Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.111029
Revised: July 31, 2025
Accepted: November 4, 2025
Published online: December 22, 2025
Processing time: 184 Days and 6.6 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease with a continually rising global prevalence and sig
Core Tip: Metabolic dysfunction-associated steatotic liver disease is associated with mood disorders such as depression and anxiety through shared pathophysiological pathways. This review links systemic inflammation, hypothalamic-pituitary-adrenal axis dysregulation, gut dysbiosis, and neurotransmitter imbalances as mediators of this bidirectional relationship. We highlight how overlapping risk factors such as insulin resistance, obesity, sarcopenia, poor sleep, and physical inactivity exacerbate both the disease processes. Recognizing the mental health burden allows for improved patient outcomes and supports a holistic, lifestyle-focused treatment model that addresses both physical and mental health.
- Citation: Sampada, Naseem M, Solanki M, Sharma R, Singh C, Sohal A. Pathophysiology of depression and anxiety in metabolic dysfunction-associated steatotic liver disease. World J Gastrointest Pathophysiol 2025; 16(4): 111029
- URL: https://www.wjgnet.com/2150-5330/full/v16/i4/111029.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i4.111029
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is characterized by the accumulation of fat in the liver and is associated with metabolic abnormalities, including obesity, insulin resistance (IR), and type 2 diabetes[1,2]. In 2023, a global Delphi consensus recommended transitioning from the term NAFLD to MASLD to better reflect the metabolic dysfunction underlying the condition and move away from stigmatizing terminology such as "nonalcoholic" and "fatty". The revised nomenclature recognizes steatosis in the presence of at least 1 of 5 cardiometabolic risk factors as a positive diagnostic criterion. It introduces related categories such as metabolic dysfunction-associated steatohepatitis (MASH) and MetALD for overlapping alcohol-related cases[3]. MASLD prevalence has increased from about 25% to nearly 34% over 18 years, highlighting its growing public health impact along with rising rates of obesity and metabolic syndrome[4-6]. In addition to its clinical burden, MASLD causes a significant financial burden. Medical costs in the United States exceed $62 billion, averaging $1584 per patient, and affecting over 39 million Americans. Once considered a condition confined to the liver, MASLD is now recognized as a complex, systemic disorder driven by metabolic dysfunction and chronic inflammation. It is associated with a variety of extrahepatic manifestations, including cardiovascular disease, type 2 diabetes, chronic kidney disease, hypothyroidism, polycystic ovarian syndrome, and psoriasis[7-10].
Studies have also reported MASLD to be linked to mental health disorders, particularly depression and anxiety[11]. Studies show that individuals with MASLD experience a significantly higher prevalence of anxiety and depression compared to the general population[12,13]. A recent meta-analysis reported that among adults with MASLD, the prevalence of depression was approximately 26%, anxiety was around 37%, and stress as high as 51%[14]. This highlights the significant psychological burden associated with the condition. While the relationship between MASLD and mental health disorders is becoming increasingly apparent, the biological mechanisms driving this relationship continue to be poorly understood. However, several hypotheses have been postulated, including gut microbiota dysbiosis as a potential contributor[15,16]. While awareness of the mental health challenges linked to MASLD is increasing, our understanding of the biological mechanisms behind these associations is still limited. This gap underscores the need for integrative research that explores how metabolic, inflammatory, and neurological pathways might connect liver dysfunction with mental health conditions like anxiety and depression. At a molecular level, mood disorders like depression and anxiety arise from complex interactions within the body’s immune, endocrine, and nervous systems. Chronic low-grade inflammation and overactivity of the hypothalamic-pituitary-adrenal (HPA) axis are key mechanisms linked to depression and anxiety[17-20]. Increasing levels of pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α), impair neuroplasticity and neurogenesis, contributing to mood disturbances[21,22]. Interestingly, these same inflammatory pathways have been implicated in the pathogenesis of MASLD[23-25].
Despite growing awareness, current treatments for MASLD and mood disorders often focus on each condition in isolation, missing the shared pathophysiological basis[26].
Even emerging therapies like HPA modulators and microbiota-based interventions show mixed results, pointing to the need for more integrated and personalized approaches[27,28]. In this review, we focus on current findings to provide an updated perspective on the mechanisms that may underlie these associations. Clarifying these connections could pave the way for earlier screening and more holistic treatment approaches that will ultimately help in improving both the physical and psychological outcomes for individuals living with MASLD.
A literature search was conducted between March and May 2025 using the databases PubMed, Scopus, and Google Scholar. Search terms included a combination of MeSH terms and keywords which were “MASLD”, “NAFLD”, “depression”, “anxiety”, “neuroinflammation”, “gut-brain axis”, “obesity”, “HPA axis”, “sarcopenia”, “cytokines”, and “insulin resistance”. Boolean operators (AND/OR) were applied as appropriate. Only articles published in English were included. We included both original research articles and review articles to contextualize and interpret findings. No formal exclusion criteria were applied. Reference lists of eligible studies were screened manually to identify additional literature that could provide mechanistic insight where direct associations were not available.
A meta-analysis study done by Younossi et al[29] which included 92 population-based studies covering over 9.36 million individuals globally, showed a striking rise in the global prevalence of MASLD. Between 1990 and 2019, global MASLD prevalence increased by 50.4%, rising from 25.26% in 1990–2006 to 38.2% in 2016–2019. The association between MASLD and mood disorders has become evident. It has been previously reported by multiple studies that patients with MASLD have a higher prevalence of depression (27.2%) than that of the general population[30-32]. A cross-sectional Brazilian study found an inverse association of MASLD with anxiety [odds ratio (OR) = 0.75, 95%CI: 0.63-0.90] and a positive association with depression (OR = 1.17, 95%CI: 1.00-1.38)[33]. A 2020 study by Labenz et al[34] conducted over a 10 years period showed that 21.2% of MASLD patients developed depression (vs 18.2% of controls), while 7.9% developed anxiety (vs 6.5%). According to Youssef et al[13], the severity of depressive symptoms increased with the severity of hepatocellular ballooning. Brodosi et al[35], interestingly, stated that disease severity did not consistently correlate with psychiatric burden, possibly due to a general lack of awareness of MASLD’s progressive nature among patients. MASLD was not independently associated with moderate/severe depression (OR = 0.34; 95%CI: 0.12–1.01) or severe trait anxiety (OR = 0.79; 95%CI: 0.27–2.34).
However, according to Tomeno et al[31], the severity of steatosis was greater in MASLD patients comorbid with depression than those without. These discrepancies may stem from a number of potential reasons. The study by Brodosi et al[35] was cross-sectional and relied on self-reported psychiatric symptoms through questionnaires. Labenz et al[34], on the other hand, conducted a longitudinal analysis using a large primary care database over 10 years, capturing incident cases of depression and anxiety. Differences in methods to diagnose MASLD such as biopsy in Tomeno et al[31] vs imaging or International Classification of Diseases coding in others can also be a reason for variability in results. Fur
The association between MASLD and mood disorders may be gender specific. A retrospective cross-sectional study conducted in a Korean population suggested that women with MASLD were more prone to suffer from anxiety and depression as the severity of steatosis increased[11]. Similarly, Jung et al[36] reported that women with MASLD had a higher prevalence of depression compared to men. A 2025 study by He et al[37] used central fat distribution as a mediator between MASLD and depression suggesting that it explains about 16.6% of the link between MASLD and depression overall. In females, this mediating effect was found to be statistically significant accounting for 17.83% of the link. Women with diabetes and women, in general, experience a higher prevalence of depression than men[38,39]. In the Brazilian study by Goulart et al[33], only men presented an association of anxiety with MASLD (OR = 0.73; 95%CI: 0.60-0.89) when sex-stratified analyses were done. The prevalence of depression is twice as high in people with type 2 diabetes (19.1%, range 6.5%-33% vs 10.7%, range 3.8%-19.4%) compared to those without[38].
This relationship has been suggested to be bidirectional. A two-step Mendelian randomization analysis concluded that people who are genetically predisposed to depression are at risk of developing MASLD (OR = 1.557; 95%CI: 1.097–2.211; P = 0.016)[40]. However, this study disproved causality in the other direction. Data from the United Kingdom Biobank, as referenced in recent longitudinal research, displayed that individuals with depression have an increased risk of developing MASLD (hazard ratio = 1.21; 95%CI: 1.09–1.34)[41]. Increased incidence of depression and anxiety was found in a cohort of adolescents with MASLD. In this cohort, the incidence of new depression and anxiety cases in MASLD was 27 and 18 per 1000 person-years, respectively. Concurrently, levels of alanine aminotransferase worsened for adolescents with MASLD who developed depression compared to those who did not develop depression[42]. A 2022 Longitudinal study done in a Southern California population exhibited that individuals with MASLD and higher levels of physical activity were at a decreased odds of having depressive symptoms [16.1% reduction (95%CI: -25.6 to -5.4%), P = 0.004], which was not observed in those without MASLD taking into account various comorbidities (i.e., age, sex, diabetes, hypertension, obesity)[43]. MASLD patients develop sarcopenia, which makes them more prone to depressive symptoms, potentially due to diminished physical capacity and fatigue[44-46]. Additional at-risk groups include women with polycystic ovary syndrome, who often experience both MASLD and high risk of depression[47-49]. The current epidemio
| Ref. | Year | Type | Population | Key findings |
| Weinstein et al[30] | 2011 | Cross-sectional study. MASLD was diagnosed through pathology and/or radiologic testing | 878 patients with chronic liver disease (MASLD, HBV, HCV) seen at a tertiary liver center | MASLD patients had a significantly higher prevalence of depression (27.2%). Independent predictors of depression for MASLD patients included hypertension, current smoking, history of lung disease, female sex, and nonAfricanAmerican ethnicity |
| Goulart et al[33] | 2023 | Cross-sectional study. MASLD was diagnosed by ultrasound imaging | 7241 working-age adults from a primary care center in Brazil. Comorbidities included metabolic syndrome components such as obesity, diabetes, dyslipidemia and hypertension | MASLD was positively associated with depression (OR = 1.17, 95%CI: 1.00-1.38). MASLD was inversely associated with anxiety (OR = 0.75, 95%CI: 0.63-0.90) and when stratified by sex, only men presented this association (OR = 0.73; 95%CI: 0.60-0.89) |
| Christian Labenz et al[34] | 2020 | Retrospective cohort study. MASLD diagnosed primarily by ICD codes in primary care records | 19871 German adults with an initial diagnosis of MASLD/MASH without liver cirrhosis matched with 19871 controls from German primary care records between years 2010–2015. Comorbidities such as type 2 diabetes, obesity, and cardiovascular disease were accounted for using the Charlson Comorbidity Index in the analysis | MASLD patients had a higher incidence of both depression (21.2% vs 18.2%) and anxiety (7.9% vs 6.5%) compared to controls. After adjusting for confounders, MASLD remained an independent risk factor, with hazard ratios of 1.21 for depression and 1.23 for anxiety |
| Youssef et al[13] | 2013 | Cross-sectional study. MASLD diagnosed by liver biopsy | 567 biopsy-proven MASLD patients enrolled in the Duke MASLD Clinical Database. Comorbidities including diabetes and obesity were reported and considered | 14% of patients were noted to have clinical depression while 23% had clinical anxiety. The severity of depressive symptoms increased with the severity of hepatocellular ballooning with adjusted cumulative odds ratio of clinical depression 3.6 (95%CI: 1.4-8.8) |
| Brodosi et al[35] | 2024 | Cross-sectional study. MASLD diagnosed by ultrasound and/or clinical criteria | 286 Italian adults out of which 223 met MASLD criteria. Comorbidities included obesity, diabetes, and hypertension reported among participants | MASLD was not independently associated with moderate/severe depression (OR = 0.34; 95%CI: 0.12–1.01) or severe trait anxiety (OR = 0.79; 95%CI: 0.27–2.34). This was attributed to awareness of MASLD progression by the patient |
| Tomeno et al[31] | 2015 | Prospective observational study. MASLD was diagnosed by biopsy | 258 Japanese adults with biopsy confirmed MASLD, out of which 32 were diagnosed with depression | Patients comorbid with depression showed higher severity of steatosis and increased levels of serum aminotransferase, γ-glutamyl transpeptidase and ferritin |
| Choi et al[11] | 2021 | Retrospective cross-sectional study. MASLD was diagnosed by ultrasonography | 25333 Korean adults among whom MASLD prevalence was 30.9%. Comorbidities including obesity, diabetes, and hypertension were reported | In women, MASLD was significantly associated with depression (adjusted OR = 1.43, 95%CI: 1.14–1.80; P = 0.002). Additionally, severe MASLD showed a significant correlation with both state anxiety (adjusted OR = 1.84, 95%CI: 1.01–3.37; P = 0.047) and trait anxiety (adjusted OR = 2.45, 95%CI: 1.08–4.85; P = 0.018) |
| He et al[37] | 2025 | Cross-sectional study. MASLD diagnosed by NHANES criteria including imaging and lab markers | 3332 ethnically diverse MASLD patients drawn from NHANES dataset | Overall, central fat mediated 16.6% of the link between MASLD and depression. In women, the mediation by central fat was 17.8%, while no mediation was found in men |
| Roy et al[38] | 2012 | Systematic review | Adults with and without type 2 diabetes from multiple studies published between 2006 and 2011 | Both, women with diabetes and also women without diabetes experience a higher prevalence of depression than men. The prevalence rate of depression is almost twice as high in people with type 2 diabetes (19.1%, range 65%-33% vs 10.7%, range 38-19.4%) than those without |
| Liang et al[40] | 2024 | Two-sample Mendelian randomization | 807553 individuals, comprising 246363 cases and 561190 controls, derived from three GWAS conducted on individuals of European ancestry | Depression increased the risk of MASLD (OR: 1.557; 95%CI: 1.097-2.211; P = 0.016) but no inverse causal relationship was found |
| Zhou et al[41] | 2024 | Prospective cohort study followed by mendelian randomisation. MASLD diagnosed using United Kingdom Biobank clinical data | 481181 United Kingdom Biobank participants after excluding participants with liver disease or alcohol/drug use disorders at baseline, without related exact date for liver disease or alcohol/drug use disorders, and new-onset severe MASLD within 5 years follow-up (median follow-up 13.5 years) | Participants with depression had a significantly higher risk of developing severe MASLD compared to those without depression (HR: 1.21, 95%CI: 1.09–1.34) |
| Noon et al[42] | 2021 | Prospective longitudinal cohort study. MASLD was diagnosed by liver biopsy | 160 adolescents (ages 12–17) with MASLD followed for a mean of 3.8 years | At baseline, 8.1% had depression and 6.3% had anxiety. Over follow-up, an additional 95% developed depression (95%CI: 4.7%-14.3%) and 6.7% developed anxiety (95%CI: 2.6%-10.7%). Those who developed depression had significantly worse ALT changes in comparison to those without depression |
| Weinstein et al[43] | 2022 | Cross-sectional study | 589 survey participants from a southern California community aged less than 70 years | After adjusting for various comorbidities, individuals with MASLD and higher levels of physical activity were at a decreased odds of having depressive symptoms [16.1% reduction (95%CI: -25.6 to -5.4%), P = 0.004], which was not observed in those without MASLD |
Even though an overwhelming amount of evidence suggests that chronic conditions like hypertension, obesity, diabetes and coronary artery disease account for shared risk factors between MASLD and mood disorders such as depression, the exact shared pathogenesis remains poorly understood[37,50]. We’ve detailed the pathophysiological factors which link the two conditions in the upcoming sections.
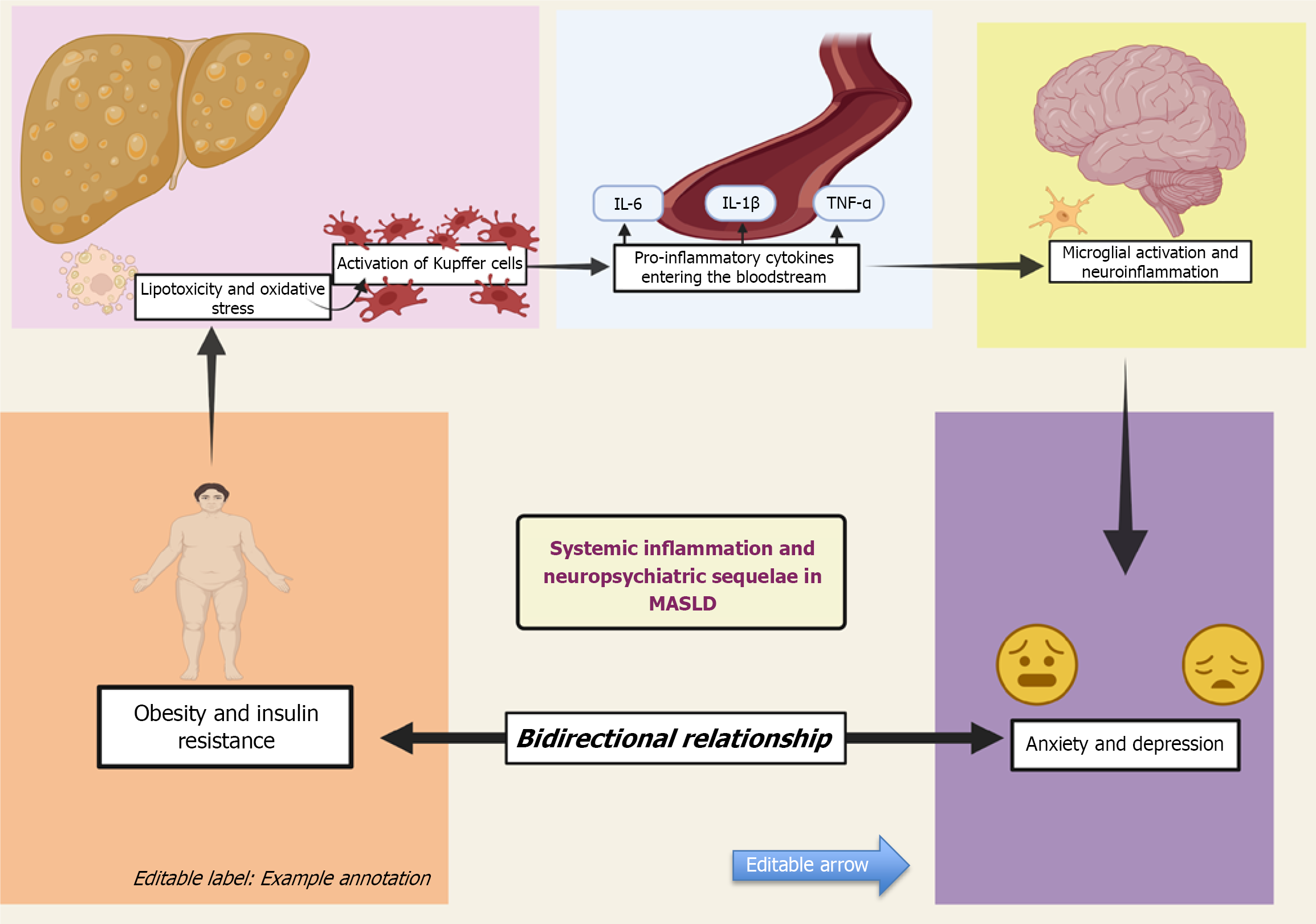
IR and type 2 diabetes: IR is defined as diminished sensitivity of target tissues to respond to insulin. This leads to a compensatory response by pancreatic beta cells to increase plasma insulin concentration resulting in hyperinsulinemia[51]. This results in increased hepatic de novo lipogenesis via the dysregulation of key transcription factors, including sterol regulatory element-binding protein 1c and carbohydrate regulatory element-binding protein[52]. As a result, there is an overproduction of free fatty acids (FFAs) and lipid accumulation in hepatocytes which leads to hepatic steatosis or MASLD. IR disrupts the process of triglycerides (TGs) synthesis from FFAs and packaging into very low-density lipoprotein (VLDL) particles for storage or export, leading to lipid accumulation in liver cells and exacerbating hepatic steatosis[53]. Thus, MASLD occurs as the metabolic consequence of IR[54]. IR promotes excessive FFAs flux to the liver, inducing lipotoxicity and activating NF-κB mediated inflammatory cascades. Insulin normally exhibits anti-inflammatory effects. In the case of IR, it paradoxically leads to development of low-grade inflammation, especially in obese individuals[55]. This pro-inflammatory state in MASLD leads to abnormal microglial activation which mediates neuroinflammation[56]. Thus, IR promotes systemic low-grade inflammation, oxidative stress, and altered immune responses, all of which have been implicated in the pathophysiology of depression. Microglial activation has been implicated in the pathophysio
Type 2 diabetes mellitus (T2DM) and MASLD usually co-occur, with more than 70% of T2DM patients presenting with MASLD[60,61]. T2DM independently increases the risk of major depressive disorder through mechanisms involving systemic inflammation, HPA axis dysfunction, and microvascular brain injury[62,63]. A study found that the prevalence rates of depression are up to two-fold higher in diabetic patients, in comparison to the general population[38]. Another study found that the generalised anxiety disorder is present in 14% and elevated symptoms of anxiety is present in 40% of patients with type 2 diabetes[64].
Obesity: In obese individuals, adipose tissue undergoes a phenotypic switch to a proinflammatory state from an anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype[65]. Infiltration of M1 macrophages results in increased secretion of cytokines such as TNFα and IL6 which inhibit insulin signalling pathways[66]. Dysregulation of adipokines and elevated stem cell growth factor-beta (SCGF-β) levels also contribute to the pro-inflammatory state[67]. Obese individuals also exhibit significantly elevated levels of reactive oxygen species, which result in impaired insulin signaling, activation of pro-inflammatory pathways, endothelial dysfunction, and ultimately contribute to the develop
Obesity and depression also share a complex, bidirectional relationship. The same pathophysiological processes that underlie obesity and MASLD are increasingly recognized as contributing to the development of depression as well. Leptin resistance and low levels of adiponectin correlate not only with obesity but also with decreased central activity and increased neuroinflammation[70,71]. Resistin and visfatin are elevated in obesity and are linked to pro-inflammatory pathways and HPA axis activation, both implicated in the pathogenesis of psychiatric disorders[72,73].
In women, visceral fat accumulation more strongly mediates the MASLD-depression relationship than in men[37]. Estrogen plays a protective role by modulating gut microbiota composition, suppressing systemic inflammation, and maintaining blood-brain barrier integrity, which limits the impact of circulating cytokines on mood regulation[74-76]. Estrogen also attenuates HPA axis reactivity, which may explain reduced stress sensitivity in premenopausal females[77]. In contrast, low testosterone in males is associated with increased adiposity and altered adipokine signaling, but lacks the neuroprotective effects seen with estrogen[78].
Beyond biological mechanisms, psychosocial factors may also be a possible link between mood disorders and obesity[79]. Chronic stress levels stimulate HPA axis activation resulting in appetite dysregulation and weight gain[80]. This promotes abdominal fat accumulation accompanied by emotional eating as a coping mechanism to reduce stress and anxiety. Stigma related to obesity, encompassing both perceived discrimination and internalized bias, has been shown to affect overall psychological well-being[81]. This stigma independently contributes to depression and anxiety symptoms in obese individuals. Overweight adolescents and young adults especially girls experiencing body image dissatisfaction are at significantly increased risk for depressive and anxiety symptoms as well[82,83].
Poor sleep, attitude towards health and exercise: Sleep is a key pillar of both physical and mental health. For people living with MASLD, poor sleep is unfortunately all too common and often overlooked[84]. Many patients experience trouble falling or staying asleep, and this chronic disruption can intensify feelings of anxiety and depression[85-87]. Obstructive sleep apnea (OSA) is known to have a strong independent association with metabolic diseases especially MASLD[88-90]. The intermittent oxygen deprivation and sleep fragmentation seen in OSA can fuel systemic inflammation and metabolic stress, further worsening both liver function and mood regulation[91-95].
Research shows that poor sleep contributes to inflammation and affects how our brain handles emotions, which helps to explain why sleep issues are strongly linked to both depression and worsening liver health in MASLD[96,97]. One cohort study conducted by Um et al[98] found that both sleep duration and sleep quality were closely related to liver disease progression, highlighting how sleep can make a major difference. Thus, prioritizing sleep health could make a meaningful difference in the holistic management of MASLD, potentially reducing the associated psychiatric burden and improving overall quality of life.
Alongside sleep, daily habits like physical activity can also play a big role in shaping mental health[99-101]. Many individuals with MASLD find it difficult to stay active, often due to fatigue, joint pain, or simply low motivation that can come with chronic illness[102-106]. But this physical inactivity can quietly contribute to emotional struggles. Regular physical activity has been shown to lower systemic inflammation, enhance neuroplasticity, and improve stress resilience, all of which protect against anxiety and depressive symptoms[107-110]. When patients disengage from physical activity and adopt more passive lifestyles, it can worsen both their physical condition and mental resilience. Botacin et al[111] highlight the link between poor lifestyle habits and elevated rates of anxiety and depression among MASLD patients. Lifestyle interventions remain central to managing both MASLD and comorbid mood disorders. Keating et al[106] describe a 24-hour integrated behavioral model that includes structured physical activity, sleep hygiene, and circadian-aligned eating. Moderate-to-vigorous aerobic and resistance training can reduce hepatic steatosis and improve mood through lower inflammation levels[112]. High-intensity interval training can be particularly effective in improving MASLD-related biomarkers[113]. However, it might not be an effective strategy in people with mood disorders due to low adherence[114]. The Mediterranean diet, rich in fruits, vegetables, whole grains, olive oil, and fish, has shown promise for MASLD. It prevents liver fat deposition and reduces IR. Its benefits may be amplified when combined with regular physical activity[115]. A randomized controlled trial found significant reductions in depression, anxiety, and stress following Mediterranean diet instructions[114]. A combined lifestyle intervention significantly reduces cortisol levels and improves liver steatosis in MASLD patients. In addition to physical benefits, these interventions also support emotional well-being. Participating in group exercise alongside psychosocial support boosts motivation and improves mood by stimulating endorphin release[116].
While lifestyle changes are first-line, pharmacologic treatments may be useful. SSRIs can relieve depression without harming liver function, but require careful monitoring[117]. Anti-inflammatory and hepatoprotective agents like vitamin E, pioglitazone, and GLP-1 receptor agonists may benefit both liver and mental health[118,119]. More research is needed to confirm their safety and effectiveness in this context.
Sarcopenia: Sarcopenia is increasingly prevalent in individuals with metabolic dysfunction, especially those with obesity, type 2 diabetes, and advancing age. Emerging evidence has shown its bidirectional association with MASLD. A recent meta-analysis showed that patients with MASLD have a significantly higher risk of developing sarcopenia[44,120]. Sarcopenia contributes to systemic inflammation, reduced insulin sensitivity, and decreased skeletal muscle glucose uptake resulting in accelerated MASLD progression[121,122].
Sarcopenia is also strongly associated with depressive and anxiety symptoms[123,124]. A 2022 meta-analysis encompassing over 25000 individuals reported a pooled depression prevalence of 28% among sarcopenic patients, with sarcopenia conferring a 71% increased risk of depression compared to non-sarcopenic controls[125]. Another study discussed the prevalence of sarcopenia and depressive symptoms among older adults, highlighting shared risk factors such as advanced age, malnutrition, obesity, diabetes, hypertension, cognitive impairment, and polypharmacy[126]. Population-based studies from Korea and Australia demonstrate that sarcopenia is independently associated with elevated anxiety scores, particularly in middle-aged and older adults[127]. These findings suggest that sarcopenia not only contributes to the systemic inflammation and sedentary behavior implicated in MASLD but also plays a central role in the emotional and psychological vulnerability observed in this population.
Systemic inflammation: Systemic inflammation was found to be one of the major common links between the patho
Out of the neurobiological systems coordinated by neuroinflammation, dysregulation of the HPA axis and depletion of brain serotonin are the primary perpetrators for development of depression[17-20]. As mentioned earlier, MASLD may occur as a predictable outcome of obesity or even as a metabolic consequence of IR. Individuals that are overweight and obese have altered serum levels of proinflammatory cytokines such as TNF-α, C-reactive protein (CRP), IL (IL-6, IL-18)[132-134]. These cytokines are also crucial in the development of IR[55,66]. A vicious cycle exists between the inflammatory response in obesity and MASLD[135]. The severity of MASLD is exacerbated due to the failure of obesity induced inflammation to resolve[136]. What’s interesting to note is that, even though these same proinflammatory cytokines are definitely involved in the development of depression, some studies have proven that the association between MASLD and depression is independent of these metabolic and lifestyle risk factors such as obesity[25,34,137,138].
It is, however, worth mentioning that these metabolic risk factors accelerate vascular disease in MASLD patients which may result in brain atrophy[139-143]. Depression patients also show similar structural and functional neuroimaging findings namely, cortical thinning and reduced gray matter[144]. According to Colognesi et al[32], the significant reduc
Reduced levels of nesfatin-1 and copine-6-associated brain-derived neurotrophic factor levels have also been considered as a probable link between MASLD and depression[147].
HPA axis hyperactivation: Stress-induced activation of the HPA axis plays a central role in maintaining homeostasis, but chronic hyperactivation has been implicated in both liver pathology and mood disorders[12]. In the context of MASLD, metabolic factors including IR, obesity, and systemic inflammation result in the hyperactivation of the HPA axis. This results in sustained cortisol secretion, which further promotes hepatic gluconeogenesis, fat accumulation, and lack of insulin sensitivity, resulting in the progression of hepatic steatosis[148-150]. Although glucocorticoids are classically anti-inflammatory, their prolonged elevation paradoxically enhances lipid deposition in hepatocytes and impairs mitochon
Simultaneously, multiple studies have proven that hyperactivity of the HPA axis is a well-established hallmark in major depressive disorder, suggesting that this shared neuroendocrine dysfunction might serve as a pathophysiological bridge between MASLD and depression[152-154]. A study expanding on the ‘emotional nervous system’ explained how the body weight of stressed individuals may be influenced by the HPA axis through cortisol regulation[155]. Prolonged cortisol elevation leads to hippocampal shrinkage (as mineralocorticoid receptors are localized to the hippocampus in the brain and cortisol binds to high affinity with these receptors), disrupting synaptic plasticity, and impairing serotonergic and dopaminergic neurotransmission—contributing to core symptoms of depression[156-158]. This desensitization occurs in both hepatocytes and the brain, impairing cortisol’s regulatory effects on inflammation and HPA axis feedback. In hepatocytes, glucocorticoid receptor (GR) desensitization weakens cortisol’s ability to suppress inflammatory cytokines like IL-6 and TNF-α, worsening hepatic inflammation[159]. In the hippocampus, chronic cytokine exposure reduces GR sensitivity, disrupts negative feedback on the HPA axis, and leads to sustained cortisol release[160,161].
Figure 2 shows how chronic HPA axis activation in MASLD raises cortisol levels, which disrupt serotonin and dopamine signaling in areas like the hippocampus[148-150]. It also shows how cortisol worsens steatosis, enhances gluconeogenesis, and worsens IR, linking metabolic dysfunction to emotional and cognitive changes[151].
While current understanding is evolving, pharmacologic interventions targeting HPA axis dysregulation have been explored in mood disorders. A meta-analysis by Ding et al[27] showed that certain HPA-modulating agents, such as GR antagonists, may benefit patients with major depression. However, inconsistent results across trials highlight the need for focused, head-to-head studies exploring this pathway.
Neurotransmitter imbalances: The liver plays a key role in tryptophan metabolism through the enzyme tryptophan 2,3-dioxygenase (TDO), which shifts tryptophan away from serotonin synthesis[162]. In MASLD, liver inflammation and oxidative stress impair this balance[163]. TDO activity becomes dysregulated and indoleamine 2,3-dioxygenase is increasingly activated by cytokines like IL-6 and TNF-α[164,165]. This leads to reduced serotonin availability in the brain. The resulting imbalance may affect mood and cognition. Animal studies have also shown disrupted dopamine and GABA signaling in MASLD-related neuroinflammation[166].
While hepatic dysfunction alters tryptophan metabolism, studies also show how gut microbial products particularly short-chain fatty acids (SCFAs) can influence brain inflammation and mood regulation. SCFAs such as acetate, pro
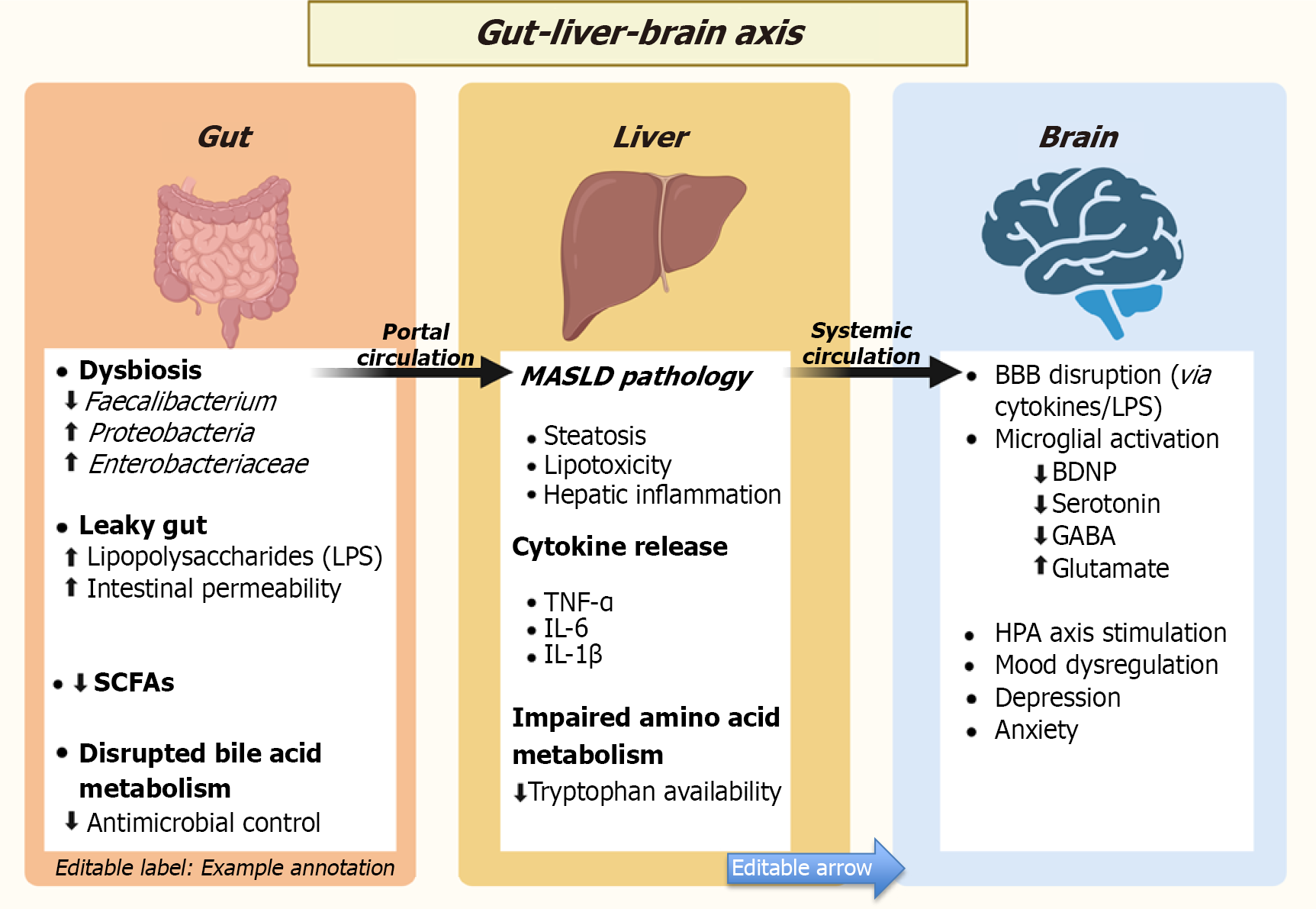
Apart from SCFAs, gut dysbiosis in MASLD can also affect tryptophan metabolism, which plays a key role in mood and brain inflammation. Under normal conditions, tryptophan is used as a precursor in serotonin synthesis in the brain. However in the context of MASLD, gut dysbiosis can push tryptophan into the kynurenine pathway[169]. This shift increases the production of neurotoxic metabolites such as quinolinic acid, which can cross the blood–brain barrier and promote oxidative stress, excitotoxicity, and microglial activation, factors known to contribute to depressive symptoms[170-174]. This shift is likely driven by gut dysbiosis in MASLD, which involves a loss of beneficial microbes and an overgrowth of pro-inflammatory ones. These microbial alterations are further detailed in the Gut dysbiosis section[175-178].
Gut dysbiosis: Emerging research continues to shed light on the intricate relationship between gut dysbiosis and MASLD, suggesting that disturbances in the gut microbiome might not only influence liver pathology but also contribute to mental health challenges. In individuals with MASLD, studies consistently report reduced microbial diversity, an increase in harmful pro-inflammatory bacteria such as Proteobacteria and Enterobacteriaceae, and a drop in beneficial species like Faecalibacterium prausnitzii[175,176]. Faecalibacterium prausnitzii supports gut health by producing butyrate, a SCFAs that reduces inflammation and maintains intestinal barrier integrity. In contrast, enterobacteriaceae are pro-inflammatory bacteria that release endotoxins like lipopolysaccharide (LPS), which trigger immune responses and worsen both hepatic and neural inflammation[176]. These changes are known to disrupt the intestinal barrier and trigger metabolic endotoxemia, which are the processes that aggravate inflammation and fat accumulation in the liver[179,180]. The disruption of bile acid metabolism in MASLD reduces microbial control, allowing harmful bacteria to thrive[181]. Combined with IR, which impairs gut integrity and immune responses, these factors significantly contribute to gut dysbiosis[182].
The disruption of bile acid metabolism in MASLD plays a more important role than it might initially seem. More than its role in aiding digestion, bile acids function as signaling molecules that help maintain microbial balance in the gut. Under healthy conditions, they exert antimicrobial effects that suppress the growth of harmful bacteria. In MASLD, bile acid synthesis and circulation become dysregulated, leading to an altered composition of the bile acid pool. This reduces their ability to regulate gut microbial populations, allowing pathogenic bacteria to grow invariably. One notable consequence is the overgrowth of gram-negative species such as Enterobacteriaceae, which can increase the release of endotoxins like LPS. These microbial products contribute to systemic inflammation and can exacerbate hepatic injury[181].
IR which is one of the hallmark features of MASLD further aggravates the problem. When insulin signaling is impaired, it affects more than just glucose and lipid metabolism. It also compromises gut epithelial integrity, increasing intestinal permeability, or what is commonly referred to as a “leaky gut”[182]. IR is also associated with impaired mucosal immunity, which reduces the host’s ability to control opportunistic pathogens. As a result, the combined effects of disrupted bile acid signaling and weakened immune defenses foster a persistent state of gut dysbiosis. Over time, this dysregulated gut–liver axis fuels a self-sustaining cycle of inflammation that drives disease progression and may even contribute to extrahepatic complications, including neuroinflammation[173,174].
But the gut’s influence doesn’t end with the liver. The gut-brain axis, a complex, two-way communication system between the central nervous system and the gastrointestinal tract is deeply influenced by the gut microbiota. Through the intricate neural, endocrine, and immune signaling pathways, these microbes can affect brain function and behavior[183-186]. For instance, Lactobacillus rhamnosus has been shown to reduce anxiety and depressive-like behavior in animal models through vagal nerve activation, highlighting the microbial impact on mood regulation[187]. Therefore, disrup
A recent study by Madabushi et al[28] highlights the promising role of gut health in improving mental well-being. Their findings suggest that interventions aimed at modulating the gut microbiota particularly through the use of probiotics may help alleviate symptoms of anxiety and depression. These benefits are thought to arise from probiotics’ ability to reduce systemic inflammation, restore microbial diversity and influence key neurochemical pathways, including the production of serotonin and gamma-aminobutyric acid. The review highlights the increasing recognition of the gut-brain axis as a therapeutic target, suggesting that mental health should not be treated in isolation from gut health. While more high-quality clinical trials are needed to define specific strains and dosages, the emerging evidence paints a hopeful picture of microbiome-focused approaches in psychiatric care.
Hence, targeting the gut microbiota through dietary interventions, probiotics, or even fecal microbiota transplants (FMT) may not only help manage MASLD directly but also ease some of the mental health difficulties that often accompany the condition. Recent studies suggest that modulating the gut environment can reduce inflammation, support neurotransmitter production, and influence mood-regulating pathways in the brain. For example, Radford-Smith and Anthony[189] describe how probiotics and prebiotics may help regulate neuroinflammation and improve emotional resilience by supporting the microbiota–gut–brain axis. Similarly, Vaghef-Mehrabany et al[190] throw light on growing evidence from randomized trials that specific strains of probiotics and synbiotics may offer measurable antidepressant effects, particularly when personalized according to the individual.
MASLD and mood disorders frequently coexist, yet there are no exclusive biomarkers available to track their combined progression. However, several overlapping markers may hold clinical value. Elevated CRP, IL-6, and TNF-α reflect the chronic inflammatory state shared by both conditions. The kynurenine-to-tryptophan ratio reflects shunting of trypto
Microbiota-targeted therapies show a promising direction towards a dual benefit. Probiotics and FMT have shown modest improvements in hepatic inflammation, steatosis, and mood symptoms. These effects are thought to occur through restoration of SCFAs production, enhanced gut barrier function, and improved serotonin signaling. The HPA axis remains another emerging therapeutic target. Since the HPA axis plays a role in both MASLD and depression, drugs like mifepristone that were mentioned earlier could be explored in future dual-targeted trials. Looking ahead, multi-omic studies are essential to understand the gut-liver-brain axis in more detail. Recent work links glutamate-producing microbes like Anaerotruncus colihominis to mood symptoms in metabolic disease[193,194]. Trials should also consider sex-specific factors like gut composition, hormonal regulation, and immune response. Alongside microbial and pharmacologic options, lifestyle changes remain crucial. Regular physical activity improves liver fat and reduces depressive symptoms by regulating stress pathways. Anti-inflammatory diets can enhance both mood and metabolic outcomes by supporting glucose control, lowering inflammation, and restoring gut microbial balance. A holistic care model including early psychiatric screening and regular monitoring using overlapping biomarkers will be beneficial. Dual-targeted treatment strategies addressing both liver and mental health may offer the most effective approach for improving long-term outcomes.
| 1. | Boccatonda A, Andreetto L, D'Ardes D, Cocco G, Rossi I, Vicari S, Schiavone C, Cipollone F, Guagnano MT. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines. 2023;11:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 2. | Mastromauro C, Polidori N, Giannini C. Metabolic dysfunction-associated fatty liver disease in obese youth with insulin resistance and type 2 diabetes. Curr Opin Pediatr. 2022;34:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1658] [Cited by in RCA: 1786] [Article Influence: 595.3] [Reference Citation Analysis (0)] |
| 4. | Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 756] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 5. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1458] [Article Influence: 364.5] [Reference Citation Analysis (1)] |
| 6. | De Roza MA, Goh GB. The increasing clinical burden of NAFLD in Asia. Lancet Gastroenterol Hepatol. 2019;4:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Wen W, Fan H, Zhang S, Hu S, Chen C, Tang J, You Y, Wang C, Li J, Luo L, Cheng Y, Zhou M, Zhao X, Tan T, Xu F, Fu X, Chen J, Dong P, Zhang X, Wang M, Feng Y. Associations between metabolic dysfunction-associated fatty liver disease and atherosclerotic cardiovascular disease. Am J Med Sci. 2024;368:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Li AA, Ahmed A, Kim D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14:168-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Velarde-Ruiz Velasco JA, García-Jiménez ES, García-Zermeño KR, Morel-Cerda EC, Aldana-Ledesma JM, Castro-Narro GE, Cerpa-Cruz S, Tapia-Calderón DK, Mercado-Jauregui LA, Contreras-Omaña R. Extrahepatic complications of non-alcoholic fatty liver disease: Its impact beyond the liver. Rev Gastroenterol Mex (Engl Ed). 2019;84:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Chacko KR, Reinus J. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Choi JM, Chung GE, Kang SJ, Kwak MS, Yang JI, Park B, Yim JY. Association Between Anxiety and Depression and Nonalcoholic Fatty Liver Disease. Front Med (Lausanne). 2020;7:585618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Shea S, Lionis C, Kite C, Atkinson L, Chaggar SS, Randeva HS, Kyrou I. Non-Alcoholic Fatty Liver Disease (NAFLD) and Potential Links to Depression, Anxiety, and Chronic Stress. Biomedicines. 2021;9:1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Youssef NA, Abdelmalek MF, Binks M, Guy CD, Omenetti A, Smith AD, Diehl AM, Suzuki A. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Shea S, Lionis C, Kite C, Lagojda L, Uthman OA, Dallaway A, Atkinson L, Chaggar SS, Randeva HS, Kyrou I. Non-alcoholic fatty liver disease and coexisting depression, anxiety and/or stress in adults: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024;15:1357664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Sharpton SR, Ajmera V, Loomba R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol. 2019;17:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 16. | Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry. 2021;78:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 501] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 17. | Richardson B, MacPherson A, Bambico F. Neuroinflammation and neuroprogression in depression: Effects of alternative drug treatments. Brain Behav Immun Health. 2022;26:100554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 18. | Tapp ZM, Godbout JP, Kokiko-Cochran ON. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front Neurol. 2019;10:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, Zhao S, Song C. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res. 2019;356:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V. Neuroinflammation and depression: A review. Eur J Neurosci. 2021;53:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 765] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 21. | Frank P, Jokela M, Batty GD, Cadar D, Steptoe A, Kivimäki M. Association Between Systemic Inflammation and Individual Symptoms of Depression: A Pooled Analysis of 15 Population-Based Cohort Studies. Am J Psychiatry. 2021;178:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 22. | Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 553] [Article Influence: 79.0] [Reference Citation Analysis (1)] |
| 23. | Xiao J, Lim LKE, Ng CH, Tan DJH, Lim WH, Ho CSH, Tan EXX, Sanyal AJ, Muthiah MD. Is Fatty Liver Associated With Depression? A Meta-Analysis and Systematic Review on the Prevalence, Risk Factors, and Outcomes of Depression and Non-alcoholic Fatty Liver Disease. Front Med (Lausanne). 2021;8:691696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Liu Q, Han M, Li M, Huang X, Feng R, Li W, Chen J, He H, Zheng W, Hu Z, Du S, Ye W. Shift in prevalence and systemic inflammation levels from NAFLD to MAFLD: a population-based cross-sectional study. Lipids Health Dis. 2023;22:185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 25. | Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 857] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 26. | Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry. 2020;20:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 27. | Ding Y, Wei Z, Yan H, Guo W. Efficacy of Treatments Targeting Hypothalamic-Pituitary-Adrenal Systems for Major Depressive Disorder: A Meta-Analysis. Front Pharmacol. 2021;12:732157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Madabushi JS, Khurana P, Gupta N, Gupta M. Gut Biome and Mental Health: Do Probiotics Work? Cureus. 2023;15:e40293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1991] [Article Influence: 663.7] [Reference Citation Analysis (3)] |
| 30. | Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, Nader F, Younossi ZM. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Tomeno W, Kawashima K, Yoneda M, Saito S, Ogawa Y, Honda Y, Kessoku T, Imajo K, Mawatari H, Fujita K, Saito S, Hirayasu Y, Nakajima A. Non-alcoholic fatty liver disease comorbid with major depressive disorder: The pathological features and poor therapeutic efficacy. J Gastroenterol Hepatol. 2015;30:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Colognesi M, Gabbia D, De Martin S. Depression and Cognitive Impairment-Extrahepatic Manifestations of NAFLD and NASH. Biomedicines. 2020;8:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Goulart AC, Bianchi LLT, Bismarchi D, Miname MH, Lourenção ACM, Henares BB, Garcia AT, de Almeida MS, Machado TAO, Syllos DH, Rienzo M, Wang YP. Sex differences in the relationship between hepatic steatosis, mood and anxiety disorders. J Psychosom Res. 2023;168:111216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Labenz C, Huber Y, Michel M, Nagel M, Galle PR, Kostev K, Schattenberg JM. Nonalcoholic Fatty Liver Disease Increases the Risk of Anxiety and Depression. Hepatol Commun. 2020;4:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Brodosi L, Stecchi M, Musio A, Bazzocchi M, Risi E, Marchignoli F, Marchesini G, Petroni ML. Anxiety and depression in metabolic-associated steatotic liver disease: relation with socio-demographic features and liver disease severity. Acta Diabetol. 2024;61:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Jung JY, Park SK, Oh CM, Chung PW, Ryoo JH. Non-Alcoholic Fatty Liver Disease and Its Association with Depression in Korean General Population. J Korean Med Sci. 2019;34:e199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | He C, Zhou L, Gao T, Cao R, Cai C, Jiang G. Sex differences in the mediation of the MASLD - Depression association by fat distribution in U.S. adults. Acta Psychol (Amst). 2025;256:105041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142 Suppl:S8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 805] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 39. | Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull. 2017;143:783-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1293] [Cited by in RCA: 1620] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 40. | Liang W, Zhong K, Lai T, Zeng Y, Huang Z, Zhou J, Huang J, Shi Z, Zhang J, Ding F. Causal relationship between depression and metabolic dysfunction-associated steatotic liver disease: a bidirectional Mendelian randomized study. Front Psychiatry. 2024;15:1384003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Zhou X, Liao J, Liu L, Meng Y, Yang D, Zhang X, Long L. Association of depression with severe non-alcoholic fatty liver disease: evidence from the UK Biobank study and Mendelian randomization analysis. Sci Rep. 2024;14:28561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Noon SL, D'Annibale DA, Schwimmer MH, Shiels J, Arin J, Durelle J, Newton KP, Goyal NP, Schwimmer JB. Incidence of Depression and Anxiety in a Cohort of Adolescents With Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2021;72:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Weinstein AA, De Avila L, Kannan S, Paik JM, Golabi P, Gerber LH, Younossi ZM. Interrelationship between physical activity and depression in nonalcoholic fatty liver disease. World J Hepatol. 2022;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Wong R, Yuan LY. Sarcopenia and metabolic dysfunction associated steatotic liver disease: Time to address both. World J Hepatol. 2024;16:871-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 45. | Iwaki M, Kobayashi T, Nogami A, Saito S, Nakajima A, Yoneda M. Impact of Sarcopenia on Non-Alcoholic Fatty Liver Disease. Nutrients. 2023;15:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 46. | Gao K, Ma WZ, Huck S, Li BL, Zhang L, Zhu J, Li T, Zhou D. Association Between Sarcopenia and Depressive Symptoms in Chinese Older Adults: Evidence From the China Health and Retirement Longitudinal Study. Front Med (Lausanne). 2021;8:755705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Paschou SA, Polyzos SA, Anagnostis P, Goulis DG, Kanaka-Gantenbein C, Lambrinoudaki I, Georgopoulos NA, Vryonidou A. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Endocrine. 2020;67:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Arvanitakis K, Chatzikalil E, Kalopitas G, Patoulias D, Popovic DS, Metallidis S, Kotsa K, Germanidis G, Koufakis T. Metabolic Dysfunction-Associated Steatotic Liver Disease and Polycystic Ovary Syndrome: A Complex Interplay. J Clin Med. 2024;13:4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Gnawali A, Patel V, Cuello-Ramírez A, Al Kaabi AS, Noor A, Rashid MY, Henin S, Mostafa JA. Why are Women With Polycystic Ovary Syndrome at Increased Risk of Depression? Exploring the Etiological Maze. Cureus. 2021;13:e13489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 50. | Cho IY, Chang Y, Sung E, Kang JH, Wild SH, Byrne CD, Shin H, Ryu S. Depression and increased risk of non-alcoholic fatty liver disease in individuals with obesity. Epidemiol Psychiatr Sci. 2021;30:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024;59 Suppl 1:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 52. | Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12 Suppl 2:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 554] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 53. | Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jenö P, Beglinger C, Peterli R, Hall MN. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 54. | Maldonado-Rojas ADC, Zuarth-Vázquez JM, Uribe M, Barbero-Becerra VJ. Insulin resistance and Metabolic dysfunction-associated steatotic liver disease (MASLD): Pathways of action of hypoglycemic agents. Ann Hepatol. 2024;29:101182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 55. | Khanna D, Khanna S, Khanna P, Kahar P, Patel BM. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus. 2022;14:e22711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 56. | Woelfer M, Kasties V, Kahlfuss S, Walter M. The Role of Depressive Subtypes within the Neuroinflammation Hypothesis of Major Depressive Disorder. Neuroscience. 2019;403:93-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. 2020;107:234-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 1502] [Article Influence: 250.3] [Reference Citation Analysis (0)] |
| 58. | Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 59. | Won E, Kim YK. Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int J Mol Sci. 2020;21:6546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 60. | Ajmera V, Cepin S, Tesfai K, Hofflich H, Cadman K, Lopez S, Madamba E, Bettencourt R, Richards L, Behling C, Sirlin CB, Loomba R. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 61. | Leith D, Lin YY, Brennan P. Metabolic Dysfunction-associated Steatotic Liver Disease and Type 2 Diabetes: A Deadly Synergy. touchREV Endocrinol. 2024;20:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 62. | Bădescu SV, Tătaru C, Kobylinska L, Georgescu EL, Zahiu DM, Zăgrean AM, Zăgrean L. The association between Diabetes mellitus and Depression. J Med Life. 2016;9:120-125. [PubMed] |
| 63. | Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1998] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 64. | Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 394] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 65. | Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 66. | Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 434] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 67. | Tarantino G, Citro V, Balsano C, Capone D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics (Basel). 2020;10:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 68. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3923] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 69. | Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 500] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 70. | Fu X, Wang Y, Zhao F, Cui R, Xie W, Liu Q, Yang W. Shared biological mechanisms of depression and obesity: focus on adipokines and lipokines. Aging (Albany NY). 2023;15:5917-5950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 71. | Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, Silva-Pereyra J. Structural Brain Changes Associated with Overweight and Obesity. J Obes. 2021;2021:6613385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 72. | Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1510] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 73. | Zhao F, Li B, Yang W, Ge T, Cui R. Brain-immune interaction mechanisms: Implications for cognitive dysfunction in psychiatric disorders. Cell Prolif. 2022;55:e13295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 74. | Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 654] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 75. | Maggioli E, McArthur S, Mauro C, Kieswich J, Kusters DHM, Reutelingsperger CPM, Yaqoob M, Solito E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. 2016;51:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (10)] |
| 76. | Sohrabji F. Guarding the blood-brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr. 2007;13:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | De Nicola AF, Saravia FE, Beauquis J, Pietranera L, Ferrini MG. Estrogens and neuroendocrine hypothalamic-pituitary-adrenal axis function. Front Horm Res. 2006;35:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 78. | Khodamoradi K, Khosravizadeh Z, Seetharam D, Mallepalli S, Farber N, Arora H. The role of leptin and low testosterone in obesity. Int J Impot Res. 2022;34:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 79. | Cifuentes L, Campos A, Silgado MLR, Kelpin S, Stutzman J, Hurtado MD, Grothe K, Hensrud DD, Clark MM, Acosta A. Association between anxiety and eating behaviors in patients with obesity. Obes Pillars. 2022;3:100021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 439] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 81. | Carr D, Friedman MA. Is obesity stigmatizing? Body weight, perceived discrimination, and psychological well-being in the United States. J Health Soc Behav. 2005;46:244-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 82. | Soares Filho LC, Batista RFL, Cardoso VC, Simões VMF, Santos AM, Coelho SJDDAC, Silva AAM. Body image dissatisfaction and symptoms of depression disorder in adolescents. Braz J Med Biol Res. 2020;54:e10397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Sharpe H, Patalay P, Choo TH, Wall M, Mason SM, Goldschmidt AB, Neumark-Sztainer D. Bidirectional associations between body dissatisfaction and depressive symptoms from adolescence through early adulthood. Dev Psychopathol. 2018;30:1447-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 84. | Bu LF, Xiong CY, Zhong JY, Xiong Y, Li DM, Hong FF, Yang SL. Non-alcoholic fatty liver disease and sleep disorders. World J Hepatol. 2024;16:304-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 85. | Chang X, Guo C, Zhou H, Liu L. Impact of rumination on sleep quality among patients with nonalcoholic fatty liver disease: a moderated mediation model of anxiety symptoms and resilience. BMC Psychiatry. 2023;23:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 86. | Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014;37:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 87. | Yasugaki S, Okamura H, Kaneko A, Hayashi Y. Bidirectional relationship between sleep and depression. Neurosci Res. 2025;211:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 92] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 88. | Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 616] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 89. | Huang J, Chen L, Li X, Chen M, Lin T, Chen G. Association Between Metabolic-Associated Fatty Liver Disease and Obstructive Sleep Apnea: A Cross-Sectional Study. Nat Sci Sleep. 2023;15:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 90. | Li M, Li X, Lu Y. Obstructive Sleep Apnea Syndrome and Metabolic Diseases. Endocrinology. 2018;159:2670-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 92. | Hayley AC, Williams LJ, Venugopal K, Kennedy GA, Berk M, Pasco JA. The relationships between insomnia, sleep apnoea and depression: findings from the American National Health and Nutrition Examination Survey, 2005-2008. Aust N Z J Psychiatry. 2015;49:156-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 93. | Grandner MA, Malhotra A. Connecting insomnia, sleep apnoea and depression. Respirology. 2017;22:1249-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Hobzova M, Prasko J, Vanek J, Ociskova M, Genzor S, Holubova M, Grambal A, Latalova K. Depression and obstructive sleep apnea. Neuro Endocrinol Lett. 2017;38:343-352. [PubMed] |
| 95. | Yamamura S, Nakano D, Hashida R, Tsutsumi T, Kawaguchi T, Okada M, Isoda H, Takahashi H, Matsuse H, Eguchi Y, Sumida Y, Nakajima A, Gerber L, Younossi ZM, Torimura T. Patient-reported outcomes in patients with non-alcoholic fatty liver disease: A narrative review of Chronic Liver Disease Questionnaire-non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2021;36:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Veler H. Sleep and Inflammation: Bidirectional Relationship. Sleep Med Clin. 2023;18:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 97. | Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 627] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 98. | Um YJ, Chang Y, Jung HS, Cho IY, Shin JH, Shin H, Wild SH, Byrne CD, Ryu S. Sleep Duration, Sleep Quality, and the Development of Nonalcoholic Fatty Liver Disease: A Cohort Study. Clin Transl Gastroenterol. 2021;12:e00417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 99. | Casanova F, O'Loughlin J, Karageorgiou V, Beaumont RN, Bowden J, Wood AR, Tyrrell J. Effects of physical activity and sedentary time on depression, anxiety and well-being: a bidirectional Mendelian randomisation study. BMC Med. 2023;21:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 100. | Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 101. | Philippot A, Dubois V, Lambrechts K, Grogna D, Robert A, Jonckheer U, Chakib W, Beine A, Bleyenheuft Y, De Volder AG. Impact of physical exercise on depression and anxiety in adolescent inpatients: A randomized controlled trial. J Affect Disord. 2022;301:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 102. | von Loeffelholz C, Roth J, Coldewey SM, Birkenfeld AL. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines. 2021;9:1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Wang S, Xia BX, Luo T, Wang P. Association between physical activity and diet quality of obese and non-obese MAFLD. Nutr Metab Cardiovasc Dis. 2024;34:75-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Li M. Association of physical activity with MAFLD/MASLD and LF among adults in NHANES, 2017-2020. Wien Klin Wochenschr. 2024;136:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 105. | Huang J, Wu Y, Zheng J, Wang M, Goh GB, Lin S. The prognostic role of diet quality in patients with MAFLD and physical activity: data from NHANES. Nutr Diabetes. 2024;14:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 106. | Keating SE, Chawla Y, De A, George ES. Lifestyle intervention for metabolic dysfunction-associated fatty liver disease: a 24-h integrated behavior perspective. Hepatol Int. 2024;18:959-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 107. | Hamer M, Endrighi R, Poole L. Physical activity, stress reduction, and mood: insight into immunological mechanisms. Methods Mol Biol. 2012;934:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Kandola A, Vancampfort D, Herring M, Rebar A, Hallgren M, Firth J, Stubbs B. Moving to Beat Anxiety: Epidemiology and Therapeutic Issues with Physical Activity for Anxiety. Curr Psychiatry Rep. 2018;20:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 109. | Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 771] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 110. | Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. 2016;73:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 111. | Botacin EC, Duarte SMB, Stefano JT, Barbosa MED, Pessoa MG, Oliveira CP. Association Between Anxiety and Depression in Metabolic Dysfunction-Associated Steatohepatitis (MASLD). Arq Gastroenterol. 2024;61:e23128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Thorp A, Stine JG. Exercise as Medicine: The Impact of Exercise Training on Nonalcoholic Fatty Liver Disease. Curr Hepatol Rep. 2020;19:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 113. | Abassi W, Ouerghi N, Hammami MB, Jebabli N, Feki M, Bouassida A, Weiss K, Knechtle B. High-Intensity Interval Training Reduces Liver Enzyme Levels and Improves MASLD-Related Biomarkers in Overweight/Obese Girls. Nutrients. 2025;17:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 114. | Radkhah N, Rasouli A, Majnouni A, Eskandari E, Parastouei K. The effect of Mediterranean diet instructions on depression, anxiety, stress, and anthropometric indices: A randomized, double-blind, controlled clinical trial. Prev Med Rep. 2023;36:102469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 115. | Sualeheen A, Tan SY, Georgousopoulou E, Daly RM, Tierney AC, Roberts SK, George ES. Mediterranean diet for the management of metabolic dysfunction-associated steatotic liver disease in non-Mediterranean, Western countries: What's known and what's needed? Nutr Bull. 2024;49:444-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 116. | Bagnato CB, Bianco A, Bonfiglio C, Franco I, Verrelli N, Carella N, Shahini E, Zappimbulso M, Giannuzzi V, Pesole PL, Ancona A, Giannelli G. Healthy Lifestyle Changes Improve Cortisol Levels and Liver Steatosis in MASLD Patients: Results from a Randomized Clinical Trial. Nutrients. 2024;16:4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 117. | Sahoo S, Mishra E, Premkumar M. Antidepressants in People With Chronic Liver Disease and Depression: When Are They Warranted and How to Choose the Suitable One? J Clin Exp Hepatol. 2024;14:101390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 118. | Gu Y, Sun L, He Y, Yang L, Deng C, Zhou R, Kong T, Zhang W, Chen Y, Li J, Shi J. Comparative efficacy of glucagon-like peptide 1 (GLP-1) receptor agonists, pioglitazone and vitamin E for liver histology among patients with nonalcoholic fatty liver disease: systematic review and pilot network meta-analysis of randomized controlled trials. Expert Rev Gastroenterol Hepatol. 2023;17:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 119. | Alharbi SH. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther Adv Endocrinol Metab. 2024;15:20420188231222367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 170] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 120. | Li X, He J, Sun Q. The prevalence and effects of sarcopenia in patients with metabolic dysfunction-associated steatotic liver disease (MASLD): A systematic review and meta-analysis. Clin Nutr. 2024;43:2005-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 121. | Welch N, Attaway A, Bellar A, Alkhafaji H, Vural A, Dasarathy S. Compound Sarcopenia in Hospitalized Patients with Cirrhosis Worsens Outcomes with Increasing Age. Nutrients. 2021;13:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 122. | Jo IH, Song DS, Chang UI, Yang JM. Change in skeletal muscle mass is associated with hepatic steatosis in nonalcoholic fatty liver disease. Sci Rep. 2023;13:6920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 123. | Szlejf C, Suemoto CK, Brunoni AR, Viana MC, Moreno AB, Matos SMA, Lotufo PA, Benseñor IM. Depression is Associated With Sarcopenia Due to Low Muscle Strength: Results From the ELSA-Brasil Study. J Am Med Dir Assoc. 2019;20:1641-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | West EC, Williams LJ, Corney KB, Pasco JA. Is sarcopenia associated with anxiety symptoms and disorders? A systematic review and meta-analysis protocol. BMJ Open. 2021;11:e054125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Li Z, Tong X, Ma Y, Bao T, Yue J. Prevalence of depression in patients with sarcopenia and correlation between the two diseases: systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:128-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 126. | Ganggaya KS, Vanoh D, Ishak WRW. Prevalence of sarcopenia and depressive symptoms among older adults: a scoping review. Psychogeriatrics. 2024;24:473-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 127. | An HE, Park HS, Yin CS. Association of sarcopenia with generalized anxiety disorder in Korean middle-aged and older adults: Results from the Korea National Health and Nutrition Examination Survey in 2022. PLoS One. 2025;20:e0323772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 128. | Rafaqat S, Gluscevic S, Mercantepe F, Rafaqat S, Klisic A. Interleukins: Pathogenesis in Non-Alcoholic Fatty Liver Disease. Metabolites. 2024;14:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 129. | Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 691] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 130. | Pipitone RM, Ciccioli C, Infantino G, La Mantia C, Parisi S, Tulone A, Pennisi G, Grimaudo S, Petta S. MAFLD: a multisystem disease. Ther Adv Endocrinol Metab. 2023;14:20420188221145549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 131. | Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, Yang J. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation. 2022;19:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 369] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 132. | McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 335] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 133. | Festa A, D'Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. 2001;25:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 513] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 134. | Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 739] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 135. | Szukiewicz D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int J Mol Sci. 2023;24:9818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 136. | Luo Y, Lin H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun Inflamm Dis. 2021;9:59-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 137. | Grygiel-Górniak B, Limphaibool N, Puszczewicz M. Cytokine secretion and the risk of depression development in patients with connective tissue diseases. Psychiatry Clin Neurosci. 2019;73:302-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Kim D, Dennis BB, Cholankeril G, Ahmed A. Association between depression and metabolic dysfunction-associated fatty liver disease/significant fibrosis. J Affect Disord. 2023;329:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 139. | Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306-13324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 140. | Khanna S, Parikh NS, VanWagner LB. Fatty liver and cerebrovascular disease: plausible association and possible mechanisms. Curr Opin Lipidol. 2022;33:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Tiehuis AM, van der Graaf Y, Mali WP, Vincken K, Muller M, Geerlings MI; SMART Study Group. Metabolic syndrome, prediabetes, and brain abnormalities on mri in patients with manifest arterial disease: the SMART-MR study. Diabetes Care. 2014;37:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 142. | Lombardi R, Fargion S, Fracanzani AL. Brain involvement in non-alcoholic fatty liver disease (NAFLD): A systematic review. Dig Liver Dis. 2019;51:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 143. | Bizino MB, Sala ML, de Heer P, van der Tol P, Smit JW, Webb AG, de Roos A, Lamb HJ. MR of multi-organ involvement in the metabolic syndrome. Magn Reson Imaging Clin N Am. 2015;23:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 144. | Han KM, Ham BJ. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J Clin Neurol. 2021;17:503-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 145. | Filipović B, Marković O, Đurić V, Filipović B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can J Gastroenterol Hepatol. 2018;2018:9638797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 146. | Ntona S, Papaefthymiou A, Kountouras J, Gialamprinou D, Kotronis G, Boziki M, Polyzos SA, Tzitiridou M, Chatzopoulos D, Thavayogarajah T, Gkolia I, Ntonas G, Vardaka E, Doulberis M. Impact of nonalcoholic fatty liver disease-related metabolic state on depression. Neurochem Int. 2023;163:105484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 147. | Ari M, Ozturk OH, Bez Y, Oktar S, Erduran D. High plasma nesfatin-1 level in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 148. | Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 149. | Kang D, Zhao D, Ryu S, Guallar E, Cho J, Lazo M, Shin H, Chang Y, Sung E. Perceived stress and non-alcoholic fatty liver disease in apparently healthy men and women. Sci Rep. 2020;10:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 150. | Kang D, Zhao D, Ryu S, Guallar E, Cho J, Lazo M, Shin H, Chang Y, Sung E. Author Correction: Perceived stress and non-alcoholic fatty liver disease in apparently healthy men and women. Sci Rep. 2020;10:21978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 151. | Demori I, Grasselli E. The Role of the Stress Response in Metabolic Dysfunction-Associated Fatty Liver Disease: A Psychoneuroendocrineimmunology-Based Perspective. Nutrients. 2023;15:795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 152. | Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and Major Depressive Disorder-Translating Findings From Humans to Animal Models and Back. Front Psychiatry. 2019;10:974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 153. | Varghese FP, Brown ES. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 154. | Almeida FB, Pinna G, Barros HMT. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int J Mol Sci. 2021;22:5495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 155. | Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 532] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 156. | Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 649] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 157. | Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1412] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 158. | Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 159. | Marino JS, Stechschulte LA, Stec DE, Nestor-Kalinoski A, Coleman S, Hinds TD Jr. Glucocorticoid Receptor β Induces Hepatic Steatosis by Augmenting Inflammation and Inhibition of the Peroxisome Proliferator-activated Receptor (PPAR) α. J Biol Chem. 2016;291:25776-25788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 160. | Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 161. | Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 162. | Klaessens S, Stroobant V, De Plaen E, Van den Eynde BJ. Systemic tryptophan homeostasis. Front Mol Biosci. 2022;9:897929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 163. | Zhou Q, Shi Y, Chen C, Wu F, Chen Z. A narrative review of the roles of indoleamine 2,3-dioxygenase and tryptophan-2,3-dioxygenase in liver diseases. Ann Transl Med. 2021;9:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 164. | Tan Q, Deng S, Xiong L. Role of Kynurenine and Its Derivatives in Liver Diseases: Recent Advances and Future Clinical Perspectives. Int J Mol Sci. 2025;26:968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 165. | Huang X, Sang Z, Liu H, Yang J, Wang K, Qu Y, Deng H. From nonalcoholic fatty liver disease to neuroinflammation: the role of chronic systemic inflammation. Metab Brain Dis. 2025;40:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 166. | Custodio RJP, Hobloss Z, Myllys M, Hassan R, González D, Reinders J, Bornhorst J, Weishaupt AK, Seddek AL, Abbas T, Friebel A, Hoehme S, Getzmann S, Hengstler JG, van Thriel C, Ghallab A. Cognitive Functions, Neurotransmitter Alterations, and Hippocampal Microstructural Changes in Mice Caused by Feeding on Western Diet. Cells. 2023;12:2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 167. | Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 752] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 168. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 3198] [Article Influence: 456.9] [Reference Citation Analysis (2)] |
| 169. | Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1385] [Article Influence: 106.5] [Reference Citation Analysis (2)] |
| 170. | Savitz J. Role of Kynurenine Metabolism Pathway Activation in Major Depressive Disorders. Curr Top Behav Neurosci. 2017;31:249-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 171. | el-Defrawy SR, Boegman RJ, Jhamandas K, Beninger RJ. The neurotoxic actions of quinolinic acid in the central nervous system. Can J Physiol Pharmacol. 1986;64:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 172. | Bansal Y, Singh R, Sodhi RK, Khare P, Dhingra R, Dhingra N, Bishnoi M, Kondepudi KK, Kuhad A. Kynurenine monooxygenase inhibition and associated reduced quinolinic acid reverses depression-like behaviour by upregulating Nrf2/ARE pathway in mouse model of depression: In-vivo and In-silico studies. Neuropharmacology. 2022;215:109169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 173. | Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, McCormick B, Sampson TR, Alam A, Ye K. Gut microbiota regulate Alzheimer's disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71:2233-2252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 174. | Baizabal-Carvallo JF, Alonso-Juarez M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson's Disease. Neuroscience. 2020;432:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 175. | Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 440] [Article Influence: 48.9] [Reference Citation Analysis (2)] |
| 176. | Grabherr F, Grander C, Effenberger M, Adolph TE, Tilg H. Gut Dysfunction and Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne). 2019;10:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 177. | Kumar A, Pramanik J, Goyal N, Chauhan D, Sivamaruthi BS, Prajapati BG, Chaiyasut C. Gut Microbiota in Anxiety and Depression: Unveiling the Relationships and Management Options. Pharmaceuticals (Basel). 2023;16:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 178. | Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1598] [Article Influence: 122.9] [Reference Citation Analysis (0)] |
| 179. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 180. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 801] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 181. | Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 992] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 182. | Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 183. | Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014;25:49-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 184. | Chattopadhyay S, Malayil L. Editorial: Reviews in microbiome in health & disease. Front Cell Infect Microbiol. 2024;14:1423386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 185. | Xiong RG, Li J, Cheng J, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, Gan RY, Li HB. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients. 2023;15:3258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 186. | Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490-15496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 673] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 187. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050-16055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2805] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 188. | Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology. 2017;65:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 189. | Radford-Smith DE, Anthony DC. Prebiotic and Probiotic Modulation of the Microbiota-Gut-Brain Axis in Depression. Nutrients. 2023;15:1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 190. | Vaghef-Mehrabany E, Maleki V, Behrooz M, Ranjbar F, Ebrahimi-Mameghani M. Can psychobiotics "mood" ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin Nutr. 2020;39:1395-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 191. | Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 2698] [Article Influence: 269.8] [Reference Citation Analysis (0)] |
| 192. | Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, Gomes-da-Costa S, Lane M, Sanches M, Diaz AP, Tseng PT, Lin PY, Berk M, Clarke G, O'Neil A, Jacka F, Stubbs B, Carvalho AF, Quevedo J, Soares JC, Fernandes BS. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol Psychiatry. 2021;26:4158-4178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 193. | Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu C, Huang H, Zhen H, Xi C, Sun Y, Wu L, Wang L, Gao X, Li Y, Fu Y, Jie Z, Li S, Zhang D, Chen Y, Zhu Y, Lu S, Lu J, Wang D, Zhou H, Yuan X, Li X, Pang L, Huang M, Yang H, Zhang W, Brix S, Kristiansen K, Song X, Nie C, Hu S. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psychiatry. 2022;27:4123-4135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 194. | Chang Z, Zhu Y, Wang P, Du L, Wu M, Wang X, Kong C, Huang D, Xie R, Ji G, Wang C, Cheng L, Yan X, Wei Q, Qin H. Multi-omic analyses of the development of obesity-related depression linked to the gut microbe Anaerotruncus colihominis and its metabolite glutamate. Sci Bull (Beijing). 2025;70:1822-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/