©The Author(s) 2025.
World J Gastrointest Pathophysiol. Dec 22, 2025; 16(4): 111550
Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.111550
Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.111550
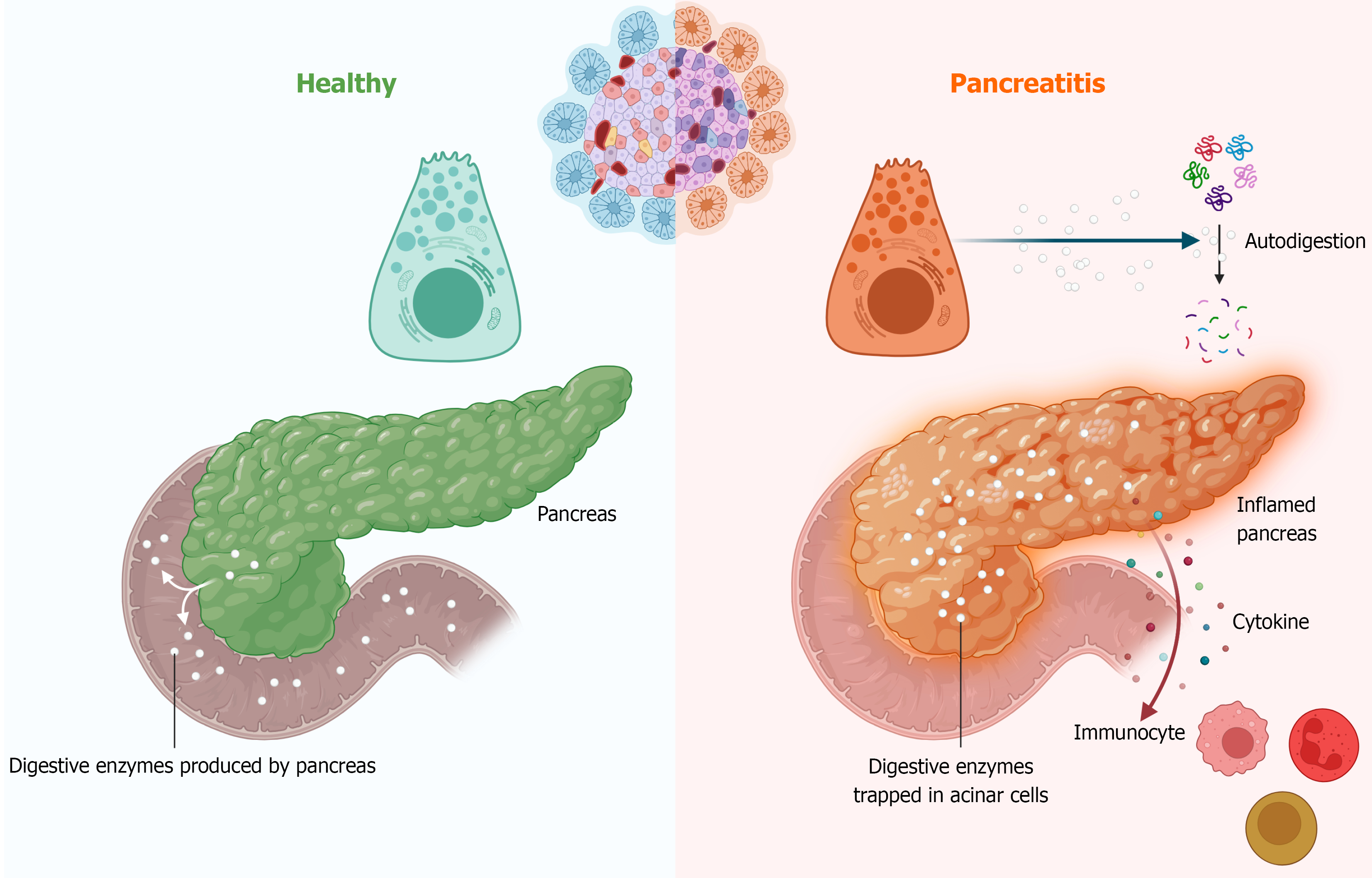
Figure 1 Pancreatic enzyme autodigestion and immunocyte-mediated inflammation.
Molecular mechanisms involved in pancreatitis. In normal conditions, pancreatic enzymes aid digestion. However, during pancreatitis, inappropriate activation of trypsinogen leads to autodigestion within the pancreas, causing inflammation. This triggers the secretion of chemokines and other cytokines, which attract and activate immune cells, further amplifying the inflammatory response and exacerbating pancreatic damage.
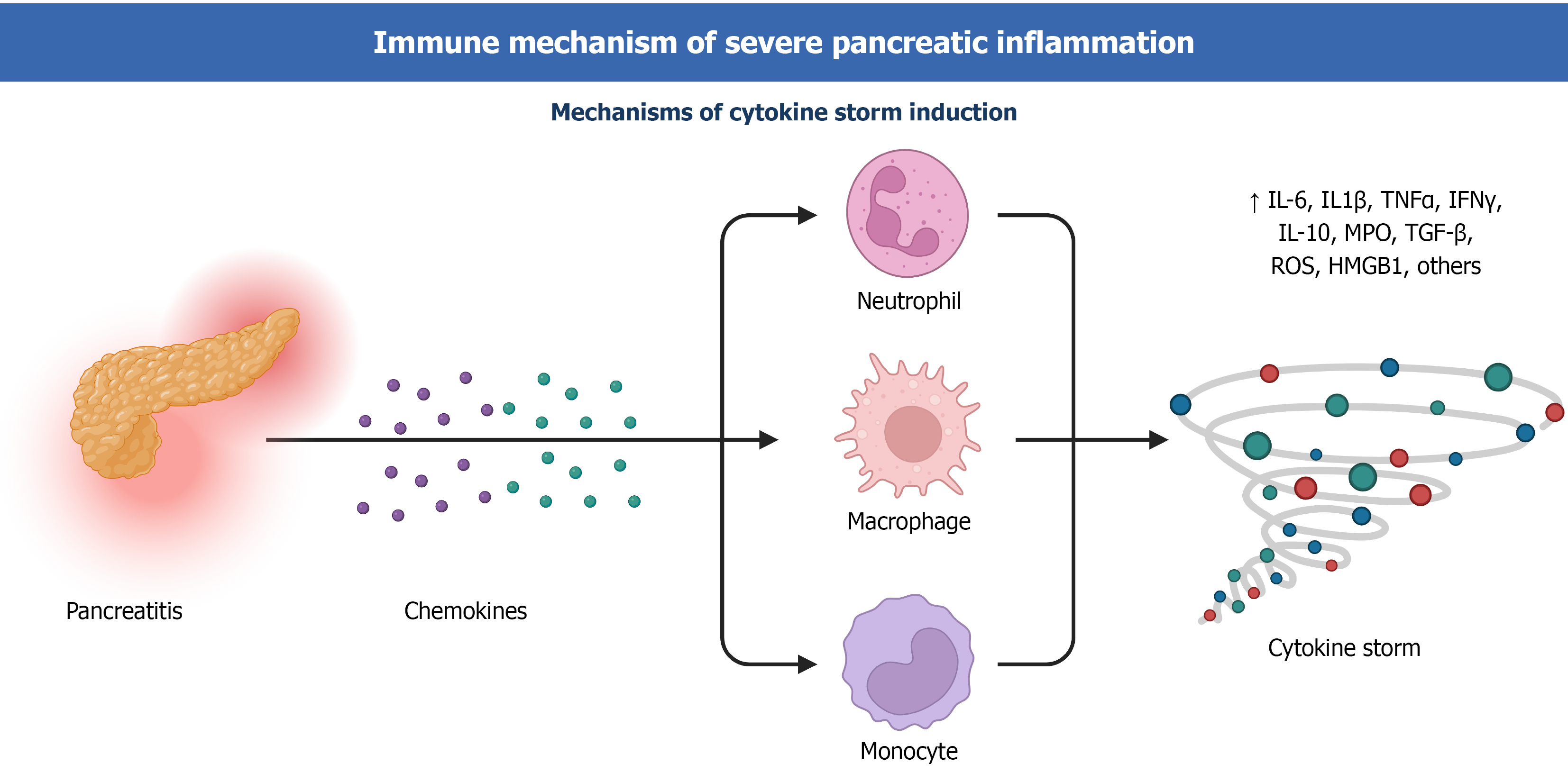
Figure 2 Immune cell recruitment and cytokine storm in pancreatitis.
During pancreatitis, chemokines attract and activate immune cells, including neutrophils, macrophages, and monocytes. The activated cells further secrete cytokines, exacerbating the inflammatory response and ultimately leading to a cytokine storm.
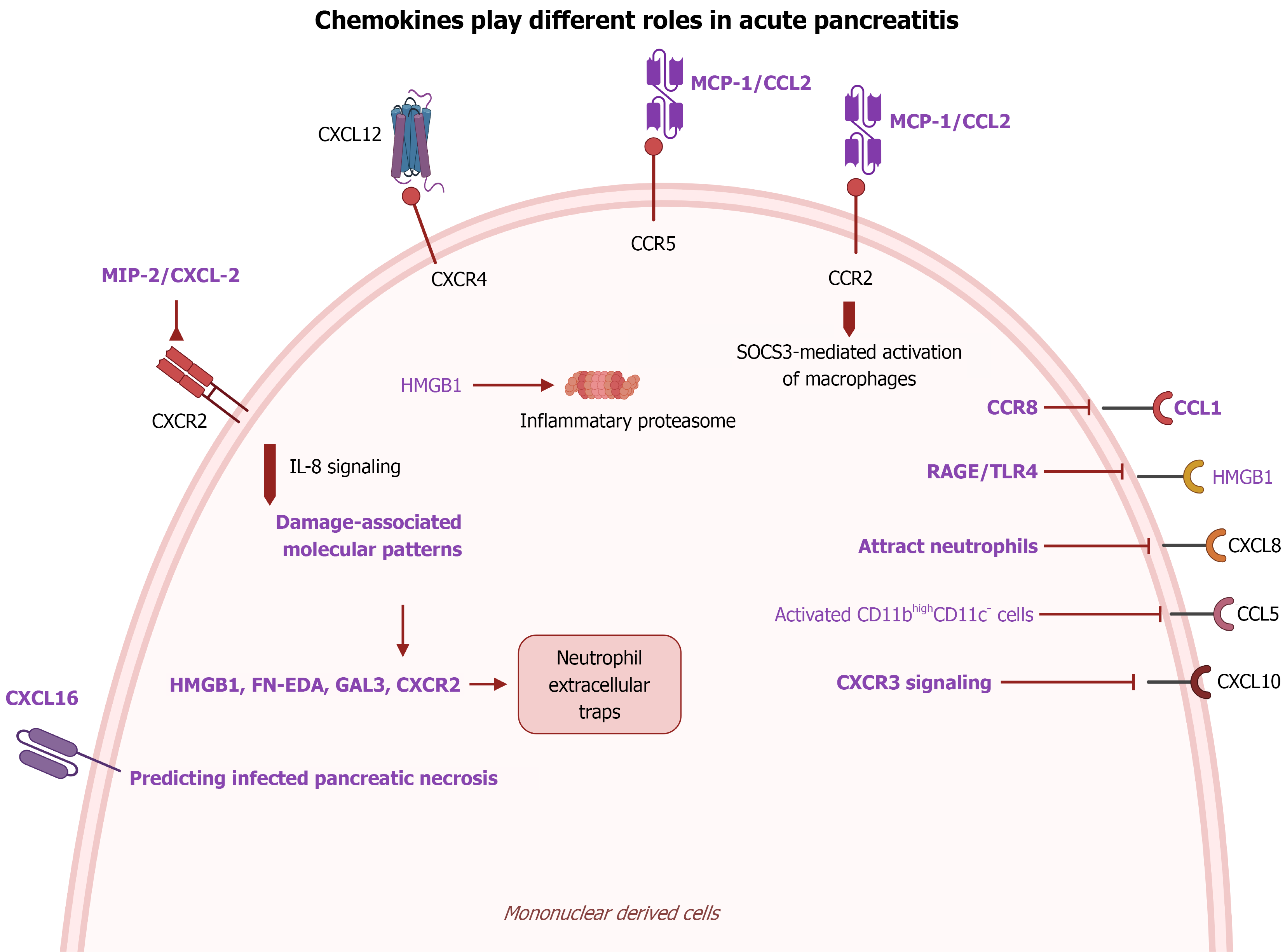
Figure 3 Chemokine-mediated activation of mononuclear cells in acute pancreatitis.
During acute pancreatitis, various chemokines are involved in activating mononuclear cells, such as monocytes, neutrophils and macrophages. These chemokines, including CCL2, CXCL10, CCL5, CXCL16, CXCL1, CXCL2, and CXCL8, play crucial roles in regulating their activities.
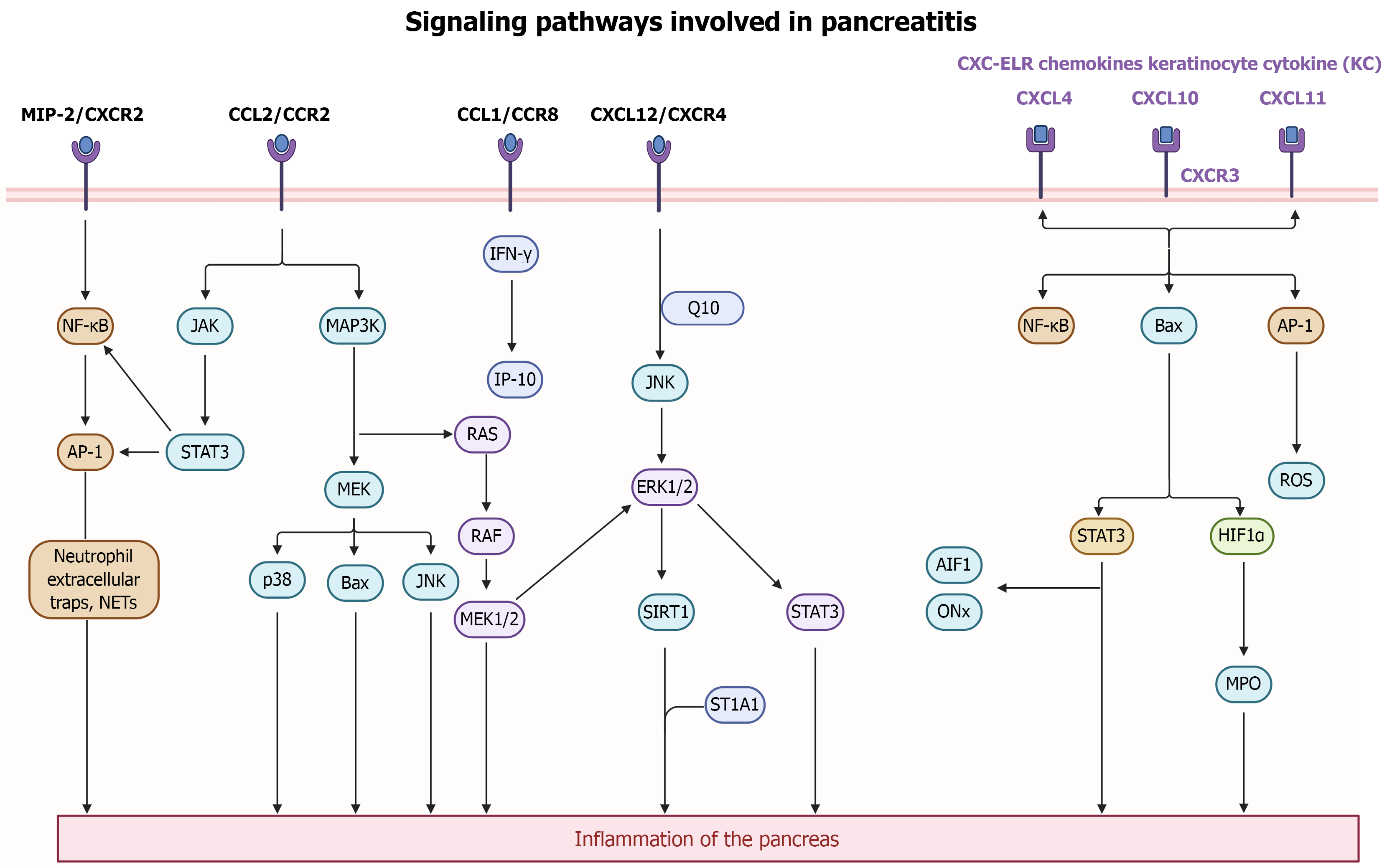
Figure 4 The signaling pathways involved in pancreatitis pathogenesis.
Upon pancreatitis onset, fatty acids released from peripancreatic fat induce expression of chemokines, including CCL2, MIP-2, and CXCL12, in pancreatic acinar cells via MAPK/JAK-mediated NF-κB and STAT3 pathways. Additionally, CXC-ELR triggers activation in mononuclear cells through CXCR3 signaling and activation of upstream regulators like pJNK, p38, and Bax, leading to the occurrence of pancreatitis. Inflammatory stimuli such as NETs or inflammation-related signaling pathways such as MAPK, JNK/STAT, INF-γ/AP-10, and ERK/SIRT1, enhance chemokine expression, accompanied by ROS, myeloperoxidase, AIF1, ONx production and NF-κB activation, exacerbating the inflammatory response. These signaling mechanisms collectively contribute to acinar cell damage, immune cell recruitment, and the progression of pancreatitis.
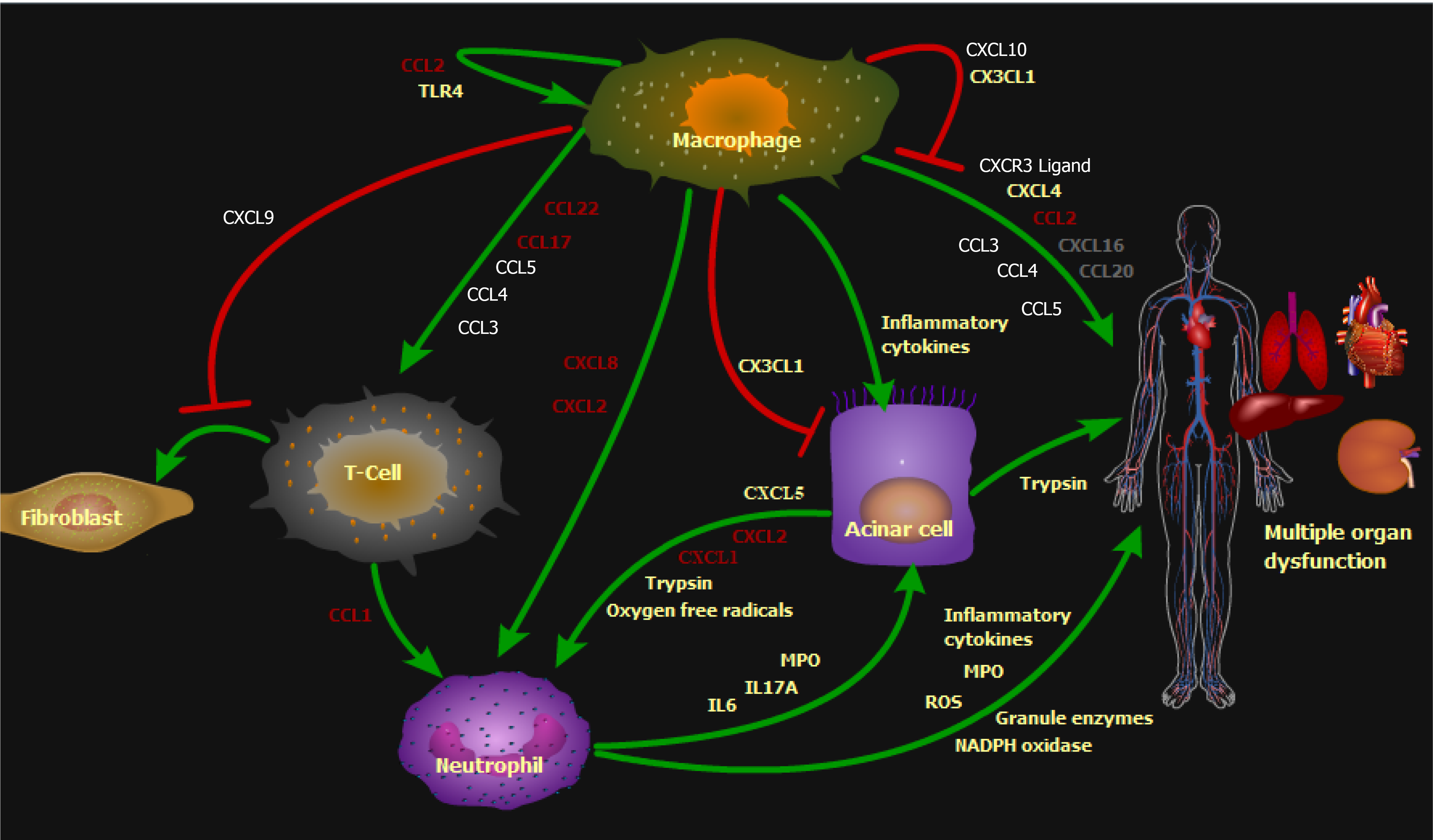
Figure 5 Molecular mechanisms involved in pancreatitis.
The figure illustrates several chemokines molecular mechanisms for the pancreatitis. These include macrophage-mediated production of inflammatory cytokines to impair pancreatic cells, causing trypsin spillage that leads to multiple organ dysfunction, and chemokines to attract immune cells, such as T-cell chemokines CCL3, CCL4, CCL5, CCL17, and CCL22, and neutrophil chemokines CXCL2 and CXCL8. Simul
- Citation: Ni WF, Qin CC. Roles of chemokines in pancreatitis: A review. World J Gastrointest Pathophysiol 2025; 16(4): 111550
- URL: https://www.wjgnet.com/2150-5330/full/v16/i4/111550.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i4.111550