Published online Sep 22, 2025. doi: 10.4291/wjgp.v16.i3.107273
Revised: April 30, 2025
Accepted: August 1, 2025
Published online: September 22, 2025
Processing time: 184 Days and 6.2 Hours
Barrett’s esophagus is a pathological process where the inflammatory milieu created within the esophagus leads to progressive changes over time that can lead eventually to frank malignancy. It is a pre-malignant condition and involves a metaplastic transformation of the distal epithelium of the esophagus. There is a conversion of the normal type of squamous epithelium into the columnar type of epithelium. There are several risk factors associated with this condition and it is typically diagnosed endoscopically. This review article provides a brief overview of this condition.
Core Tip: Barrett’s esophagus is a clinical condition that is diagnosed endoscopically and is a premalignant condition. It involves the metaplastic transformation of the lining of the esophagus due to various risk factors. This review describes the pathogenesis as well as diagnosis and management.
- Citation: Chela HK, Gandhi M, Hazique M, Ertugrul H, Gangu K, Daglilar E. Barrett’s esophagus: A review. World J Gastrointest Pathophysiol 2025; 16(3): 107273
- URL: https://www.wjgnet.com/2150-5330/full/v16/i3/107273.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i3.107273
Over the years, chronic inflammation has been recognized as an underlying key component of many disease states. Barrett’s esophagus (BE) is a pathological process where the inflammatory milieu created within the esophagus leads to progressive changes over time that can lead eventually to frank malignancy. It is a pre-malignant condition that is enco
This condition is named after Norman Rupert Barrett who was a London based surgeon at a hospital called St. Thomas. In 1950, he described ulcerations lined by columnar type epithelium in the distal portion of what we know to be the esophagus. Barrett argued that because the esophagus, by definition, must be lined by squamous epithelium, the ulce
This review article provides a brief synopsis of the epidemiology, pathogenesis, clinical features, diagnosis, and mana
The criteria for diagnosis and thus the definition of BE differs across the world. In the United States, Barrett's esophagus is described as a metaplastic process resulting in a change of the lining of the distal esophagus from one that is a normal squamous epithelium to a columnar-lined epithelium containing goblet cells[4]. The definition does require that at least 1 cm of metaplastic columnar epithelium be replacing the lining of the distal esophagus which is normally stratified squamous epithelium[4]. The segments that are less than 1 cm in length are categorized as specialized intestinal meta
BE is being detected more readily in clinical practice given the increased awareness about BE and the long-term risks associated with it, especially EAC[7]. It is reported to be seen in about 2.3% to 8.3% of people with GERD and about 1.2% to 5.6% of those without GERD[8]. In the United States, it is known to impact about 5% of people overall and about 1% globally[8]. A meta-analysis of 51 studies on the prevalence of BE in Asian countries found that the pooled prevalence of endoscopic BE was 7.8% and of BE that was histologically confirmed was 1.3%[9]. Additionally, from 1991 to 2004, there was a trend of increasing prevalence of BE, especially in eastern Asia, with the pooled prevalence of low-grade dysplasia (LGD), high-grade dysplasia (HGD), and adenocarcinoma of the esophagus being 6.9%, 3.0%, and 2.0%, respectively[9]. A systematic review published in 2020 analyzed 103 on the global prevalence of BE and found that the pooled prevalence of BE in Western, Eastern, and Latin American countries was 2.30%, 0.59%, and 0.51% respectively[10]. The incidence of not only GERD but BE and EAC has been increasing over the preceding few decades and is suggested to be partly due to increased endoscopic procedures being performed likely for GERD[11]. Studies have suggested that the absolute risk of having EAC is about 0.1%–0.5% annually in patients with non-dysplastic BE, for those with LGD, the annual EAC risk has a wide range from 1% to 43% while for HGD the annual risk is much higher at 23%–60% with about 5% of patients with BE going on to develop cancer[11]. Esophageal cancer is the sixth most common etiology of cancer related mortality and is the eighth most prevalent malignancy across the globe[12]. Hence the timely recognition of risk factors for BE and en
There are several well established risk factors linked to the emergence of BE and it is through recognition of these risk factors that a clinician can identify those patients using their clinical judgment. Table 1 provides a list of these risk factors.
| Chronic (≥ 5 years) GERD symptoms |
| Advancing age (> 50 years) |
| Male gender |
| Caucasian race |
| Tobacco usage |
| Central obesity (waist circumference > 102 cm or waist–hip ratio > 0.9) |
| Family history of BE |
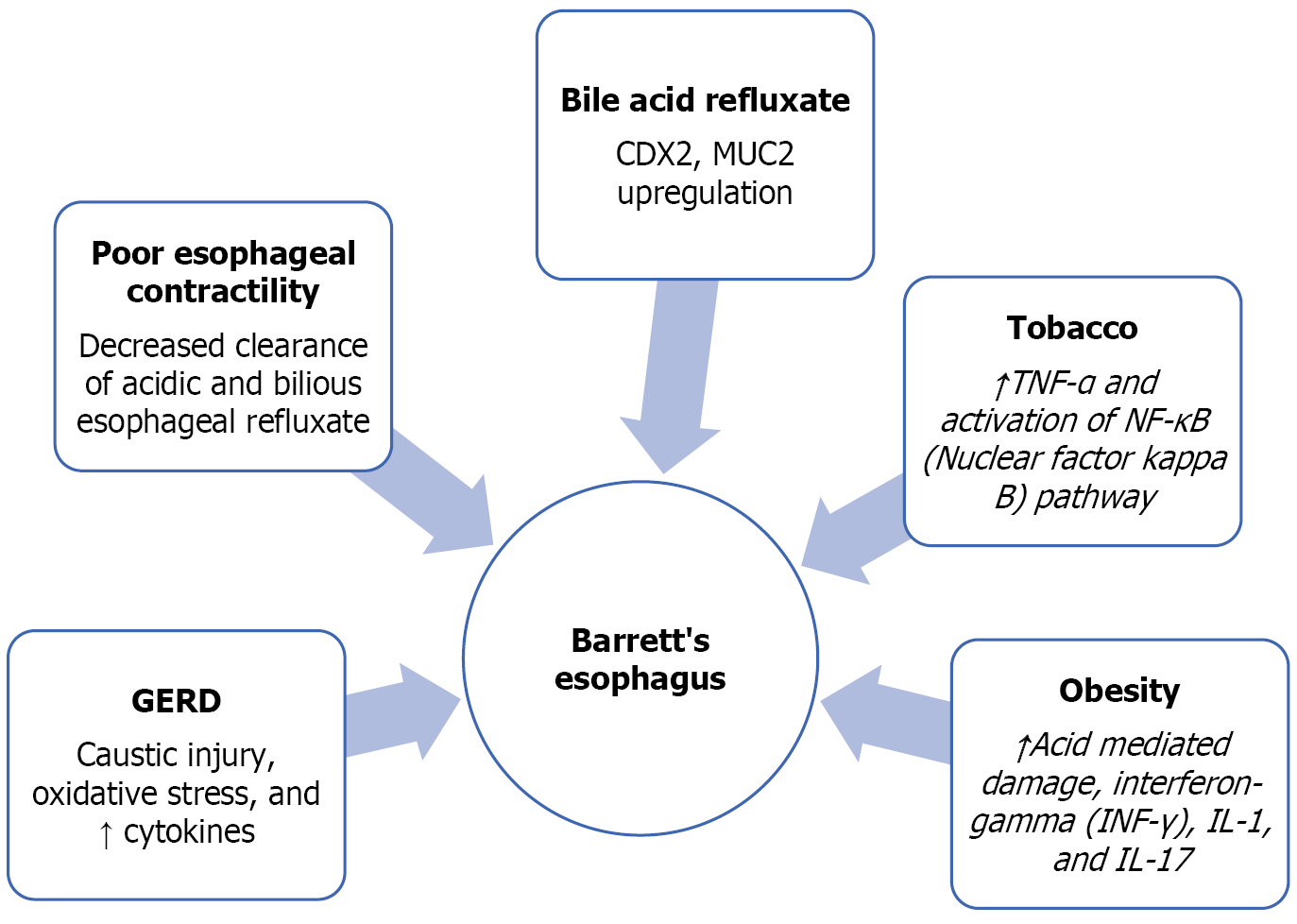
GERD is a risk factor that is implicated in the pathogenesis of BE. However, the duration of the GERD carries relevance as it is chronic GERD defined as symptoms of GERD for 5 years or more with frequent weekly symptoms[4]. A common symptom associated with GERD is a retrosternal burning sensation termed heartburn which is especially worse after food consumption. Patients may also complain of difficulty with swallowing, a ’lump’ like sensation in the throat, nausea, or indigestion. It is important to remember that GERD can also be asymptomatic or present as ‘silent reflux’ which may present with a chronic cough and repeated throat clearing. Longstanding and untreated acid reflux stimulates acid-related damage to the mucosa of the esophagus due to chronic inflammation. This can be seen initially with the changes of erosive esophagitis due to acid reflux-mediated damage[7]. This eventually leads to the metaplastic changes known as BE. This specialized intestinal epithelium which contains the columnar and goblet cells is thought to have less susceptibility to acid reflux-related damage[14]. The acid-mediated chronic inflammation leads to ongoing exposure of the esophageal lining to the deleterious effects of cytokines, chemokines, and reactive oxygen species[15]. These mediators of a chronic inflammatory state promote the growth of cells as well as vasculature and stimulate invasion[15]. The exposure to acid leads to caustic injury and oxidative damage to the DNA with decreased growth factor secretion as well[16]. Poor contractility of the esophagus and decreased clearance of acidic and bilious esophageal refluxate are also reported to occur[16]. Acid stimulates the squamous epithelial cells to produce pro-inflammatory cytokines like interleukin 8 and interleukin 1b[11]. These cytokines create an inflammatory milieu by attracting the T lymphocytes and neutrophils into the epithelium[11]. Bile acid refluxate upregulates an intestinal differentiation factor called CDX2 and MUC2 which is the goblet cell specific gene[11]. Bile acids also tend to lead to DNA damage and activation of nuclear factor kappa B (NF-κB) (prevents apoptosis and death of damaged cells)[17-19]. Hence it is the prolonged exposure of the distal esophagus to noxious stimuli that causes the alteration of the squamous epithelium typical of the esophagus into the metaplastic intestinal-type columnar epithelium and then eventually the dysplastic type.
Smoking tobacco is linked to the causation of BE as well as to the formation of adenocarcinoma of the esophagus[12]. The risk increases with an increased number of pack years of smoking and tends to be higher in male smokers as compared to females[20]. It stimulates the formation of pro-inflammatory cytokines like interleukin (IL)-1, IL-6, and IL-8 as well as tumor necrosis factor-α (TNF-α)[21]. Furthermore, it leads to reduced amount of IL-10, which is a mediator that combats inflammation[21]. One of the main pathways that leads to the formation of a pro-inflammatory response to smoking is the NF-κB[22]. Smoking tobacco also leads to oxidative damage due to the production of free radicals for example reactive nitrogen and oxygen species[23]. These free radicals cause the stimulation of the NF-κB pathway and a pro-inflammatory milieu with resultant harm to proteins, lipids and nucleic acids ultimately predisposing to malignant changes[24]. Lipid peroxidation generates products that injure the DNA and proteins[25]. Another phenomenon that has been described to be caused by smoking is the momentary relaxation of the lower esophageal sphincter (LES)[26]. An increase in the period of smoking causes a proportional increase in the mean hourly rate of the LES relaxation in asymptomatic smokers as well as smokers with reflux symptoms[26]. These transient relaxations of the LES can result in further acid reflux and drive metaplastic changes in the lower esophagus. Through these deleterious processes, tobacco lends itself to promoting the changes necessary for the development of BE. It seems that smoking and GERD work together to raise the risk of Barrett's esophagus. The International BEACON (Barrett’s Oesophagus and Esophageal Ade
Obesity is linked to the development of BE utilizing increasing acid-mediated damage as it raises the intra-abdominal pressure[28]. By increasing acid reflux, it directly augments the possibility of BE in a patient with untreated reflux disease. It is also linked to an ongoing state of low levels of inflammation involving the adipocytes resulting in increased levels of C-reactive protein[28]. Pro-inflammatory substances such as cytokines are produced by inflammatory cells (T-lymphocytes and macrophages) as well as the adipocytes themselves[29]. Interferon-gamma, IL-1, and IL-17 are generated by the T-lymphocytes and the macrophages generate IL-12 and TNF-α[30]. Certain cytokines are speculated to have carcinogenic potential such as insulin-like growth factor-1, transforming growth factor-beta, and vascular endo
Some studies have shown that the male gender is linked with a higher prevalence of BE (about 6.8%) and so is a family history of BE or EAC (about 23%)[35]. Those over the age of 50 years are also shown to have about 6.1% prevalence of BE[35]. Of all individuals with BE, it is reported that about 67% are males and 33% are females[4,36]. The possibility of transformation of BE into EAC is significantly increased in men as compared to women with an odds ratio of about 2.2[36]. This is portrayed in the disproportionate difference in that 89% of EAC is seen in males[4,36].
As a result of this gender disparity, BE screening in women is anticipated to have a modest yield in terms of lowering EAC incidence. However, screening women with numerous risk factors for BE and EAC may be justified after discussing the pros and cons with the patient. The Caucasian race is another risk factor associated with the development of BE[4]. As there is an accumulation of a number of risk factors, the risk of development of BE further increases as compared to having long-standing GERD alone[4] (Figure 1).
Several factors have been studied for their association with higher rates of development from BE to adenocarcinoma of the esophagus. Studies demonstrate that male gender, older age, a history of smoking, and low-grade dysplasia (also known as low-grade intraepithelial neoplasia) are associated with a higher chance of transformation of BE to EAC[36]. There was also a proportional increase in the risk of development of EAC with a longer length of BE segment on endo
Studies have been undertaken to evaluate the effectiveness of chemo-preventative strategies for limiting the trans
Prolonged use of PPIs alters the nature of reflux rather than eliminating it by altering the pH of refluxed material. Under PPI treatment, the usually inactive bile salts become aggressive toward the esophageal mucosa when reflux is alkalinized, increasing the risk of damage to the esophageal lining and potentially exacerbating conditions like Barrett's Esophagus. This highlights a significant limitation of PPI therapy and signifies the necessity for meticulous management and monitoring of patients on long-term PPI therapy, as alkaline reflux can adversely affect disease progression and risk of complications.
Long term use of proton pump inhibitors has been linked to adverse effects such as osteoporosis, renal dysfunction, dementia and risk for infections particularly pneumonias and clostridium difficile infection. However, many of these potential effects are not conclusive as different studies have shown inconsistent results. Given the limitations and potential adverse effects of long-term use of proton pump inhibitors, the use in clinical practice is based on evidence from the guidelines and discussion with the patient.
The American College of Gastroenterology (ACG) and the American Gastroenterological Association do advocate the limitation of acid exposure to the esophagus using proton pump inhibitors to decrease the progression of dysplastic changes[13,39]. The ACG does discourage the routine use of aspirin and other cyclooxygenase inhibitors for chem
The symptomatology of BE is typically associated with GERD and reflux-associated symptoms and related complications. The change in the lining of the esophagus to intestinal columnar type of metaplasia typical of BE itself does not lead to symptoms but it is the reflux that does so[40]. Patients may complain of long-standing retrosternal burning sensation termed heartburn or even dysphagia related to underlying peptic stricture or erosive esophagitis. BE can often lead to complications such as ulceration and stricture formation which presents as dysphagia and odynophagia while bleeding of the gastrointestinal tract rarely occurs[41]. In patients with GERD, atypical symptoms such as nausea, hoarseness, wheezing, chest discomfort, and globus sensation may be seen[42]. In such cases, BE may be discovered as an incidental finding during an endoscopy performed for other reasons[11].
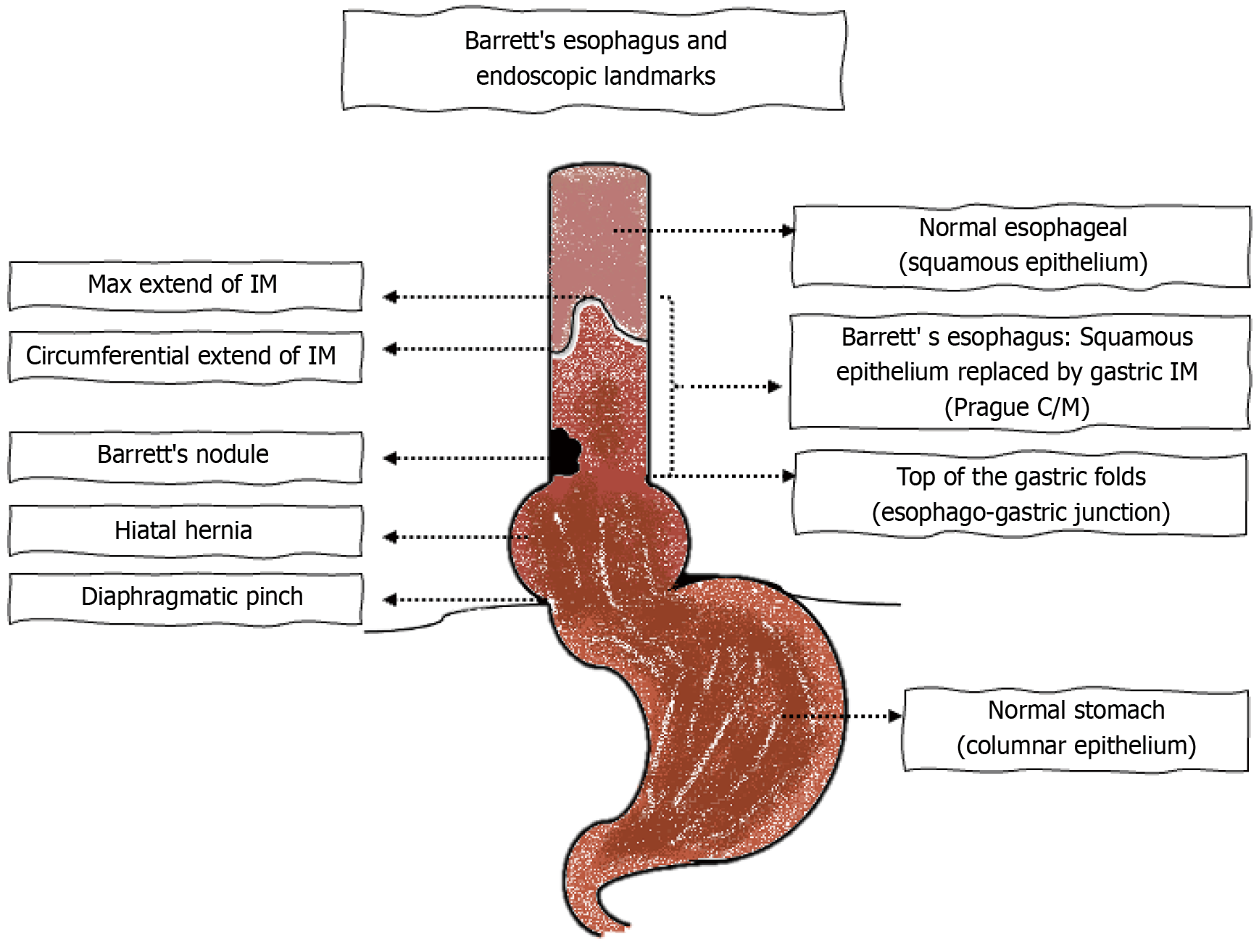
Screening refers to the evaluation of individuals with GERD for BE. The gold standard for diagnosis of BE is via direct visualization of the aberrant segment of the esophagus[4]. This is performed with the use of conventional per-oral endoscopy[4]. The Prague classification is one of the best-validated methods for defining BE and involves measuring the maximal and the circumferential extent of the columnar epithelium above the proximal margin of the gastric folds[43]. At least 1 cm of columnar mucosa is required for classification as BE and to perform biopsies and it is not recommended to biopsy < 1 cm length of proximal displacement of the Z line from the top of the gastric folds[4]. This screening criteria improves the specificity of the testing so as to minimize the number of false positive results that would confound dia
However, conventional endoscopy does have limitations as the routine application in the general population is not cost-effective for screening[44]. Especially as there is a significant burden of GERD in the overall population and a wide-scale application of screening endoscopies would result in a large health care burden and cost as it would lead to surve
Hence as per the latest Barrett’s guidelines, it is advised that a single screening endoscopy be implemented for certain high-risk patients[4]. This includes patients who have symptoms of chronic GERD in addition to ≥ 3 risk factors connected with the formation of BE[3]. The factors linked with the progression to BE include age more than 50 years, male gender, Caucasian race, tobacco use, obesity, and those with a strong family history of BE or esophageal adenocarcinoma in a first-degree family member[4].
A less invasive modality known as unsedated Trans-Nasal Endoscopy can also potentially be employed for screening for BE. The advantages include that is it less expensive and less invasive without anesthesia-related risks[45]. It has comparable sensitivity and specificity to endoscopy for the diagnosis of BE[45]. Providers other than physicians can be trained which helps to reduce the cost[45]. However, it is not readily utilized due to limitations such as discomfort for the patient especially as it is unsedated[4].
A non-endoscopic technique that is available is the swallowable capsule sponge device and when used in conjunction with a biomarker it is considered an acceptable substitute for endoscopic evaluation[4]. These devices are composed of dissolvable gelatin or vegetable capsules that have a spherical sponge made of polyurethane. The sponge is connected to a string that enlarges into a spherical shape once the outer capsule dissolves or they are made of an inflatable silicone balloon. The devices are swallowed in order to obtain esophageal cells which are used for cytology. The cells collected are then analyzed for the presence of certain biomarkers that are seen in IM (trefoil factor 3) or methylated DNA markers associated with BE[4].
Advantages include being able to be conducted as an outpatient without any sedation or anesthesia and being less expensive than endoscopy. It is minimally invasive and does not require a physician[4]. Patient discomfort can occur with the swallowing of the device and potential dislodgement can occur in which case the device can be removed endoscopically[4].
Esophageal video capsule endoscopy is another non-invasive technique that can be well tolerated. However, it has limited diagnostic yield with limited accuracy and low sensitivity as well as specificity hence not recommended for routine use[13].
Another technique that is being created is a device that evaluates the volatile organic substances that are exhaled by a metal oxide sensor and the assessment is confected by artificial neural networks[4]. However, this is not yet available in the United States.
In routine practice, the most utilized modality is high-definition white light endoscopy. A repeat endoscopy is not recommended for screening for BE after an initial negative endoscopic evaluation of BE[4]. However, if erosive eso
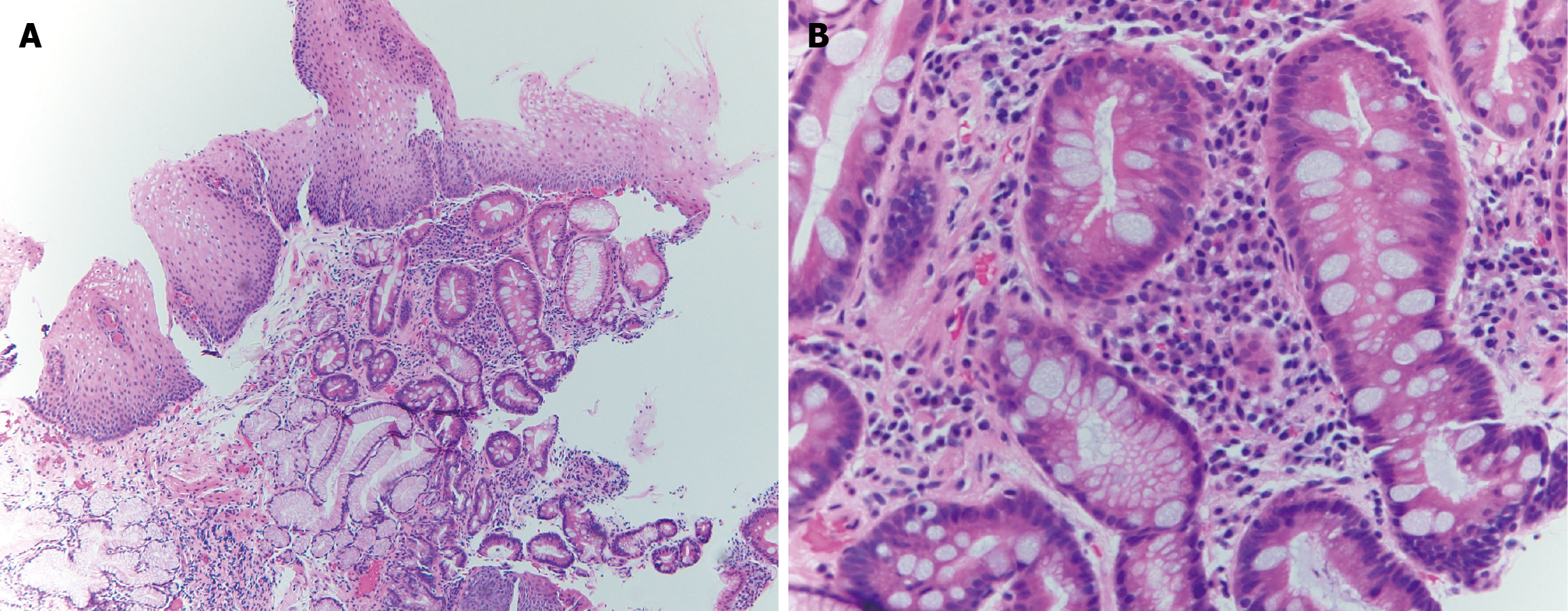
Once BE is suspected on endoscopy, a minimum of 8 endoscopic biopsies are recommended. In cases of short segments of 1-2 cm where 8 biopsies are not feasible then 4 biopsies/cm of circumferential columnar mucosa and 1 biopsy per cm of tongues of suspected Barrett’s mucosa is recommended[4]. For segments longer than 4 cm, the Seattle protocol is recommended for sampling[4]. The Seattle protocol involves obtaining 4 quadrant biopsies every 1-2 cm in the columnar lined mucosa and sampling areas of irregular appearing mucosa separately[39]. Any dysplasia that is identified on biopsy specimens is mandated to be verified by a second pathologist who has expertise in gastrointestinal pathology[4]. If there is salmon-colored mucosa that extends ≥ 1 cm above the top of the gastric folds and the biopsies come back negative for intestinal metaplasia, then a repeat endoscopy is advised in 1-2 years[4]. Figure 3A and B show histologic findings of BE on biopsies obtained during endoscopy.
In contrast to screening, surveillance refers to the periodic evaluation of those with a proven diagnosis of BE in order to identify malignancy in the esophagus at the earliest. Once the diagnosis of BE is established, a discussion needs to be held with the patient regarding surveillance programs. The rationale for performing endoscopic surveillance is to identify dysplasia or carcinoma at an initial and potentially treatable stage[4]. Given the need for repeat endoscopic assessments and associated risks as well as the potential detection of dysplastic mucosa in the future and the implications associated with that. The overall patient profile and comorbidities also need to be taken into consideration when implementing surveillance programs.
Endoscopic evaluation with both white light endoscopy, as well as chromoendoscopy, is advised when performing surveillance of BE. Initial assessment of the BE should start with high-definition white light endoscopy which should incorporate a retroflexed view of the cardia. This should be followed by chromoendoscopy which can be used to augment the efforts to identify dysplastic changes and even carcinoma[4]. Vital dyes such as acetic acid can be used or through electronic chromoendoscopy. Dye based chromoendoscopy can be performed by applying diluted acetic acid to the BE causing a whitening of the area and neoplastic tissue loses this whitening effect more quickly than non-dysplastic BE[4]. Electronic chromoendoscopy systems provide an enhanced assessment of the mucosa and the vascular patterns and abnormal mucosa (dysplastic or even harboring carcinoma) will have irregular mucosal or vascular changes with narrow-band imaging. Chromoendoscopy-guided biopsies are not a replacement for standardized biopsies and chromoendoscopy is recommended to be used in combination with high-definition white light-based biopsy protocols[4].
Other imaging techniques are available to further improve the detection of dysplastic changes and cancer. Confocal laser endomicroscopy involves the intravenous administration of fluorescein and utilizes blue laser light to visualize the esophageal tissue[4]. This helps to obtain targeted biopsies with real-time high-magnification imaging.
Volumetric laser endomicroscopy uses optical coherence tomography and is a probe-based method that can perform a 6 cm circumferential scan of the esophagus[46]. It has the ability to visualize the mucosa and submucosa of the esophagus in a 2-dimensional method and to a depth of 3 mm[46]. There is also work underway on utilizing artificial intelligence as well in the detection of dysplastic BE and detection of carcinoma, utilizing computer-based techniques[3].
The surveillance intervals vary depending on the length of the segment, presence and degree of dysplasia and pre
If there is no dysplasia detected and the length of the BE segment is ≥ 3 cm then surveillance is recommended in 3 years and if the segment is < 3 cm then surveillance can be extended up to 5 years[4]. If the samples are indefinite for dysplasia, then this needs to be verified by a second pathologist who has expertise in the field of gastrointestinal path
The mainstay of non-endoscopic treatment is centered around acid suppression with proton pump inhibitor (PPI) therapy. Once daily PPI therapy is advised in patients that do not have contraindications to their use to prevent ongoing acid-mediated damage to the esophageal mucosa[4]. The latest guidelines do not make any recommendations for the combination of ASA with PPI to decrease the chances of advancement of BE to HGD or even EAC[4].
Once HGD or intramucosal carcinoma (T1a) is detected on biopsies then this needs to be treated and can potentially be done endoscopically. If a patient with LGD also opts for treatment, then they would also be candidates for endoscopic approaches. A risks and benefits discussion needs to be held before embarking on this path as it also lends to future repeat endoscopies and surveillance programs. Endoscopic management is recommended for LGD, HGD, or intra
The modalities of endoscopic resection include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). They are cap-assisted EMR, multiband EMR, and ESD. Both EMR techniques (cap-assisted, multiband) have comparable efficacy and safety[4]. ESD has a higher risk for complications as compared to EMR given the increased depth of resection.
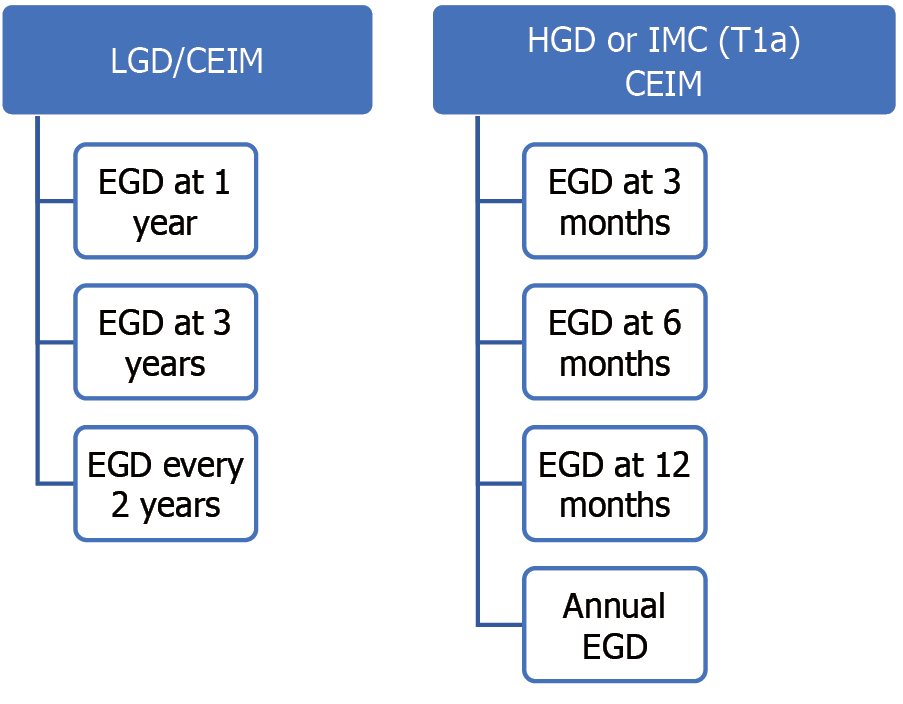
Radiofrequency ablation (RFA) is the most efficacious and safe eradication technique for ablation and is the preferred one routinely[4]. RFA uses thermal energy to obliterate the precancerous cells and the treated tissue usually sloughs off over 48-72 hours[13]. After about 6-8 weeks, this treated area will be replaced by a new, normal esophageal lining[13]. Once CIEM is attained, then a surveillance program is initiated in which endoscopies are performed at intervals to detect the recurrence of BE (Figure 5).
For those with involvement of the submucosa, T1b lesions and beyond surgical treatment are advised. The possibility for lymph node metastasis in T1b disease is significantly higher and hence patients should be referred for surgical con
As research in this area evolves, there are advances in techniques for detection and diagnosis of BE. More non-invasive markers are being developed for use as alternatives to endoscopy. Another upcoming area that is being studied is the use of artificial intelligence (AI) to evaluate for BE. AI is being used to detect neoplastic lesions with the development of computer aided detection[47]. Additionally, it can then also help characterize the neoplastic lesion and hence assist with the diagnosis and further management (referred to as Computer aided diagnosis)[47]. It is a powerful tool that has many advantages as it is not subject to fatigue or the observer bias that an endoscopist can encounter[47]. It can also serve as a quality control mechanism as it can improve the quality of the procedure performed by giving feedback to the endo
BE is a common pathology encountered in clinical practice by both gastroenterologists and general practitioners. It deserves recognition and careful consideration when considering patients who require screening and the implications of the subsequent steps that may arise as a consequence. The early detection of dysplastic changes in BE is paramount so that treatment can be ensured to prevent progression to EAC and drastic surgeries.
| 1. | Barrett NR. Chronic peptic ulcer of the oesophagus and 'oesophagitis'. Br J Surg. 1950;38:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 553] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Maret-Ouda J, Markar SR, Lagergren J. Gastroesophageal Reflux Disease: A Review. JAMA. 2020;324:2536-2547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 3. | Gindea C, Birla R, Hoara P, Caragui A, Constantinoiu S. Barrett esophagus: history, definition and etiopathogeny. J Med Life. 2014;7 Spec No. 3:23-30. [PubMed] |
| 4. | Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, Wani S. Diagnosis and Management of Barrett's Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117:559-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 5. | Riddell RH, Odze RD. Definition of Barrett's esophagus: time for a rethink--is intestinal metaplasia dead? Am J Gastroenterol. 2009;104:2588-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett's oesophagus. Gut. 2006;55:442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Chela HK, Gangu K, Ertugrul H, Juboori AA, Daglilar E, Tahan V. The 8th Wonder of the Cancer World: Esophageal Cancer and Inflammation. Diseases. 2022;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Sharma P. Barrett Esophagus: A Review. JAMA. 2022;328:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 9. | Shiota S, Singh S, Anshasi A, El-Serag HB. Prevalence of Barrett's Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015;13:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Marques de Sá I, Marcos P, Sharma P, Dinis-Ribeiro M. The global prevalence of Barrett's esophagus: A systematic review of the published literature. United European Gastroenterol J. 2020;8:1086-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Khieu M, Mukherjee S. Barrett Esophagus. StatPearls. Published online August 8, 2022. Accessed December 5, 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430979/. |
| 12. | Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933-7943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 554] [Cited by in RCA: 788] [Article Influence: 71.6] [Reference Citation Analysis (24)] |
| 13. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1086] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 14. | Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus. An ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 240] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Abdel-Latif MM, Duggan S, Reynolds JV, Kelleher D. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol. 2009;9:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 16. | Mikhael M, Pasha B, Chela H, Tahan V, Daglilar E. Immunological and Metabolic Alterations in Esophageal Cancer. Endocr Metab Immune Disord Drug Targets. 2022;22:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett's esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Stachler MD, Taylor-Weiner A, Peng S, McKenna A, Agoston AT, Odze RD, Davison JM, Nason KS, Loda M, Leshchiner I, Stewart C, Stojanov P, Seepo S, Lawrence MS, Ferrer-Torres D, Lin J, Chang AC, Gabriel SB, Lander ES, Beer DG, Getz G, Carter SL, Bass AJ. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nat Genet. 2015;47:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 19. | Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and Cells of Origin of Barrett's Esophagus. Gastroenterology. 2019;157:349-364.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Dong J, Thrift AP. Alcohol, smoking and risk of oesophago-gastric cancer. Best Pract Res Clin Gastroenterol. 2017;31:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258-J265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 689] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 22. | Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60:409-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Rom O, Kaisari S, Aizenbud D, Reznick AZ. Identification of possible cigarette smoke constituents responsible for muscle catabolism. J Muscle Res Cell Motil. 2012;33:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Rom O, Avezov K, Aizenbud D, Reznick AZ. Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol. 2013;187:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Łuczaj W, Gęgotek A, Skrzydlewska E. Antioxidants and HNE in redox homeostasis. Free Radic Biol Med. 2017;111:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Cook MB, Shaheen NJ, Anderson LA, Giffen C, Chow WH, Vaughan TL, Whiteman DC, Corley DA. Cigarette smoking increases risk of Barrett's esophagus: an analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, Leighton P, Quesenberry C, Rumore GJ, Buffler PA. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34-41; quiz 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Conroy MJ, Dunne MR, Donohoe CL, Reynolds JV. Obesity-associated cancer: an immunological perspective. Proc Nutr Soc. 2016;75:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Mazzarella L. Why does obesity promote cancer? Epidemiology, biology, and open questions. Ecancermedicalscience. 2015;9:554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Singh S, Sharma AN, Murad MH, Buttar NS, El-Serag HB, Katzka DA, Iyer PG. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1399-1412.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 33. | Elliott JA, Reynolds JV. Visceral Obesity, Metabolic Syndrome, and Esophageal Adenocarcinoma. Front Oncol. 2021;11:627270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L, Wu AH, Ward MH, Casson AG, Murray LJ, Corley DA, Nyrén O, Pandeya N, Vaughan TL, Chow WH, Gammon MD. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 35. | Qumseya BJ, Bukannan A, Gendy S, Ahemd Y, Sultan S, Bain P, Gross SA, Iyer P, Wani S. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Krishnamoorthi R, Singh S, Ragunathan K, Visrodia K, Wang KK, Katzka DA, Iyer PG. Factors Associated With Progression of Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1046-1055.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Parasa S, Vennalaganti S, Gaddam S, Vennalaganti P, Young P, Gupta N, Thota P, Cash B, Mathur S, Sampliner R, Moawad F, Lieberman D, Bansal A, Kennedy KF, Vargo J, Falk G, Spaander M, Bruno M, Sharma P. Development and Validation of a Model to Determine Risk of Progression of Barrett's Esophagus to Neoplasia. Gastroenterology. 2018;154:1282-1289.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks C, Attwood S, Harrison R, Barr H, Moayyedi P; AspECT Trial Team. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 39. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology. 2011;140:e18-52; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 812] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 40. | DeMeester SR, DeMeester TR. Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann Surg. 2000;231:303-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Spechler SJ. Barrett's esophagus. Semin Gastrointest Dis. 1996;7:51-60. [PubMed] |
| 42. | Vaezi MF. Atypical manifestations of gastroesophageal reflux disease. MedGenMed. 2005;7:25. [PubMed] |
| 43. | Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 745] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 44. | Moriarty JP, Shah ND, Rubenstein JH, Blevins CH, Johnson M, Katzka DA, Wang KK, Wongkeesong LM, Ahlquist DA, Iyer PG. Costs associated with Barrett's esophagus screening in the community: an economic analysis of a prospective randomized controlled trial of sedated versus hospital unsedated versus mobile community unsedated endoscopy. Gastrointest Endosc. 2018;87:88-94.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Shariff MK, Bird-Lieberman EL, O'Donovan M, Abdullahi Z, Liu X, Blazeby J, Fitzgerald R. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc. 2012;75:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Houston T, Sharma P. Volumetric laser endomicroscopy in Barrett's esophagus: ready for primetime. Transl Gastroenterol Hepatol. 2020;5:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Jong MR, de Groof AJ. Advancement of artificial intelligence systems for surveillance endoscopy of Barrett's esophagus. Dig Liver Dis. 2024;56:1126-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/