©The Author(s) 2025.
World J Clin Oncol. Oct 24, 2025; 16(10): 112392
Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.112392
Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.112392
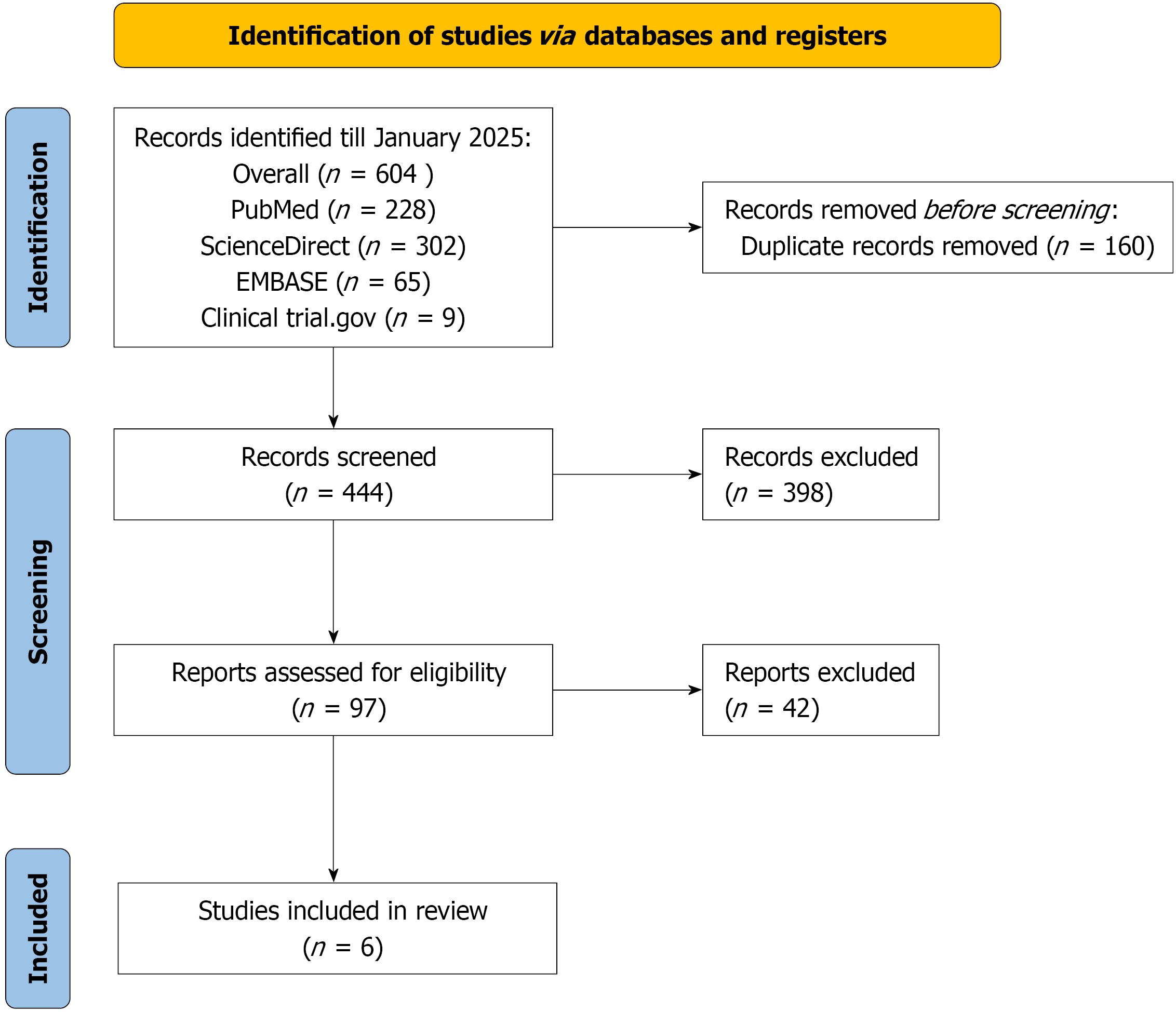
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart outlining the literature screening process, study selection, and exclusion criteria.
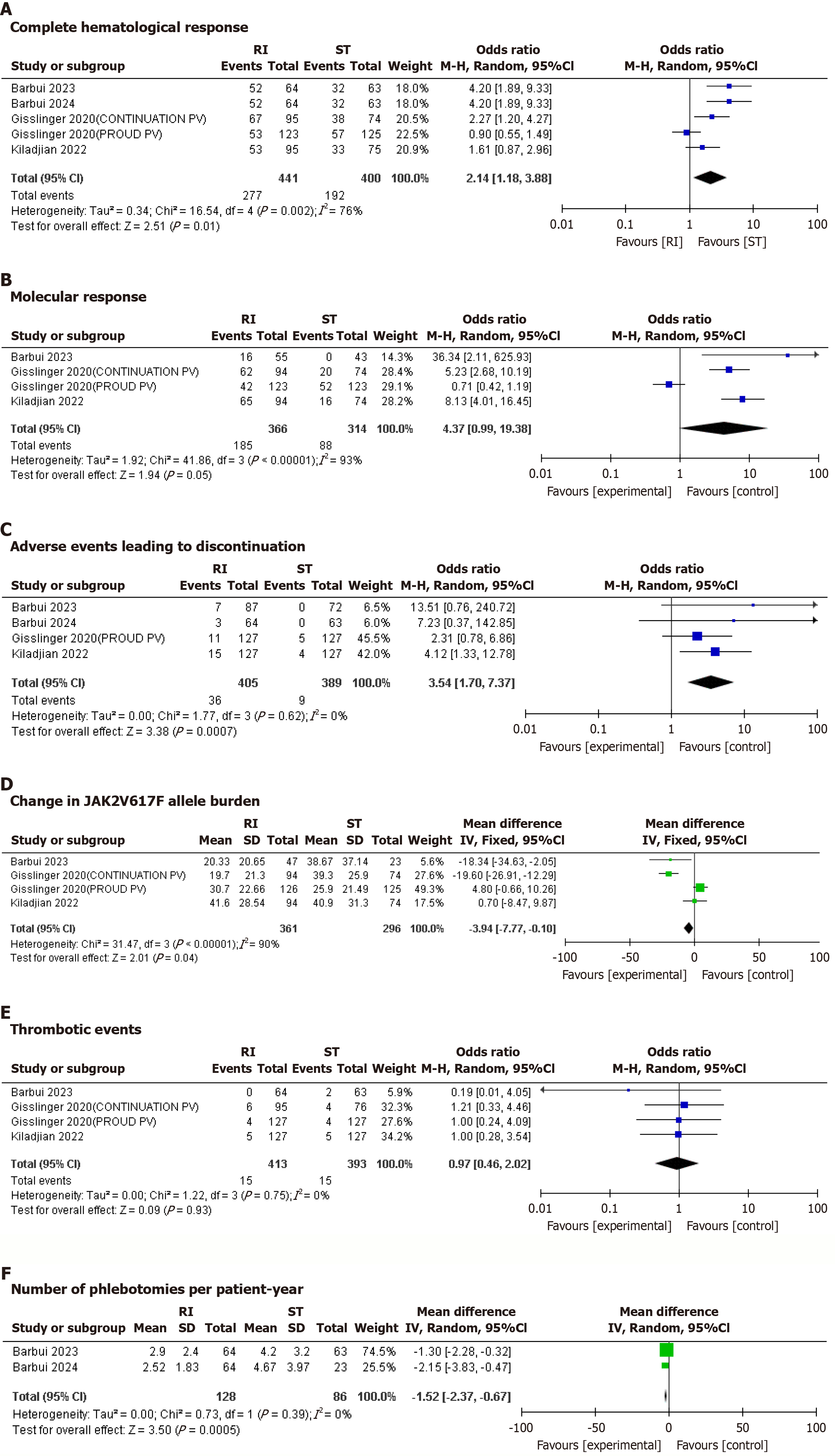
Figure 2 Forest plots of clinical outcomes.
A: Complete hematological response; B: Molecular response; C: Adverse effects leading to discontinuation; D: JAK2V617F allele burden; E: Thrombotic events; F: Number of phlebotomies per patient per year. RI: Ropeginterferon alfa-2b; ST: Standard therapy; 95%CI: 95% confidence interval.
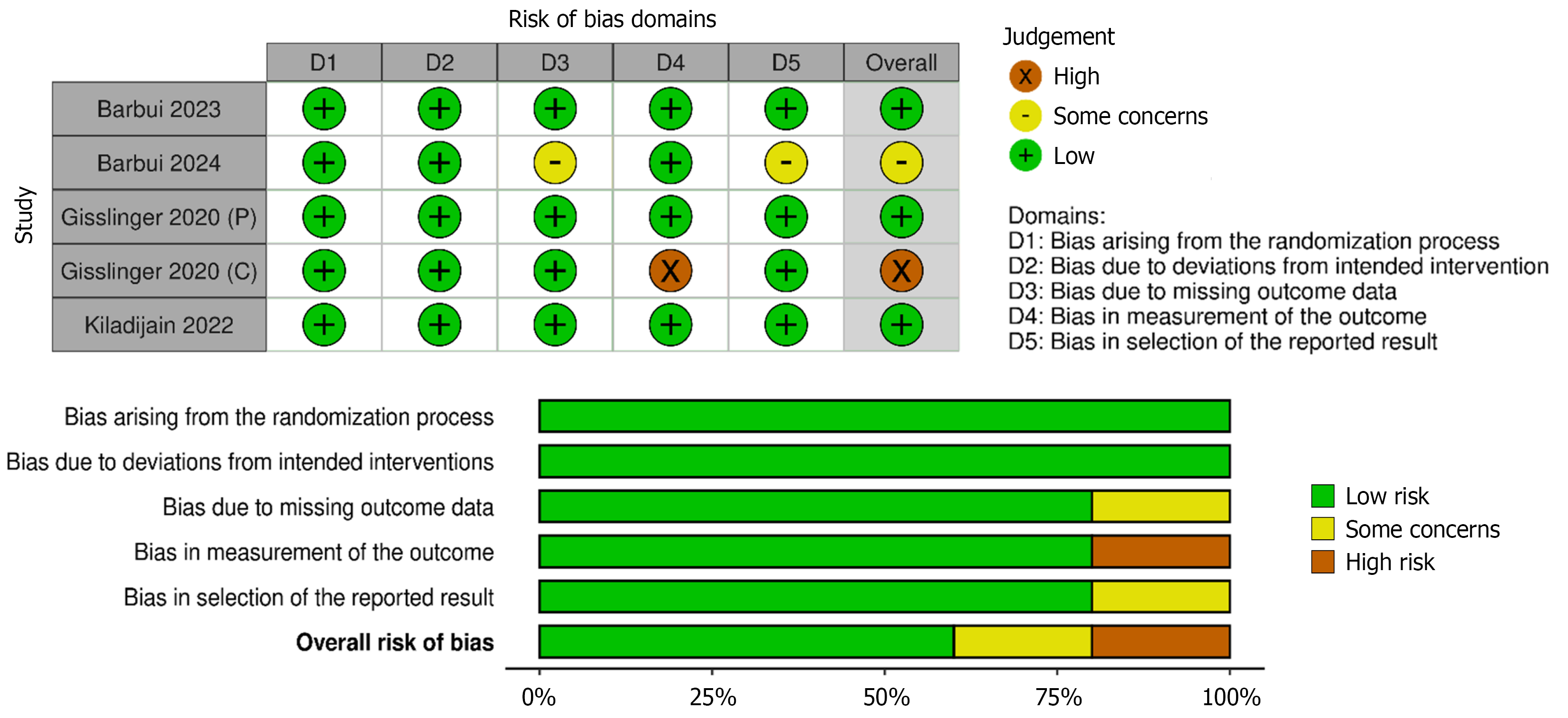
Figure 3 Traffic light plot and Summart plot of risk of bias assessment for included studies using the ROB 2.
0 tool. P: PROUD-PV phase; C: CONTINUATION-PV phase.
- Citation: Tom L, Mani S, Rawat A, Nan M, Mundada SM, Al Khatib B, Gopinath A, Taj O, Salahuddin N, Shaju F, Parsa S, Abdurrahman M, Das A, Khawar MMH, Sher A. Ropeginterferon alfa-2b vs standard therapy in polycythemia vera: A meta-analysis of efficacy and safety outcomes. World J Clin Oncol 2025; 16(10): 112392
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/112392.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.112392