Published online Oct 24, 2024. doi: 10.5306/wjco.v15.i10.1342
Revised: August 16, 2024
Accepted: August 21, 2024
Published online: October 24, 2024
Processing time: 174 Days and 3.1 Hours
Liver cancer treatment is characterized by multidisciplinary participation and coexistence of multiple treatment methods. Hypofractionated and intensity-modulated radiotherapy is a new precise radiotherapy technique applied to the treatment of systemic malignant tumors. There is a lack of understanding of hypofractionated and intensity-modulated radiotherapy combined with systemic therapy in metastatic hepatocellular carcinoma (HCC).
We report a case of metastatic HCC treated with hypofractionated and intensity-modulated radiotherapy combined with systemic therapy. A 41-year-old man was diagnosed with metastatic HCC (T3N1M1 stage IVB). Because it was found to be in the late stage of cancer and had already metastasized, it was impossible to undergo surgical treatment. In addition to aggressive comprehensive treatment for the primary lesion, local treatment for metastatic cancer can improve the patient's survival potential. Hypofractionated and intensity-modulated radiotherapy can provide a larger single treatment dose within a shorter overall treatment time, and improve the local control rate of the tumor. Follow-up examination demonstrated that the tumor and metastatic lesions had shrunk after therapy. The treatment has showed good efficacy. The patient survived for 18 months without disease progression and stable disease persisted for > 38 months.
Targeted therapy and immunotherapy followed by hypofractionated and intensity-modulated radiotherapy are also effective for advanced metastatic HCC.
Core Tip: We report a 41-year-old man diagnosed with metastatic hepatocellular carcinoma (HCC) (T3N1M1 stage IVB), and discuss its imaging and hypofractionated and intensity-modulated radiotherapy combined with systemic therapy. The treatment showed good efficacy. The patient survived for 18 months without disease progression and stable disease persisted for > 38 months. These findings demonstrated that targeted therapy and immunotherapy followed by hypofractionated and intensity-modulated radiotherapy is a promising treatment regimen for advanced metastatic HCC, which is safe and controllable.
- Citation: Chen QQ, Chen CQ, Liu JK, Huang MY, Pan M, Huang H. Hypofractionated and intensity-modulated radiotherapy combined with systemic therapy in metastatic hepatocellular carcinoma: A case report. World J Clin Oncol 2024; 15(10): 1342-1350
- URL: https://www.wjgnet.com/2218-4333/full/v15/i10/1342.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i10.1342
Hepatocellular carcinoma (HCC) is the most common primary liver malignant tumor. HCC has no obvious clinical symptoms in the early stage and is often found to be locally advanced or metastatic. It has a high degree of malignancy, poor treatment efficacy and poor prognosis[1]. Research has reported that in HCC patients with metastasis, in addition to actively comprehensive treatment of the primary lesion, local treatment of metastatic cancer can also improve survival potential[2].
Hypofractionated and intensity-modulated radiotherapy is a new precise radiotherapy technique for treatment of systemic malignant tumors. It can provide a larger single treatment dose within a shorter overall treatment time, change the traditional dose segmentation mode, improve the local control rate of tumors, and have better economic benefits[3]. Hypofractionated and intensity-modulated radiotherapy can enhance the effectiveness of tumor treatment by releasing tumor antigens, altering the expression of immune-related molecules, altering the tumor microenvironment, and generating distant effects[4,5]. There is a lack of understanding of hypofractionated and intensity-modulated radio
A 41-year-old man presented with a complaint of being unable to walk, a history of lower back pain with bilateral hip numbness and weight loss of 5 kg in January 2020.
Initial symptoms included lower back pain with bilateral hip numbness and weight loss of 5 kg. He had symptomless hepatitis B and had started antiviral treatment with entecavir.
He had a past medical history of hypertension. He denied a history of diabetes, coronary heart disease and tuberculosis.
The patient denied any family history of malignant tumors.
Physical examination revealed mental, appetite, sleep, normal urine and feces, and weight loss of 5 kg. Eastern Cooperative Oncology Group score was 3 and Karnofsky performance status (PS) score was 50. His physical examination revealed no signs of adenopathy or organomegaly.
Laboratory tests showed a-fetoprotein (AFP) > 1210.00 ng/mL; sugar antigen 19934.50 U/mL; absolute T helper cell number 462 cells/uL; hepatitis B virus DNA 1793 IU/mL; hepatitis B surface antigen > 250.00 IU/mL. Urinalysis, liver and kidney function, and electrolytes showed no significant abnormalities.
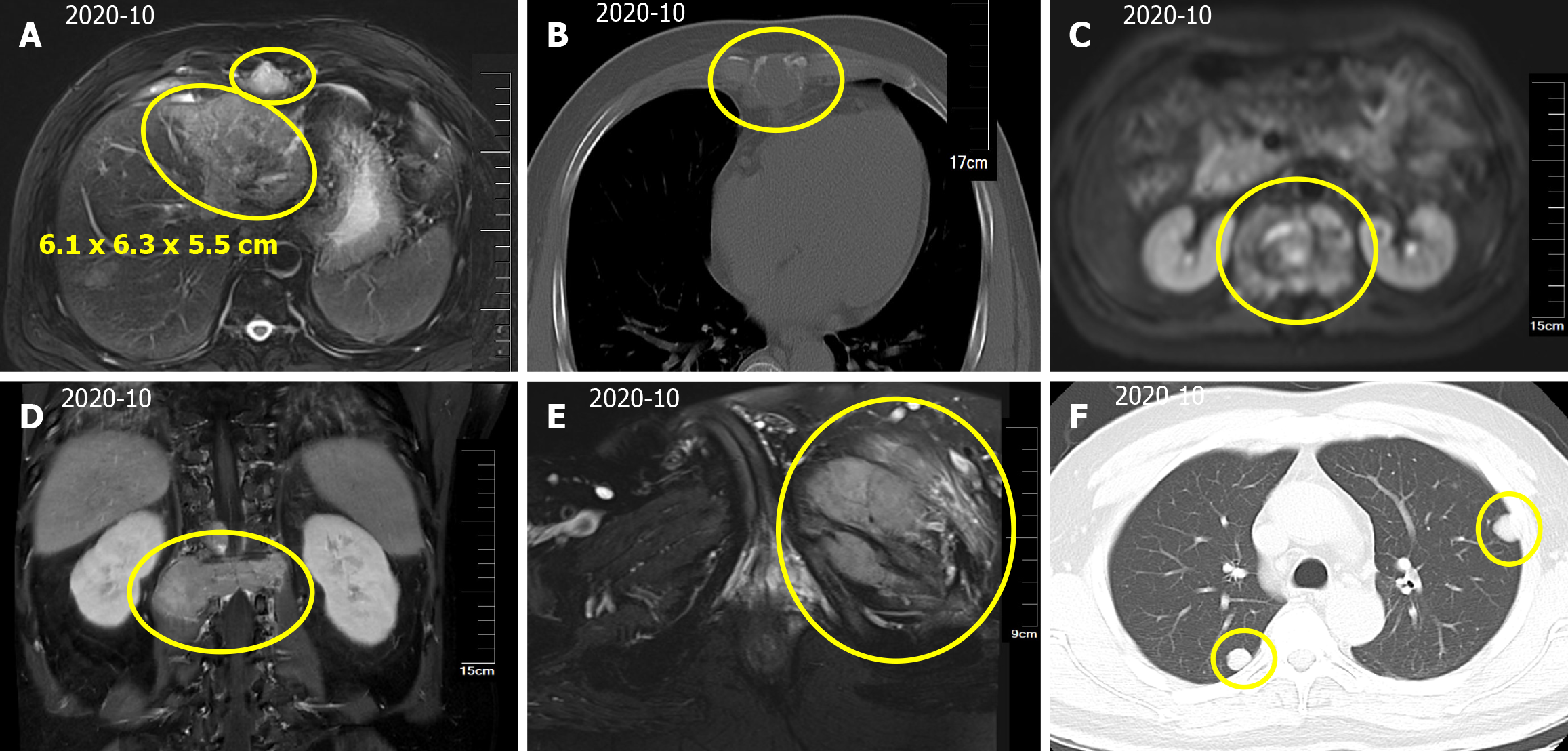
Magnetic resonance imaging (MRI) of the upper abdomen indicated a mass in the left lobe of the liver, with a size of approximately 6.1 cm × 6.3 cm × 5.5 cm (Figure 1A). In addition, scattered circular abnormal signal shadows with blurred boundaries were seen in the liver parenchyma, suspecting that lymph node metastasis was present in the hepatic hilum and abdominal cavity (Figure 1A). Thoracic and lumbar MRI suggested multiple bone and paravertebral soft tissue metastases (Figure 1B and C), L1 vertebral compression fracture, and spinal cord compression (Figure 1D). Pelvic MRI suggested metastasis to the left iliac bone (Figure 1E). Computed tomography (CT) showed multiple nodules in both lungs (Figure 1F), suggesting metastasis. Brain MRI showed no signs of brain metastasis.
In summary, the patient was diagnosed with primary HCC with intrahepatic and abdominal lymph nodes, and multiple bone and lung metastases (T3N1M1 stage IVB).
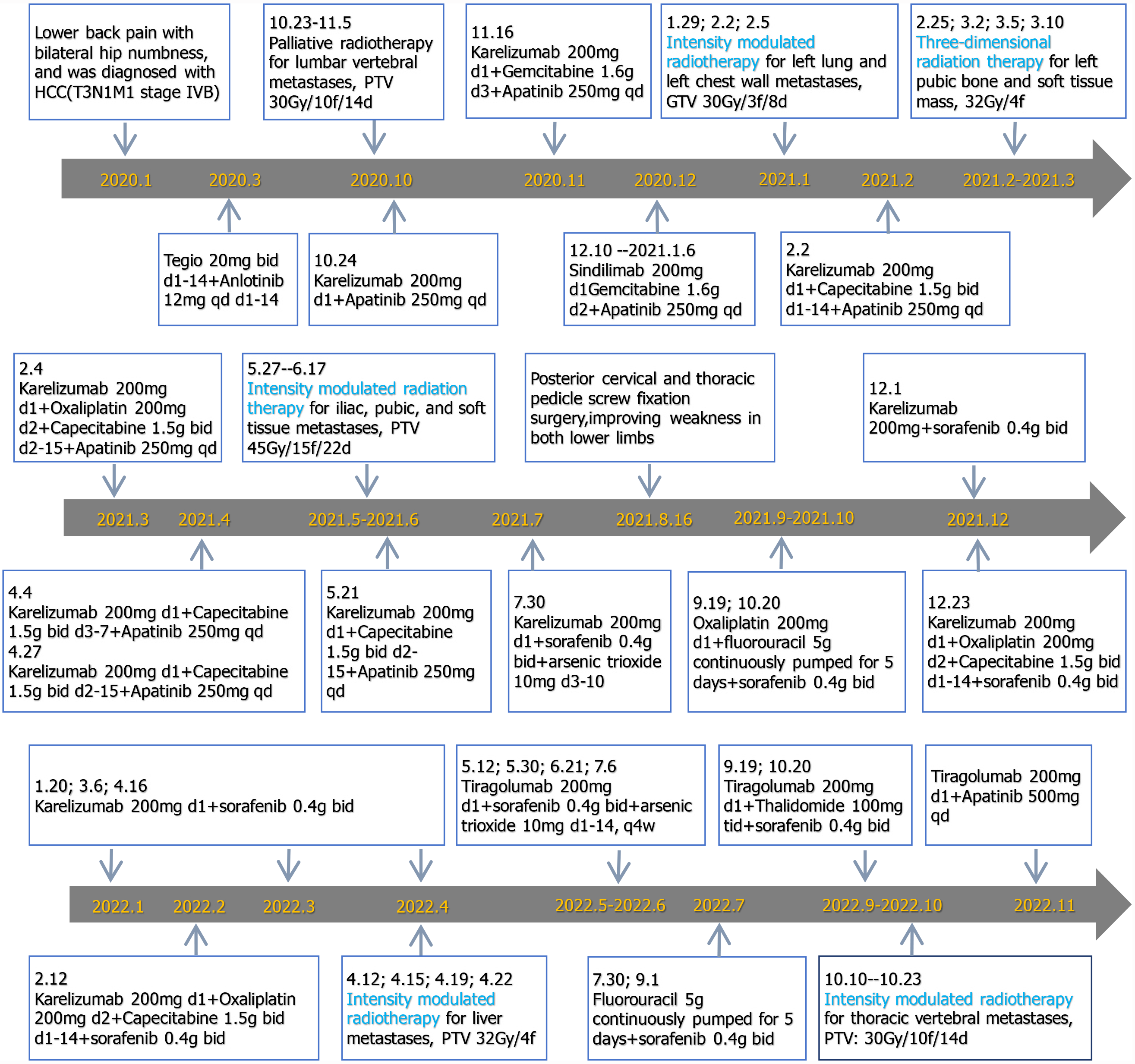
Surgical treatment was impossible because HCC was in the late stage and had already metastasized. The patient had been given other active treatments. The treatment schedule is shown in Figure 2 and can be roughly divided into three stages. From January 2020 to January 2021, he received intensity-modulated radiotherapy for metastatic pathological fractures of the L1 vertebral, with a planning target volume (PTV) of 30 Gy/10 fractions/14 days. Synchronous immunotherapy, antivascular therapy, and chemotherapy were administered during palliative radiotherapy but the effect was not ideal, so the treatment plan was adjusted. From February 2021 to June 2022, in addition to the original treatment plan, he was treated with hypofractionated and intensity-modulated radiotherapy. The radiotherapy included hypofractionated radiotherapy for liver, lung and metastatic tumors (large dose radiotherapy for lung and pleural metastases), and 3D radiotherapy for pelvic metastases. He received intensity-modulated radiotherapy for left lung and left chest wall metastases, gross tumor volume (GTV) 30 Gy/3 fractions/8 days; 3D radiotherapy for left pubic bone and soft tissue masses, with a dose of 32 Gy/4 fractions; intensity-modulated radiotherapy for iliac, pubic and soft tissue metastases, PTV 45 Gy/15 fractions/22 days; and intensity-modulated radiotherapy for liver metastases, PTV 32 Gy/4 fractions. In September 2022, the patient experienced weakness in both lower limbs again. Chest CT showed an enlarged nodule in the right upper lobe of the lung, while upper abdominal MRI showed an enlarged lesion in the left outer lobe of the liver and an increased nodule in the right lobe. The therapeutic effect was evaluated as progressive disease. However, the patient refused chemotherapy, so immunotherapy and antivascular therapy were used to continue treatment.
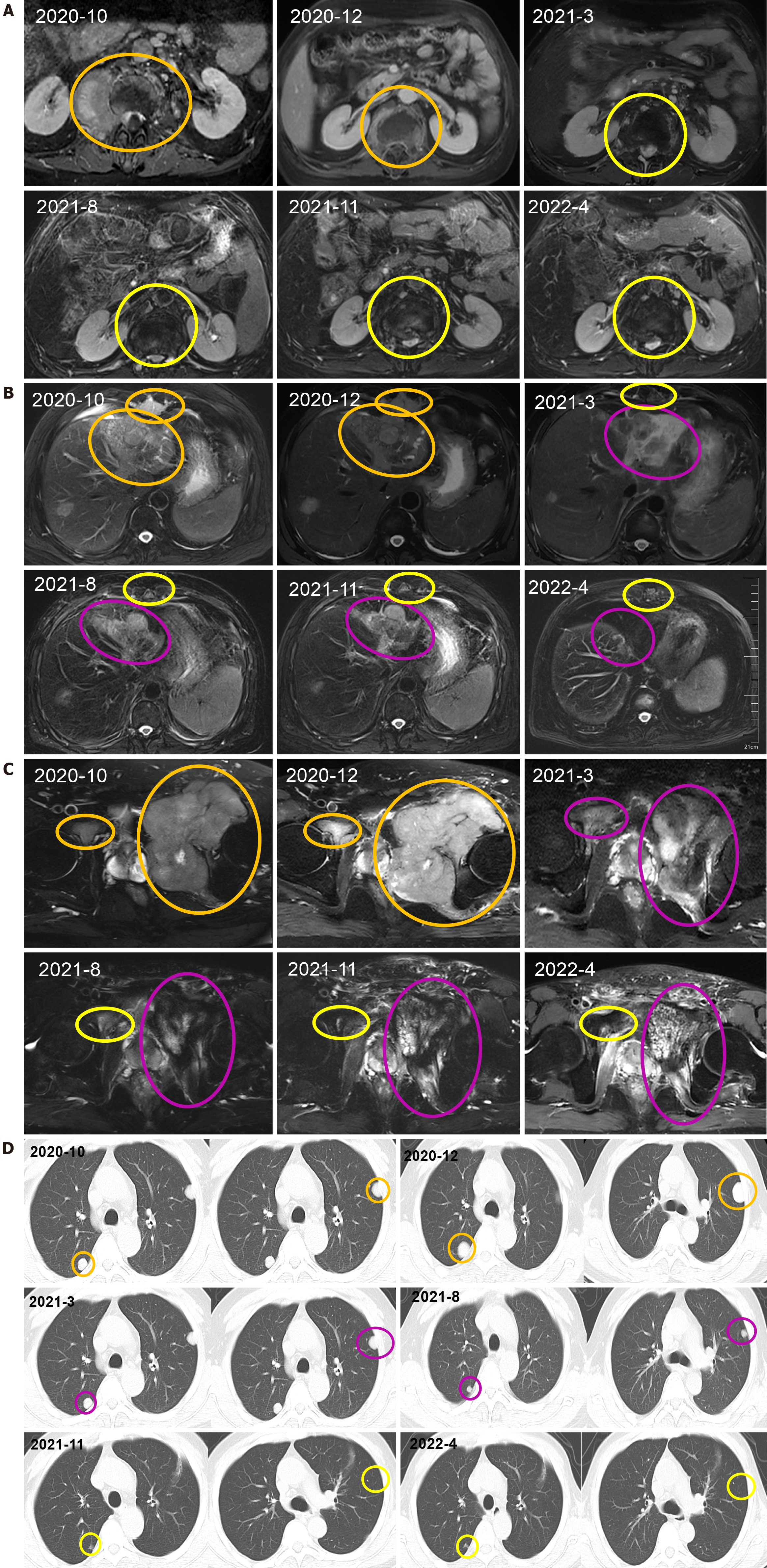
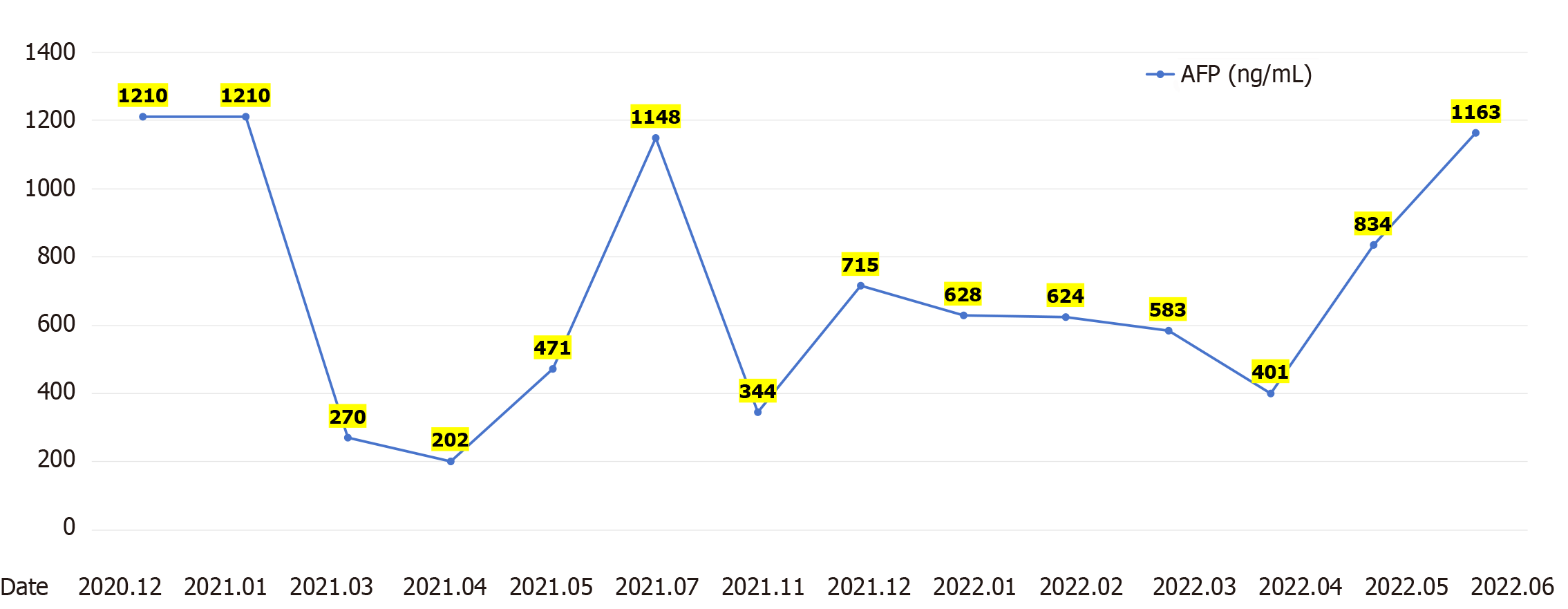
After receiving palliative radiotherapy, immunotherapy, antivascular therapy and chemotherapy from January 2020 to January 2021, the patient's local spinal cord compression symptoms significantly improved, and the vertebral body and right paravertebral mass shadows significantly reduced (Figure 3A) (2020-10 and 2020-12). However, the lesions in the left lobe of the liver, abdominal lymph nodes, intrahepatic region (Figure 3B) (2020-10 and 2020-12), sacrum, left and right iliac bones, left iliac fossa (Figure 3C) (2020-10 and 2020-12), sternum, and bilateral lungs (Figure 3D) (2020-10) were not significantly changed, or were slightly increased. Treatment outcome suggested unsatisfactory lesion control. The patient reported with numbness below the waist and weakness of the lower limbs, and AFP increased to 1148.00 ng/mL on re-examination (Figure 4). MRI showed that the intrahepatic metastases were larger than before, accompanied with leukopenia and thrombocytopenia.
Combination with hypofractionated and intensity-modulated radiotherapy from February 2021 to June 2022 showed that the vertebral body and right paravertebral mass shadows significantly reduced [the therapeutic effect was evaluated as complete response (CR)] (Figure 3A) (2021-3-2022-4); continuous reduction of intrahepatic lesions [the therapeutic effect was evaluated as partial response (PR)] and resolution of sternal lesions (CR) (Figure 3B) (2021-3-2022-4); continuous reduction of pelvic metastases (PR) and resolution of right pubic bone lesions (CR) (Figure 3C) (2021-3-2022-4); metastasis in the left lung and left chest wall had subsided (CR), while the metastasis in the right lung continued to shrink to resolution (CR) (Figure 3D) (2021-3-2022-4); and AFP level decreased to 401 ng/mL (Figure 4). After comprehensive treatment, the patient survived for 18 months without disease progression. At that stage, follow-up examination showed that the lesions in the left lobe of the liver and intrahepatic metastases had shrunk, and AFP level had significantly decreased and remained stable. The main adverse effects were thrombocytopenia and anemia.
After September 2022, follow-up revealed that the patient died of systemic failure in March 2023, with a total overall survival (OS) of 38 months.
With the continuous development of medicine, combination therapy has become a comprehensive method for most advanced tumors. The characteristics of liver cancer treatment are multidisciplinary participation and coexistence of multiple treatment methods. Common methods include liver resection, liver transplantation, ablation therapy, transcatheter arterial chemoembolization, radiotherapy, and systemic antitumor therapy[2]. Choosing a reasonable treatment method for liver cancer patients of different stages can maximize the therapeutic effect. In addition, in terms of the current medical level, in addition to ultrasound, angiography, CT, MRI and other imaging for diagnosis, tumor markers and serum indicators are also important methods for the diagnosis of liver cancer, and AFP is the most commonly used serological marker for the clinical diagnosis of liver cancer[6]. AFP mainly promotes lymphocyte apoptosis and other pathways, leading to immunosuppression in patients. It can also stimulate cancer cell membrane receptors, further inducing abnormal proliferation of cancer cells[7]. AFP is currently the most commonly used specific tumor marker for liver cancer and can be used for early screening. In our patient, who developed primary HCC (T3N1M1 stage IVB) with metastasis, resulting in inability to walk in both lower limbs, accompanied by lower back pain and numbness in both buttocks. Laboratory tests showed AFP > 1210.00 ng/mL. The PS score was > 4 and the patient was unable to take care of himself. Adopting palliative radiotherapy with synchronous immunotherapy, antivascular therapy and chemotherapy, the vertebral body and right paravertebral mass shadow were significantly reduced, and the quality of life was improved. However, the lesions in the left lobe of the liver, abdominal lymph nodes, intrahepatic region, bilateral lungs, other vertebrae and appendages, sternum, sacrum, left and right iliac bones, and left iliac fossa were not significantly changed, or were even slightly increased. Therefore, we need to actively explore better solutions.
Unlike conventional radiotherapy, hypofractionated and intensity-modulated radiotherapy shortens the course of treatment by increasing a single dose, taking into account both the re-proliferation of tumor cells during radiotherapy and the protection of normal tissues, in accordance with the principles of radiation biology[8]. However, the survival benefits of combined hypofractionated and intensity-modulated radiotherapy in HCC are still being explored. In recent years, research has reported that the synergistic effect of hypofractionated and intensity-modulated radiotherapy combined with other treatments includes the following effects: enhancing tumor immunogenicity[9], promoting maturation of antigen-related immune cells[10], changing the tumor microenvironment[11], and distance effects[8]. A prospective multicenter study[12] confirmed that stereotactic radiotherapy is an effective alternative for treatment of liver metastases. After receiving 100 Gy irradiation, the control rates were 84.9% and 78.4% at 24 and 48 months, respectively; while the OS was 27.7 months. In the present case, intensity-modulated radiotherapy had a dose of GTV 30 Gy/3 fractions/8 days for left lung and left chest wall metastases, PTV 45 Gy/15 fractions/22 days for iliac, pubic and soft tissue metastases, and PTV 32 Gy/4 fractions for liver metastases. Progression-free survival (PFS) of the patient from February 2021 to June 2022 reached 11 months.
There is great potential for the combination of hypofractionated and intensity-modulated radiotherapy and immunotherapy. Compared with chemotherapy, hypofractionated and intensity-modulated radiotherapy is focal, has good toxicity and local control, and does not cause significant systemic adverse reactions. The combination of hypofractionated and intensity-modulated radiotherapy and anti PD-1/PD-L1 therapy can achieve palliative therapy for a single site, which can control local symptoms and may trigger a stronger systemic immune response[13]. In addition, combination therapy can consolidate the efficacy of all tumor sites and may alter the tumor microenvironment, achieving long-term local tumor control[14]. Su et al's study included 197 patients with HCC[15]. Patients who treated with combined PD-1 inhibitors and antiangiogenic therapy and intensity-modulated radiotherapy triple therapy showed better PFS (8.7 vs 5.4 months) and OS (18.5 vs 12.6 months) than control patients with PD-1 inhibitors plus antiangiogenic therapy, and the adverse effects were not significantly different. Liang et al[16] reported that combined with administration of pembrolizumab and bevacizumab, the objective response rates of hypofractionated radiotherapy for intrahepatic HCC and HCC without radiotherapy were 34.8% and 10.0%, and the disease control rates were 91.3% and 70.0%, respectively. PFS reached 6.6 months and OS reached 18.3 months after radiotherapy. The results suggest that combining intrahepatic HCC-directed moderately hypofractionated radiotherapy with pembrolizumab and bevacizumab was an effective regimen for advanced HCC. At present, there are many studies that combine conventional radiotherapy with single-agent chemotherapy or immunotherapy for primary tumor lesions. In this case, we adopted a combination of hypofractionated and intensity-modulated radiotherapy with immunotherapy, antivascular therapy and chemotherapy for primary and metastatic lesions, to improve quality of life and prolong survival.
Most patients with HCC are already in the advanced stage, therefore, they are not suitable for definitive treatment such as resection or transplantation. Thus, various alternative local and regional therapies have been used to prevent disease progression, alleviate symptoms, and delay liver failure. Similar to those studies, our patient underwent synchronous hypofractionated and intensity-modulated radiotherapy on the basis of immunotherapy, targeted therapy and che
We have reported a case of HCC treated with radiotherapy combined with targeted therapy and immunotherapy. It is important for us to propose a personalized radiotherapy and immunotherapy strategy, which can provide the best treatment plan according to the specific tumor environment of the patient. Although the combination of hypofractionated and intensity-modulated radiotherapy and immunotherapy has great potential, we still need more carefully designed high-quality clinical combination therapy data to clarify the specific plans, doses, and schedules for different combination modes in different stages or comorbid risk factors of HCC, and provide personalized solutions for HCC management.
We have reported a case of advanced HCC with multiple metastases and discussed its imaging and treatment. This study suggests that targeted therapy and immunotherapy therapy followed by hypofractionated and intensity-modulated radiotherapy for advanced metastatic HCC are also effective, and can prolong PFS and OS.
| 1. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 540] [Article Influence: 180.0] [Reference Citation Analysis (2)] |
| 2. | Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Her EJ, Ebert MA, Kennedy A, Reynolds HM, Sun Y, Williams S, Haworth A. Standard versus hypofractionated intensity-modulated radiotherapy for prostate cancer: assessing the impact on dose modulation and normal tissue effects when using patient-specific cancer biology. Phys Med Biol. 2021;66:045007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Pitroda SP, Chmura SJ, Weichselbaum RR. Integration of radiotherapy and immunotherapy for treatment of oligometastases. Lancet Oncol. 2019;20:e434-e442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 932] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 6. | Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. 2023;21:415-423.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 7. | Samban SS, Hari A, Nair B, Kumar AR, Meyer BS, Valsan A, Vijayakurup V, Nath LR. An Insight Into the Role of Alpha-Fetoprotein (AFP) in the Development and Progression of Hepatocellular Carcinoma. Mol Biotechnol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Kennedy AS. Radiation oncology approaches in liver malignancies. Am Soc Clin Oncol Educ Book. 2014;e150-e155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Pierini S, Mishra A, Perales-Linares R, Uribe-Herranz M, Beghi S, Giglio A, Pustylnikov S, Costabile F, Rafail S, Amici A, Facciponte JG, Koumenis C, Facciabene A. Combination of vasculature targeting, hypofractionated radiotherapy, and immune checkpoint inhibitor elicits potent antitumor immune response and blocks tumor progression. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, Dephoure N, Clement CC, Santambrogio L, Zhou XK, Formenti SC, Demaria S. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 11. | Alimohammadi M, Ghaffari-Nazari H, Alimohammadi R, Bakhshandeh M, Jalali SA, Rezaei N. Radiotherapy Combination: Insight from Tumor Immune Microenvironment (TIME). Avicenna J Med Biotechnol. 2023;15:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Rodríguez MR, Chen-Zhao X, Hernando O, Flamarique S, Fernández-Letón P, Campo M, López M, Rodríguez M, Zucca D, Martínez D, Sánchez-Saugar E, Mañeru F, Ruiz-Zorrilla JG, de Acilu PG, Valero J, Montero A, Ciérvide R, Alvarez B, García-Aranda M, Alonso R, de la Casa MA, Alonso L, Nuñez M, Martí J, Arias F. SBRT-SG-01: final results of a prospective multicenter study on stereotactic body radiotherapy for liver metastases. Clin Transl Oncol. 2024;26:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Li JX, Deng WX, Huang ST, Lin XF, Long MY, Zhang J, Su TS, Li LQ, Pang YD, Liang CF, Zhou HM, Lu HY, Liang SX, Xiang BD. Efficacy and safety of radiotherapy plus anti-PD1 versus transcatheter arterial chemoembolization plus sorafenib for advanced hepatocellular carcinoma: a real-world study. Radiat Oncol. 2022;17:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Su K, Guo L, Ma W, Wang J, Xie Y, Rao M, Zhang J, Li X, Wen L, Li B, Yang X, Song Y, Huang W, Chi H, Gu T, Xu K, Liu Y, Chen J, Wu Z, Jiang Y, Li H, Zeng H, Wang P, Feng X, Chen S, Yang B, Jin H, He K, Han Y. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: A propensity score matching study. Front Immunol. 2022;13:972503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Liang X, Jiang Y, Yao W, Deng Y, Yang S, Liu Q. Liver-directed moderately hypo-fractionated radiotherapy combined with pembrolizumab and bevacizumab for advanced hepatocellular carcinoma: a retrospective observational study of 23 cases. Transl Cancer Res. 2024;13:1508-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/