biomarker. Elevated ESR levels have been associated with more advanced disease, increased tumor burden, and worse OS, reflecting the pro-inflammatory TME that supports cancer progression. While ESR alone is insufficient for diagnosis due to its elevation in numerous benign and malignant conditions, it can contribute to risk stratification when used alongside other markers such as CA19-9 or CRP. Addi
Plasma viscosity, a measure of blood flow resistance influenced by plasma proteins and inflammation, has potential clinical utility in PC primarily as a prognostic biomarker. While not specific enough for diagnostic use on its own, elevated plasma viscosity levels have been linked to systemic inflammation and hypercoagulability commonly seen in cancer patients, including those with pancreatic malignancies. Higher plasma viscosity may correlate with advanced disease stage, greater tumor burden, and poorer overall prognosis. Some studies suggest it could also serve as a predictive marker, with elevated levels potentially indicating reduced responsiveness to treatment or increased risk of postoperative complications. When combined with other inflammatory or tumor-specific markers, plasma viscosity may enhance risk stratification and guide therapeutic decision-making, offering a low-cost, non-invasive adjunct in the clinical management of PC.
PCT, a biomarker typically elevated in bacterial infections, has emerging clinical relevance in PC, particularly in distinguishing infection-related complications from cancer-related inflammation. While its diagnostic utility for PC itself is limited, elevated PCT levels can aid in identifying infectious complications such as cholangitis or pancreatic abscesses, which are common in advanced disease or post-interventional settings. Prognostically, higher PCT levels have been associated with worse clinical outcomes, reflecting a heightened systemic inflammatory response or occult infection that may complicate cancer progression. As a predictive biomarker, PCT may help guide antibiotic stewardship and monitor treatment response in infected patients, but its role in predicting chemotherapy response or long-term survival remains unclear. Overall, PCT is a valuable adjunctive biomarker in the supportive care of PC patients, especially for managing infectious complications and tailoring clinical interventions.
CP, a calcium-binding protein released predominantly by activated neutrophils, has shown potential clinical utility in PC, particularly as a marker of inflammation associated with tumor progression. Although its diagnostic specificity is limited, elevated serum or fecal CP levels have been observed in patients with PC and may help differentiate malignant from benign pancreatic conditions when used in conjunction with established markers like CA19-9. As a prognostic biomarker, higher CP levels correlate with more aggressive disease, systemic inflammation, and poorer OS, reflecting the tumor-promoting role of chronic inflammation. Emerging evidence also suggests a potential predictive role, as elevated CP may indicate resistance to treatment or a higher likelihood of disease recurrence. Overall, while not yet widely adopted in clinical practice, CP holds promise as a non-invasive adjunctive biomarker for risk assessment and monitoring in PC.
Hp, an acute-phase glycoprotein involved in binding free haemoglobin and modulating inflammation, has demonstrated potential clinical utility in PC, particularly as a prognostic and possibly diagnostic biomarker. Elevated serum Hp levels have been observed in PC patients and are associated with tumor progression, systemic inflammation, and poorer OS, supporting its role as a prognostic indicator. While Hp alone lacks the specificity required for reliable diagnosis, when combined with established markers like CA19-9, it may enhance diagnostic accuracy, especially in distinguishing malignant from benign pancreatic conditions. Additionally, changes in Hp levels during treatment may offer predictive insight into therapeutic response and disease monitoring. Overall, Hp reflects the inflammatory and metabolic alterations associated with PC and holds promise as part of a multi-marker panel for improving clinical assessment and management.
SAA, an acute-phase reactant produced predominantly by the liver in response to inflammation, has demonstrated promising clinical utility in PC, particularly as a prognostic and potential diagnostic biomarker. Elevated SAA levels have been consistently associated with advanced disease stages, increased tumor burden, systemic inflammation, and reduced OS, underscoring its value in prognostication. While not specific enough for standalone diagnosis, SAA, when used in combination with conventional markers like CA19-9, may enhance diagnostic sensitivity, especially in early or am
Ferritin, an intracellular iron-storage protein and acute-phase reactant, has shown clinical relevance in PC, primarily as a prognostic biomarker with potential diagnostic and predictive implications. Elevated SF levels are frequently observed in PC patients and are associated with systemic inflammation, greater tumor burden, and poorer OS, making it a useful indicator of disease severity and prognosis. Although ferritin lacks specificity for diagnostic use, when combined with other markers such as CA19-9, it may improve diagnostic accuracy in differentiating malignant from benign pancreatic conditions. Additionally, rising ferritin levels during treatment may reflect disease progression or therapeutic resistance, indicating a possible predictive role. Overall, ferritin serves as a cost-effective, non-specific marker that reflects the inflammatory and metabolic alterations in PC, with potential utility in comprehensive biomarker panels for risk assessment and clinical monitoring.
Ferritin serves as a clinically important biomarker for the assessment of various iron-related disorders. It is routinely employed in the diagnostic evaluation of common conditions such as iron-deficiency anemia, as well as hereditary and acquired iron-overload syndromes, including hereditary hemochromatosis and iron accumulation secondary to chronic transfusion therapy. SF is typically included as part of a broader panel of laboratory tests used to diagnose and monitor these conditions and is often considered the most informative single marker in many clinical contexts. However, its interpretation must be approached with caution due to potential confounding factors. Additionally, markedly elevated SF levels may serve as an important diagnostic indicator of rare but severe autoimmune or systemic inflammatory diseases[66].
Fib, a key coagulation factor and acute-phase protein, has demonstrated significant clinical utility as a biomarker in PC, primarily in prognostic and emerging predictive contexts. Although its diagnostic specificity is limited when used alone, elevated plasma Fib levels, especially when combined with CA19-9, may enhance diagnostic accuracy. More robustly, Fib serves as a prognostic biomarker, with higher pre-treatment levels correlating with advanced disease stage, greater tumor burden, metastasis, and poorer overall and PFS. Additionally, elevated Fib may predict reduced responsiveness to chemotherapy and higher recurrence rates post-surgery, suggesting a potential predictive role. Mechanistically, Fib promotes tumor progression through angiogenesis, immune evasion, and a pro-thrombotic TME. Thus, while not suitable as a standalone diagnostic tool, Fib is a valuable marker for risk stratification and treatment planning in PC.
As of now, there are no inflammation-specific biomarkers that are approved for routine clinical use in the diagnosis, prognosis, or monitoring of PC. While markers such as IL-6 and CRP have been extensively studied and are often elevated in patients with PDAC, they lack the specificity and validation required for clinical approval. These inflammatory markers are influenced by a variety of non-malignant conditions, including infections and systemic inflammatory responses, which limits their diagnostic accuracy. In contrast, CA19-9 remains the only FDA-approved serum biomarker currently used in clinical practice for PC, primarily for monitoring disease progression, treatment response, and re
Also, elevated levels of inflammatory markers, including NLR, CRP, and SAA, have been associated with poor prognosis, advanced disease stage, and reduced OS in PDAC patients. Dynamic changes in inflammatory biomarkers during treatment may reflect tumor response or resistance to chemotherapy, targeted therapy, or immunotherapy, aiding in real-time monitoring of therapeutic efficacy. Inflammatory profiles can help stratify patients for specific therapies, such as immunomodulatory treatments, by indicating the degree of immune suppression or systemic inflammation. These biomarkers provide insight into the inflammatory landscape of the TME, which is essential for developing novel therapeutic strategies targeting stromal or immune components. Furthermore, a deeper understanding of the molecular mechanisms underlying PC development and progression is essential for the identification of novel targets and the development of more effective, personalized treatment approaches[90].
PC cells, stromal cells, and cytokines collectively contribute to the establishment of an inflammatory and immunosuppressive TME. Emerging evidence underscores the importance of the intricate crosstalk between inflammatory processes and stromal elements within the pancreatic TME. Tumor-associated inflammation encompasses complex interactions among diverse immune cell populations, inflammatory cells, cancer cells, chemokines, and cytokines. Inflammation plays a critical role in the initiation, progression, and metastasis of PC. Inflammatory infiltration within the TME of PDAC plays a significant role in tumor growth and metastasis. Both systemic and localized chronic inflammation have been im
| 1. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2226] [Article Influence: 139.1] [Reference Citation Analysis (2)] |
| 2. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1612] [Article Influence: 230.3] [Reference Citation Analysis (1)] |
| 3. | Goral V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac J Cancer Prev. 2015;16:5619-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Ren B, Liu X, Suriawinata AA. Pancreatic Ductal Adenocarcinoma and Its Precursor Lesions: Histopathology, Cytopathology, and Molecular Pathology. Am J Pathol. 2019;189:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48716] [Article Influence: 3247.7] [Reference Citation Analysis (12)] |
| 6. | Shou S, Liu R, He J, Jiang X, Liu F, Li Y, Zhang X, En G, Pu Z, Hua B, Pang B, Zhang X. Current and projected incidence rates of pancreatic cancer in 43 countries: an analysis of the Cancer Incidence in Five Continents database. BMJ Open Gastroenterol. 2025;12:e001544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Pergamo M, Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017;24:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology. 2023;164:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 316] [Reference Citation Analysis (1)] |
| 9. | Pourhoseingholi MA, Ashtari S, Hajizadeh N, Fazeli Z, Zali MR. Systematic review of pancreatic cancer epidemiology in Asia-Pacific Region: major patterns in GLOBACON 2012. Gastroenterol Hepatol Bed Bench. 2017;10:245-257. [PubMed] |
| 10. | Ranganath R, Chu Q. Global trends in pancreas cancer among Asia-Pacific population. J Gastrointest Oncol. 2021;12:S374-S386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | Hesami Z, Olfatifar M, Sadeghi A, Zali MR, Mohammadi-Yeganeh S, Habibi MA, Ghadir MR, Houri H. Global Trend in Pancreatic Cancer Prevalence Rates Through 2040: An Illness-Death Modeling Study. Cancer Med. 2024;13:e70318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 13. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12562] [Article Influence: 6281.0] [Reference Citation Analysis (6)] |
| 14. | Filho AM, Laversanne M, Ferlay J, Colombet M, Piñeros M, Znaor A, Parkin DM, Soerjomataram I, Bray F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2025;156:1336-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 358] [Article Influence: 358.0] [Reference Citation Analysis (4)] |
| 15. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13298] [Article Influence: 1662.3] [Reference Citation Analysis (4)] |
| 16. | Moshayedi N, Escobedo AL, Thomassian S, Osipov A, Hendifar AE. Race, sex, age, and geographic disparities in pancreatic cancer incidence. J Clin Oncol. 2022;40:520-520. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Chandar AK, Al Armashi AR, Alkrekshi A. S87 Rural–Urban Disparities in Pancreatic Cancer Survival: A SEER Database Study From 2000–2020. Am J Gastroenterol. 2023;118:S70-S71. [DOI] [Full Text] |
| 18. | Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, Li J, Huang K. Pancreatic Cancer Epidemiology, Detection, and Management. Gastroenterol Res Pract. 2016;2016:8962321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014;345:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 20. | Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21:481-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 21. | Rafaqat S, Khurshid H, Hafeez R, Arif M, Zafar A, Gilani M, Ashraf H, Rafaqat S. Role of Interleukins in Pancreatic Cancer: A Literature Review. J Gastrointest Cancer. 2024;55:1498-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190:1367-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Li Y, Wang J, Wang H, Zhang S, Wei Y, Liu S. The Interplay Between Inflammation and Stromal Components in Pancreatic Cancer. Front Immunol. 2022;13:850093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Tod J, Jenei V, Thomas G, Fine D. Tumor-stromal interactions in pancreatic cancer. Pancreatology. 2013;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Boyle KA, James MA, Tsai S, Evans DB, Dwinell MB. Stromal Inflammation in Pancreatic Cancer: Mechanisms and Translational Applications. In: Neoptolemos J, Urrutia R, Abbruzzese J, Büchler M, editors. Pancreatic Cancer. New York: Springer, 2017: 1-28. |
| 26. | Brenner DR, Scherer D, Muir K, Schildkraut J, Boffetta P, Spitz MR, Le Marchand L, Chan AT, Goode EL, Ulrich CM, Hung RJ. A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev. 2014;23:1729-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Shi J, Xue J. Inflammation and development of pancreatic ductal adenocarcinoma. Chin Clin Oncol. 2019;8:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Padoan A, Plebani M, Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int J Mol Sci. 2019;20:676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 29. | Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 242] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Singh N, Gupta S, Rashid S, Saraya A. Association of inflammatory markers with the disease & mutation status in pancreatic cancer. Indian J Med Res. 2022;155:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Grote VA, Kaaks R, Nieters A, Tjønneland A, Halkjær J, Overvad K, Skjelbo Nielsen MR, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Teucher B, Becker S, Pischon T, Boeing H, Trichopoulou A, Cassapa C, Stratigakou V, Palli D, Krogh V, Tumino R, Vineis P, Panico S, Rodríguez L, Duell EJ, Sánchez MJ, Dorronsoro M, Navarro C, Gurrea AB, Siersema PD, Peeters PH, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Allen NE, Travis RC, Fedirko V, Jenab M, Michaud DS, Chuang SC, Romaguera D, Bueno-de-Mesquita HB, Rohrmann S. Inflammation marker and risk of pancreatic cancer: a nested case-control study within the EPIC cohort. Br J Cancer. 2012;106:1866-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Sollie S, Michaud DS, Sarker D, Karagiannis SN, Josephs DH, Hammar N, Santaolalla A, Walldius G, Garmo H, Holmberg L, Jungner I, Van Hemelrijck M. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer. 2019;19:858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Neumann CCM, Schneider F, Hilfenhaus G, Vecchione L, Felsenstein M, Ihlow J, Geisel D, Sander S, Pratschke J, Stintzing S, Keilholz U, Pelzer U. Inflammation-Based Prognostic Scores in Pancreatic Cancer Patients-A Single-Center Analysis of 1294 Patients within the Last Decade. Cancers (Basel). 2023;15:2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 34. | Gu Y, Hua Q, Li Z, Zhang X, Lou C, Zhang Y, Wang W, Cai P, Zhao J. Diagnostic value of combining preoperative inflammatory markers ratios with CA199 for patients with early-stage pancreatic cancer. BMC Cancer. 2023;23:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Nurmi AM, Mustonen HK, Stenman UH, Seppänen HE, Haglund CH. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci Rep. 2021;11:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Shirakawa T, Makiyama A, Shimokawa M, Otsuka T, Shinohara Y, Koga F, Ueda Y, Nakazawa J, Otsu S, Komori A, Arima S, Fukahori M, Taguchi H, Honda T, Shibuki T, Nio K, Ide Y, Ureshino N, Mizuta T, Mitsugi K, Akashi K, Baba E. C-reactive protein/albumin ratio is the most significant inflammatory marker in unresectable pancreatic cancer treated with FOLFIRINOX or gemcitabine plus nab-paclitaxel. Sci Rep. 2023;13:8815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Bao Y, Giovannucci EL, Kraft P, Qian ZR, Wu C, Ogino S, Gaziano JM, Stampfer MJ, Ma J, Buring JE, Sesso HD, Lee IM, Rifai N, Pollak MN, Jiao L, Lessin L, Cochrane BB, Manson JE, Fuchs CS, Wolpin BM. Inflammatory plasma markers and pancreatic cancer risk: a prospective study of five U.S. cohorts. Cancer Epidemiol Biomarkers Prev. 2013;22:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Aktekin A, Torun M, Ustaalioğlu BBO, Ozkara S, Cakır O, Muftuoglu T. The effects of systemic inflammatory response on prognosis of pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. 2019;23:155-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J Gastroenterol. 2019;25:3-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Plebani M, Piva E. Erythrocyte sedimentation rate: use of fresh blood for quality control. Am J Clin Pathol. 2002;117:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Shi N, Xing C, Chang X, Dai M, Zhao Y. Pancreatic carcinoma masked as fever of unknown origin: A case report and comprehensive review of literature. Medicine (Baltimore). 2016;95:e4643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Thompson D, Milford-Ward A, Whicher JT. The value of acute phase protein measurements in clinical practice. Ann Clin Biochem. 1992;29 (Pt 2):123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Rubiano A, Delitto D, Han S, Gerber M, Galitz C, Trevino J, Thomas RM, Hughes SJ, Simmons CS. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 2018;67:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Wang WZ, Xu LJ, Hu DM, Tang W. Case of an abnormal procalcitonin-producing metastatic pancreatic neuroendocrine tumor. Clin Case Rep. 2020;8:2269-2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Nijsten MW, Olinga P, The TH, de Vries EG, Koops HS, Groothuis GM, Limburg PC, ten Duis HJ, Moshage H, Hoekstra HJ, Bijzet J, Zwaveling JH. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med. 2000;28:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE. Calprotectin: from biomarker to biological function. Gut. 2021;70:1978-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (1)] |
| 47. | Shibuki T, Mitsunaga S, Kan M, Kimura G, Umemoto K, Watanabe K, Sasaki M, Takahashi H, Hashimoto Y, Imaoka H, Ohno I. Circulating calprotectin, innate inflammatory protein, was decreased under disease control during first-line chemotherapy for advanced pancreatic cancer. J Clin Oncol. 2019;37. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Pezzilli R, Barassi A, Morselli-Labate AM, Fantini L, Tomassetti P, Campana D, Casadei R, Finazzi S, d'Eril GM, Corinaldesi R. Fecal calprotectin and elastase 1 determinations in patients with pancreatic diseases: a possible link between pancreatic insufficiency and intestinal inflammation. J Gastroenterol. 2007;42:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Holub M, Bartáková E, Stráníková A, Koblihová E, Arientová S, Blahutová M, Máca J, Ryska M. Calprotectin and Calgranulin C as Biomarkers of Pancreatic Tumors: Baseline Levels and Level Changes after Surgery. Mediators Inflamm. 2019;2019:6985703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 2001;6:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Firpo MA, Gay DZ, Granger SR, Scaife CL, DiSario JA, Boucher KM, Mulvihill SJ. Improved diagnosis of pancreatic adenocarcinoma using haptoglobin and serum amyloid A in a panel screen. World J Surg. 2009;33:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Okuyama N, Ide Y, Nakano M, Nakagawa T, Yamanaka K, Moriwaki K, Murata K, Ohigashi H, Yokoyama S, Eguchi H, Ishikawa O, Ito T, Kato M, Kasahara A, Kawano S, Gu J, Taniguchi N, Miyoshi E. Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer. 2006;118:2803-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 53. | Morishita K, Ito N, Koda S, Maeda M, Nakayama K, Yoshida K, Takamatsu S, Yamada M, Eguchi H, Kamada Y, Miyoshi E. Haptoglobin phenotype is a critical factor in the use of fucosylated haptoglobin for pancreatic cancer diagnosis. Clin Chim Acta. 2018;487:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Ito N, Yamada M, Morishita K, Nojima S, Motooka K, Sakata N, Asuka T, Otsu R, Takamatsu S, Kamada Y, Mori S, Akita H, Eguchi H, Morii E, Miyoshi E. Identification of fucosylated haptoglobin-producing cells in pancreatic cancer tissue and its molecular mechanism. Glycoconj J. 2021;38:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Lin Z, Simeone DM, Anderson MA, Brand RE, Xie X, Shedden KA, Ruffin MT, Lubman DM. Mass spectrometric assay for analysis of haptoglobin fucosylation in pancreatic cancer. J Proteome Res. 2011;10:2602-2611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv Clin Chem. 2019;90:25-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 57. | Ye RD, Sun L. Emerging functions of serum amyloid A in inflammation. J Leukoc Biol. 2015;98:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Kushner I. The acute phase response: an overview. Methods Enzymol. 1988;163:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Moshkovskii SA. Why do cancer cells produce serum amyloid A acute-phase protein? Biochemistry (Mosc). 2012;77:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Ding H, Yang Q, Mao Y, Qin D, Yao Z, Wang R, Qin T, Li S. Serum Amyloid a Predicts Prognosis and Chemotherapy Efficacy in Patients with Advanced Pancreatic Cancer. J Inflamm Res. 2023;16:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 62. | Yokoi K, Raber MN, Shih LN, Li D, Hamilton SR, Fidler IJ, Abbruzzese JL. Serum Amyloid A (SAA) in Patients with Pancreatic Carcinoma. J Clin Oncol. 2004;22:9649-9649. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Takamuku Y, Lemecha M, Itakura K. Abstract C031: Differential growth of pancreatic ductal adenocarcinoma cells in serum amyloid A (SAA) deficient mouse model through immune modulation. Cancer Res. 2024;84:C031-C031. [DOI] [Full Text] |
| 64. | Djurec M, Graña O, Lee A, Troulé K, Espinet E, Cabras L, Navas C, Blasco MT, Martín-Díaz L, Burdiel M, Li J, Liu Z, Vallespinós M, Sanchez-Bueno F, Sprick MR, Trumpp A, Sainz B Jr, Al-Shahrour F, Rabadan R, Guerra C, Barbacid M. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci U S A. 2018;115:E1147-E1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 65. | Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90:4979-4986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 66. | Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010;1800:760-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 602] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 67. | Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 68. | Nitti D, Fabris C, Del Favero G, Farini R, Grassi F, Farini A, Baccaglini U, Pedrazzoli S, Piccoli A, Lise M, Naccarato R. Serum ferritin in pancreatic disease. An accurate test of malignancy? Digestion. 1982;25:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Plebani M, Fabris C, Basso D, Del Favero G, Angonese C, Leandro G, Di Mario F, Burlina A, Naccarato R. Limits of CEA and ferritin in the diagnosis of pancreatic cancer. Int J Pancreatol. 1988;3 Suppl 1:S113-S117. [PubMed] |
| 70. | Basso D, Fabris C, Del Favero G, Meggiato T, Panozzo MP, Vianello D, Plebani M, Naccarato R. Hepatic changes and serum ferritin in pancreatic cancer and other gastrointestinal diseases: the role of cholestasis. Ann Clin Biochem. 1991;28 (Pt 1):34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Ren G, Xia T, Wang Y, Zhu F, Wu W. Elevated Serum Ferritin Levels Predict Poor Prognosis in Pancreatic Cancer With Hypofractionated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;96:E185. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Zubritsky L, Alkhateeb A, Leitzel K, Ali SM, Campbell-Baird C, Connor J, Lipton A. Pretreatment serum ferritin and overall survival in metastatic pancreatic cancer. J Clin Oncol. 2013;31:171-171. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Zhuge X, Zhou H, Chen L, Chen H, Chen X, Guo C. The association between serum ferritin levels and malignant intraductal papillary mucinous neoplasms. BMC Cancer. 2021;21:1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Park JM, Mau CZ, Chen YC, Su YH, Chen HA, Huang SY, Chang JS, Chiu CF. A case-control study in Taiwanese cohort and meta-analysis of serum ferritin in pancreatic cancer. Sci Rep. 2021;11:21242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Alkhateeb A, Zubritsky L, Kinsman B, Leitzel K, Campbell-Baird C, Ali SM, Connor J, Lipton A. Elevation in multiple serum inflammatory biomarkers predicts survival of pancreatic cancer patients with inoperable disease. J Gastrointest Cancer. 2014;45:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 469] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 77. | Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, Skjønsberg OH. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 78. | Chung KH, Lee JC, Lee J, Cho IK, Kim J, Jang W, Yoo BC, Hwang JH. Serum fibrinogen as a diagnostic and prognostic biomarker for pancreatic ductal adenocarcinoma. Pancreatology. 2020;20:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Li C, Fan Z, Guo W, Liang F, Mao X, Wu J, Wang H, Xu J, Wu D, Liu H, Wang L, Li F. Fibrinogen-to-prealbumin ratio: A new prognostic marker of resectable pancreatic cancer. Front Oncol. 2023;13:1149942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 80. | Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Chowdhury NN, Yang Y, Shen Y, Gampala S, Babb O, Han B, Wolberg AS, Flick MJ, Fishel ML. Abstract 2447: Unraveling the role of the fibrinolytic system in pancreatic cancer progression. Cancer Res. 2022;82:2447-2447. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, Muro-Cacho CA, Yeatman TJ. Fibrinogen gamma overexpression in pancreatic cancer identified by large-scale proteomic analysis of serum samples. Cancer Res. 2006;66:2592-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Chang S, Zou Y, Huang J, Li Z, Liang Y, Gao S. Fibrinogen to pre-albumin ratio is an independent prognostic index for patients with pancreatic ductal adenocarcinoma after radical resection. World J Surg Oncol. 2024;22:284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Wang HY, Xiu DR, Li ZF, Wang G. Coagulation function in patients with pancreatic carcinoma. Chin Med J (Engl). 2009;122:697-700. [PubMed] |
| 85. | Igarashi Y, Shirai Y, Tanji Y, Hamura R, Yanagaki M, Abe K, Onda S, Furukawa K, Matsumoto M, Tsunematsu M, Ikegami T. The Impact of Fibrinogen to Prognostic Nutritional Index Rate on Prognosis of Pancreatic Ductal Adenocarcinoma. Am Surg. 2023;89:4255-4261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Tomita K, Ochiai S, Gunji T, Hikita K, Kobayashi T, Sano T, Chiba N, Kawachi S. Prognostic Significance of Plasma Fibrinogen/Serum Albumin Ratio in the Postoperative Outcome of Pancreatic Ductal Adenocarcinoma. Anticancer Res. 2020;40:7017-7023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Cao J, Fu Z, Gao L, Wang X, Cheng S, Wang X, Ren H. Evaluation of serum D-dimer, fibrinogen, and CA19-9 for postoperative monitoring and survival prediction in resectable pancreatic carcinoma. World J Surg Oncol. 2017;15:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Fang L, Yan FH, Liu C, Chen J, Wang D, Zhang CH, Lou CJ, Lian J, Yao Y, Wang BJ, Li RY, Han SL, Bai YB, Yang JN, Li ZW, Zhang YQ. Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer. Cancer Res Treat. 2021;53:131-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Lin Y, Liu Z, Qiu Y, Zhang J, Wu H, Liang R, Chen G, Qin G, Li Y, Zou D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | Kumar L, Kumar S, Sandeep K, Patel SKS. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines. 2023;11:1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 91. | Hayat U, Croce PS, Saadeh A, Desai K, Appiah J, Khan S, Khan YI, Kumar K, Hanif A. Current and Emerging Treatment Options for Pancreatic Cancer: A Comprehensive Review. J Clin Med. 2025;14:1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 92. | Overman M, Javle M, Davis RE, Vats P, Kumar-Sinha C, Xiao L, Mettu NB, Parra ER, Benson AB, Lopez CD, Munugalavadla V, Patel P, Tao L, Neelapu S, Maitra A. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J Immunother Cancer. 2020;8:e000587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 93. | Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Muñoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Vainstein Haras A, Von Hoff DD, Hidalgo M. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 414] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 94. | June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 2256] [Article Influence: 282.0] [Reference Citation Analysis (0)] |
| 95. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4979] [Article Influence: 622.4] [Reference Citation Analysis (0)] |
| 96. | Yoon JH, Jung YJ, Moon SH. Immunotherapy for pancreatic cancer. World J Clin Cases. 2021;9:2969-2982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 97. | Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 440] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 98. | Arshad A, Chung WY, Steward W, Metcalfe MS, Dennison AR. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford). 2013;15:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Schmidt J, Welsch T, Jäger D, Mühlradt PF, Büchler MW, Märten A. Intratumoural injection of the toll-like receptor-2/6 agonist 'macrophage-activating lipopeptide-2' in patients with pancreatic carcinoma: a phase I/II trial. Br J Cancer. 2007;97:598-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Mayanagi S, Kitago M, Sakurai T, Matsuda T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, Hamamoto Y, Takaishi H, Okamoto M, Sunamura M, Kawakami Y, Kitagawa Y. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015;106:397-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 101. | Farhangnia P, Khorramdelazad H, Nickho H, Delbandi AA. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J Hematol Oncol. 2024;17:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 129] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 102. | Cai H, Li X, Liu Y, Ke J, Liu K, Xie Y, Xie C, Zhou D, Han M, Ji B. Decitabine-based nanoparticles for enhanced immunotherapy of hepatocellular carcinoma via DNA hypermethylation reversal. Chem Eng J. 2024;492:152175. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 103. | Sharma V, Sachdeva N, Gupta V, Nada R, Jacob J, Sahni D, Aggarwal A. IL-6 is associated with expansion of myeloid-derived suppressor cells and enhanced immunosuppression in pancreatic adenocarcinoma patients. Scand J Immunol. 2021;94:e13107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 104. | Cai H, Wang R, Guo X, Song M, Yan F, Ji B, Liu Y. Combining Gemcitabine-Loaded Macrophage-like Nanoparticles and Erlotinib for Pancreatic Cancer Therapy. Mol Pharm. 2021;18:2495-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 105. | Cai H, Dai X, Guo X, Zhang L, Cao K, Yan F, Ji B, Liu Y. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. 2021;127:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 106. | Spadi R, Brusa F, Ponzetti A, Chiappino I, Birocco N, Ciuffreda L, Satolli MA. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol. 2016;7:27-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/