Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.110961
Revised: July 13, 2025
Accepted: October 22, 2025
Published online: December 22, 2025
Processing time: 185 Days and 14.8 Hours
Over 150000 new diagnoses of colorectal cancer (CRC) are diagnosed yearly, and 1 in 5 patients have distant metastases on diagnosis. Previous estimates appro
To describe the updated literature about the incidence and risk factors of BM in CRC as well as their treatment with surgery, chemotherapy, and radiation.
We systematically searched the literature published between January 1, 2010 and April 1, 2025 in PubMed, Cochrane, Scopus, and EMBASE. All studies about BM from CRC were included. Studies only containing information about the treat
Our primary search resulted in 1648 articles that were eventually screened to 147. These articles were analyzed to provide the state of current literature on incidence and risk factors of BM from CRC as well as how these metastases are treated with chemotherapy, radiation, and surgery.
Prognosis is influenced by tumor burden, performance status, and emerging mo
Core Tip: Brain metastases (BM) from colorectal cancer are becoming more common as overall survival increases. So
- Citation: Hutchinson HJ, Gonzalez M, Feier D, Welch CE, Lucke-Wold B. Colorectal cancer metastasis to the brain: A scoping review of incidence, treatment, and outcomes. World J Gastrointest Pathophysiol 2025; 16(4): 110961
- URL: https://www.wjgnet.com/2150-5330/full/v16/i4/110961.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i4.110961
The increased survival of patients with colorectal cancer (CRC) has increased the likelihood of late complications, in
This scoping review provides information describing the updated literature on the incidence and risk factors of BM in CRC as well as their treatment with surgery, chemotherapy, and radiation.
We systematically searched the literature on the incidence and risk factors of BM in CRC, as well as their treatment with surgery, chemotherapy, and radiation published between January 1, 2010 and April 1, 2025 in PubMed, Cochrane, Scopus, and EMBASE databases. Our PubMed search strategy included search query ("Colorectal Neoplasms"[Mesh] OR "Colorectal Cancer" OR "Colorectal Tumors" OR "Colorectal Carcinoma") AND ("Neoplasm Metastasis"[Medical Subject Headings (Mesh)] OR "Metastases" OR "Metastatic" OR "Secondary") AND ("Central Nervous System"[Mesh] OR "CNS" OR "Brain"). Our Cochrane search strategy included the search query (MeSH descriptor: [Colorectal Neoplasms] OR "Colorectal Cancer" OR "Colorectal Tumors" OR "Colorectal Carcinoma") AND (MeSH descriptor: [Neoplasm Metastasis] OR "Metastases" OR "Metastatic" OR "Secondary") AND (MeSH descriptor: [Central Nervous System] OR "CNS" OR "Brain"). Our Scopus search strategy included the search query (TITLE-ABS("Colorectal Neoplasms" OR "Colorectal Cancer" OR "Colorectal Tumors" OR "Colorectal Carcinoma")) AND (TITLE-ABS("Neoplasm Metastasis" OR "Metastases" OR "Metastatic" OR "Secondary")) AND (TITLE-ABS("Central Nervous System" OR "CNS" OR "Brain")). Our EMBASE search strategy included the search terms ('colorectal neoplasm'/exp OR 'colorectal cancer': Ti,ab OR 'colorectal tumors': Ti,ab OR 'colorectal carcinoma': Ti,ab) AND ('neoplasm metastasis'/exp OR metastases: Ti,ab OR metastatic: Ti,ab OR secondary: Ti,ab) AND ('central nervous system'/exp OR cns: Ti,ab OR brain: Ti,ab). In EMBASE, articles were filtered to only include articles of type “Medicine”. All clinical trials, clinical studies, meta-analyses, and systematic reviews about BM from CRC written in English were included. Two authors (Hutchinson HJ and Gonzalez M) screened all articles to determine which ones were relevant to the review. Studies only containing information about the treatment of primary CRC or primary brain tumors were not included. Articles were categorized and described as incidence, surgery, chemotherapy, or radiation to provide an overview of the state of research on BM from CRC. Each category was assigned to an author for appraisal of the current literature, risk of bias, and gaps in need of future research.
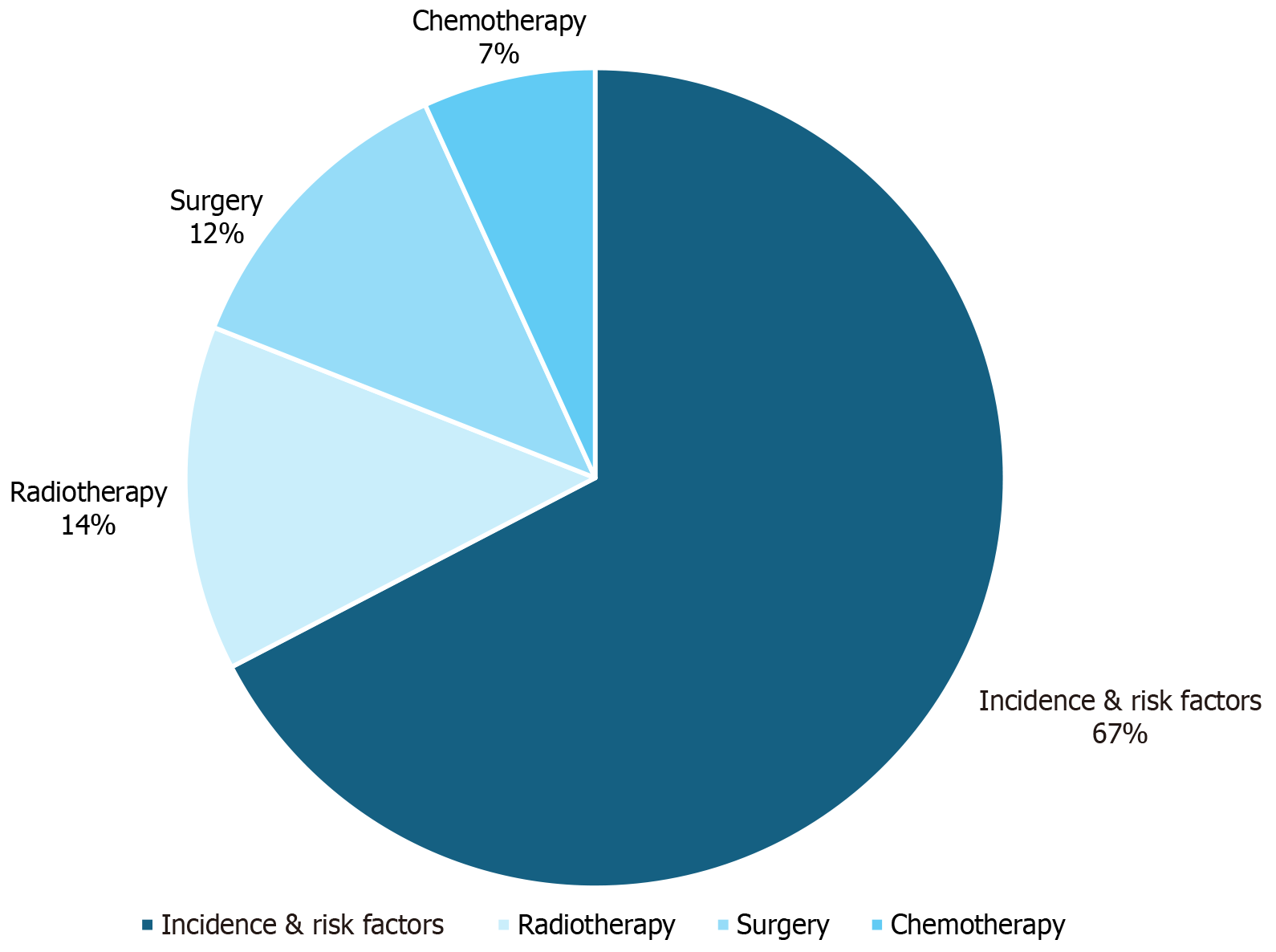
Our primary search resulted in 1648 articles that were eventually screened to 147. These articles were analyzed to provide the state of the current literature on incidence and risk factors of BM from CRC as well as how these metastases are treated with chemotherapy, radiation, and surgery. Figure 1 diagrams the results of the search process. Figure 2 displays the proportion of screened articles in each category. Of the studies included in the review, 99 (67.3%) were related to incidence and risk factors, 20 (13.6%) were related to radiotherapy, 18 were related to surgery (12.2%), and 10 (6.6%) were related to chemotherapy.
De novo metastasis to the brain is correlated with a 10-fold increased risk of mortality[15]. BM from CRC portend a poor prognosis with an estimated survival of less than 1 year[3,6,16]. The initial diagnosis of BM is often delayed due to the di
| Factor | Incidence | Prognosis | Ref. |
| Advanced age | Inconclusive | Inconclusive | [3,20-30] |
| Sex | Inconclusive | Inconclusive | [13,22,24,28,30-33] |
| African American race | Inconclusive | Worse | [15,25] |
| Poor access to health insurance | Inconclusive | Worse | [33] |
| Residence in urban areas | Inconclusive | Worse | [35] |
| Synchronicity with primary tumor | Lower | Inconclusive | [39-41] |
| Rectal primary tumor | Higher | Worse | [4,17,20,25,26,27,33,39,43] |
| Right-sided primary tumor | Higher | Worse | [4,17,25,33,43] |
| Tumor stage | Inconclusive | Worse | [5,25,35,44,45] |
| Adenocarcinoma histology | Higher | Worse | [20,22,24,25,26,46-49] |
| CEA levels | Higher | Inconclusive | [26,46,50] |
| HCMV infection | Inconclusive | Worse | [2,34,51] |
| Size of primary tumor | Higher | Inconclusive | [2] |
| Size of BM | - | Worse | [3,28,47,52,54] |
| Number of BMs | - | Inconclusive | [16,23,41,54] |
| Location of BM | - | Inconclusive | [25,30,34,56-59] |
| Multiple extracranial metastatic lesions | - | Worse | [60] |
| Concomitant lung metastasis | Higher | Worse | [18,24,22,38,44,52] |
| Concomitant liver metastasis | Higher | Inconclusive | [3,18,23,24,30,38,41,62,63] |
| Concomitant bone metastasis | Higher | Inconclusive | [5] |
| KPS > 70 | - | Worse | [41,42,51,52,65,66,68,69] |
| Higher RPA score | - | Worse | [42,64] |
The influence of demographic and socioeconomic factors on the prognosis of CRC BM remains controversial. Advanced age is associated with BM and may serve as an indicator of overall survival[3,20]. However, the definition of advanced age in the setting of CRC BM is not well established and has led to inconclusive findings. Most studies report prolonged survival and favorable prognosis in patients with CRC BM under the age of 65[21-24], whereas older age groups expe
CRC is a heterogeneous disease characterized by individual differences in primary tumor location, metastatic preference, histological, and molecular features, which complicates standardization of prognostic scoring[33]. CRC metastatic patterns to the brain can be further classified as metachronous or synchronous. Metachronous is the development of BM months after the primary CRC, whereas synchronous is the simultaneous development of CRC and BM in the patient at the time of diagnosis[37]. Although metachronous metastatic disease is more closely associated with rectal primaries[38] and an increased risk of developing BM[39]. Meta-analyses have revealed no significant difference in survival outcomes between synchronous and metachronous BM[40,41]. The location of the primary tumor plays a significant role in the tumor’s metastatic pattern due to innate differences in the vascular anatomy between the proximal and distal segments of the colon[28]. The most common primary tumor site for CRC with subsequent BM was the distal colon, which is com
An array of CRC histological findings have also been linked to the development and prognosis of BM. Higher grade tumors with advanced T and N staging were predictors of BM development[5,25,44], with N staging having the highest correlation to increased mortality[35,45]. Of note, research indicates that differences in cellular differentiation (i.e. undif
The evolution and prognosis of BM in the clinical setting relies on tumor size, number of BM, and lesion location. Tumor size is considered a risk factor for early death and poorer prognosis amongst CRC BM subgroups[2,34,51]. CRC BM are more likely to occur in patients with larger primary tumor size[2], frequently ranging from 4 cm to 7 cm[2]. The number of BM lesions has been linked to cancer survival[41,48], with longer survival times observed in patients with single BM[21-23,52,53]. The presence of multiple BM is a poor prognostic factor indicative of advanced CRC requiring aggressive treatment[3,47,52]. Few studies dispute this stance due to findings of minimal survival differences between solitary and multiple BM lesions[28,54]. Although the cerebellum was the most frequently involved site for BM[55], cerebral BM lesions convey favorable prognosis with prolonged overall survival[16]. There remains controversy in the prognostic validity of supratentorial and infratentorial sites of BM, with limited data suggesting that supratentorial BM are favorable[23], whereas others describe no survival differences based solely on brain lesion sites[41,54].
The isolated incidence of solitary BM is relatively rare in CRC[5] and more commonly presents with extracranial metastases to the lung[17], liver[35], and bone[5,42]. Inversely, having three or more sites of metastasis significantly increases the odds of developing BM[20,26]. Multiple extracranial metastatic lesions have been consistently linked with a worse prognosis[25,30,56] and shorter survival[34,57-59], especially concomitant metastasis to the lung and liver[18,24,38]. The presence of lung metastasis in CRC is an independent risk factor for developing BM[3,5,20,39] and negatively impacts overall survival[60]. Large liver metastases and bone metastases are underrepresented in the CRC BM literature and provide inconsistent data on prognostic value[22,44,52].
In addition to the prognostic variables, performance status assessments continue to be the most validated prognostic tool for BM[41,61]. The Karnofsky performance status (KPS) is the most frequently implemented tool[41] because of its accu
Many genetic mutations are implicated in CRC BM. The molecular profile of CRC BM is distinct from other BM such as those from lung and breast cancers[70,71]. The molecular profile of CRC BM is also different from other CRC metastases[72]. Infratentorial CRC BM are most common with no predilection for vascular distribution[73]. Recent efforts have shifted towards categorizing and phenotyping clusters of co-occurring mutations in CRC into consensus molecular sub
The rat sarcoma (RAS) protooncogene is mutated in approximately 50% of CRC tumors and until recently was con
Human epidermal growth factor 2 (HER2) mutations are another area of exploration in CRC BM, initiated because of the analogous evidence that HER2 mutations increase the risk of BM from breast cancer. Current evidence supports a similar relationship in CRC. The HEROES study found HER2+ CRC BMs in 4 of 22 resected tumors, and 3 of these 4 had HER2+ primary CRC tumors using next-generation sequencing[91]. The connection of HER2 to CRC BM is exciting because of the potential therapeutics already available for other HER2+ solid tumors[92]. Trastuzumab, a monoclonal antibody targeting HER2/neu, provided an 11% absolute risk reduction of death at 1 year in patients with BM from breast cancer when used in conjunction with chemotherapy vs chemotherapy alone[93,94]. Other genetic analyses of pa
Other molecular markers have less evidence supporting their link to CRC BM. A single nucleotide polymorphism array of patient primary CRC tumors and their metastases found matching 20q11.1 gains in all 4 patient tumors with BM[100]. This mutation is found on 20% of human pluripotent stem cells and is amplified in 20% of human cancers[101]. O6-methylguanine-DNA methyltransferase methylation is also found in more than half of primary CRC tumors and CRC BM[102]. Two studies found an association between V600E B-rapidly accelerated fibrosarcoma (BRAF) gene mutations and incidence of CRC BM[103,104], which is promising because immunotherapies targeting V600E BRAF are already being explored in treating metastatic CRC; however, more randomized controlled trials are needed to prove its efficacy[105]. In
Primary colorectal tumors have a low radiosensitivity[8-11]. The primary treatment modality of BM and CRC BM is external beam radiation therapy, including whole-brain radiotherapy (WBRT) and stereotactic ablative radiotherapy (SABR)[12]. There has been little research into radiopharmaceutical therapy for treating BM, but a Phase I trial demon
WBRT is the former mainstay of treating CRC BM and the preferred current treatment of large BM (maximum diameter of ≥ 2-4 cm or a volume of ≥ 4-15 cm3) or multiple metastases, and involves fractionated radiation therapy, with the stan
A study by Meyners et al[8], which identified prognostic factors in those who received only WBRT for BM from radio
Rades et al[111] developed a score explicitly based on CRC BM and patients who were only treated with 10 × 3 Gy WBRT (WBRT-30-CRC), which appeared precise in identifying patients with CRC BM who will die within and survive past 6 months following WBRT. Tsao et al[109] examined 54 published trials with a total of 11898 participants, not limited to radioresistant BM, on augmenting WBRT used in treatment of newly diagnosed multiple BM with lower and higher doses, radiosensitizers, and WBRT in addition to SABR. Overall, there were no improvements to outcomes with any aug
SABR is recommended and preferred in those whose prognosis is greater than 3 months and have either a single brain metastasis larger than 3 cm to 4 cm if surgically removed, a single metastasis smaller than 3 cm to 4 cm, or 2 cm to 3 metastasis if all are smaller than 3 cm to 4 cm[11,12,112-117]. SABR is a high Gy dose, which is dependent on each clinical scenario, over a single or few fractions[10-12,115,116,118-120]. Specifically for CRC BM, SABR has a proven effectiveness with reported excellent overall survival, with some studies reporting higher Gy doses required in CRC BM[10-12,115,116,118-121].
Akin to WBRT, prognostic factors are critical in selecting SABR treatment, with Paix et al[117], Matsunaga et al[10], and Taori et al[121] outlining the following for patients with CRC BM: KPS; number of BM; surgical resection of BM; and the control of extracranial disease, and age. Improved OS was associated with KPS > 70 or > 80 KPS, less than five BM, no extracranial metastases, and if possible, a surgical resection[10,117,121,122]. For local tumor control (LTC), there has been variability between studies; however, in patients with CRC, the tumor volume and margin dose have been significantly associated with LTC, and Taori et al[121] found that those with ≥ 3 BM was significantly correlated with the risk of developing additional BM after initial SABR[121]. However, prognostic factors such as previous WBRT status, number of BM, margin dose, and active systemic disease had no effect on overall survival or LTC[121]. Similar to WBRT, several scores can be employed in the usage of SABR: RPA, RPA, II, Graded Prognostic Assessment (GPA), disease-specific GPA, and basic score for BM[115].
For augmenting SABR, a study by Li et al[120] focusing exclusively on CRC BM found that simultaneous bevacizumab had significant improvements in quality of life and OS along with improved symptoms of radiation-induced brain necrosis[120]. Rades et al[112] developed a scoring tool to predict which patients with SABR may benefit from supplemental WBRT, with those with a score of 2 benefiting from additional WBRT, a score of 3 requiring individual treat decisions, and a score of 4 not benefiting from additional WBRT[112]. Gorovets et al[123] developed a nomogram to select which patients may benefit from upfront SABR alone vs WBRT in addition to SABR, which could prevent erroneous neurocognitive toxicities of WBRT[123]. Mondaca et al[122], a retrospective analysis of 1154 patients, found that treatment of CRC BM with surgery, when possible, followed by SABR, can provide longer survival times[122].
The selection of WBRT or SABR is highly dependent on the clinical scenario, with those having fewer and smaller CRC BM likely benefiting from SABR while those with larger and more numerous BM likely benefiting from WBRT. Some studies have shown that higher doses of WBRT may have better outcomes in CRC BM. Figure 3 diagrams the process for determining which radiotherapy modality to use for CRC BM.
Surgical resection remains one of the core treatment modalities for metastasis to the brain. Multiple studies support its efficacy in treatment, often in combination with radiotherapy. Resection of tumors can help provide relief of symptoms caused by increased intracranial pressure and mass effect including headache, dizziness, change in mental status, and aphasia[124]. In fact, Kim et al[125] found surgery to provide a greater rate of symptom relief at 6 months, compared with gamma-knife radiosurgery (72.7% vs 18.5%; P = 0.005)[125]. Brain resection also includes the opportunity to conduct molecular testing on the tumor[124]. In today's age of increasingly individualized care, molecular markers can guide treatment plans.
Multiple factors may be considered when determining if a patient is a candidate for surgery including tumor size, location, number of BM, and performance status. Studies have found that patients with improved performance scores experience greater benefit from surgical resection; performance scores include RPA, KPS, ECOG status, and Katz score[126,127]. However, performance status may also be a limitation in assessing the effectiveness of surgical interventions, since it is used as an exclusion criterion in studies[126-128]. For instance, one retrospective study noted that only 1.6% of the patients who received surgery were in RPA class 3[129]. Therefore, any retrospective studies that assess surgical treatment must take this factor into account.
Extracranial metastases are associated with worse outcomes and survival in patients with BM[13,126,130-134]. Gui et al[130] found that those who underwent surgery were likely to have fewer extracranial metastases (P = 0.048)[130]. This may be connected to performance scores in that extensive metastases impact patients’ activities and need for assistance.
For tumors > 3 cm in diameter, surgical resection remains the most common treatment[136]. Gui et al[130] found that patients with BM from CRC who underwent surgery had larger lesions than those who received stereotactic radiosurgery (SRS) alone (diameter of 3.1 cm and 0.8 cm, respectively; P < 0.001)[130]. Similar results were found by Kim et al[125], where those who underwent surgery were more likely to have lesions > 3 cm, compared with those who had gamma-knife radiosurgery[125]. Interestingly, the morphology of the brain lesion can also impact prognosis. Although rarer, Zancana et al[129] found that cystic lesion morphology was associated with a worse overall survival compared to solid morphology (6.66 months vs 22.4 months; P = 0.001)[127].
Although sometimes used alone, surgery is frequently employed with adjuvant radiotherapy as treatment for BM. Radiotherapy is conventionally used postoperatively to treat the resection site for microscopic tumor remnants and prevent future recurrence. However, there has been interest in assessing the use of preoperative SRS, as there has been evidence of reduced adverse radiation effect and meningeal disease[135]. In a study that compared preoperative and postoperative dosimetry plans of patients, Cheok et al[136] found the preoperative plans to be more conformal to the lesion (P < 0.001), along with having a sharper dose drop-off (P = 0.0018)[136]. There are clinical trials in process to assess the timing for SRS pre-operatively and post-operatively (NCT05438212, NCT04474925, NCT05124236).
Patients with BM often receive systemic therapy to treat primary CRC disease. In a meta-analysis, it was reported that patients who received chemotherapy before BM ranged from 45% to 82%, whereas those who received chemotherapy after BM diagnosis ranged from 24% to 75%[126]. Biological therapy is also increasing in use; Mondaca et al[122] reported that 50% of patients had received biological therapeutics prior to BM diagnosis[122].
Multiple studies have demonstrated that patients with BM secondary to CRC who undergo surgical resection have better survival outcomes compared with those who do not[13,122,126,130-132,137-139]. Results from regression analyses of factors associated with overall survival are described in Table 3. Chang et al[126] and Wong et al[137] offered valuable in
| Ref. | Group 1 | Group 2 | Outcome | HR/OR | 95%CI | Statistical significance | Analysis method |
| Chang et al[126], 2022 | Surgery | Radiotherapy | Survival outcomes | HR = 0.53 | 0.47-0.6 | I2 = 0% | Univariate analysis |
| Gui et al[130], 2025 | Surgical resection | No resection | Overall survival | HR = 0.58 | 0.39-0.86 | P = 0.006 | Univariate analysis |
| Gui et al[130], 2025 | Surgical resection | No resection | Overall survival | HR = 0.81 | 0.52-1.25 | P = 0.3 | Multivariate analysis |
| Suzuki et al[131], 2014 | Surgical resection | No resection | Overall survival | HR = 0.26 | 0.17-0.41 | P < 0.0001 | Multivariate analysis |
| Kye et al[132], 2012 | Conservative management | Treatment modality | Overall survival | HR = 7.973 | 1.487-42.761 | P = 0.015 | Multivariate analysis |
| Bonadio et al[13], 2021 | Surgery alone | No local therapy | Overall survival | HR = 0.56 | 0.34-0.90 | P = 0.018 | Multivariate analysis |
| Bonadio et al[13], 2021 | Surgery + radiotherapy | No local therapy | HR = 0.27 | 0.16-0.43 | P < 0.001 | Multivariate analysis | |
| Wong et al[137], 2024 | Surgery | WBRT | Overall survival | OR = 0.540 | 0.233-1.250 | P = 0.150 | Multivariate analysis |
| Wong et al[137], 2024 | Surgery + WBRT | WBRT | OR = 0.232 | 0.107-0.504 | P < 0.001 | Multivariate analysis | |
However, this finding was not always consistent. The significant survival benefit associated with surgical resection by Gui et al[130] was lost upon multivariate analysis, where P = 0.3 (Table 3)[132]. Furthermore, while Suzuki et al[131] reported improved overall survival for patients who received neurosurgical intervention compared with those who did not (Table 3), there was no significant difference in prognosis between patients who received SRS vs those who had curative neurosurgical resection (P = 0.13)[132].
It is important to note that most of these cited studies are retrospective; the indications for surgery discussed above may influence the studies’ results, since surgery is more frequently performed on patients with better performance scores and fewer extracranial metastases. Furthermore, one retrospective study reported that those who underwent surgical re
In a Cochrane systematic review comparing surgery with SRS for patients with single or solitary BM, only two randomized controlled trials, with 85 total participants, met the inclusion criteria[128]. No difference was found in overall survival, progression-free survival, or adverse events between the treatment modalities[128]. However, Fuentes et al[128] rated the certainty of the evidence to be very low or low due to risk of bias and imprecision of the data. This lack of strong evidence supports the need for improved randomized controlled trials, especially in the setting of BM from CRC, since these patients have a worse prognosis than patients with BM from other primary tumors[133]. Nonetheless, while stronger studies are required, surgery still plays a crucial role in treatment for BM.
The current literature specific to chemotherapy options for CRC BM is limited. Adjuvant chemotherapy treatment regimens in metastatic CRC often consists of a cytotoxic chemotherapy regimen of 5-fluorouracil, leucovorin, and capecitabine with either oxaliplatin or camptothecin[141]. Immunotherapy is also being investigated in CRC BM[142]. Although regorafenib, a multi-kinase inhibitor, has shown efficacy as a late-line agent in metastatic CRC, it has shown reduced efficacy and contraindications in patients with BM or liver metastases[143]. Clinical trials have also found dab
Data regarding novel chemotherapies for CRC BM were not found in our search of the current literature, despite a re
Overall, conclusive data regarding prognosis and factors predisposing patients to CRC BM are scarce. Distal primary CRC tumors tend to develop more BM and are more likely to be metachronous. Outcomes in metachronous vs syn
Treatment of CRC BM is multi-faceted, with tumors less than 3 cm to 4 cm currently being treated with SABR. Larger tumors are surgically resected and treated with WBRT, which can also be used palliatively. The optimal combination and indications for radiotherapy and surgery are yet to be discovered, as high-quality randomized controlled trials add
BM from CRC remain rare but increasingly recognized due to improved overall survival from primary disease. Prognosis is influenced by socioeconomoic status tumor burden, performance status, and emerging molecular markers. Stereotactic radiotherapy and surgical resection provide favorable outcomes for select patients, whereas chemotherapy and immunotherapy remain areas of limited evidence. Additional prospective studies are needed to develop better tools to predict the incidence and prognosis of CRC BM and targeted chemotherapies. Better treatments for CRC BM require new chemo
| 1. | Singh S, Amirtham U, Premalata CS, Lakshmaiah KC, Viswanath L, Kumar RV. Spectrum of metastatic neoplasms of the brain: A clinicopathological study in a tertiary care cancer centre. Neurol India. 2018;66:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Zhang Q, Li B, Zhang S, Huang Q, Zhang M, Liu G. Prognostic impact of tumor size on patients with metastatic colorectal cancer: a large SEER-based retrospective cohort study. Updates Surg. 2023;75:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 3. | Müller S, Köhler F, Hendricks A, Kastner C, Börner K, Diers J, Lock JF, Petritsch B, Germer CT, Wiegering A. Brain Metastases from Colorectal Cancer: A Systematic Review of the Literature and Meta-Analysis to Establish a Guideline for Daily Treatment. Cancers (Basel). 2021;13:900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Luo T, Wang Y, Shan X, Bai Y, Huang C, Li G, Wang H. Nomogram based on homogeneous and heterogeneous associated factors for predicting distant metastases in patients with colorectal cancer. World J Surg Oncol. 2021;19:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Miccio JA, Tian Z, Mahase SS, Lin C, Choi S, Zacharia BE, Sheehan JP, Brown PD, Trifiletti DM, Palmer JD, Wang M, Zaorsky NG. Estimating the risk of brain metastasis for patients newly diagnosed with cancer. Commun Med (Lond). 2024;4:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Gu XD, Cai YT, Zhou YM, Li ZY, Xiang JB, Chen ZY. Prognostic factors and multidisciplinary treatment modalities for brain metastases from colorectal cancer: analysis of 93 patients. BMC Cancer. 2015;15:902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Wang X, Mao M, Xu G, Lin F, Sun P, Baklaushev VP, Chekhonin VP, Peltzer K, Zhang J, Zhang C. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int J Colorectal Dis. 2019;34:1189-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Meyners T, Heisterkamp C, Kueter JD, Veninga T, Stalpers LJ, Schild SE, Rades D. Prognostic factors for outcomes after whole-brain irradiation of brain metastases from relatively radioresistant tumors: a retrospective analysis. BMC Cancer. 2010;10:582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D. A survival score for patients with brain metastases from less radiosensitive tumors treated with whole-brain radiotherapy alone. Strahlenther Onkol. 2014;190:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Matsunaga S, Shuto T, Kawahara N, Suenaga J, Inomori S, Fujino H. Gamma Knife surgery for brain metastases from colorectal cancer. Clinical article. J Neurosurg. 2011;114:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Fokas E, Henzel M, Hamm K, Surber G, Kleinert G, Engenhart-Cabillic R. Multidisciplinary treatment of brain metastases derived from colorectal cancer incorporating stereotactic radiosurgery: analysis of 78 patients. Clin Colorectal Cancer. 2011;10:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Navarria P, Minniti G, Clerici E, Comito T, Cozzi S, Pinzi V, Fariselli L, Ciammella P, Scoccianti S, Borzillo V, Anselmo P, Maranzano E, Dell'acqua V, Jereczek-Fossa B, Giaj Levra N, Podlesko AM, Giudice E, Buglione di Monale E Bastia M, Pedretti S, Bruni A, Bossi Zanetti I, Borghesi S, Busato F, Pasqualetti F, Paiar F, Scorsetti M. Brain metastases from primary colorectal cancer: is radiosurgery an effective treatment approach? Results of a multicenter study of the radiation and clinical oncology Italian association (AIRO). Br J Radiol. 2020;93:20200951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Bonadio RC, Freitas GF, Batista DN, Moreira OAN, Dias CAR, Castria TB, Sabbaga J, Hoff PM. Epidemiology and Outcomes of Patients With Brain Metastases From Colorectal Cancer-Who Are These Patients? Clin Colorectal Cancer. 2021;20:e195-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Tosi F, Sartore-Bianchi A, Lonardi S, Amatu A, Leone F, Ghezzi S, Martino C, Bencardino K, Bonazzina E, Bergamo F, Fenocchio E, Martinelli E, Troiani T, Siravegna G, Mauri G, Torri V, Marrapese G, Valtorta E, Cassingena A, Cappello G, Bonoldi E, Vanzulli A, Regge D, Ciardiello F, Zagonel V, Bardelli A, Trusolino L, Marsoni S, Siena S. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2020;19:256-262.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Akinyemiju T, Sakhuja S, Waterbor J, Pisu M, Altekruse SF. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med. 2018;7:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Randrian V, Portales F, Bouché O, Thezenas S, Chibaudel B, Mabro M, Terrebonne E, Garnier-Tixidre C, Louvet C, André T, Aparicio T, Dubreuil O, Bouché G, Ychou M, Tougeron D. The METACER national cohort study of brain metastases in gastrointestinal cancers prospectively establishes prognostic factors. J Neurooncol. 2025;172:229-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Gao Z, Jin X, Wu S. Clinical features and prognostic factors of brain metastases from colorectal cancer: a single center experience. Int J Colorectal Dis. 2023;38:198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Esmaeilzadeh M, Majlesara A, Faridar A, Hafezi M, Hong B, Esmaeilnia-Shirvani H, Neyazi B, Mehrabi A, Nakamura M. Brain metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract. 2014;68:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Yang L, He W, Xie Q, Liu S, Kong P, Jiang C, Zhang B, Xia L. Brain metastases in newly diagnosed colorectal cancer: a population-based study. Cancer Manag Res. 2018;10:5649-5658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Jeri-Yabar A, Vittini-Hernandez L, Benites-Meza JK, Prado-Nuñez S. Survival Analysis, Clinical Characteristics, and Predictors of Cerebral Metastases in Patients with Colorectal Cancer. Med Sci (Basel). 2024;12:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Roussille P, Auvray M, Vansteene D, Lecomte T, Rigault E, Maillet M, Locher C, Dior M, Hautefeuille V, Artru P, Mabro M, Touchefeu Y, Marthey L, Moulin V, Louafi S, Lecaille C, Chautard R, Lièvre A, Zaanan A, Bennouna J, Berger A, Emambux S, Randrian V, Tougeron D. Prognostic factors of colorectal cancer patients with brain metastases. Radiother Oncol. 2021;158:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117:3630-3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Pietrantonio F, Aprile G, Rimassa L, Franco P, Lonardi S, Cremolini C, Biondani P, Sbicego EL, Pasqualetti F, Tomasello G, Niger M, Casagrande M, Ghidini M, Muni R, Montrone S, Bergamo F, Berenato R, Fontanella C, Bozzarelli S, Moretto R, Battaglin F, Di Bartolomeo M, de Braud F, Miceli R. A new nomogram for estimating survival in patients with brain metastases secondary to colorectal cancer. Radiother Oncol. 2015;117:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Quan JC, Guan X, Ma CX, Liu Z, Yang M, Zhao ZX, Sun P, Zhuang M, Wang S, Jiang Z, Wang XS. Prognostic scoring system for synchronous brain metastasis at diagnosis of colorectal cancer: A population-based study. World J Gastrointest Oncol. 2020;12:195-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Liu Z, Xu Y, Xu G, Baklaushev VP, Chekhonin VP, Peltzer K, Ma W, Wang X, Wang G, Zhang C. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021;21:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Ge X, Li D, Ye X, Ma R, Yuan Y. A clinical prediction model for the presence of brain metastases from colorectal cancer. Int J Colorectal Dis. 2022;37:2469-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer--Incidence and patient characteristics. BMC Cancer. 2016;16:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Damiens K, Ayoub JP, Lemieux B, Aubin F, Saliba W, Campeau MP, Tehfe M. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012;19:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Mege D, Ouaissi M, Fuks D, Metellus P, Peltier J, Dufour H, Regimbeau JM, Dahan L, Sielezneff I, Sastre B. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res. 2013;33:5645-5648. [PubMed] |
| 30. | Quan J, Ma C, Sun P, Wang S, Zhuang M, Liu Z, Jiang Z, Chen H, Yang M, Zhao Z, Guan X, Wang X. Brain metastasis from colorectal cancer: clinical characteristics, timing, survival and prognostic factors. Scand J Gastroenterol. 2019;54:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Shindorf ML, Jafferji MS, Goff SL. Incidence of Asymptomatic Brain Metastases in Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2020;19:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6:38658-38666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 33. | Lei S, Ge Y, Tian S, Cai B, Gao X, Wang N, Wang G, Wang L, Wang Z. Colorectal Cancer Metastases to Brain or Bone and the Relationship to Primary Tumor Location: a Population-Based Study. J Gastrointest Surg. 2020;24:1833-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Wang Q, Shen K, Fei B, Luo H, Li R, Wang Z, Wei M, Xie Z. A predictive model for early death in elderly colorectal cancer patients: a population-based study. Front Oncol. 2023;13:1278137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Thompson E, Banerjee S, Thompson S, Silva R, Muse A, Arif-Tiwari H, Scott AJ, Nfonsam V. Incidence and predictors of brain metastasis in colorectal cancer patients. Int J Colorectal Dis. 2022;37:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Michl M, Thurmaier J, Schubert-Fritschle G, Wiedemann M, Laubender RP, Nüssler NC, Ruppert R, Kleeff J, Schepp W, Reuter C, Löhe F, Karthaus M, Neumann J, Kirchner T, Engel J, Heinemann V. Brain Metastasis in Colorectal Cancer Patients: Survival and Analysis of Prognostic Factors. Clin Colorectal Cancer. 2015;14:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Pan SY, Huang CP, Chen WC. Synchronous/Metachronous Multiple Primary Malignancies: Review of Associated Risk Factors. Diagnostics (Basel). 2022;12:1940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 38. | Thurmaier J, Heinemann V, Engel J, Schubert-Fritschle G, Wiedemann M, Nüssler NC, Ruppert R, Kleeff J, Schepp W, Löhe F, Karthaus M, Neumann J, Kumbrink J, Taverna F, Stahler A, Heinrich K, Westphalen CB, Holch JW, Kirchner T, Michl M. Patients with colorectal cancer and brain metastasis: The relevance of extracranial metastatic patterns predicting time intervals to first occurrence of intracranial metastasis and survival. Int J Cancer. 2021;148:1919-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Christensen TD, Palshof JA, Larsen FO, Høgdall E, Poulsen TS, Pfeiffer P, Jensen BV, Yilmaz MK, Christensen IJ, Nielsen D. Risk factors for brain metastases in patients with metastatic colorectal cancer. Acta Oncol. 2017;56:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Tsai TC, Song J, Chi KY, Lin HM, Chang Y. Comparative survival outcome of synchronous and metachronous brain metastasis from colorectal cancer: A metaanalysis. Oncol Lett. 2025;29:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Imaizumi J, Shida D, Narita Y, Miyakita Y, Tanabe T, Takashima A, Boku N, Igaki H, Itami J, Kanemitsu Y. Prognostic factors of brain metastases from colorectal cancer. BMC Cancer. 2019;19:755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Baek JY, Kang MH, Hong YS, Kim TW, Kim DY, Oh JH, Lee SH, Park JH, Kim JH, Kim SY. Characteristics and prognosis of patients with colorectal cancer-associated brain metastases in the era of modern systemic chemotherapy. J Neurooncol. 2011;104:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Bergen ES, Scherleitner P, Ferreira P, Kiesel B, Müller C, Widhalm G, Dieckmann K, Prager G, Preusser M, Berghoff AS. Primary tumor side is associated with prognosis of colorectal cancer patients with brain metastases. ESMO Open. 2021;6:100168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Byrne BE, Geddes T, Welsh FK, John TG, Chandrakumaran K, Rees M. The incidence and outcome of brain metastases after liver resection for colorectal cancer metastases. Colorectal Dis. 2012;14:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Boysen AK, Ording AG, Astradsson A, Høyer M, Spindler KL. Metastasis directed treatment of brain metastases from colorectal cancer - a Danish population-based cohort study. Acta Oncol. 2020;59:1118-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Chen Q, He L, Li Y, Zuo C, Li M, Wu X, Pu C, Xu X, Tang R, Xiong Y, Li J. Risk Factors on the Incidence and Prognostic Effects of Colorectal Cancer With Brain Metastasis: A SEER-Based Study. Front Oncol. 2022;12:758681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Kim BH, Park HJ, Kim K, Han SW, Kim TY, Jeong SY, Park KJ, Chie EK. Novel graded prognostic assessment for colorectal cancer patients with brain metastases. Int J Clin Oncol. 2018;23:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, Yamada T, Fujiwara Y, Ohigashi H, Yano M, Ishikawa O. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. 2012;106:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Shi X, Lu L, Wang Z, Dai Y, Hu S, Wu Z, Yu R, Liu T, Jiang Y, Ma Y, Shen B, Zhou G, Chen EY, Chen C, Zhao L, Shi Y, Wang X. The potential role of tumor deposits in the prognosis and TNM staging for colorectal cancer. J Gastrointest Oncol. 2024;15:2473-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Taher C, Frisk G, Fuentes S, Religa P, Costa H, Assinger A, Vetvik KK, Bukholm IR, Yaiw KC, Smedby KE, Bäcklund M, Söderberg-Naucler C, Rahbar A. High prevalence of human cytomegalovirus in brain metastases of patients with primary breast and colorectal cancers. Transl Oncol. 2014;7:732-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Zhang Y, Zhang Z, Wei L, Wei S. Construction and validation of nomograms combined with novel machine learning algorithms to predict early death of patients with metastatic colorectal cancer. Front Public Health. 2022;10:1008137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 52. | Duan H, He ZQ, Guo CC, Li JH, Wang J, Zhu Z, Sai K, Chen ZP, Jiang XB, Mou YG. Bone metastasis predicts poor prognosis of patients with brain metastases from colorectal carcinoma post aggressive treatment. Cancer Manag Res. 2018;10:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Tokoro T, Okuno K, Hida JC, Ueda K, Yoshifuji T, Daito K, Sugiura F. Prognostic factors for patients with advanced colorectal cancer and symptomatic brain metastases. Clin Colorectal Cancer. 2014;13:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Pramateftakis MG, Hatzigianni P, Kanellos D, Vrakas G, Kanellos I, Agelopoulos S, Ouzounidis N, Lazaridis C. Brain metastases in colorectal cancer. Tech Coloproctol. 2010;14 Suppl 1:S67-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Tevlin R, Larkin JO, Hyland JM, O'Connell PR, Winter DC. Brain metastasis from colorectal carcinoma: a single cancer centre experience. Ir J Med Sci. 2015;184:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Del Carpio Huerta L, Virgili Manrique AC, Szafranska J, Martin-Richard M, Paez Lopez-Bravo D, Sebio Garcia A, Espinosa Mariscal I, Gomila Pons P, Andres Granyo M, Barba Joaquin A, Barnadas Molins A, Tobeña Puyal M. Brain metastases in colorectal cancer: prognostic factors and survival analysis. Int J Colorectal Dis. 2018;33:1517-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Rades D, Dahlke M, Gebauer N, Bartscht T, Hornung D, Trang NT, Phuong PC, Khoa MT, Gliemroth J. A New Predictive Tool for Optimization of the Treatment of Brain Metastases from Colorectal Cancer After Stereotactic Radiosurgery. Anticancer Res. 2015;35:5515-5518. [PubMed] |
| 58. | Sander C, Frydrychowicz C, Prasse G, Taubenheim S, Arlt F, Meixensberger J, Fehrenbach MK. The impact of neurological performance and volumetrics on overall survival in brain metastasis in colorectal cancer: a retrospective single-center case series. BMC Cancer. 2022;22:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Wang H, Shan X, Zhang M, Qian K, Shen Z, Zhou W. Nomograms for predicting overall survival in colorectal cancer patients with metastasis to the liver, lung, bone, and brain. Cancer Causes Control. 2023;34:1059-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Tanriverdi O, Kaytan-Saglam E, Ulger S, Bayoglu IV, Turker I, Ozturk-Topcu T, Cokmert S, Turhal S, Oktay E, Karabulut B, Kilic D, Kucukzeybek Y, Oksuzoglu B, Meydan N, Kaya V, Akman T, Ibis K, Saynak M, Sen CA, Uysal-Sonmez O, Pilancı KN, Demir G, Saglam S, Kocar M, Menekse S, Goksel G, Yapar-Taskoylu B, Yaren A, Uyeturk U, Avci N, Denizli B, Ilis-Temiz E. The clinical and pathological features of 133 colorectal cancer patients with brain metastasis: a multicenter retrospective analysis of the Gastrointestinal Tumors Working Committee of the Turkish Oncology Group (TOG). Med Oncol. 2014;31:152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Magni E, Santoro L, Ravenda PS, Leonardi MC, Bonomo G, Monfardini L, Nolè F, Zampino MG. Brain metastases from colorectal cancer: main clinical factors conditioning outcome. Int J Colorectal Dis. 2014;29:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Nieder C, Hintz M, Grosu AL. Colorectal cancer metastatic to the brain: analysis of prognostic factors and impact of KRAS mutations on presentation and outcome. Clin Transl Oncol. 2016;18:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Imaizumi J, Shida D, Boku N, Igaki H, Itami J, Miyakita Y, Narita Y, Takashima A, Kanemitsu Y. Prognostic factors associated with the transition in treatment methods for brain metastases from colorectal cancer. Int J Clin Oncol. 2023;28:1043-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 64. | Fountzilas C, Chang K, Hernandez B, Michalek J, Crownover R, Floyd J, Mahalingam D. Clinical characteristics and treatment outcomes of patients with colorectal cancer who develop brain metastasis: a single institution experience. J Gastrointest Oncol. 2017;8:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Jung M, Ahn JB, Chang JH, Suh CO, Hong S, Roh JK, Shin SJ, Rha SY. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol. 2011;101:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Kimura M, Tanishima H, Tatsubayashi T, Iwakura S, Tominaga T, Horiuchi T. [A Case with Solitary Brain Metastatic Tumor from Colon Cancer]. Gan To Kagaku Ryoho. 2017;44:1388-1390. [PubMed] |
| 67. | Randrian V, Desette A, Emambux S, Derangere V, Roussille P, Frouin E, Godet J, Karayan-Tapon L, Ghiringhelli F, Tougeron D. New Artificial Intelligence Score and Immune Infiltrates as Prognostic Factors in Colorectal Cancer With Brain Metastases. Front Immunol. 2021;12:750407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Rades D, Nguyen T, Janssen S, Schild SE. An Easy-To-Use Survival Score Compared to Existing Tools for Older Patients with Cerebral Metastases from Colorectal Cancer. Cancers (Basel). 2020;12:833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Ge H, Yan Y, Xie M, Guo L, Tang D. Construction of a nomogram to predict overall survival for patients with M1 stage of colorectal cancer: A retrospective cohort study. Int J Surg. 2019;72:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Jiang Z, Huang Y, Zhang P, Han C, Lu Y, Mo Z, Zhang Z, Li X, Zhao S, Cai F, Huang L, Chen C, Shi Z, Zhang Y, Ling F. Characterization of a pathogenic variant in GBA for Parkinson's disease with mild cognitive impairment patients. Mol Brain. 2020;13:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Nunes L, Aasebø K, Mathot L, Ljungström V, Edqvist PH, Sundström M, Dragomir A, Pfeiffer P, Ameur A, Ponten F, Mezheyeuski A, Sorbye H, Sjöblom T, Glimelius B. Molecular characterization of a large unselected cohort of metastatic colorectal cancers in relation to primary tumor location, rare metastatic sites and prognosis. Acta Oncol. 2020;59:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Michl M, Taverna F, Woischke C, Li P, Klauschen F, Kirchner T, Heinemann V, von Bergwelt-Baildon M, Stahler A, Herold TM, Jurinovic V, Engel J, Kumbrink J, Neumann J. Identification of a gene expression signature associated with brain metastasis in colorectal cancer. Clin Transl Oncol. 2024;26:1886-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Cardinal T, Pangal D, Strickland BA, Newton P, Mahmoodifar S, Mason J, Craig D, Simon T, Tew BY, Yu M, Yang W, Chang E, Cabeen RP, Ruzevick J, Toga AW, Neman J, Salhia B, Zada G. Anatomical and topographical variations in the distribution of brain metastases based on primary cancer origin and molecular subtypes: a systematic review. Neurooncol Adv. 2022;4:vdab170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3826] [Article Influence: 347.8] [Reference Citation Analysis (8)] |
| 75. | Irmer B, Wlochowitz D, Krekeler C, Richter KM, Chandrabalan S, Bayerlova M, Wolff A, Lenz G, Conradi LC, Schildhaus HU, Stadelmann C, Rohde V, Proescholdt M, Salinas G, Homayounfar K, Kuhlmann T, Hailfinger S, Pukrop T, Menck K, Beissbarth T, Bleckmann A. Consensus molecular subtyping of colorectal carcinoma brain metastases reveals a metabolic signature associated with poor patient survival. Mol Oncol. 2025;19:614-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 76. | Patelli G, Tosi F, Amatu A, Mauri G, Curaba A, Patanè DA, Pani A, Scaglione F, Siena S, Sartore-Bianchi A. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open. 2021;6:100156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 77. | Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, Leal TA, Bekaii-Saab TS, Paweletz CP, Heavey GA, Christensen JG, Velastegui K, Kheoh T, Der-Torossian H, Klempner SJ. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;388:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 355] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 78. | United States Food and Drug Administration. FDA grants accelerated approval to adagrasib with cetuximab for KRAS G12C-mutated colorectal cancer. FDA [Internet]. Jun 21, 2024. [cited 26 May 2025]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-cetuximab-kras-g12c-mutated-colorectal-cancer. |
| 79. | Kim SH, Lee YS, Lee SH, Sung YE, Lee A, Kang J, Park JS, Jeun SS, Lee YS. Single-center study on clinicopathological and typical molecular pathologic features of metastatic brain tumor. J Pathol Transl Med. 2023;57:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Desette A, Guichet PO, Emambux S, Masliantsev K, Cortes U, Ndiaye B, Milin S, George S, Faigner M, Tisserand J, Gaillard A, Brot S, Wager M, Tougeron D, Karayan-Tapon L. Deciphering Brain Metastasis Stem Cell Properties From Colorectal Cancer Highlights Specific Stemness Signature and Shared Molecular Features. Cell Mol Gastroenterol Hepatol. 2023;16:757-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Lavacchi D, Fancelli S, Roviello G, Castiglione F, Caliman E, Rossi G, Venturini J, Pellegrini E, Brugia M, Vannini A, Bartoli C, Cianchi F, Pillozzi S, Antonuzzo L. Mutations matter: An observational study of the prognostic and predictive value of KRAS mutations in metastatic colorectal cancer. Front Oncol. 2022;12:1055019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Nicolazzo C, Barault L, Caponnetto S, Macagno M, De Renzi G, Gradilone A, Belardinilli F, Cortesi E, Di Nicolantonio F, Gazzaniga P. Circulating Methylated DNA to Monitor the Dynamics of RAS Mutation Clearance in Plasma from Metastatic Colorectal Cancer Patients. Cancers (Basel). 2020;12:3633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Yanai Y, Hayashi T, Akazawa Y, Yatagai N, Tsuyama S, Yao T, Saito T. Clinicopathological and mutational differences between tumors with multiple metastases and single lung metastasis in colorectal cancer. Oncol Lett. 2020;20:541-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Roussille P, Tachon G, Villalva C, Milin S, Frouin E, Godet J, Berger A, Emambux S, Petropoulos C, Wager M, Karayan-Tapon L, Tougeron D. Pathological and Molecular Characteristics of Colorectal Cancer with Brain Metastases. Cancers (Basel). 2018;10:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Liu J, Zeng W, Huang C, Wang J, Yang D, Ma D. Predictive and Prognostic Implications of Mutation Profiling and Microsatellite Instability Status in Patients with Metastatic Colorectal Carcinoma. Gastroenterol Res Pract. 2018;2018:4585802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Aprile G, Casagrande M, De Maglio G, Fontanella C, Rihawi K, Bonotto M, Pisa FE, Tuniz F, Pizzolitto S, Fasola G. Comparison of the molecular profile of brain metastases from colorectal cancer and corresponding primary tumors. Future Oncol. 2017;13:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | El-Deiry WS, Vijayvergia N, Xiu J, Scicchitano A, Lim B, Yee NS, Harvey HA, Gatalica Z, Reddy S. Molecular profiling of 6,892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol Ther. 2015;16:1726-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 88. | Michl M, Heinemann V, Jung A, Engel J, Kirchner T, Neumann J. Expression of cancer stem cell markers in metastatic colorectal cancer correlates with liver metastasis, but not with metastasis to the central nervous system. Pathol Res Pract. 2015;211:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Lipsyc M, Yaeger R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J Gastrointest Oncol. 2015;6:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 90. | Yaeger R, Cowell E, Chou JF, Gewirtz AN, Borsu L, Vakiani E, Solit DB, Rosen N, Capanu M, Ladanyi M, Kemeny N. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 91. | Prete AA, Angerilli V, Bergamo F, Vettore V, De Toni C, Intini R, Cerma K, Ricagno G, Cerantola R, Perissinotto E, De Rosa A, Ceccon C, Gasparello J, Denaro L, D'Amico A, Chioffi F, Carcea E, Fassan M, Lonardi S. HER2 expression and genOmic characterization of rESected brain metastases from colorectal cancer: the HEROES study. Br J Cancer. 2024;130:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 92. | Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 93. | Pasquier D, Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Petit A, Mouret-Reynier MA, Goncalves A, Dalenc F, Deluche E, Fresnel JS, Augereau P, Ferrero JM, Geffrelot J, Fumet JD, Lecouillard I, Cottu P, Petit T, Uwer L, Jouannaud C, Leheurteur M, Dieras V, Robain M, Mouttet-Audouard R, Bachelot T, Courtinard C. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer. 2020;125:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8266] [Article Influence: 330.6] [Reference Citation Analysis (11)] |
| 95. | Lasota J, Kaczorowski M, Chłopek M, Miłek-Krupa J, Szczepaniak M, Ylaya K, Chodyna M, Iżycka-Świeszewska E, Scherping A, Czapiewski P, Dziuba I, Kato Y, Hałoń A, Kowalik A, Miettinen M. An immunohistochemical and molecular genetic study of 60 colorectal carcinoma brain metastases in pursuit of predictive biomarkers for cancer therapy. Hum Pathol. 2025;155:105717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Chen PC, Yeh YM, Chu CT, Su PF, Chiu PH, Lin BW, Chen SH, Lin PC, Lee CT, Chen HHW, Chen CC. HER2 amplification in colorectal cancer with brain metastasis: A propensity score matching study. Eur J Cancer. 2023;181:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 97. | Mitra D, Clark JW, Shih HA, Oh KS, Brastianos PK, Wo JY, Strickland MR, Curry WT, Parikh AR, Corcoran RB, Ryan DP, Iafrate AJ, Borger DR, Lennerz JK, Hong TS. Enrichment of HER2 Amplification in Brain Metastases from Primary Gastrointestinal Malignancies. Oncologist. 2019;24:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Zhou Y, Yang Y, Wang ZB, Wang X, Xiang J, Chen Z. Neurotrophin-3 upregulates HER-2 to promote the growth of brain metastasis from colorectal cancer. Int J Clin Exp Pathol. 2016;9:6867-6876. |
| 99. | Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Brandt VP, Holland H, Wallenborn M, Koschny R, Frydrychowicz C, Richter M, Holland L, Nestler U, Sander C. SNP array genomic analysis of matched pairs of brain and liver metastases in primary colorectal cancer. J Cancer Res Clin Oncol. 2023;149:18173-18183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 101. | Krivec N, Ghosh MS, Spits C. Gains of 20q11.21 in human pluripotent stem cells: Insights from cancer research. Stem Cell Reports. 2024;19:11-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | De Maglio G, Casagrande M, Guardascione M, Fontanella C, Lutrino SE, Rihawi K, Pisa FE, Tuniz F, Fasola G, Pizzolitto S, Aprile G. MGMT promoter methylation status in brain metastases from colorectal cancer and corresponding primary tumors. Future Oncol. 2015;11:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, Morris V, Janku F, Dasari A, Chung W, Issa JP, Gibbs P, James B, Powis G, Nolop KB, Bhattacharya S, Saltz L. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33:4032-4038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 566] [Article Influence: 51.5] [Reference Citation Analysis (1)] |
| 104. | Phillips WJ, Marginean H, Alrehaili M, Abdelrahim AA, Asmis T, Vickers M, Yeung B, Lo B, Goodwin R. Real-world evaluation of treatment patterns and clinical outcomes in patients with BRAF-V600E metastatic colorectal cancer (mCRC) in Canada. Cancer Treat Res Commun. 2025;43:100896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Aiman W, Qirem M, Alkhlaifat O, Garcia J, Asfeen U, Jumean S, Rayad MN, Veeraballi S, Dacosta T, Guron GK, Shaaban HS. Efficacy of vemurafenib based regimens in colorectal cancer: A systematic review of clinical trials. J Clin Oncol. 2023;41:e15566-e15566. [DOI] [Full Text] |
| 106. | Slack RJ, Macdonald SJF, Roper JA, Jenkins RG, Hatley RJD. Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov. 2022;21:60-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 107. | Schittenhelm J, Klein A, Tatagiba MS, Meyermann R, Fend F, Goodman SL, Sipos B. Comparing the expression of integrins αvβ3, αvβ5, αvβ6, αvβ8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors. Int J Clin Exp Pathol. 2013;6:2719-2732. [PubMed] |
| 108. | Akhavan D, Yazaki P, Yamauchi D, Simpson J, Frankel PH, Bading J, Colcher D, Poku K, Chen YJ, Lim D, Cristea M, Wu A, Shively J, Wong JYC. Phase I Study of Yttrium-90 Radiolabeled M5A Anti-Carcinoembryonic Antigen Humanized Antibody in Patients with Advanced Carcinoembryonic Antigen Producing Malignancies. Cancer Biother Radiopharm. 2020;35:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, Rakovitch E, Chow E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018;1:CD003869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 110. | Lee JH, Kim IY, Jung S, Jung TY, Moon KS, Kim YJ, Park SJ, Lim SH. Two-Day Fraction Gamma Knife Radiosurgery for Large Brain Metastasis. J Korean Neurosurg Soc. 2024;67:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 111. | Rades D, Hansen HC, Janssen S, Schild SE. Diagnosis-specific WBRT-30-CRC Score for Estimating Survival of Patients Irradiated for Brain Metastases from Colorectal Cancer. Anticancer Res. 2019;39:2569-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Rades D, Dziggel L, Blanck O, Gebauer N, Bartscht T, Schild SE. A Score to Identify Patients with Brain Metastases from Colorectal Cancer Who May Benefit from Whole-brain Radiotherapy in Addition to Stereotactic Radiosurgery/Radiotherapy. Anticancer Res. 2018;38:3111-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Gavriilidis P. Solitary brain metastasis from colorectal cancer without other systemic metastases. Surg Chron. 2013;18:31-33. |
| 114. | Kann BH, Park HS, Johnson SB, Chiang VL, Yu JB. Radiosurgery for Brain Metastases: Changing Practice Patterns and Disparities in the United States. J Natl Compr Canc Netw. 2017;15:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 115. | Paix A, Antoni D, Adeduntan R, Noël G. Stereotactic radiation therapy of brain metastases from colorectal cancer: A single institution cohort. Cancer Radiother. 2017;21:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Bin-Alamer O, Abou-Al-Shaar H, Singh R, Mallela AN, Legarreta A, Bowden G, Mathieu D, Perlow HK, Palmer JD, Elhamdani S, Shepard M, Liang Y, Nabeel AM, Reda WA, Tawadros SR, Abdelkarim K, El-Shehaby AMN, Emad Eldin R, Elazzazi AH, Warnick RE, Gozal YM, Daly M, McShane B, Addis-Jackson M, Karthikeyan G, Smith S, Picozzi P, Franzini A, Kaisman-Elbaz T, Yang HC, Hess J, Templeton K, Zhang X, Wei Z, Pikis S, Mantziaris G, Simonova G, Liscak R, Peker S, Samanci Y, Chiang V, Kersh CR, Lee CC, Trifiletti DM, Niranjan A, Hadjipanayis CG, Lunsford LD, Sheehan JP. Local control and survival after stereotactic radiosurgery for colorectal cancer brain metastases: an international multicenter analysis. J Neurosurg. 2024;140:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Doyle E, Killean AJ, Harrow S, Phillips ID. Systematic review of the efficacy of stereotactic ablative radiotherapy for oligoprogressive disease in metastatic cancer. Radiother Oncol. 2024;196:110288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Skeie BS, Enger PØ, Ganz JC, Skeie GO, Parr E, Hatteland S, Ystevik B, Heggdal JI, Pedersen PH. Gamma knife surgery of colorectal brain metastases: a high prescription dose of 25 Gy may improve growth control. World Neurosurg. 2013;79:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 119. | Page BR, Wang EC, White L, McTyre E, Peiffer A, Alistar A, Mu F, Loganathan A, Bourland JD, Laxton AW, Tatter SB, Chan MD. Gamma Knife radiosurgery for brain metastases from gastrointestinal primary. J Med Imaging Radiat Oncol. 2017;61:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Li Y, Wu J, Liu F, Shao X, Liang X, Zhang F, Meng Y, Shen M, Pan M. Single-fraction SRS and multiple-fraction SRT for brain metastases from colorectal cancer. Front Oncol. 2022;12:1060570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 121. | Taori S, Wei Z, Deng H, Lunsford LD, Niranjan A. The Role of Stereotactic Radiosurgery in Patients With Brain Metastases From Colorectal Cancers. Neurosurgery. 2024;94:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 122. | Mondaca S, Hornig V, Munoz-Schuffenegger P, Acevedo F, Garrido M, Nervi B. Central nervous system metastasis secondary to colorectal cancer: a retrospective cohort study of 20 cases. Ecancermedicalscience. 2016;10:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 123. | Gorovets D, Ayala-Peacock D, Tybor DJ, Rava P, Ebner D, Cielo D, Norén G, Wazer DE, Chan M, Hepel JT. Multi-institutional Nomogram Predicting Survival Free From Salvage Whole Brain Radiation After Radiosurgery in Patients With Brain Metastases. Int J Radiat Oncol Biol Phys. 2017;97:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Proescholdt MA, Schödel P, Doenitz C, Pukrop T, Höhne J, Schmidt NO, Schebesch KM. The Management of Brain Metastases-Systematic Review of Neurosurgical Aspects. Cancers (Basel). 2021;13:1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 125. | Kim HJ, Huh JW, Jung TY, Kim IY, Kim HR, Jung S, Kim YJ. Clinical outcome with gamma-knife surgery or surgery for brain metastases from colorectal cancer. J Clin Neurosci. 2013;20:1417-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Chang Y, Wong CE, Lee PH, Huang CC, Lee JS. Survival Outcome of Surgical Resection vs. Radiotherapy in Brain Metastasis From Colorectal Cancer: A Meta-Analysis. Front Med (Lausanne). 2022;9:768896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | Zancana G, Armocida D, Capobianco M, Corvino S, Cofano F, Garbossa D, Santoro A, Frati A. Clinical, Radiologic, and Surgical Features of Brain Metastases in Colorectal Cancer. A Strong Correlation Between Surgical Patterns and Outcome. World Neurosurg. 2024;189:e1040-e1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 128. | Fuentes R, Osorio D, Expósito Hernandez J, Simancas-Racines D, Martinez-Zapata MJ, Bonfill Cosp X. Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. Cochrane Database Syst Rev. 2018;8:CD012086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Lim CS, Grundy PL. Effectiveness and outcomes of surgery for cerebral metastases. Br J Neurosurg. 2013;27:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 130. | Gui C, Walch HS, Mueller KD, Boe LA, Ilica AT, Strong J, Eichholz JE, Yu KKH, Wilcox JA, Manca P, Yu Y, Yamada Y, Imber BS, Maron SB, Foote MB, Yaeger R, Schultz N, Pike LRG. Clinicogenomic predictors of survival and intracranial progression after stereotactic radiosurgery for colorectal cancer brain metastases. J Neurosurg. 2025;142:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 131. | Suzuki Y, Yamaguchi T, Matsumoto H, Nakano D, Honda G, Shinoura N, Karasawa K, Takahashi K. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. 2014;57:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 132. | Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis. 2012;14:e378-e385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Kavouridis VK, Harary M, Hulsbergen AFC, Lo YT, Reardon DA, Aizer AA, Iorgulescu JB, Smith TR. Survival and prognostic factors in surgically treated brain metastases. J Neurooncol. 2019;143:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 134. | Krist DT, Naik A, Thompson CM, Kwok SS, Janbahan M, Olivero WC, Hassaneen W. Management of brain metastasis. Surgical resection versus stereotactic radiotherapy: a meta-analysis. Neurooncol Adv. 2022;4:vdac033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 135. | Prabhu RS, Dhakal R, Vaslow ZK, Dan T, Mishra MV, Murphy ES, Patel TR, Asher AL, Yang K, Manning MA, Stern JD, Patel AR, Wardak Z, Woodworth GF, Chao ST, Mohammadi A, Burri SH. Preoperative Radiosurgery for Resected Brain Metastases: The PROPS-BM Multicenter Cohort Study. Int J Radiat Oncol Biol Phys. 2021;111:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 136. | Cheok SK, Yu C, Feng JJ, Briggs RG, Chow F, Hwang L, Ye JC, Attenello FJ, Tran D, Chang E, Zada G. Comparison of preoperative versus postoperative treatment dosimetry plans of single-fraction stereotactic radiosurgery for surgically resected brain metastases. Neurosurg Focus. 2023;55:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 137. | Wong CE, Chang Y, Huang CC, Hsu HH, Lai YH, Chang KY, Huang CY, Wang LC, Lee JS, Lee PH. Surgical excision and radiotherapy for brain metastasis from colorectal cancer: How frailty and comorbidity indices influence outcome. Kaohsiung J Med Sci. 2024;40:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 138. | Kim DY, Ryu CG, Jung EJ, Paik JH, Hwang DY. Brain metastasis from colorectal cancer: a single center experience. Ann Surg Treat Res. 2018;94:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Tapia Rico G, Price TJ, Karapetis C, Piantadosi C, Padbury R, Roy A, Maddern G, Moore J, Carruthers S, Roder D, Townsend AR. Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC) registry. Cancer Biol Med. 2017;14:371-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Silva SB, Barreto RB, de Oliveira FCG, Martin GSD, Takiguchi OMY, Chirichela IA, Miranda MHF, Bodnar D, Alves Reis LA, Pereira GCB, Miranda IL, Pereira BR, Arruda GV, Peria FM. Radiotherapy for Brain Metastases Near the End of Life: Characterizing Patients and Tumor Features. JCO Glob Oncol. 2023;9:e2300143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 141. | Recchia F, Candeloro G, Necozione S, Bratta M, Bisegna R, Rea S. Alternating XELFOX and XELFIRI in patients with metastatic colorectal cancer. Am J Clin Oncol. 2008;31:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Amin S, Baine MJ, Meza JL, Lin C. Association of Immunotherapy With Survival Among Patients With Brain Metastases Whose Cancer Was Managed With Definitive Surgery of the Primary Tumor. JAMA Netw Open. 2020;3:e2015444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 143. | Hsu HC, Huang KC, Chen WS, Jiang JK, Yang SH, Wang HS, Chang SC, Lan YT, Lin CC, Lin HH, Huang SC, Cheng HH, Yang TS, Chen CC, Chao Y, Teng HW. Preference criteria for regorafenib in treating refractory metastatic colorectal cancer are the small tumor burden, slow growth and poor/scanty spread. Sci Rep. 2021;11:15370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, Hamid O, Infante JR, Millward M, Pavlick AC, O'Day SJ, Blackman SC, Curtis CM, Lebowitz P, Ma B, Ouellet D, Kefford RF. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 735] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 145. | Berghoff AS, Breckwoldt MO, Riedemann L, Karimian-Jazi K, Loew S, Schlieter F, Furtner J, Cinci M, Thomas M, Strowitzki MJ, Marmé F, Michel LL, Schmidt T, Jäger D, Bendszus M, Preusser M, Wick W, Winkler F. Bevacizumab-based treatment as salvage therapy in patients with recurrent symptomatic brain metastases. Neurooncol Adv. 2020;2:vdaa038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Finkelmeier F, You SJ, Waidmann O, Wolff R, Zeuzem S, Bähr O, Trojan J. Bevacizumab in Combination with Chemotherapy for Colorectal Brain Metastasis. J Gastrointest Cancer. 2016;47:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 147. | Chen CW, Ou TS, Chen WS, Jiang JK, Yang SH, Wang HS, Chang SC, Lan YT, Lin CC, Lin HH, Huang SC, Cheng HH, Yang YW, Lin YZ, Chao Y, Wang LW, Teng HW. Anti-VEGF Therapy Possibly Extends Survival in Patients With Colorectal Brain Metastasis by Protecting Patients From Neurologic Disability. Clin Colorectal Cancer. 2023;22:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 148. | Chahine G, Ibrahim T, Felefly T, El-Ahmadie A, Freiha P, El-Khoury L, Khalife-Saleh N, Saleh K. Colorectal cancer and brain metastases: An aggressive disease with a different response to treatment. Tumori. 2019;105:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 149. | Nieblas-Bedolla E, Nayyar N, Singh M, Sullivan RJ, Brastianos PK. Emerging Immunotherapies in the Treatment of Brain Metastases. Oncologist. 2021;26:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 150. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 2056] [Article Influence: 342.7] [Reference Citation Analysis (0)] |
| 151. | Chen N, Pu C, Zhao L, Li W, Wang C, Zhu R, Liang T, Niu C, Huang X, Tang H, Wang Y, Yang H, Jia B, Jiang X, Han G, Wang W, Chen D, Wang Y, Rowinsky EK, Kennedy E, Lu VX, Cui G, Wu Z, Xiao L, Cui J. Chimeric Antigen Receptor T Cells Targeting CD19 and GCC in Metastatic Colorectal Cancer: A Nonrandomized Clinical Trial. JAMA Oncol. 2024;10:1532-1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 152. | Liu J, Wang Y, Song Z, Zhang Y. Nanoengineered immune check point inhibitors delivery for targeted brain cancer treatment: Current status and future perspectives. Biochem Pharmacol. 2025;233:116789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 153. | Liu HJ, Xu P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv Drug Deliv Rev. 2022;191:114619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 154. | Hersh AM, Alomari S, Tyler BM. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int J Mol Sci. 2022;23:4153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 155. | Yuzhalin AE, Yu D. Brain Metastasis Organotropism. Cold Spring Harb Perspect Med. 2020;10:a037242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 156. | Li Y, Yang SY, Zhang YR, Wang Y. Decoding the neuroimmune axis in colorectal cancer: From neural circuitry to therapeutic innovation. Cytokine Growth Factor Rev. 2025;83:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/