Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1333
Revised: August 25, 2024
Accepted: August 29, 2024
Published online: October 24, 2024
Processing time: 187 Days and 13.9 Hours
Large abdominal wall defect (LAWD) measures > 20 cm in width. LAWD can easily lead to intestinal necrosis, peritonitis, other complications, and even mul
This article summarizes the nursing experience of a patient with sigmoid carci
After more than 7 months of careful care, the patient's physical fitness has been well recovered, local inflammation is well controlled, which wins the opportunity for the operation, and the postoperative recovery is good.
Core Tip: Multiple intestinal fistulas are high-flow fistulas, which may lead to severe water and electrolyte disorder and malnutrition in patients. After nutritional support treatment, care of the abdominal wall defect and intestinal fistula, con
- Citation: Zhu YL, Li R, Cheng YG, Wang YF. Perioperative management of postoperative sigmoid colon cancer complicated by a large abdominal wall defect: A case report. World J Clin Oncol 2024; 15(10): 1333-1341
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1333.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1333
Large abdominal wall defect (LAWD) measures > 20 cm in width[1]. LAWD can easily lead to intestinal necrosis, peri
A 78-year-old male patient who had sigmoid colon cancer with intestinal obstruction underwent "radical sigmoid re
Postoperative pathology showed a moderately differentiated adenocarcinoma of the sigmoid colon. On July 20, 2018, the stoma fistula was found to be retracted into the abdominal cavity, and fecal-like liquid leaked around the left lower abdominal drainage tube and incision. Therefore, the patient underwent emergency “exploratory laparotomy + abdo
The patient has a history of appendectomy (1996) and coronary heart disease for over a month, but has not taken any medication orally.
The patient's family has no history of similar diseases or familial genetic illnesses.
The patient’s clear consciousness, poor mental state, moderate malnutrition (height 175 cm, body weight 50 kg, body mass index (BMI) 16.3 kg/m2. NRS2002 nutritional risk screening score is 5 points), no obvious yellowing of the skin and sclera, no enlargement of superficial lymph nodes throughout the body, soft neck, coarse respiratory sounds in both lungs, no obvious dry and wet rales heard, regular heart rhythm, no obvious pathological murmurs.
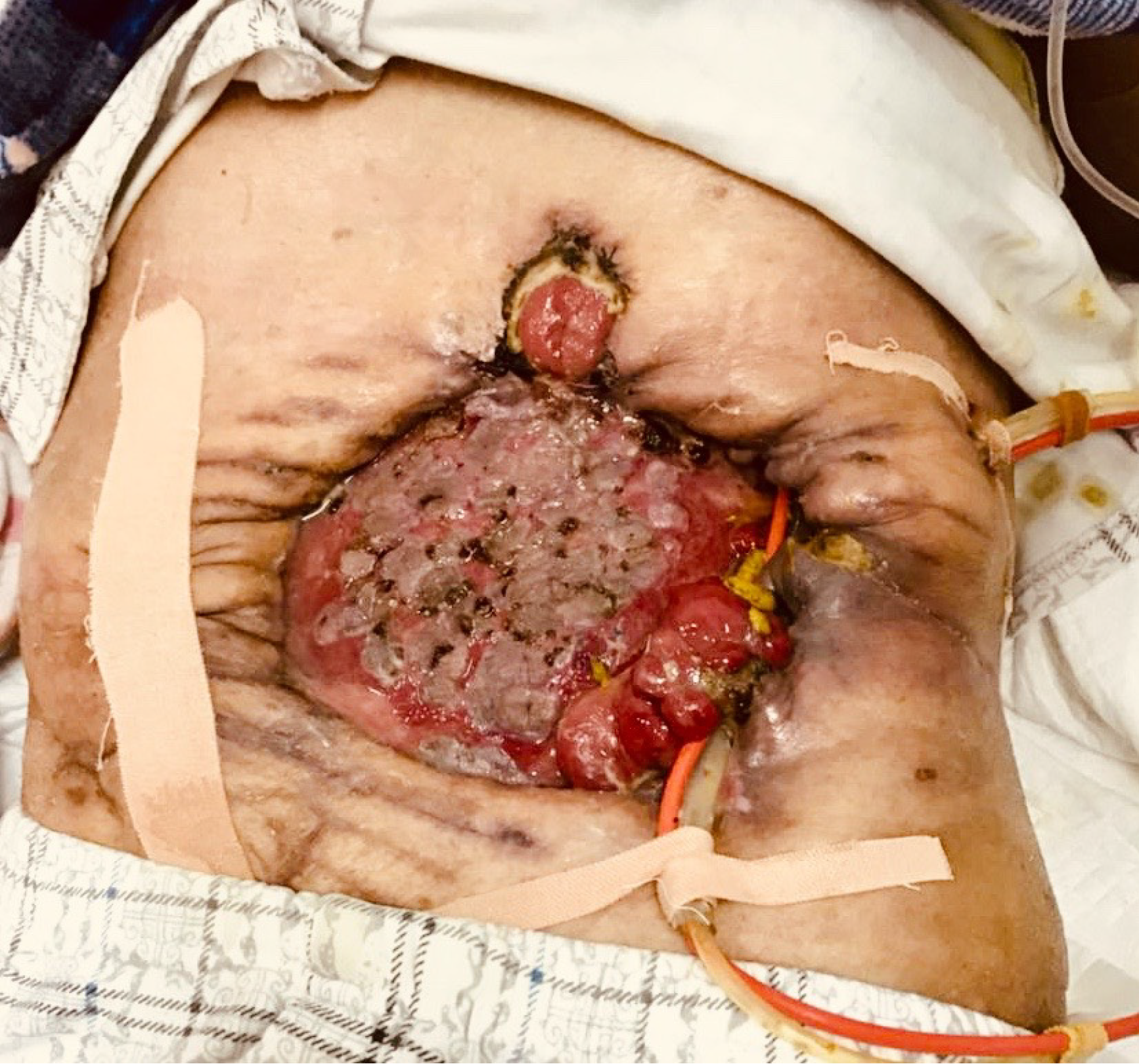
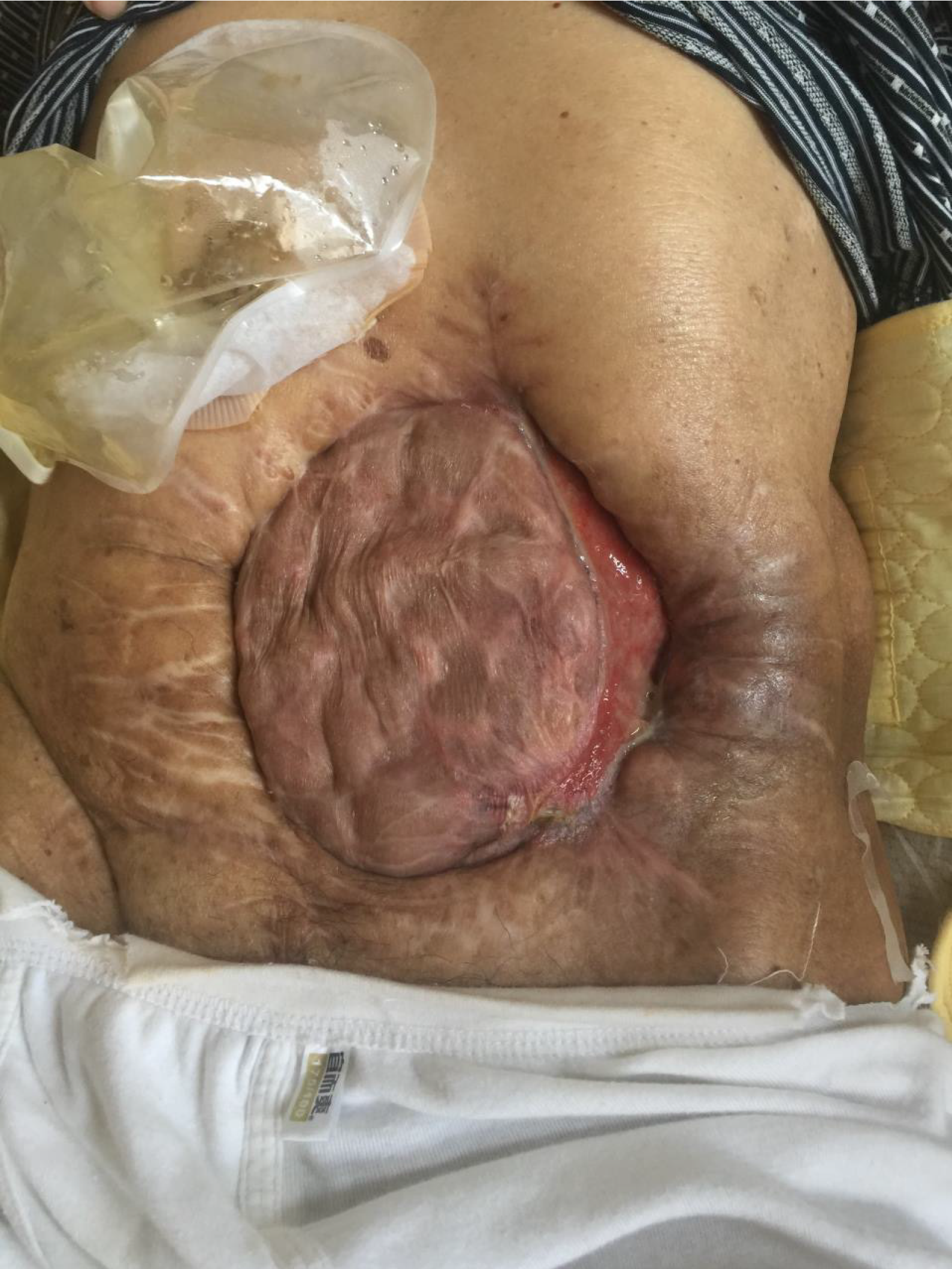
The patient had stable vital signs and moderate malnutrition (175 cm height, 50 kg body mass, BMI 16.3 kg/m2). The score of NRS2002 nutritional risk screening table was 5 points)[6]. There was an open wound of 22 cm × 18 cm in the middle abdomen, with the visible intestinal wall, bright red in color, good blood supply, no active bleeding, and a small part of relatively thin skin graft covering the surface. There were one stoma on the upper side and one on the left side of the wound, which were red in color, with good blood circulation, and without stenosis or slip. There were yellow formed stools discharged from the left stoma, and four intestinal fistula could be seen on the intestinal wall of the wound, with yellow feces discharged. The patient was placed with drainage tubes on the upper and lower sides of the left abdomen and subjected to continuous low-pressure flushing (Figure 1). There is no obvious edema in both lower limbs.
Blood routine: White blood cell count 6.62 × 109/L, neutrophil percentage 71.7%, red blood cell count 3.78 × 1012/L, hemoglobin 112 g/L, platelets 179 × 109/L, C-reactive protein 51.83 mg/L. Urine routine: PH 5, 42 red blood cells/μL, 1842 white blood cells/μL, 42 fungi/LPF. Blood biochemistry test: Liver function: Albumin 34.1 g/L, prealbumin 113 g/L, glutamyl transpeptidase 182 μL, cholinesterase 1389 u/L, all other values are within normal range. Renal function is normal. Carbon dioxide binding capacity is 19.9 mmol/L, glucose (non fasting) is 6.8 mmol/L, and cystatin C is 1.96 mg/L. Electrolytes are within the normal range. Myocardial enzyme examination: Creatine kinase 29.64 μ/L, lactate dehydrogenase 606 μ/L, and high-sensitivity troponin 126.4 μg/L. Blood B-type natriuretic peptide measurement: 228.9 pmol/L. Sputum culture: Klebsiella pneumoniae. Urine culture: Smooth Candida resistant. Coagulation function: Antithrombin III 64.9%, fibrin degradation products 10.97 μg/mL, D-dimer 2.31 mg/L.
Head, chest, and abdomen computed tomography scan: (1) Postoperative absence of part of the left lower abdominal wall following sigmoid colon cancer surgery, with a tube shadow in the left lower abdominal wall, and residual contrast agent visible in the right hemicolon; (2) Multiple nodular lesions in both lungs, considered as possible fibroproliferative lesions metastatic tumors to be excluded; please compare with old images, and a follow-up is recommended; (3) Inflammation in the upper lobe of the left lung and both lower lobes; (4) Right pleural effusion with atelectasis of the right lower lung lobe, and thickening and adhesion of the left pleura; (5) Multiple lymph node shadows in the mediastinum; (6) Multiple cysts in the liver and both kidneys; (7) Enlargement of the prostate with an irregular anterior margin; follow-up and re-examination are advised; (8) Age-related brain changes; and (9) Sphenoid sinusitis.
The final diagnosis was multiple intestinal fistulas, abdominal infection, LAWD with abdominal opening, and moderate malnutrition secondary to incisional laceration and debridement, autologous skin transplantation status, radical resection of the sigmoid colon cancer, enterostomy, colostomy, and appendectomy. He was also diagnosed with coronary heart di
Infection, homeostasis imbalance, and malnutrition are the three major pathophysiological changes in patients with intestinal fistulas, and these changes interact with each other. Malnutrition-related sepsis is the primary cause of death in patients with external intestinal fistulas. Active nutritional support can help reduce the incidence and mortality of com
EN helps prevent intestinal atrophy, maintain intestinal function, and support immune function[7]. If conditions permit, EN should be used as much as possible. The patient was admitted to the hospital with a nutritional tube, and yellow formed stools were leaking from both the stoma and the intestinal fistula. Considering that the patient’s gastrointestinal tract maintained to be partially functional, 1500 mL of EN suspension was administered daily through a gastrointestinal nutritional tube after heating and dripping. This EN suspension was the enteral nutrition suspension (SP), a short peptide-type EN preparation that is directly absorbed in the intestine without digestion. It is especially suitable for pa
During the EN period, owing to abdominal pain, vomiting, and diarrhea, the patient experienced significant fluctuations in body temperature, reaching 39.2 ℃. At one point, his plasma albumin and hemoglobin levels decreased to 22.13 and 109 g/L, respectively. After consultation with the nutrition department, the patient was considered to have intestinal nutrition tolerance disorders; hence, the nutrition plan was adjusted. EN was changed to PN using a fully integrated PN mixture (50% glucose, 250 mL; 10% glucose, 250 mL; 10% potassium chloride, 40 mL; 10% sodium chloride, 40 mL; multi-trace elements injection (II), 10 mL; 50% glucose, 100 mL; water-soluble vitamin, 10 mL; insulin, 32 units; 11.4% com
After the gastrointestinal symptoms such as abdominal pain and diarrhea were eliminated and the body temperature normalized, PN was gradually transitioned to EN under the guidance of the nutrition department according to the patient’s condition, to stimulate the growth of intestinal mucosal epithelial cells and prevent intestinal mucosal atrophy. The abovementioned PN formula should be halved, and adding 1000 mL of SP, which should be heated and dripped through a nutrient tube, per day was necessary. As the gastrointestinal function improved, the patient was progressively transitioned to total EN, discontinuing the PN infusion.
After more than half a year of nutritional support treatment, before the 7th operation in Hefei First People’s Hospital, the patient’s albumin level, hemoglobin count, BMI, and NRS2002 score were 37.1 g/L, 128 g/L, 18.7 kg/m2, and 2 points, respectively, which laid a good foundation for subsequent surgical treatment.
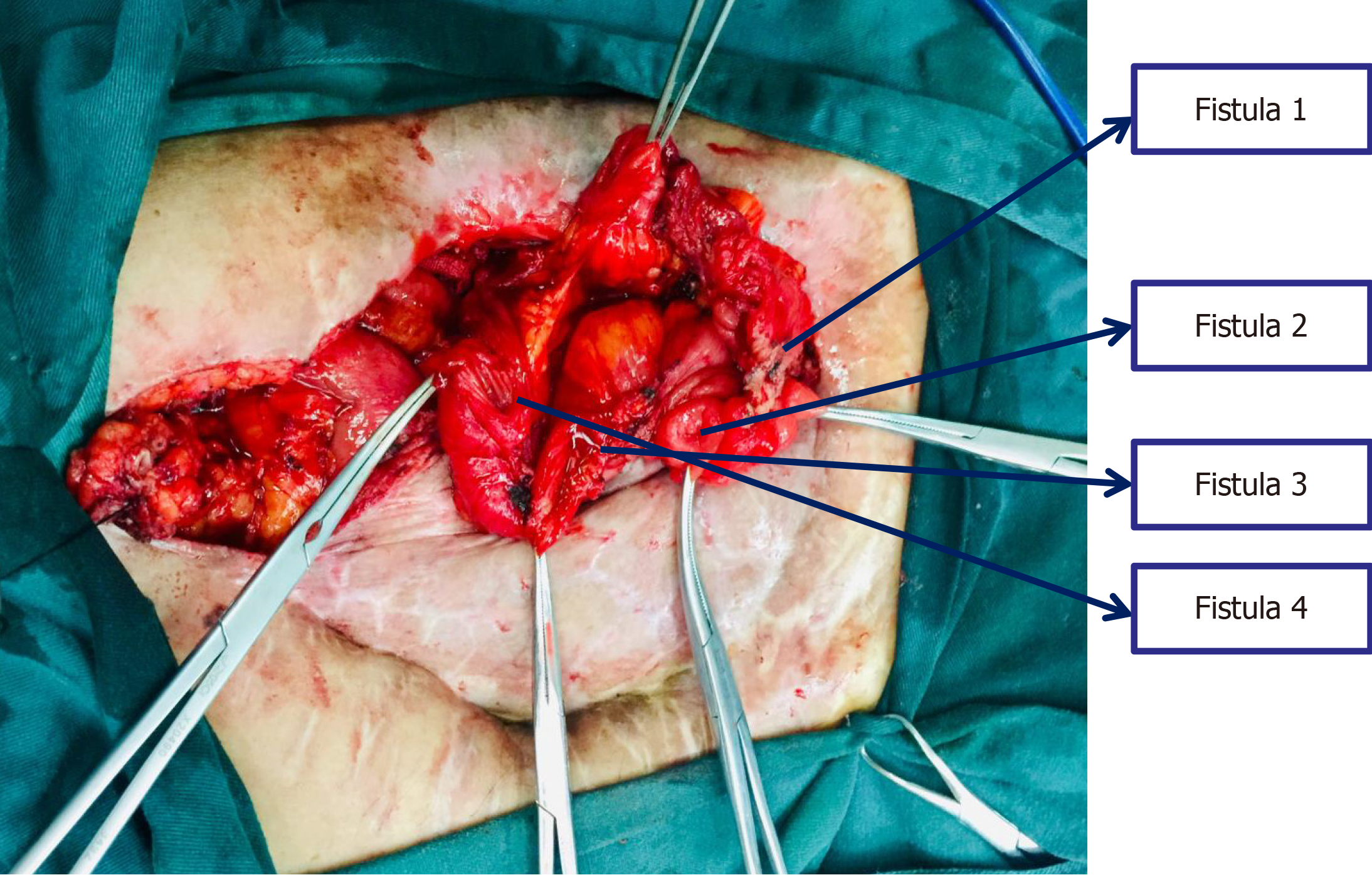
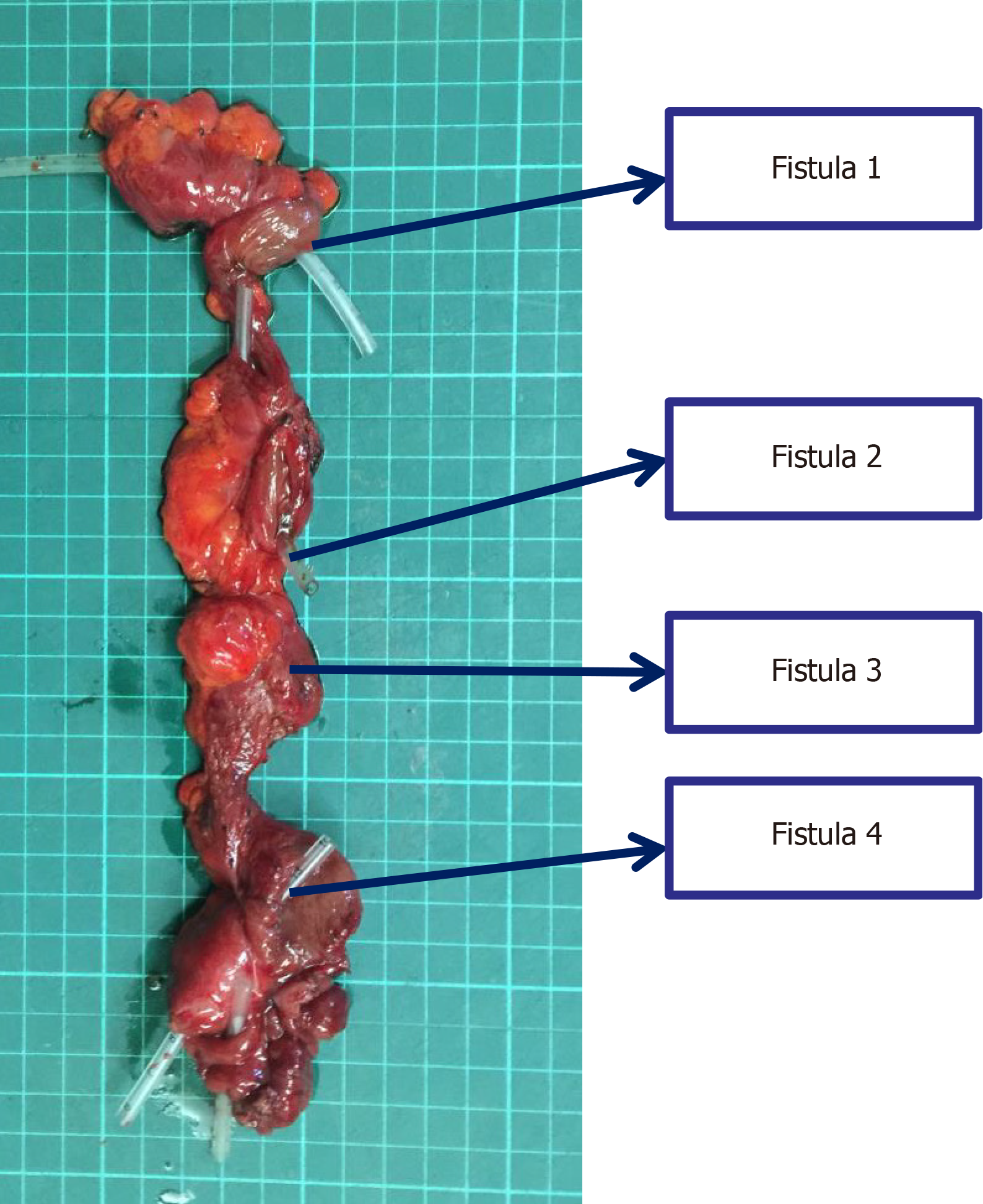
The patient underwent the 7th operation of "complex intestinal fistulectomy + colostomy" in Hefei First People’s Hospital. Four small intestinal fistulas were completely removed, and anastomosis of the small intestine end was performed. Furthermore, the original colostomy orifice was removed, and the transverse colon stump was dragged out of the ab
On admission, the patient had LAWD measuring 22 cm × 18 cm in the midabdomen, with a bright red intestinal wall, good blood supply, and no active bleeding. Part of the intestinal wall surface was covered with gray and white skin grafts, which were discontinuous and patchy, and the skin flap was relatively thin. One stoma was found on the upper side and another on the left side of the wound, appearing red and displaying a good blood supply, without stenosis or detachment. Additionally, yellow formed stools expelled from the left stoma. Four intestinal fistulas were also observed in the intestinal wall of the affected area, also discharging yellow stools. The skin around the defect area was red, swollen, and eroded, indicating inflammation, owing to the long-term exposure to intestinal content leakage. After admission, the following nursing measures were taken after consultation with a wound ostomy specialist. First, the dressing should be changed timely to remove the necrotic tissue. The color of the intestinal mucosa should be monitored closely, and the necrotic tissue from the LAWD should be removed to promote wound healing. After 12 days of treatment, the redness and swelling of the surrounding tissue of the LAWD clearly subsided, and the inflammation improved. Second, the skin around the fistula should be protected. The patient had multiple intestinal fistulas, which were high-flow fistulas. The spilled intestinal fluid could easily corrode the adjacent tissues and the abdominal wall skin, causing serious tissue edema. Therefore, the spilled intestinal fluid should be sucked by a negative-pressure suction device in time. The su
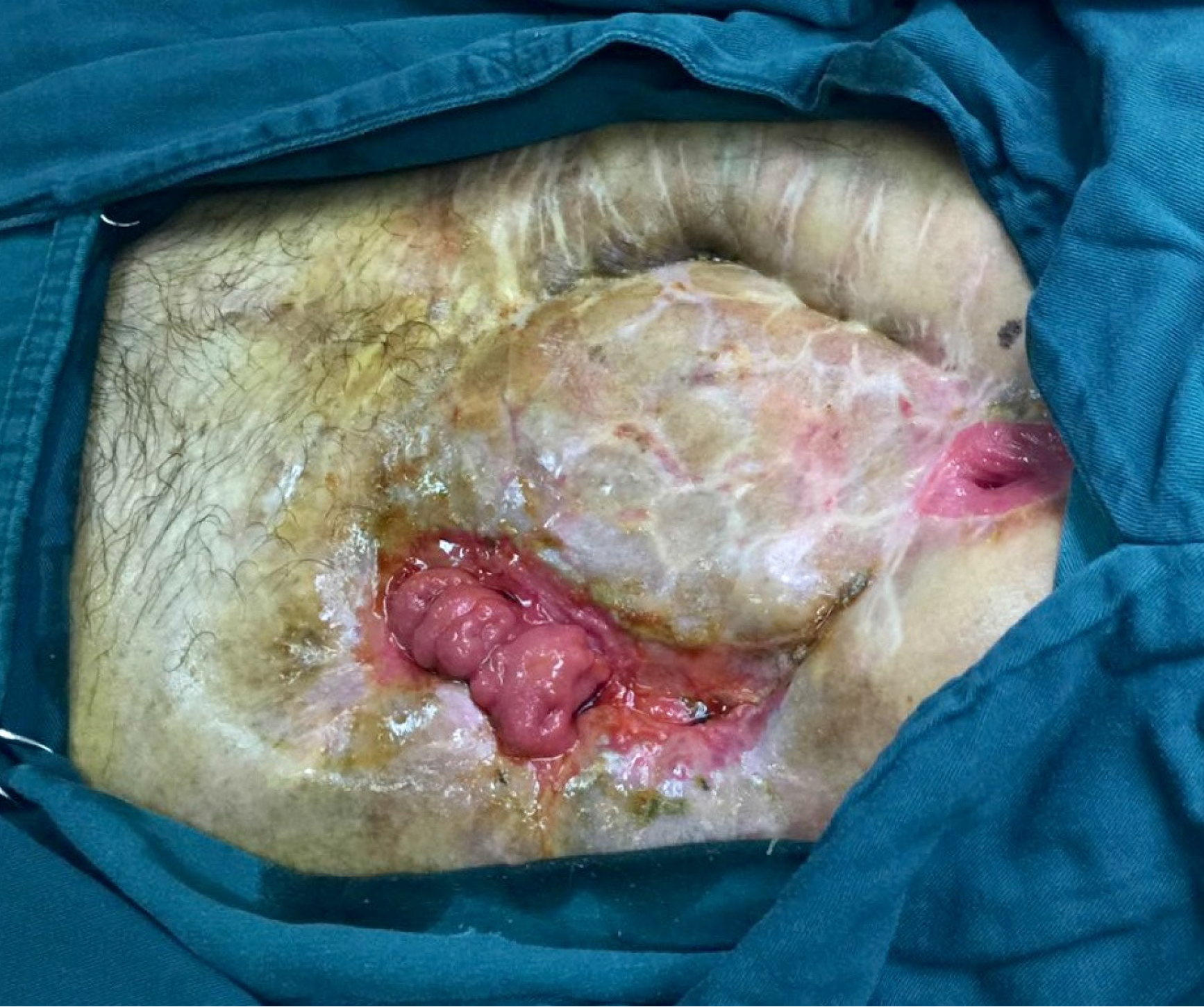
At the follow-up visit after 63 postoperative days, the skin graft had completely covered the wound.
Continuous flushing of peritoneal drainage tube with isotonic saline and low negative-pressure drainage. The patient had multiple intestinal fistulas and a large amount of leakage, which may cause serious abdominal infection if the drainage was incomplete. Negative-pressure drainage could be used in abdominal wall defects with severe contamination or infection within the abdominal cavity, or as a transitional treatment before clear repair and reconstruction of such defects. It could significantly reduce intra-abdominal pressure, alleviate edema, enable granulation tissue for better and faster formation, and strengthen the coverage of abdominal wall defects[8]. During admission, the abdominal drainage tubes were placed in the upper and lower parts of the left abdomen and were continuously flushed with isotonic saline combined with low negative-pressure suction (irrigation speed within 60-80 drops/min; negative drainage pressure between -110 and -95 mmHg). The negative-pressure drainage tube drew the flushing fluid, exudates, and necrotic tissue out of the wound, promoting wound healing. After the inflammation was controlled, the irrigation rate was changed to 40 drops/min, and the negative-pressure drainage was adjusted between −80 and −55 mmHg. After 10 weeks of treatment, the abdominal infection was controlled and the skin inflammation subsided.
On admission, the patient’s Autar deep vein thrombosis (DVT) risk score was 13, and he could not get out of bed and move outside because of his illness, thereby at risk of developing DVT. The nurse instructed the patient to execute bed activities daily, assisted the patient to turn over, regularly massaged the leg muscles, encouraged the use of lower-limb compression elastic socks, and provided lower-limb pressure therapy (30 minutes/session), ankle pump exercises of both lower limbs, knee extension and bending exercises, hip-lifting exercises, and others. After the patient had recovered his strength, he was encouraged to get out of bed in time, and the blood coagulation index was monitored twice a week. Anticoagulant drugs were used when necessary. After active prevention, the patient did not develop DVT during hospitalization.
Physical exercise can promote patients’ intestinal peristalsis and overall muscle strength recovery, contribute to the gradual restoration of the intestinal function, and prevent and control pulmonary complications[9]. After patient admi
The patient was an older adult, and his condition had fluctuated repeatedly since the disease onset. He had undergone multiple surgeries and dressing changes in a short period of time. The wound surface was large, and the condition was serious, resulting in changes in external appearance and bowel movements. The secondary infection after the operation, wound pain, worries regarding the disease prognosis, and the burden on the family and other situations had led to his unstable mental state, as manifested by depression, unwillingness to communicate, pessimistic attitude toward disease development and prognosis, and deep concern about his future life. Considering his psychological state, the supervising physician, nurse, and wound specialist nurse all showed understanding and provided supportive psychological inter
In this case, the patient had LAWD accompanied with multiple intestinal fistulas. On admission, he had poor nutritional status, severe intra-abdominal infection, and evident inflammation and edema in the abdominal wall, exposed tissue, and surrounding tissue of the LAWD. Through multidisciplinary consultation preoperatively, the medical team formulated individualized diagnosis and treatment plan according to the patient’s condition, corrected the patient’s nutritional status, and significantly improved the skin status around the fistula. Consequently, the patient’s tissue, organ, and im
The authors thank all the participants and investigators for their efforts and are grateful for the support provided by the Hefei First People’s Hospital.
| 1. | Gu Y, Wang P, Li H, Tian W, Tang J. Chinese expert consensus on adult ventral abdominal wall defect repair and reconstruction. Am J Surg. 2021;222:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Wei Z, Wang B, Lin T, Zhu J, Yang X, Fang X, Zhu Y, Cheng J. Acellular allogenic dermis combined with VSD for repair of abdominal wall defect: a case series. Am J Transl Res. 2023;15:2233-2240. [PubMed] |
| 3. | Wanjala NF, Dan K. Local/regional flaps for extensive abdominal wall defects: Case series. Int J Surg Case Rep. 2020;74:10-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Ghimire P. Management of Enterocutaneous Fistula: A Review. JNMA J Nepal Med Assoc. 2022;60:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ribeiro-Junior MAF, Yeh DD, Augusto SS, Elias YGB, Néder PR, Costa CTK, Maurício AD, Saverio SD. The role of fistuloclysis in the treatment of patients with enteroatmospheric fistulas. Arq Bras Cir Dig. 2021;34:e1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Zeilstra D, Younes JA, Brummer RJ, Kleerebezem M. Perspective: Fundamental Limitations of the Randomized Controlled Trial Method in Nutritional Research: The Example of Probiotics. Adv Nutr. 2018;9:561-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Doley J. Enteral Nutrition Overview. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 8. | Li Y, Li PY, Sun SJ, Yao YZ, Li ZF, Liu T, Yang F, Zhang LY, Bai XJ, Huo JS, He WB, Ouyang J, Peng L, Hu P, Zhu YA, Jin P, Shao QF, Wang YF, Dai RW, Hu PY, Chen HM, Wang GF, Wang YG, Jin HX, Zhu CJ, Zhang QY, Shao B, Sang XG, Yin CL. Chinese Trauma Surgeon Association for management guidelines of vacuum sealing drainage application in abdominal surgeries-Update and systematic review. Chin J Traumatol. 2019;22:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, Boutros M, McClane J, Feldman LS, Steele SR. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017;60:761-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/