Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.108876
Revised: May 30, 2025
Accepted: September 2, 2025
Published online: October 24, 2025
Processing time: 182 Days and 11.2 Hours
Internal mammary (IM) lymphadenopathies in breast cancer indicate a worse prognosis than axillary metastases, yet they are rarely assessed. Accurate staging is essential for treatment planning. Robotic biopsy offers a promising alternative to video-assisted thoracic surgery for precise histological sampling. This article outlines a systematic robot-assisted dissection approach to enhance staging ac
At our institution, robotic lymphadenectomy of the IM chain was performed in 5 patients between July 2020 and December 2024. Patients were positioned in a 30° semi-supine position with a roll under the shoulder to elevate the chest. The camera port was inserted in the fifth intercostal space along the mid-axillary line, allowing a 0°, 12 mm robotic camera to inspect the chest cavity; CO2 insufflation
Robotic biopsy of IM lymph nodes is safe, feasible, and provides key information on breast cancer management, with very rare contraindications
Core Tip: Robotic-assisted surgery for internal mammary lymph node dissection is a highly effective, minimally invasive technique that overcomes the limitations of video-assisted thoracic surgery. It offers superior three-dimensional visualization, greater instrument maneuverability, and improved ergonomics for the surgeon. The panoramic view improves staging and specimen biopsy, while meticulous dissection prevents vascular injury. Proper incision placement is crucial in patients with breast implants. One-lung ventilation may limit eligibility, and high body mass index may increase complexity.
- Citation: Pardolesi A, Ferrari M, Leuzzi G, Stanzi A, Calderoni M, Uslenghi C, Scarci M, Raveglia F, Cioffi U, Solli P. Robotic approach for lymphadenectomy of internal mammary lymph nodes in breast cancer: Five case reports. World J Clin Oncol 2025; 16(10): 108876
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/108876.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.108876
The internal mammary (IM) or internal thoracic lymph node (LN) chain is responsible for up to 30% of breast lymphatic drainage and it is the second most common site of nodal metastases in breast cancer patients. The involvement of IM-LNs represents significantly advanced disease and is well known to be associated with a worse prognosis than patients with axillary LN metastases[1]. However, the clinical value of IM-LN biopsy remains controversial. American Joint Committee on Cancer guidelines include the assessment of both axillary and internal thoracic LNs, yet routine surgical staging is habitually limited to the axillary chain[2]. Factors that potentially limit routine biopsy or dissection of IM-LNs in breast cancer patients can be summarized as follow: (1) The perception that the identification of IM-LN metastases may not influence or change adjuvant treatment strategies[3]; (2) The clinical benefits of IM-LN dissection are still under investigation[4]; and (3) Morbidity of IM-LN biopsy[5].
In patients with IM adenopathy with positron emission tomography (PET)-positive uptake, tissue sampling should be performed to confirm the suspicion of malignancy. Moreover, differential diagnosis with inflammatory or granulomatous reaction should be considered in women with breast cancer who have undergone mammoplasty with silicone implants. Rupture or leakage of silicone breast prosthesis can cause lymphatic spread which involves frequently axillary LNs and more rarely internal thoracic LNs. Numerous cases of post-mastectomy patients with silicone-induced IM adenopathy mimicking cancer recurrence on computed tomography (CT) or PET scan have been reported in literature[6,7]. In these patients, the histological diagnosis is a key prognostic factor in case of planning adjuvant treatment. LN biopsy can be performed percutaneously with ultrasound-guided fine-needle aspiration. When performing ultrasound-guided fine-needle aspiration, the experience of the operator is critical to successfully place the needle inside or through the target LN and obtain a satisfactory cytological specimen. IM-LNs are usually located lateral to the IM vessels; however, sometimes they are placed medially or between them, making the procedure more dangerous, as the needle would have to cross just over the vessels. Moreover, in patients with elevated body mass index (BMI), thick muscles, or narrow intercostal spaces, ultrasound identification of the LNs can be more demanding, and placing the needle safely is more challenging with a consequent higher risk of complications such as pleura breach, pneumothorax, and vascular injury[8]. The surgical approach to the IM-LN chain can be performed in two ways: The traditional open trans-pectoral and the video-assisted thoracic surgery (VATS) approach. The trans-pectoral approach allows access to the IM-LNs through the mastectomy incision or, when this option is not available, through a separate incision parallel to the sternum extended 3-4 cm lateral to its margin. This approach offers a comprehensive view of the intercostal spaces and allows safe dissection of the LN chain can be conducted without opening the pleural space. This procedure should be considered as a valid approach when performing extended radical mastectomy[9]. Otherwise, a minimally invasive technique should be the preferred surgical strategy for IM-LN biopsy. Several authors have recently reported and demonstrated the feasibility and safety of the VATS approach in patients with internal thoracic lymphadenopathy[10]. The VATS approach offers reduced surgical trauma with a lower rate of postoperative complications. It is a “simple” and rapid procedure that allows achieving high accuracy in diagnosis with good cosmetics results. However, the VATS approach is not exempt from some potential limitations[11]. In our experience, robotic manipulation of the IM pedicle may offer an improved minimally invasive approach for the diagnosis and management of IM lymphadenopathy and overcome some technical limitations of VATS. Specifically, a combination of three-dimensional (3D) video imaging and the “EndoWrist” features of the instruments, with multiple degrees of freedom, allows to perform a safe and precise dissection of the IM-LN chain.
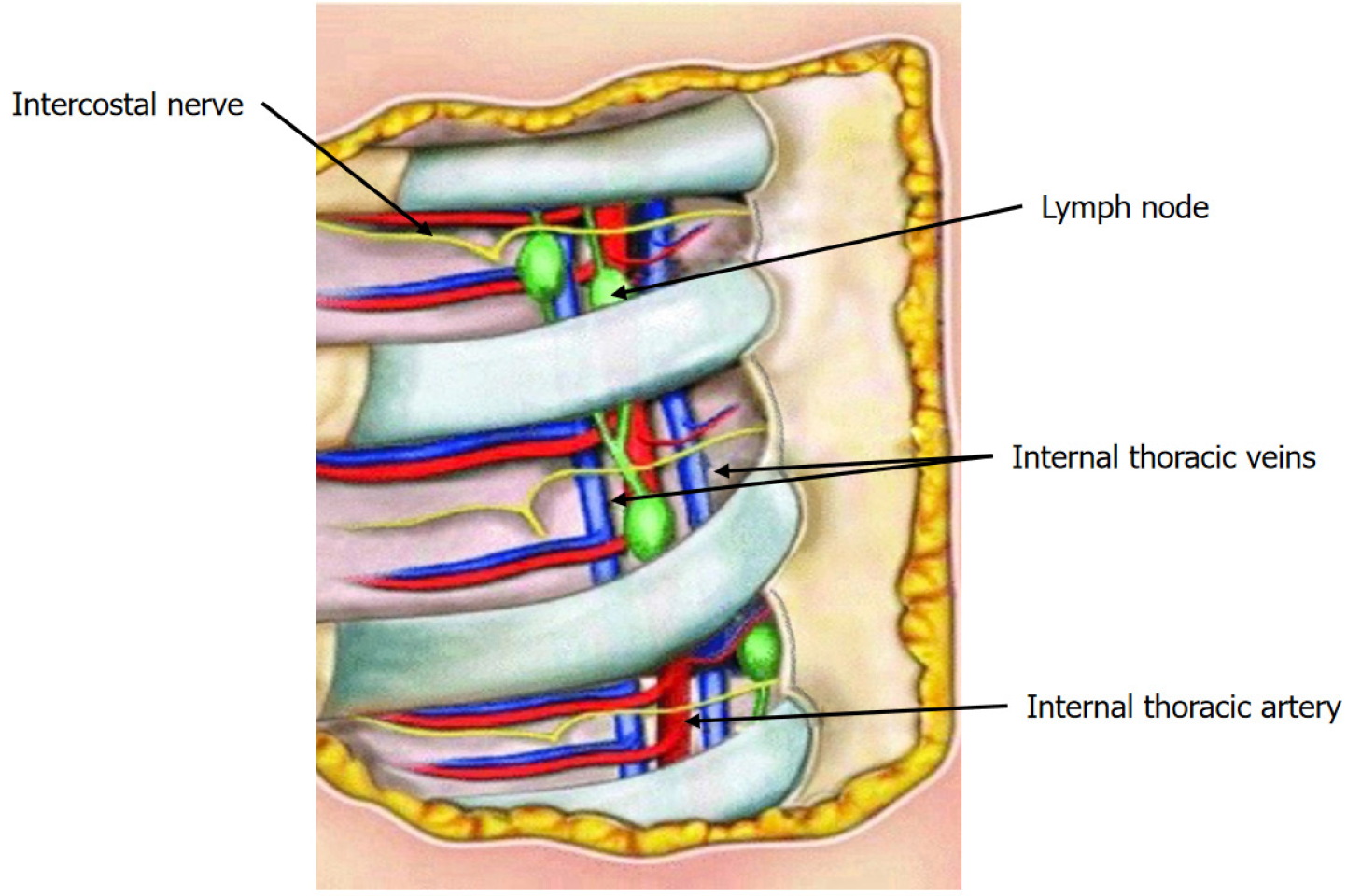
The lymphatic vessels of the medial and deep portion of the breast lead to the IM lymph glands. The lymphatic vessels that pass through the medial aspect of the pectoralis major muscle and the internal intercostal muscle enter into the IM-LN. These LNs are usually located medially to the IM artery and vein, embedded in the adipose tissue in the same plane or anteriorly to the vessels. There are usually 3 to 6 LNs on each side, distributed in a variable manner in each intercostal space. IM vessels and LNs lean on the transverse thoracic muscle, on an extrapleural plane. Intercostal arteries arise from the IM artery two for each intercostal space. Two intercostal veins run along the artery, one laterally and one medially (Figure 1)[12,13].
Five female patients presented with PET-positive adenopathy involving the IM-LN chain, identified during routine oncologic follow-up. These cases represent a heterogeneous cohort of patients with suspected IM-LN involvement, either in the context of previous malignancy or as an isolated finding. Between July 2020 and December 2024, all five patients underwent robotic-assisted lymphadenectomy of the IM chain at our institution for diagnostic and therapeutic purposes.
Case 1: A 77-year-old woman with a prior history of stage I A2 Lung adenocarcinoma and invasive ductal breast carcinoma was found to have PET-positive lymphadenopathy in the right IM chain.
Case 2: A 47-year-old woman previously treated for breast cancer presented with PET-positive adenopathy in the IM region.
Case 3: A 34-year-old woman diagnosed with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2+, high-proliferative (Ki67 60%) invasive ductal carcinoma of the breast showed PET-positive LN involvement in the left IM chain.
Case 4: A 50-year-old woman with a dual history of stage I A2 Lung adenocarcinoma and pT2 invasive ductal breast carcinoma presented with PET-positive adenopathy in the right IM chain.
Case 5: A 49-year-old woman with no prior oncologic history was found to have PET-positive lymphadenopathy in the left IM chain.
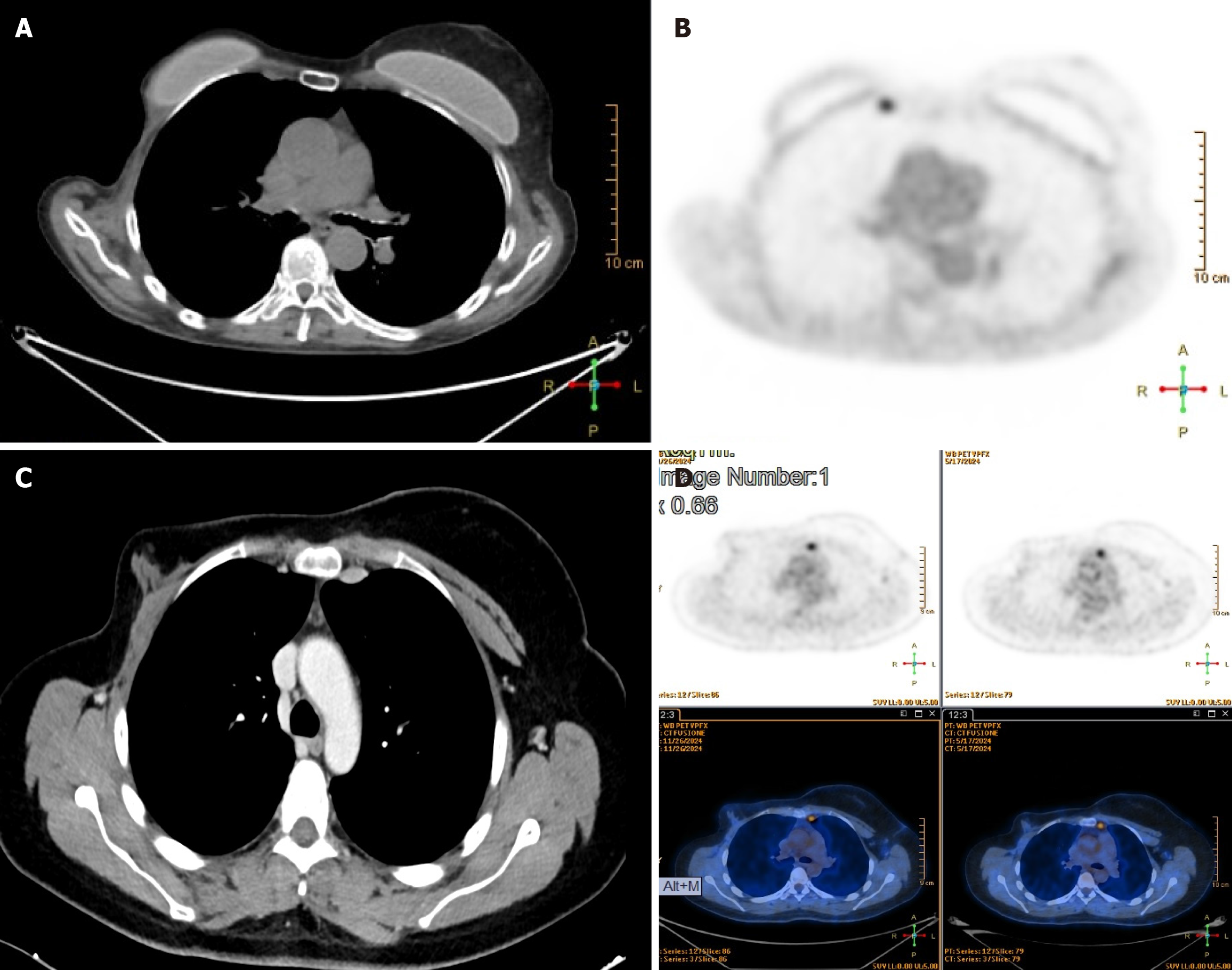
In all five cases, hypermetabolic LNs within the IM chain were identified during routine oncologic surveillance or diagnostic imaging. PET/CT scans revealed increased uptake, with standardized uptake value maximum (SUVmax) values ranging from 2.6 to 5.0 (Figure 2), raising clinical suspicion for metastatic recurrence. The patients, with a median age of 51.4 years, had previously undergone multimodal treatment for either breast or lung cancer, including surgery, chemotherapy, and/or radiation therapy. Given their oncologic history, the findings were considered potentially in
The clinical background of the five patients varied in terms of oncologic history and previous treatments.
Case 1: The patient had previously been diagnosed with both infiltrating ductal breast carcinoma and stage I A2 Lung adenocarcinoma. She remained disease-free for three years following multimodal treatment. The patient had a dual oncologic history, with breast and lung cancer, and had ER-positive breast carcinoma treated with letrozole and stage I lung adenocarcinoma, with disease-free intervals of three years.
Case 2: A history of breast cancer treated with surgery and adjuvant systemic therapy was documented.
Case 3: The patient had ER-positive invasive ductal breast carcinoma and underwent comprehensive multimodal therapy, including endocrine treatment.
Case 4: This patient had synchronous diagnoses of stage I A2 Lung adenocarcinoma and pT2 infiltrating ductal breast carcinoma, both treated with curative intent. The patient had a dual oncologic history, with breast and lung cancer, and had ER-positive breast carcinoma treated with letrozole and stage I lung adenocarcinoma, with disease-free intervals of four years.
Case 5: No prior history of malignant disease was reported.
In patients with a previous diagnosis of breast cancer (cases 1-4), the primary tumors measured between 1.5 cm and 2.0 cm. Axillary LN dissection was not performed in any of these cases, as sentinel LN biopsies were negative at the time of initial treatment.
Case 1: No relevant family history.
Cases 2-4: Maternal history of breast cancer.
Case 5: No family or personal history of malignancy.
No remarkable findings at the time of surgical evaluation in cases 1-5.
Cases 1 and 4: Elevated cancer antigen (CA) 15-3 (> 30 U/mL).
Case 2: CA15-3 showed a progressive increase compared with previous measurements, but remained below 30 U/mL.
Cases 3 and 5: Tumor markers within normal range.
PET/CT imaging confirmed the presence of metabolically active LNs within the IM chain in all five patients.
Case 1: Right IM-LN, 10 mm × 11 mm, SUVmax: 3.9.
Case 2: IM-LN, 12 mm × 18 mm, SUVmax: 2.7.
Case 3: Left IM-LN, 12 mm × 15 mm, SUVmax: 5.0.
Case 4: Right IM-LN, 18 mm × 16 mm, SUVmax: 4.0.
Case 5: Left IM-LN, 12 mm × 16 mm, SUVmax: 2.8. A CT scan was initially performed due to unexplained weight loss and night sweats. The findings prompted a subsequent PET scan, which revealed suspicious lymphadenopathy in the IM chain. In this context, a lymphoproliferative disorder was considered as a differential diagnosis, rather than recurrence of previously treated malignancy.
All five cases were reviewed by the institutional multidisciplinary tumor board. The board included radiologists, medical oncologists, and surgeons, who collectively discussed the optimal diagnostic strategy for each patient. In two patients (cases 1 and 2), percutaneous biopsy was attempted but was non-diagnostic. The radiologist’s assessment highlighted the inconclusive nature of the results, while the medical oncologist emphasized the need for a definitive tissue diagnosis to guide therapeutic decisions, given the suspected disease progression. In the remaining three cases (cases 3-5), the procedure was deemed technically unsafe. The surgeons and radiologists collaboratively determined the high risk associated with percutaneous biopsy due to the deep anatomical location of the LNs and their proximity to major vascular structures. Given that the IM LNs represented the only sites of suspected disease progression - particularly in patients already receiving endocrine therapy - the tumor board reached a consensus. The medical oncology team argued for the critical need for a definitive histological diagnosis to adjust the treatment plan, and the surgical team confirmed that a robotic-assisted surgical excision was the safest and most effective way to obtain the required tissue sample. This approach was recommended not only for diagnostic purposes but also to guide further management.
Metastatic breast carcinoma. Surgical excision was deemed necessary due to the limitations of ultrasound-guided biopsy and the clinical importance of accurate histological characterization in determining subsequent therapeutic strategies.
Granulomatous adenopathy. Surgical excision was deemed necessary due to the limitations of ultrasound-guided biopsy and the clinical importance of accurate histological characterization in determining subsequent therapeutic strategies.
Robotic-assisted right IM lymphadenectomy, operative time 98 minutes.
Robotic-assisted IM lymphadenectomy, operative time 95 minutes.
Robotic-assisted left IM lymphadenectomy, operative time 140 minutes.
Robotic-assisted right IM lymphadenectomy, operative time 128 minutes.
Robotic-assisted left IM lymphadenectomy, operative time 130 minutes.
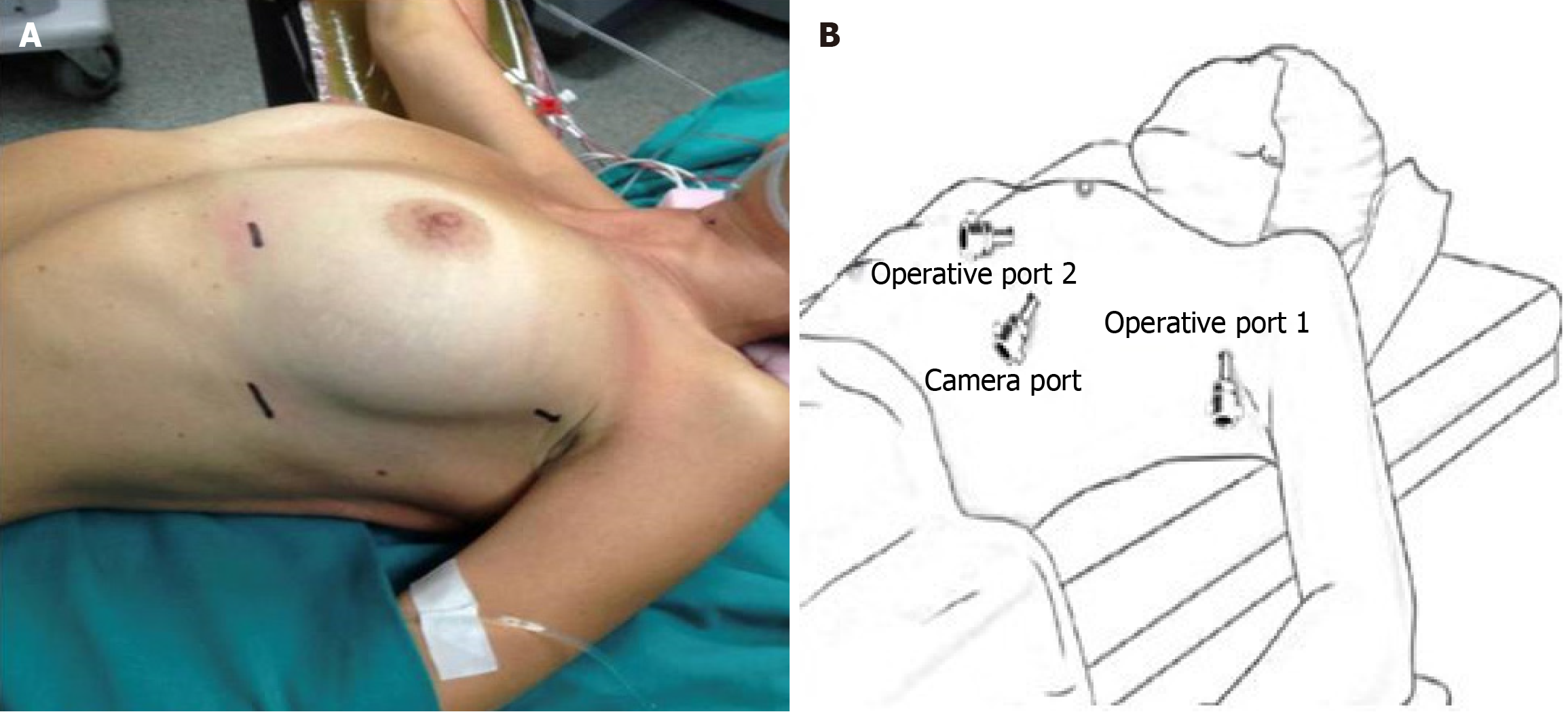
Patient position: Patients positioning and port placement do not change whether the surgery is performed on the left or right chest. Patients are placed in a 30° semi-supine position, aligned with the edge of the side of the operating room table. A roller or pillow is placed under the shoulder, providing an approximately 30° of ipsilateral chest elevation. The same sidearm is held over a padded bar below the level of the table. Once the patient is positioned, the operating table can be elevated for about 30° into the reverse Trendelenburg position and rotated with the ipsilateral side up approximately 10° to 15°. The chest is then supported including the lower neck, the sternum, and the upper part of the abdomen providing space for possible median sternotomy (Figure 3A).
Anaesthetic management: Under general anesthesia, a double-lumen endotracheal tube is placed to achieve single lung ventilation, thus facilitating mediastinal exposition. Invasive (intra-arterial) blood pressure monitoring is generally used for patients with chronic atrial fibrillation or previous history of coronary artery diseases and myocardial infarction. As a rule, we never use epidural anesthesia in this type of procedure. Alternatively, we prefer to perform paravertebral/intercostal nerve blocks that offer an analgesic effect equivalent to the epidural anesthesia with a lower incidence of side effects (hypotension, nausea, and vomiting)[14].
Port placement and docking: Operating room staff set up the robotic console and the vision system. We performed this procedure with the Intuitive da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, United States). The camera port with its trocar is placed in the fifth intercostal space at the level of the mid-axillary line. We introduce the 0°, 12 mm robotic camera and start inspection of the chest cavity and mediastinum. At this time via the same trocar, CO2 is insufflated with a flow of 8 L/minute and a pressure of about 8 mmHg to 10 mmHg. CO2 insufflation helps to collapse lung parenchyma and creates pneumo-mediastinum facilitating dissection of the mediastinal fat tissue surrounding the IM chain. Under direct vision, we place two operating ports: One in the third intercostal space on the anterior axillary line, the other at the level of the fifth intercostal space at about 3 cm to 4 cm lateral to the parasternal line (Figure 3B). The robot docking starts when skin incisions are completed, and all trocars are seated.
The docking procedure is slightly different based on the robotic device model. DaVinci Si system: The robot is positioned behind the patient’s head on the right upper corner of the surgical table for the left side procedure and the left upper corner when approaching the right chest. DaVinci Xi system: The robot is always placed in the same position on the left side of the table independently from which side of the chest will be operated. The procedure is performed with a three-arm approach (camera + two operative arms).
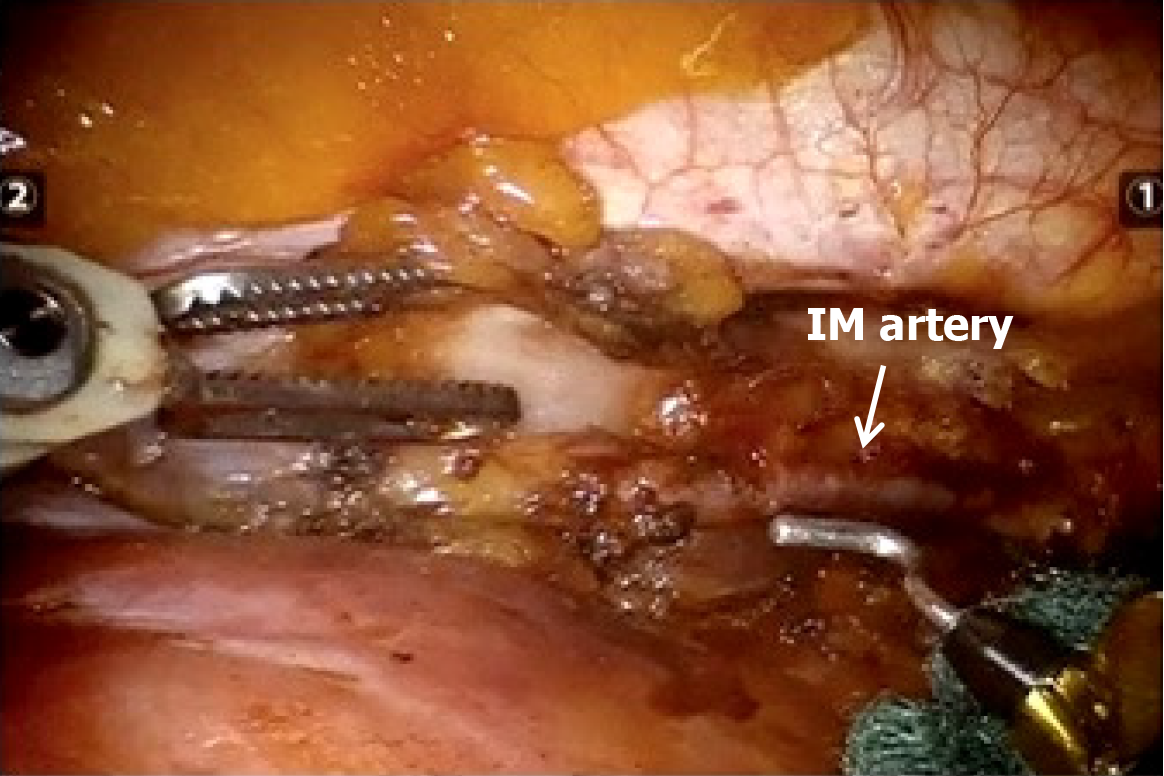
Surgical steps: Port placement and robot setup take usually 8 minutes to 10 minutes. After the introduction of the camera, the two operative robotics arms are introduced under direct vision. A Cadiere forceps is inserted through the parasternal trocar and normally used by the surgeon’s left hand; it allows lung retraction and better exposure of the target area. The fenestrated bipolar forceps and/or the cautery hook are placed on the second operative arm, controlled by the surgeon’s right hand. We prefer to use the bipolar forceps to open the pleura and identify the IM vessels and the cautery hook to dissect mediastinum tissue and the isolation of the LNs (Figure 4).
After inspection of the pleural surface to confirm the absence of metastases, we proceed by opening the pleura laterally to the IM vessels. Dissection is conducted in a cranio-caudal and lateral-medial direction, starting approximately 2 cm below the subclavian vein on the left side and below the origin of the IM vein on the right side. A gentle and careful dissection of the fat tissue around the IM vessels is performed and all the LNs are isolated and removed. At the end of the procedure, a 20 Fr chest tube is introduced from the camera trocar and is usually removed on the 1st postoperative day.
Preference cards: (1) Stereoscopic 3D high-definition camera; (2) One fenestrated bipolar forceps, and a permanent cautery hook (EndoWrist monopolar cautery), operative port 1 (surgeon right hand); (3) One Cadiere forceps, operative port 2 (surgeon left hand); (4) Sponge (or rolled up sponge); and (5) EndoWrist Hem-o-lock clip applier.
All five procedures were successfully completed without intraoperative complications. Postoperative recovery was uneventful across the cohort: Chest tubes were removed on the first postoperative day, and all patients were discharged within 48 hours. Robotic-assisted LN dissection was performed using the DaVinci Xi surgical system under general anesthesia with single-lung ventilation. Operative times ranged from 95 minutes to 140 minutes, with a mean duration of 118 minutes. Histopathological examination confirmed metastatic breast carcinoma in three patients (cases 1, 2, and 4), while the remaining two (cases 3 and 5) exhibited benign findings consistent with granulomatous or reactive adenopathy.
Patients with confirmed malignancy were referred for further oncologic management, including systemic therapy and tailored follow-up. Those with benign pathology resumed routine surveillance without additional intervention. These cases highlight the clinical utility of robotic-assisted IM-LN dissection as a safe, minimally invasive, and diagnostically effective approach in patients with PET-positive adenopathy and a history of malignancy. The technique offers precise histological characterization while minimizing surgical morbidity, thereby informing and optimizing subsequent treatment planning (Table 1).
| Age | Primary tumor | Site of involvement | IM-LN dimension (SUVmax) | Histopatological examination | Operation time (minute) |
| 77 | Lung (adenocarcinoma stage I A2) and breast cancer (infiltrating ductal carcinoma) | Right IM-LN | 10 mm × 11 mm (3.9) | Breast carcinoma | 98 |
| 47 | Breast cancer | IM-LN | 12 mm × 18 mm (2.7) | Breast carcinoma | 95 |
| 34 | Breast cancer [infiltrating ductal carcinoma ER+ (50%), HER2+, Ki67 (60%)] | Left IM-LN | 12 mm × 15 mm (5.0) | Granulomatous adenopathy | 140 |
| 50 | Lung (adenocarcinoma stage I A2) and breast cancer (infiltrating ductal carcinoma pT2) | Right IM-LN | 18 mm × 16 mm (4.0) | Breast carcinoma | 128 |
| 49 | No oncological disease | Left IM-LN | 12 mm × 16 mm (2.8) | Granulomatous adenopathy | 130 |
Robotic-assisted surgery for the IM chain is a highly effective, minimally invasive technique for LN dissection in cases of suspected malignant IM adenopathy. Compared to VATS, robotic technology overcomes various technical and ope
Furthermore, robotic-assisted surgery facilitates a more radical and extended LN dissection of the IM chain, from the second intercostal space along the entire parasternal course. In contrast, VATS is often limited to excisional or incisional biopsies, making robotic technology a valid alternative for comprehensive oncologic management. Importantly, while robotic IM-LN dissection provides key histological information, it should be emphasized that not all patients with PET-positive IM adenopathy necessarily require immediate surgical intervention. In some cases, a cost-effective and clinically appropriate strategy might include delayed PET-CT surveillance to monitor for progression before proceeding with surgery. However, this approach may delay diagnosis and treatment in patients with early recurrence or isolated nodal involvement.
Moreover, percutaneous biopsy - although less invasive - has significant limitations, particularly in the IM region. Technical challenges include deep anatomical location, proximity to critical vascular structures, and reduced visibility in patients with high BMI or unfavorable thoracic anatomy. In our series, percutaneous biopsy was either considered unsafe or yielded non-diagnostic results in all five patients. Therefore, surgical excision was recommended after multidisciplinary tumor board evaluation, especially given that histological confirmation was crucial to inform treatment decisions, including systemic therapy.
Finally, we would like to highlight that all robotic procedures were performed by board-certified thoracic surgeons with extensive experience in minimally invasive thoracic oncology. The procedures were performed in a high-volume cancer center with dedicated robotic infrastructure. This level of surgical expertise was critical to achieving the safety and efficiency outcomes reported in this series. While robotic IM lymphadenectomy is technically demanding, we believe the procedure is reproducible in similarly equipped centers with experienced thoracic robotic teams. Including this information may help guide other institutions seeking to adopt this approach and assess its feasibility within their own clinical settings.
These findings underline the importance of careful patient selection, where robotic IM-LN dissection is reserved for cases with isolated, suspicious adenopathy not amenable to non-invasive sampling, and where a tissue diagnosis could significantly influence clinical management. When performing this procedure, we highlight some tips and key points to pay attention to: (1) The robotic approach also provides a further diagnostic advantage due to the excellent panoramic view of the entire pleural cavity and its recesses. All suspicious areas on the pericardium, pleura, mediastinum, and lung can be identified for biopsy. It also allows the management of concomitant pathological conditions such as pleural effusion by performing talc pleurodesis (improved intra-operative staging of the disease); (2) Careful and accurate dissection of the LNs chain should be performed to avoid injuries to internal thoracic vessels. In case of accidental rupture of the IM artery, we usually prefer to control the bleeding by placing Hem-o-lock clips with the appropriate robotic instrument (Hem-o-lock clip applier); (3) Meticulous coagulation of the tissue surrounding the LNs is mandatory to avoid damage of small lymphatic vessels which may result in prolonged pleural effusion in the post-operative setting; (4) A sponge (typically rolled up) should always be available at all times during the procedure. This offers a means of applying direct pressure in case of vascular injuries and helps keep the working area clean from small “annoying” bleeding. The sponge should be preferred to the sucker which can reduce/alter the effect of the CO2 insufflation; (5) Special care should be taken when performing the skin incision in a patient with a silicone breast prosthesis to avoid rupture/damage to the prosthesis. In this situation, the camera port can be placed more laterally than in patients without a prosthesis, preferably not lower the fifth intercostal space to keep the heart out of the camera view; (6) Conversion to an open approach should be always recommended in case of uncontrolled bleeding or if a safe dissection plane cannot be found in the presence of widespread and tenacious adhesions; and (7) Robotic IM-LN biopsy may be contraindicated in patients with com
In our experience, the robotic biopsy/dissection of the IM-LNs is a safe and feasible technique that could provide significant information for the management of breast cancer patients with very few rare contraindications and complications.
We gratefully thank Gerardo Cioffi, native speaker, for reviewing the English language.
| 1. | Paredes P, Vidal-Sicart S, Zanón G, Pahisa J, Fernández PL, Velasco M, Santamaría G, Ortín J, Duch J, Pons F. Clinical relevance of sentinel lymph nodes in the internal mammary chain in breast cancer patients. Eur J Nucl Med Mol Imaging. 2005;32:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Caudle AS, Yi M, Hoffman KE, Mittendorf EA, Babiera GV, Hwang RF, Meric-Bernstam F, Sahin AA, Hunt KK. Impact of identification of internal mammary sentinel lymph node metastasis in breast cancer patients. Ann Surg Oncol. 2014;21:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Heuts EM, van der Ent FW, Hulsewé KW, von Meyenfeldt MF, Voogd AC. Results of tailored treatment for breast cancer patients with internal mammary lymph node metastases. Breast. 2009;18:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Johnson N, Soot L, Nelson J, Franzini MD, Vea H, Gruner S, Kulawiak L, Young K. Sentinel node biopsy and internal mammary lymphatic mapping in breast cancer. Am J Surg. 2000;179:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Estourgie SH, Tanis PJ, Nieweg OE, Valdés Olmos RA, Rutgers EJ, Kroon BB. Should the hunt for internal mammary chain sentinel nodes begin? An evaluation of 150 breast cancer patients. Ann Surg Oncol. 2003;10:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Steinke K, Brook P, Ramuz O. Radiological pitfall: Siliconoma in internal mammary lymph node mimics breast cancer recurrence. Radiol Case Rep. 2011;6:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Lee Y, Song SE, Yoon ES, Bae JW, Jung SP. Extensive silicone lymphadenopathy after breast implant insertion mimicking malignant lymphadenopathy. Ann Surg Treat Res. 2017;93:331-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Fornage BD, Dogan BE, Sneige N, Staerkel GA. Ultrasound-guided fine-needle aspiration biopsy of internal mammary nodes: technique and preliminary results in breast cancer patients. AJR Am J Roentgenol. 2014;203:W213-W220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Sacchini G, Borgen PI, Galimberti V, Veronesi P, Zurrida S, Luini A, Spaggiari L, Cody HS 3rd, Veronesi U. Surgical approach to internal mammary lymph node biopsy. J Am Coll Surg. 2001;193:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Gareer WY, Elsebaie H, Gareer H, Ahmed H, Wafa M, Soliman H. Thoracoscopic internal mammary lymph nodes dissection: a staging tool for internal mammary lymph nodes in breast cancer. Chin-Ger J Clin Oncol. 2011;10:580-583. [DOI] [Full Text] |
| 11. | Ogawa Y, Ishikawa T, Sawada T, Chung SH, Osaka H, Takashima T, Onoda N, Kato Y, Ochi H, Hirakawa K. Thoracoscopic internal mammary sentinel node biopsy for breast cancer. Surg Endosc. 2003;17:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Tanis PJ, Nieweg OE, Valdés Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001;192:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 187] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Barros AC, Mori LJ, Nishimura D, Jacomo AL. Surgical anatomy of the internal thoracic lymph nodes in fresh human cadavers: basis for sentinel node biopsy. World J Surg Oncol. 2016;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/