Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1324
Revised: August 26, 2024
Accepted: September 19, 2024
Published online: October 24, 2024
Processing time: 189 Days and 22.8 Hours
Cervical myeloid sarcoma (MS) is a rare hematological malignancy characterized by the formation of extramedullary soft tissue masses in the cervical region. Due to its uncommon presentation in the female reproductive system, cervical MS poses significant diagnostic and therapeutic challenges. Consequently, there is a pressing need for more research and clinical experience to better understand, diagnose, and manage this condition effectively.
This report details four cases, the diagnostic process, treatment strategy, and outcomes, discussing cervical MS as an initial clinical manifestation. The disease exhibits varied clinical presentations, such as irregular vaginal bleeding and pa
Immunohistochemical diagnosis and tailored therapeutic strategies are essential. Further research is crucial in improving outcomes and developing effective treatment protocols.
Core Tip: Cervical myeloid sarcoma is rare but can indicate the presence of acute myeloid leukemia, myelodysplastic syndrome, or myeloproliferative neoplasms. Immunohistochemistry is pivotal in diagnosing and differentiating myeloid sarcoma and the absence of cluster of differentiation (CD) 20 and CD3 can help distinguish it from lymphoma. Systemic chemotherapy is the most common treatment with some patients also undergoing surgery or radiation therapy. The literature indicates that allogeneic hematopoietic stem cell transplantation is a critical strategy for long-term survival.
- Citation: Li JK, Wang XX, Fu JJ, Zhang DD. Cervical myeloid sarcoma as an initial clinical manifestation: Four case reports. World J Clin Oncol 2024; 15(10): 1324-1332
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1324.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1324
Malignant tumors of the hematopoietic system can manifest as extramedullary soft tissue masses, and myeloid sarcoma (MS) is a rare clinical presentation. It refers to the formation of tumors outside the bone marrow by immature myeloid cells, with or without concurrent marrow involvement[1]. In most cases, it is associated with myeloid disorders, such as acute myeloid leukemia (AML), but there are instances where it presents solely as an extramedullary myeloid tumor. MS can affect multiple tissues and organs throughout the body, with rare occurrences in the cervix and reproductive organs[2,3].
This report presents four cases of cervical MS diagnosed at our hospital and provides an in-depth analysis. Ad
Case 1: A 49-year-old woman presented with a 2-month history of irregular vaginal bleeding.
Case 2: A 54-year-old postmenopausal woman presented with irregular bleeding.
Case 3: A 46-year-old woman presented with irregular bleeding after menstruation.
Case 4: A 48-year-old female presented with 4 months of irregular vaginal bleeding.
Case 1: The ultrasound examination performed at the local hospital indicated a hypoechoic cervical mass. A colposcopic biopsy indicated diffuse infiltration of small cells in the cervical stroma. Immunohistochemistry (IHC) results were as follows: Cytokeratin (CK) (-), P63 (-), cluster of differentiation (CD) 20 (-), CD3 (-), CD43 (+), and myeloperoxidase (MPO) (-). The pathological diagnosis was cervical MS.
Case 2: The patient presented with irregular vaginal bleeding for 2 months after being postmenopausal for 1 year, with a volume less than that of previous menstrual periods and a dark red color.
Case 3: The patient experienced vaginal bleeding for 2 months after menopause. Examination revealed a mass on the anterior lip of the cervix, and a biopsy performed under colposcopy showed severe chronic inflammation with erosion and lymphoid tissue hyperplasia in the cervical tissue.
Case 4: The patient was found to have a cervical mass, and a cervical biopsy suggested a diagnosis of undifferentiated carcinoma, which suggested undifferentiated carcinoma. IHC results were as follows: Ki-67 60% (+), high molecular weight-CK, carcinoembryonic antigen, P40, CK5/6, P16, CD56, and chromogranin A were all negative. Despite undergoing subsequent chemotherapy (uterine artery interventional chemotherapy: Cisplatin 40 mg, vincristine 2 mg; Intravenous chemotherapy: Paclitaxel 135 mg/m² on day 1, cisplatin 70 mg/m² on day 1), there was no significant change in the tumor.
Cases 1, 3, and 4: Generally healthy.
Case 2: The patient had a 3-year history of hypertension without antihypertensive treatment, and no other medical history.
The patient denied a family history of malignant tumors or hematologic disorders.
Case 1: Gynecological examination revealed that the vaginal vault was absent, the cervix was markedly enlarged, firm with contact bleeding.
Case 2: Gynecological examination revealed a firm, 6 cm mass on the posterior lip of the cervix with limited mobility.
Case 3: Gynecological examination revealed a firm, 3 cm mass on the cervix with surface erosion, and bleeding on palpation.
Case 4: Gynecological examination showed the disappearance of the anterior vaginal vault and a firm, 7.0 cm × 5.0 cm mass on the posterior lip of the cervix extending into the lumen. The tissue was gray-white and friable, with thickening of the parametrium.
Case 1: Peripheral blood tests and cervical cytology were unremarkable. The patient declined a bone marrow biopsy procedure (BMP).
Case 2: Cervical cytology was normal. Blood tests showed an elevated white blood cell (WBC) count of 50.8 × 10^9/L. Peripheral blood morphology analysis revealed decreased neutrophils (15%), lymphocytes (7%), and monocytes (1%), with 76% abnormal WBCs, suggesting acute leukemia. BMP showed highly active marrow proliferation (90%) and diffuse proliferation of blast cells, with medium-sized cells and abundant cytoplasm. Scattered late erythroblasts were observed, and megakaryocytes were common, predominantly with segmented nuclei. Reticulin staining showed a fibrosis stage of modified fibrosis grade 1; Periodic acid-Schiff staining was positive. IHC results showed MPO (+), CD34 (-), CD117 (+), CD20 (-), CD3 (-), and terminal deoxynucleotidyl transferase (TdT) (-), consistent with AML. Chromosome analysis showed a karyotype of 46XX with no clonal abnormalities; fusion gene testing was negative for the promyelocytic leukemia/retinoic acid receptor alpha gene fusion. Flow cytometry identified 74.09% abnormal myeloid blasts, consistent with the AML phenotype, with AML-with maturation (M2) as the likely diagnosis after excluding recurrent genetic abnormalities.
Case 3: Peripheral blood tests and cervical cytology were unremarkable.
Case 4: The squamous cell carcinoma antigen was 5.35 mg/mL. Cervical cytology did not show any abnormalities.
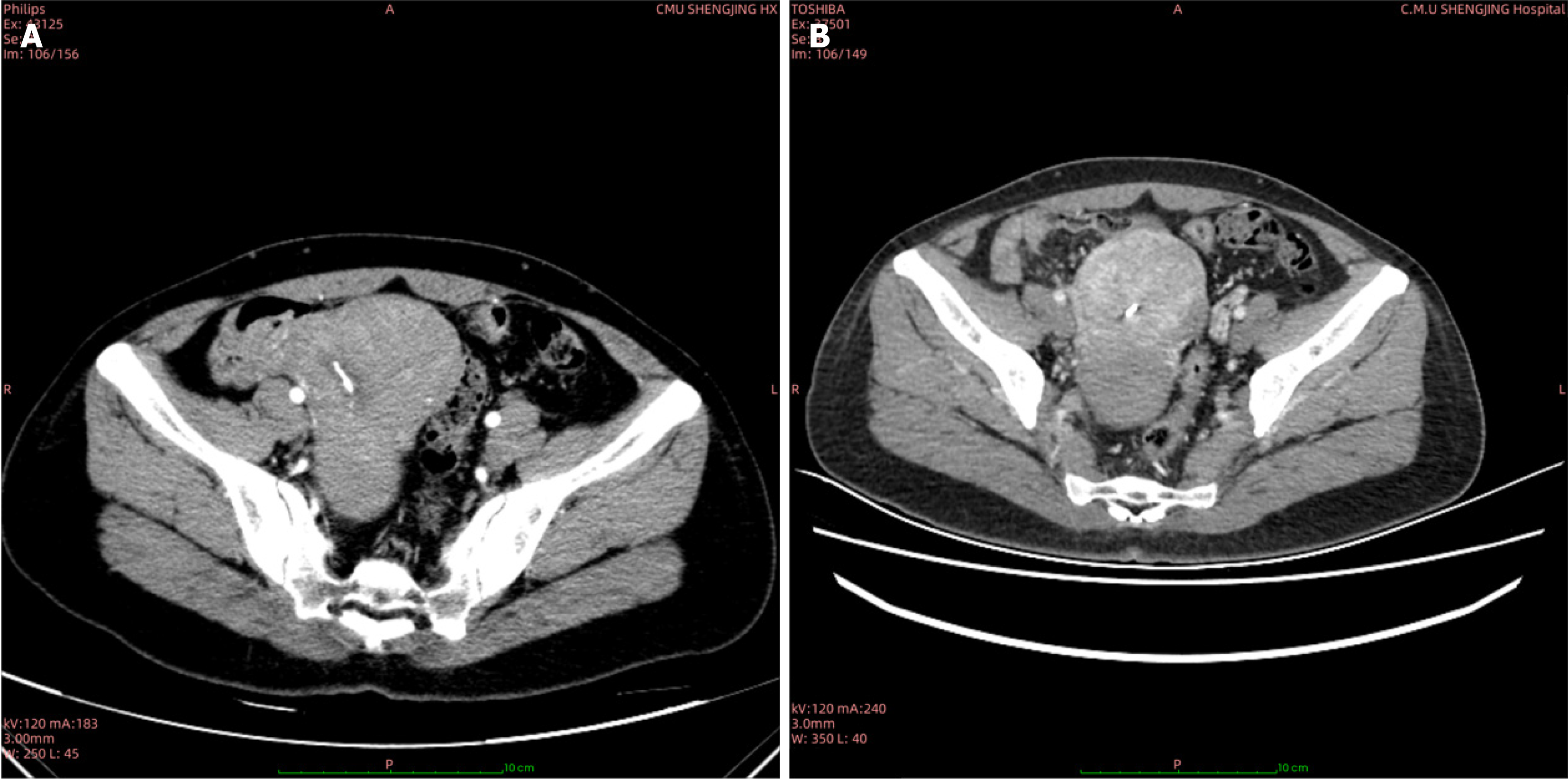
Case 1: Computed tomography (CT) identified an 8.8 cm × 7.4 cm cervical mass (Figure 1A).
Case 2: Ultrasound revealed a full cervical shape with a hypoechoic mass in the posterior lip, measuring approximately 6.4 cm × 5.1 cm × 6.1 cm.
Case 3: Ultrasound examination revealed a cervical mass, showing a hypoechoic lesion measuring 3.0 cm × 2.5 cm.
Case 4: Pelvic enhanced magnetic resonance imaging (MRI) showed a significantly enlarged cervical canal containing a mass measuring 7.2 cm × 4.5 cm. There was involvement of endometrium and the upper one-third of the vagina, with multiple enlarged lymph nodes adjacent to the bilateral iliac vessels.
Based on physical examination, imaging studies, and pathological evaluation, the diagnosis was cervical MS.
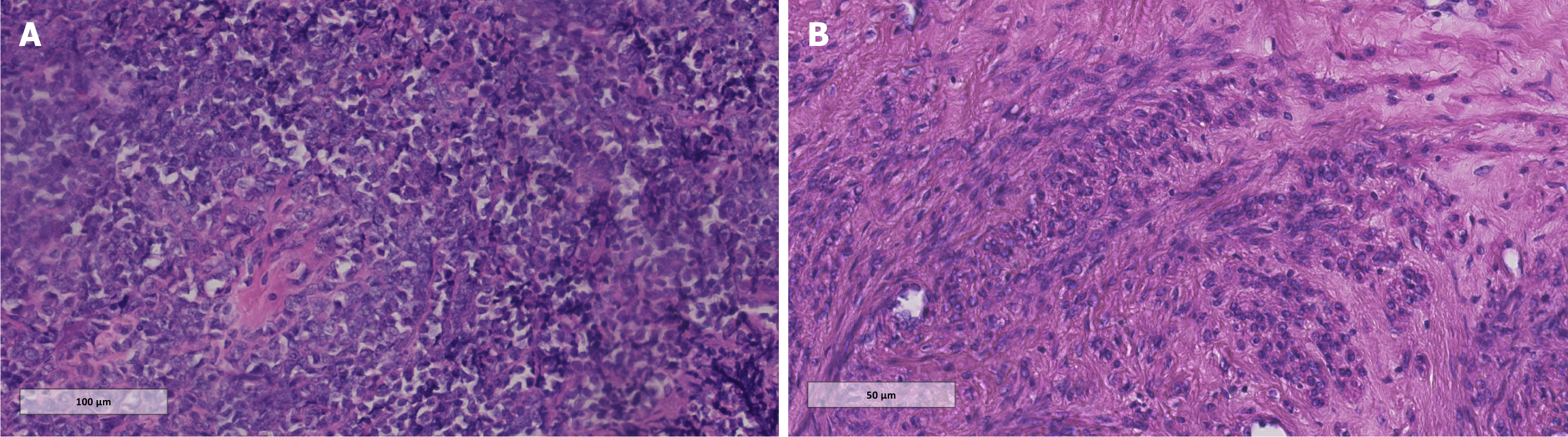
The initial diagnosis was a cervical mass of uncertain nature, leading to a cervical biopsy. Histopathological evaluation (Figure 2) and IHC (positive for CD117 and MPO; negative for CD34, TdT, CD20, PAX5, CD3, CD7, Cg-A, CK, and Syn) confirmed the diagnosis of cervical MS, potentially resulting from AML.
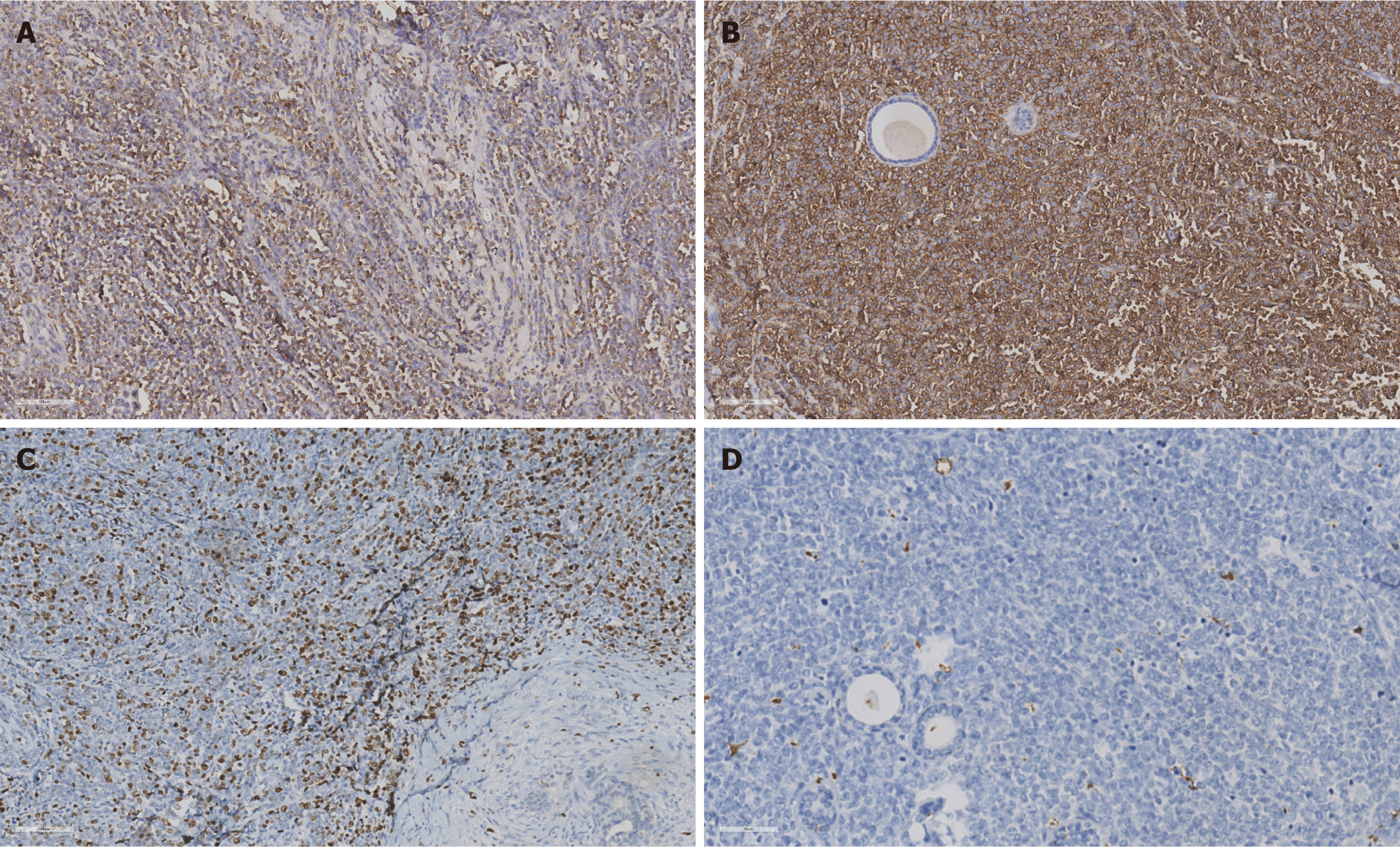
A conization was performed, which removed the mass entirely. Postoperative pathological examination showed diffuse proliferation of atypical cells with large cell bodies, moderate to abundant cytoplasm, and oval or slightly irregular nuclei with fine chromatin. IHC results were: CD43 (+), CD117 (+), MPO (70% +), lysozyme (focal +), Ki-67 (50% +), and negative for PAX5, CD34, CD7, TdT, and CD2 (Figure 3). These findings suggest a hematological malignancy consistent with MS, but further BMP showed no abnormalities.
The patient underwent tumor debulking surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and enlarged lymph node biopsy). Postoperative pathology revealed diffuse infiltration of atypical lymphoid cells throughout the cervical tissue. The cells were medium-sized with pale-staining cytoplasm, round and irregular nuclei, fine chromatin, occasional small nucleoli, and frequent mitotic figures. Tumor emboli were observed in lymphatic vessels but not in blood vessels. Pelvic lymph nodes showed metastasis: 1/9 on the left and 1/4 on the right. IHC showed lymphocyte common antigen (+), P16 (+), CD68 (+), and CD117 (+), with CK5/6, CK panel, PAX5, TdT, CD3, CD20, CD15, and CD34 all negative, consistent with cervical MS. Further BMP showed no abnormalities.
After initial chemotherapy with the DA regimen (daunorubicin on days 1-3 and cytarabine on days 1-7, for two cycles), a reduction in the mass’s size was observed (Figure 1B). Subsequent surgical management included a radical hysterectomy and pelvic lymph node dissection. The gross pathology revealed a solid, finely textured, green lesion infiltrating the entire cervical wall, and microscopic examination showed medium-sized tumor cells with irregular nuclei and frequent mitotic figures. IHC results were as follows: TdT (focal +), MPO (+), CD34 (-), CD68 (+), and Ki-67 (> 50% +). The diagnosis of cervical MS was confirmed. A BMP performed postoperatively was unremarkable.
The patient was treated with cytarabine to lower the WBC count, which decreased to 11.56 × 109/L. However, the patient refused further chemotherapy.
11 days postoperatively, a recurrent cervical mass was detected. The patient received chemotherapy with the DA regimen for two cycles, followed by total hysterectomy and lymph node dissection. Pathological examination of the resected tissue revealed an irregular, slightly protruding mass with a gray-white to gray-brown soft, delicate texture, infiltrating the entire cervical wall, the cervical canal, and the cervical dome. No tumor emboli were found in the vessels, and pelvic lymph nodes showed no metastasis. IHC was positive for lysozyme, CD43, CD117, CD34, and CD99, with focal positivity for MPO and Ki-67 at 50%. The diagnosis of cervical MS was confirmed. Postoperatively, the patient continued with 4 cycles of DA regimen chemotherapy.
The patient received four cycles of mitoxantrone 10 mg on days 1-3 and cytarabine 240 mg on days 1-7 intravenous chemotherapy postoperatively.
Despite further chemotherapy (cytarabine and etoposide), the patient developed metastatic disease in the breast, vulva, and vagina and succumbed to the disease 22 months after diagnosis.
The patient declined recommended chemotherapy and tragically passed away 1.5 months after discharge.
The patient continued with four additional cycles of DA regimen chemotherapy and remains disease-free after 3 years of follow-up.
The tumor progressed with pulmonary metastases. The patient chose to discontinue treatment and died 9 months later.
MS, or granulocytic sarcoma, is a neoplasm arising from primitive or immature granulocytic cells outside the bone marrow or bone tissue. This condition can precede, coincide with, or manifest as the initial sign of AML, myelodysplastic syndrome, or myeloproliferative neoplasms. It may also herald an AML relapse, sometimes with a considerable time lag between the occurrence of MS and the onset of AML, which can range from months to years. While MS commonly affects sites, such as the skull, paranasal sinuses, sternum, ribs, vertebrae, pelvic bones’ subperiosteal regions, lymph nodes, and skin, its occurrence in the cervix is exceedingly rare, particularly when it presents as an isolated mass without accom
We conducted a retrospective analysis, searching for clinical and pathological data, treatment protocols, and prognosis of patients with cervical MS reported in the domestic and international literature over the past 10 years. A total of 21 complete cases were included in the comprehensive data (Table 1)[4-21]. In terms of treatment, 17 cases received systemic chemotherapy, with seven of these also undergoing concurrent surgical intervention, either in the form of total or radical hysterectomy. Additionally, three cases received radiotherapy. The follow-up periods varied widely, extending from 1 to 91 months, with a median follow-up duration of 8 months. At the conclusion of the follow-up period, 12 patients were alive, while eight had unfortunately passed away. Immunohistochemical analysis of the 20 patients revealed a high po
| Ref. | Published year | Age | Tumor size | Symptoms | With/without AML at diagnosis or previous | Initial treatment | Progression and subsequent treatment | Follow-up (months) | Outcome |
| Case 1 | - | 49 | 8.8 | Vaginal bleeding | No | DA chemotherapy + surgery + chemotherapy | Multi-site recurrence | 22 | Death |
| Case 2 | - | 54 | 6.4 | Vaginal bleeding | Yes | Withdrawing treatment | - | 1.5 | Death |
| Case 3 | - | 46 | 3 | Vaginal bleeding | No | DA chemotherapy + surgery + DA chemotherapy | - | 36 | Survival |
| Case 4 | - | 48 | 7.2 | Vaginal bleeding | No | Chemotherapy + surgery + MA chemotherapy | Lung metastasis | 9 | Death |
| Jiang et al[4] | 2015 | 66 | 1.2 | Vaginal bleeding | No | Surgery + IA chemotherapy + radiotherapy | - | 6 | Survival |
| Wu and He[5] | 2014 | 25 | 8.4 | Vaginal bleeding | No | IA chemotherapy | - | 3 | Survival |
| Liu et al[6] | 2013 | 27 | 8.5 | Contact bleeding | No | Abandoning treatment after surgery | - | 15 | Survival |
| Wang et al[7] | 2014 | 43 | 6 | Contact bleeding | No | DA chemotherapy + intrathecal injection | - | 7 | Survival |
| Peng et al[8] | 2019 | 39 | 11 | Vaginal bleeding | No | Surgery + chemotherapy | - | 2 | Survival |
| Zuo et al[9] | 2016 | 42 | 4 | Vaginal bleeding | No | Surgery + DA chemotherapy | Transformation into AML during chemotherapy | 12 | Death |
| Zuo et al[9] | 2016 | 51 | 4.5 | No | No | E-CHOP chemotherapy + CHEP chemotherapy + surgery + DA chemotherapy | Gingival metastases at 55 months, with radiation and chemotherapy + bone marrow transplant | 91 | Survival |
| Zhang and Cai[10] | 2017 | 23 | 6.9 | Vaginal bleeding | Diagnosed with AML-M2 5 years ago | MA chemotherapy | - | 1 | Survival |
| Jiang et al[11] | 2017 | 28 | - | Vaginal bleeding | No | Chemotherapy | Progressed into AML-M2a within 5 months | 20 | Death |
| Jiang et al[12] | 2017 | 49 | - | Vaginal bleeding | No | Withdrawing treatment | - | 5 | Death |
| Jiang et al[12] | 2017 | 43 | - | Vaginal bleeding | No | Radiotherapy + chemotherapy | - | 7 | Death |
| Feng et al[13] | 2017 | 57 | 2.5 | Contact bleeding | No | Withdrawing treatment | - | 6 | Survival |
| Yu and Ye[14] | 2013 | 28 | 4.5 | Vaginal bleeding | No | IA chemotherapy | - | 18 | Death |
| Gill et al[15] | 2013 | 36 | 2 | Vaginal bleeding | No | Chemotherapy | - | 16 | Survival |
| Abu Saadeh et al[16] | 2015 | 40 | 5 | No | Diagnosed with AML 11 years ago | Surgery + chemotherapy + radiotherapy | - | 21 | Survival |
| Mishra et al[17] | 2018 | 36 | 2 | Increased menstrual flow | Yes | Chemotherapy | Multiple lymph node infiltrations detected on the 31st day of chemotherapy | 1.5 | Death |
| Ucar et al[18] | 2014 | 23 | - | Vaginal bleeding | Diagnosed with AML 3 years ago | Chemotherapy | - | 2 | Survival |
| Gui et al[19] | 2019 | 46 | 6 | Contact bleeding | No | Surgery + chemotherapy | Multi-site recurrence, without AML | 21 | Death |
| Gui et al[19] | 2019 | 39 | 6.9 | Vaginal bleeding | No | Surgery + chemotherapy | Progressed into AML within 5 months | 26 | Death |
| Shatilova et al[20] | 2021 | 49 | 7.6 | Vaginal bleeding | Uncertain | DA chemotherapy | Bone marrow transplantation | 4 | Survival |
| Choi et al[21] | 2022 | 49 | 3.4 | Vaginal bleeding | Diagnosed with MDS, but not AML | Surgery | - | - | - |
The clinical presentation of cervical MS often includes vaginal bleeding (71.41% of cases in this series), palpable cervical masses, or cervical enlargement. A salient example is the first case in this report, where the patient presented with a bleeding, enlarged cervix, and the gross specimen exhibited a characteristic grass-green coloration. This color is due to MPO within the tumor tissue, which fades rapidly in air but reappears upon exposure to hydrogen peroxide, leading to the term “green sarcoma”.
Microscopically, MS is composed of a heterogeneous population of primitive granulocytic cells, neutrophils, and their precursors. It can mimic lymphoma, presenting with a diffuse pattern of small, lymphocyte-like cells. These cells vary in size, show slight pleomorphism, have minimal cytoplasm, and often display eosinophilia. The nuclei are typically irregular, round, or oval with a prominent nuclear membrane, fine chromatin, and indistinct nucleoli, alongside frequent mitotic figures. Immature eosinophilic granulocytes may be sparsely interspersed among the tumor cells. IHC is pivotal in diagnosing and differentiating MS, with markers, such as CD68/KP1, MPO, CD117, CD99, CD68/PG-M1, lysozyme, CD34, TdT, CD56, and CD30 being commonly utilized[22]. MPO, a marker for MS, may be negative in poorly differentiated tumors. The absence of CD20 and CD3, which are markers for T and B cells, can help distinguish MS from lym
No one-size-fits-all treatment protocol for MS exists, encompassing systemic chemotherapy, local therapy, hema
In essence, cervical MS is a rare hematological malignancy affecting the female reproductive system. While it may present as a localized condition, it is recognized as a manifestation of a more extensive systemic disease. The therapeutic management of cervical MS diverges from the standard surgical and radiation treatments typically employed for cervical squamous cell carcinoma, with a primary focus on systemic chemotherapy. Owing to the rarity of this disease, our knowledge regarding its optimal treatment strategies and prognostic indicators remain limited, highlighting the imperative for additional research to expand our understanding and improve patient outcomes.
| 1. | Magdy M, Abdel Karim N, Eldessouki I, Gaber O, Rahouma M, Ghareeb M. Myeloid Sarcoma. Oncol Res Treat. 2019;42:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid Sarcoma: Presentation, Diagnosis, and Treatment. Clin Lymphoma Myeloma Leuk. 2017;17:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Shallis RM, Gale RP, Lazarus HM, Roberts KB, Xu ML, Seropian SE, Gore SD, Podoltsev NA. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: A tale of misnomers, controversy and the unresolved. Blood Rev. 2021;47:100773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Jiang QM, Lu P, Zhou WW, Ye XZ, Li J. [Clinicopathologic study of 6 patients with myeloid sarcoma]. Linchuang Zhongliuxue Zazhi. 2015;20:351-356. |
| 5. | Wu L, He J. [Clinical and Pathological Analysis of Granulocytic Sarcoma of Uterine Cervix]. Yixue Xinxi. 2014;27:23-23. [DOI] [Full Text] |
| 6. | Liu QY, Shi YJ, He H, Zhang MY, Li Z, Hao YR, Kong LF. [Clinical pathology research of granulocytic sarcoma of uterine cervix]. Zhongliu Jichu Yu Linchuang. 2013;26:471-475. [DOI] [Full Text] |
| 7. | Wang Y, Zhou Y, Lou L, Liu MH, Zuo XL. [Primary granulocytic sarcoma of cervix: a case report and review of the literature]. Wuhan Daxue Xuebao. 2014;35:628-631. [DOI] [Full Text] |
| 8. | Peng JF, Wang HR, Wang JT, Jiang HL. [MRI diagnosis and literature review of primary granulocytic sarcoma of cervix]. Guoji Yixue Fangshexue Zazhi. 2019;42:470-473. |
| 9. | Zuo J, Cheng M. Li Z, Song Y, Wu LY. [Primary cervical hematopoietic malignancy: a clinicopathologic analysis and literature review]. Aizheng Jinzhan. 2016;14:444-448. [DOI] [Full Text] |
| 10. | Zhang XN, Cai YQ. [Partial differentiation of acute myeloid leukemia complicated with cervical granulocytic sarcoma: a case report]. Zhongguo Shiyong Xiangcunyisheng Zazhi. 2013;20:59-60. [DOI] [Full Text] |
| 11. | Jiang YJ, Wang HX, Zhuang WC, Chen H, Zhang C, Li XM, Zhu GH, He Y. [Clinical and Pathologic Features of Myeloid Sarcoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Jiang WH, Chen YP, Xu CW, Long LW, Wang JC, Huang RF, Hu D, Lu JP, He S, Chen G. [Clinicopathologic analysis of myeloid sarcoma]. Linchuang Yu Bingli Zazhi. 2017;37:2118-2124. [DOI] [Full Text] |
| 13. | Feng HL, Hou L, He LB, Jin W, Song X, Liu AJ, Yu G. [Primary granulocytic sarcoma of cervix: a clinicopathological analysis]. Zhenduan Binglixue Zazhi. 2017;24:267-269. [DOI] [Full Text] |
| 14. | Yu XH, Ye L. [A case of cervical granulocytic sarcoma]. Linchuang Yu Shiyan Binglixue Zazhi. 2013;29:116-118. [DOI] [Full Text] |
| 15. | Gill H, Loong F, Mak V, Chan K, Au WY, Kwong YL. Myeloid sarcoma of the uterine cervix presenting as missed abortion. Arch Gynecol Obstet. 2012;286:1339-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Abu Saadeh F, Collins V, Al-Saadi M, Gleeson N. An unusual relapse in acute myeloid leukaemia. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Mishra K, Muralidaran C, Jandial A, Mittal BR, Varma S. Uterine Mass and Menorrhagia: A Rare Presentation of Acute Myeloid Leukemia with Arduous Clinical Course. Balkan Med J. 2018;35:282-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ucar M, Guryildirim M. Granulocytic Sarcoma of the Uterus: A Rare Presentation of Extramedullary Relapse of AML and Importance of MRI. Case Rep Radiol. 2014;2014:501342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Gui W, Li J, Zhang Z, Wang L, Zhao J, Ma L, Su L. Primary hematological malignancy of the uterine cervix: A case report. Oncol Lett. 2019;18:3337-3341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Shatilova A, Girshova L, Zaytsev D, Budaeva I, Mirolyubova Y, Ryzhkova D, Grozov R, Bogdanov K, Nikulina T, Motorin D, Zammoeva D, Efremova S, Ivanov V, Petukhov A, Alekseeva Y, Zaritskey A. The myeloid sarcoma treated by Venetoclax with hypomethylating agent followed by stem cell transplantation: rare case report. BMC Womens Health. 2021;21:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Choi HY, Pak MG, Park JW. Myeloid sarcoma arising at the uterine cervix in a patient with intestinal Behçet's disease and concurrent myelodysplastic syndrome: A case report. Medicine (Baltimore). 2022;101:e31559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Meyer HJ, Pönisch W, Schmidt SA, Wienbeck S, Braulke F, Schramm D, Surov A. Clinical and imaging features of myeloid sarcoma: a German multicenter study. BMC Cancer. 2019;19:1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, Cytogenetic, and Prognostic Analysis of 131 Myeloid Sarcoma Patients. Am J Surg Pathol. 2016;40:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Kaur V, Swami A, Alapat D, Abdallah AO, Motwani P, Hutchins LF, Jethava Y. Clinical characteristics, molecular profile and outcomes of myeloid sarcoma: a single institution experience over 13 years. Hematology. 2018;23:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Ramia de Cap M, Chen W. Myeloid sarcoma: An overview. Semin Diagn Pathol. 2023;40:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Lee D, Omofoye OA, Nuño MA, Riestenberg RA, Shahlaie K. Treatment Outcomes of Intracranial Myeloid Sarcomas: A Meta-Analysis. World Neurosurg. 2021;148:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Loscocco GG, Vannucchi AM. Myeloid sarcoma: more and less than a distinct entity. Ann Hematol. 2023;102:1973-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |