Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.111432
Revised: July 26, 2025
Accepted: October 27, 2025
Published online: December 22, 2025
Processing time: 176 Days and 8.6 Hours
Helicobacter pylori (H. pylori) is a gram-negative, spiral-shaped, microaerophilic bacterium that infects over 43% of the global population, with higher prevalence in regions of low socioeconomic status and poor sanitation. It is transmitted main
Core Tip: Helicobacter pylori (H. pylori) is a gastric pathogen that infects more than 40% of the world’s population. Patho
- Citation: Costa JMC, Aguiar CEO, Oliveira MMGL, Cenci Dietrich V, Lima PHM, Freire JPC, Lemos FFB, Queiroz DMM, de Melo FF. Update on the pathogenesis and clinical management of Helicobacter pylori gastric infection and associated diseases. World J Gastrointest Pathophysiol 2025; 16(4): 111432
- URL: https://www.wjgnet.com/2150-5330/full/v16/i4/111432.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i4.111432
Helicobacter pylori (H. pylori) is a microaerophilic, spiraled, gram-negative bacterium that infects the stomach and leads to chronic inflammation[1]. It is transmitted mainly through the fecal-oral and oral-oral routes, and less frequently by the gastric-oral pathway, especially in patients prone to vomiting[2]. These routes correlate to its prevalence in populations with lower socioeconomic status, lack of sanitation and poor household conditions[3,4], due to the transmission between family members and the contamination of water and utensils.
Although present in more than 43% of individuals and often causing no symptoms[5], H. pylori is still considered a gastric pathogen, since it not only disrupts the gastric microbiota, but also potentially leads to severe diseases such as peptic ulcers and gastric cancer (GC)[6]. The genesis of these conditions and their differential presentation is directly linked to the host immunological response, as well as H. pylori virulence factors, such as cytotoxin-associated antigen A (CagA) and vacuolating cytotoxin A (VacA)[7]. In this regard, a recent discovery of a small non-coding RNA, NikS, has demonstrated the inner regulation of H. pylori virulence program is extremely intricate[8], allowing its persistence on the gastric environment, and also correlating to the severity of clinical outcomes.
Proper diagnosis and treatment of H. pylori-positive patients has helped prevent severe complications[9], however, the extensive use of antibiotics has compromised the efficacy of standard empirical therapies, renewing the interest in tailored therapy guided by susceptibility tests and novel adjuvants for treatment, such as potassium-competitive acid blockers (P-CABs) and probiotics, although further evidence is needed to actually ensure the implementation of these strategies[10,11], along with the remaining need for the development of prophylactic and therapeutic vaccines[12]. Overall, clinical research on H. pylori has focused on optimizing treatment strategies, while also minimizing the rise of antibiotic resistance.
This review aims to provide a comprehensive and up-to-date overview of H. pylori pathogenesis and clinical manage
After encountering the gastric environment, H. pylori employs a variety of mechanisms to assure its survival and adhere to the gastric epithelium. In order to deal with the stomach's low pH, the bacterium resorts mainly to urease, a nickel-dependent enzyme capable of catalyzing the hydrolysis of urea into ammonia and carbon dioxide, thereby increasing the pH around the bacterial cells[13]. Ammonia production also serves as a wide source of nitrogen, and has shown corre
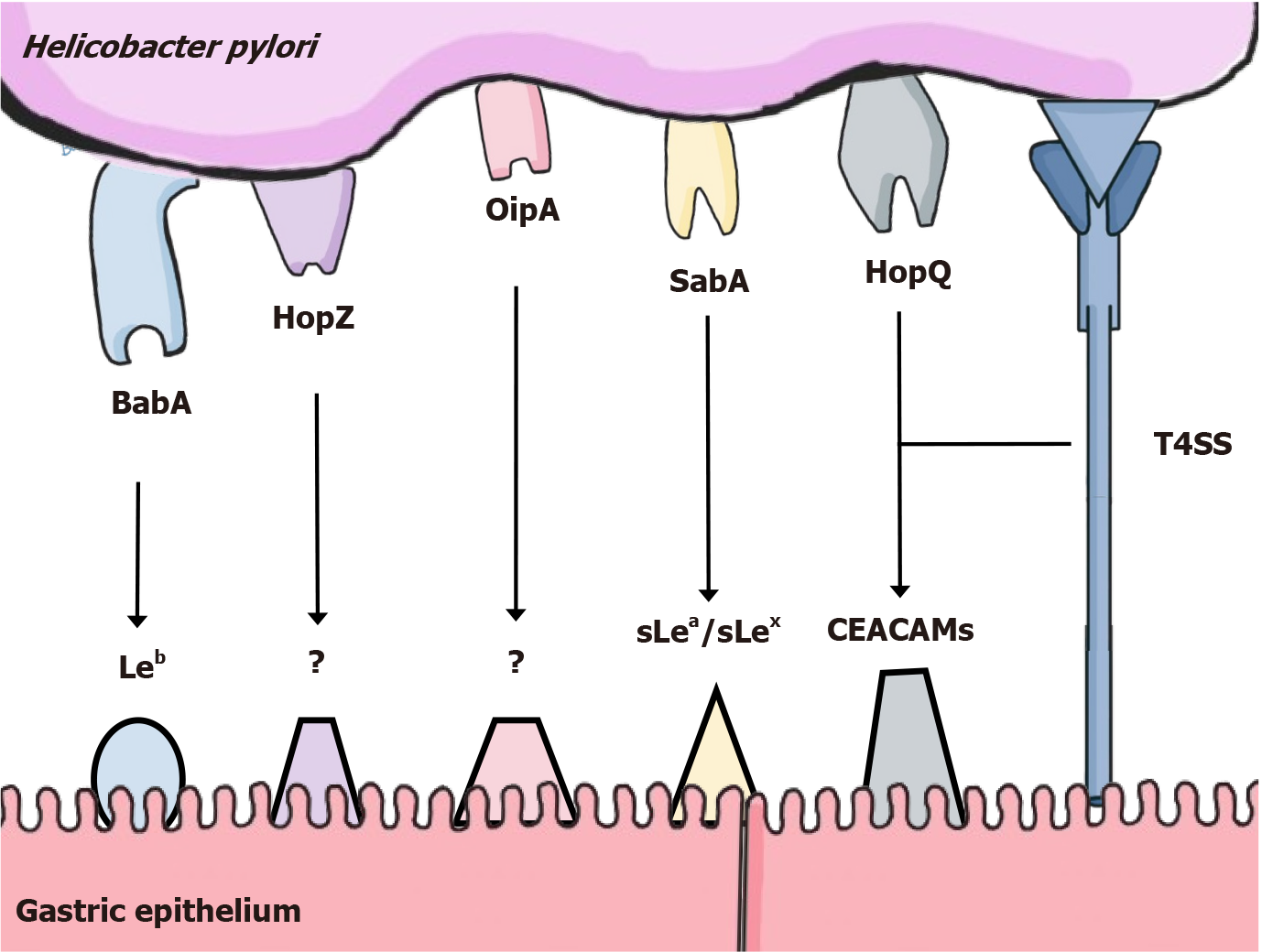
The reaching and adherence of H. pylori to the gastric mucosa is especially mediated by two different types of outer membrane proteins: Chemoreceptors and adhesins. Chemoreceptors, especially transducer-like proteins sense attractants and repellents in order to direct the flagellar movement towards regions with higher pH, closer to the epithelium and away from harmful chemicals[16], while adhesins mediate binding to antigens present on the gastric tissue. The intricate adaptation of H. pylori has enabled the bacterium to adhere to the healthy tissue on a first encounter, but also to survive within the inflamed environment induced by the infection itself, since it differentially expresses adhesins such as BabA[17] and HopZ[18], important for adherence with the healthy epithelium, and others like SabA[17], OipA[19] and HopQ[20], that bind to antigens overexpressed in inflamed environments. Figure 1 summarizes the most important adhesin-receptor interactions.
Adhesion of H. pylori to the gastric epithelium is also critical for the effective functioning of its type IV secretion system (T4SS). The proper assembly and positioning of the T4SS machinery is specially mediated by HopQ and BabA, allowing the bacterium to inject effector molecules into host cells, the main one being the oncoprotein CagA[21].
CagA: Cytotoxin-associated gene A (cagA), contained within the cag pathogenicity island, encodes the CagA oncoprotein, extremely important virulence factor of H. pylori. It is translocated into gastric epithelial cells via type IV secretion system and undergoes tyrosine phosphorylation at specific sequences known as EPIYA (Glu-Pro-Ile-Tyr-Ala) motifs located at the C-terminal of the protein[22]. These motifs are variable, consisting of different segments (A, B, C, D). While EPIYA-A and EPIYA-B are common in most H. pylori strains, EPIYA-C and EPIYA-D are characteristic of Western and East Asian strains, respectively, and the presence of EPIYA-D or multiple EPIYA-C segments is associated with an increased risk of GC development[23-26].
Older data has reported that, in Western countries, approximately 60%-70% of isolates are CagA-positive. However, in East Asian populations, notably Japan, China, and South Korea, nearly 100% of H. pylori strains contain the cagA gene[27]. Although more recent and broader studies regarding cagA prevalence in the west are scarce, a recent meta-analysis from 2024 further supports this data, reporting an 83% CagA positivity rate among H. pylori strains associated with gast
Upon phosphorylation, CagA binds to SHP-2 phosphatase, leading to hyperactivation of Ras/ERK signaling and inducing cytoskeletal rearrangement, which promotes the “hummingbird phenotype”, as well as increased cell proliferation and motility through Rac1 and MAPK pathways[29,30]. Non-phosphorylated CagA can also disrupt tight jun
VacA: Another important virulence factor is the vacuolating cytotoxin A (VacA), encoded by the vacA gene, found in virtually all strains. It presents variations mainly on the signal (s), middle (m), and intermediate (i) regions[24]. The s1, i1, and m1 vacA genotypes are linked to an increased risk of GC or precancerous conditions when compared to strains carrying the type s2, i2, or m2 of vacA[34], and the s1 and m1 strains have also been correlated with greater occurrence of severe gastric inflammation and ulceration[35].
VacA is able to insert into host epithelial cell membranes and form anion-selective pores that disrupt intracellular ionic gradients and induce the formation of large acidic vacuoles[36]. It also targets organelles like mitochondria, where it induces the release of cytochrome c and activates caspase-dependent apoptosis, and the endoplasmic reticulum, trig
NikS: A small nickel regulated non-coding RNA (sRNA) molecule that acts as a post-transcriptional regulator of genes related to H. pylori virulence factors, such as VacA and CagA[8]. Like other trans-encoded sRNAs, NikS acts by pairing complementary bases in regions of mRNA genes (usually closer to the 5' end in the translation initiation region), which prevents contact with ribosomes and downregulates gene expression[8,39]. The influence of nickel on NikS activity is directly related to the transcriptional regulator NikR, which responds to the availability of nickel in the environment[40]. An increased concentration of nickel induces NikR to bind to the promoter region of NikS, suppressing its expression[40,41]. Therefore, the higher the nickel concentration and the greater the suppressive action of NikR on NikS, which favors the expression of virulence factors.
Furthermore, Eisenbart et al[8] demonstrated that a variation in the number of thymine bases (Ts) in the hypermutable simple sequence repeats region can alter the transcription rate of NikS. This sequence of Ts is located immediately before the promoter, near the TATAAT box (box -10), so changes in the number of Ts alter the distance between box -10 and the binding sites of the NikR transcription factor, which can alter the affinity with RNA polymerase and, consequently, alter the transcription of NikS[8].
The modulation of CagA expression by NikS appears to be favorable for a better prognosis of H. pylori infection, since it down regulates its production[41]. Accordingly, it has been observed that GC patients have reduced NikS expression when compared to a non-cancer group[42].
Recently explored virulence aspects: In addition to the already described virulence factors, recent studies have high
Additionally, Sharafutdinov et al[46] found that a specific single-nucleotide polymorphism of the gene that encodes the High-temperature requirement A (HtrA), a bacterial serine protease that cleaves E-cadherin and occludin, is present in GC-associated strains. This gene variant promotes HtrA trimerization and enhances its proteolytic activity, leading to in
The immunological response against H. pylori is initiated when toll-like receptors (TLRs) detect pathogen-associated molecular patterns[47-50], triggering the activation of nuclear factor-kappaB (NF-κB), activator protein-1, and interferon regulatory factors, resulting in the expression of pro-inflammatory cytokines[51], antimicrobial peptides, type I interferons, and the recruitment of neutrophils, macrophages, and dendritic cells[47]. In this regard, H. pylori evasion mechanisms take place: Bacterial ligands like lipopolysaccharide (LPS/Lipid A) for TLR4[52] and flagellins for TLR5[53], have evolved to exhibit low intrinsic activation potential[54], antigen presentation is also impaired by metabolites like ADP-heptose, which activate microRNAs that suppress the antigen-presenting HLA-II expression[55], and modulates phagocytic destruction by interfering with reactive oxygen species mediated and nitric oxide mediated processes[56]. Together, these mechanisms help H. pylori evade the initial immune response and persist in the gastric environment.
As mentioned, NF-κB activation is a hallmark of H. pylori infection. It is closely linked to gastric inflammation and also plays important roles in carcinogenesis[57], either by impairing apoptosis, allowing gastric epithelial cells to survive and accumulate mutations, eventually contributing to malignant transformations[58-61] and also by modulating the infiltration of leukocytes and cytokine expression in the tumor microenvironment[62]. Moreover, Frauenlob et al[63], found that H. pylori triggers a form of innate immune memory in monocytes that leads to hypersensitivity to future challenges via NF-κB pathway amplification, which may worsen conditions like gastritis and GC.
The establishment of chronic inflammation is also linked to a complex interplay of different T helper cell profiles. While Th1 cells secrete interferon-gamma and IL-12, promoting macrophage activation and contributing to mucosal damage[64,65], Th17 cells produce IL-17A, IL-21, and IL-22, which recruit neutrophils and sustain chronic inflammation. Elevated levels of these cytokines were associated with increased gastric mucosal damage and progression to atrophic gastritis[64,65]. Moreover, Tumor necrosis factor-alpha (TNF-α) was also linked to an amplification of the inflammatory response and correlates with the severity of gastric lesions[66]. Nonetheless, regulatory T cells (Treg) are also pivotal for H. pylori establishment, as a balanced immunosuppression impairs bacterial clearance[67]. A predominance of Treg in detriment to the pro-inflammatory profiles in pediatric patients has been proposed as an explanation for the higher susceptibility to infection[68], along with the milder manifestations[69], and may even play a role in the down regulation of allergic reactions[70].
Variations in the balance between Th1 and Th17 responses have also been correlated to differences in disease pro
Lastly, regarding the humoral response against H. pylori, antibodies are incapable of clearing the infection, due to the bacterium’s evasion mechanisms and localization within the gastric mucus layer[73]. Anti-H. pylori IgG is also not the first choice as a diagnostic tool, as it shows limited sensitivity and cannot distinguish active from past infection[74]. As for biomarker use, Anti-CagA antibodies have been studied as possible indicators of cagA-positive strains and therefore, cancer risk, with good outcomes regarding sensibility and specificity[75,76], but further research is needed for its use to be included in guidelines.
H. pylori infection is the most important cause of chronic gastritis, characterized by persistent gastric inflammation[74]. The lesions typically begin in the antrum, causing non-atrophic gastritis, and may progress to atrophic gastritis, marked by the loss of gastric glands and the predisposition to intestinal metaplasia[77]. To better stratify cancer risk, the Operative Link on Gastritis Assessment staging system integrates the severity and distribution of atrophy into a stage-based model, from stage 0 to IV, with higher stages strongly associated with GC development[78]. H. pylori-induced gastritis is dynamic, and is influenced by bacterial virulence, host response, and environmental factors[79]. Its importance lies on its role as a precursor to peptic ulcer disease and gastric malignancies, making accurate assessing and classification essential for effective management.
The treatment for H. pylori related gastritis is multifaceted. Bacterial eradication by antibiotic regimen is an essential first step, however, antimicrobial therapy alone often cannot restore the gastric mucosa and resolve active inflammation[80], which is why the standard H. pylori eradication regimens also include proton pump inhibitors for managing gastric acidity[81]. While H2 receptor blockers and mucosal protective agents such as rebamipide are not part of standard eradication therapy, they may be useful for symptom relief following eradication, seeing they do not impact H. pylori and thus, do not interfere with short-term follow-up for treatment success[74]. Even so, mainly on atrophic variations, the gastric acid secretion may be compromised, and the administration of proton pump inhibitors may worsen problems associated with low stomach acidity, such as alterations in the gastric microbiota or reduced absorption of nutrients[82].
Even with successful eradication of H. pylori, residual atrophic changes or intestinal metaplasia often persist, high
For optimal accuracy in diagnosing H. pylori infection, current guidelines recommend at least two positive results in distinct diagnostic tests. However, achieving this standard can be challenging in routine clinical practice, and it is there
Invasive methods: H. pylori culture is a complex and time-consuming procedure that is generally considered unnecessary in routine clinical practice, seen that other diagnostic methods, including invasive and non-invasive tests, are capable of detecting the organism in most patients with greater efficiency[85,86].
Endoscopic imaging of H. pylori-infected patients shows mucosal edema, patchy redness, diffuse erythema, nodularity, and loss of regular arrangement of collecting venules pattern (RAC), an important parameter, as recent data suggests that the integrity of RAC in endoscopic imaging can accurately rule out H. pylori infection on the gastric corpus[87-90]. The Kyoto classification of gastritis is also implied, it is a score based on 5 endoscopic findings: Atrophy, intestinal metaplasia (IM), enlarged folds, nodularity, and diffuse redness, developed to evaluate risk of GC[91]. These scores provide high specificity and accuracy in identifying current H. pylori infections[92-96].
Histopathological examination is the most informative test in case of gastroscopy. The histological characteristics of chronic active gastritis, combined with the distinctive morphology of H. pylori and associated to diagnostic methods pro
This approach also facilitates a detailed evaluation of the associated inflammatory changes, enabling predictions about treatment efficacy, infection prognosis, and individual risk of developing GC[84]. Biopsies should ideally be obtained from areas of the gastric mucosa that appear even in apparently endoscopic normal aspects, seen that mucosal alterations, such as ulceration, erosions, atrophy, or intestinal metaplasia, may be associated with a reduced H. pylori density, which may compromise diagnostic accuracy[98-102].
The rapid urease test (RUT) leverages the high urease production of H pylori as a surrogate marker for bacterial detection in gastric biopsies[85]. Biopsies obtained from both the antrum and body of the stomach for RUT are preferred, as this approach demonstrates superior sensitivity compared to using antral biopsies alone[103]. Several diagnostic tech
Polymerase chain reaction (PCR) testing is another good alternative for diagnosis, because infection can be reliably detected using PCR, even at very low bacterial densities, where other diagnostic methods may yield negative results[107-109]. There are several CE-certified kits available for routine use, and, among biopsy-based tests, is considered one of the most accurate methods, particularly in patients with active bleeding[108]. PCR is not only used for detecting infection, but also for identification and analysis of pathogenic genes and specific mutations linked to antimicrobial resistance, such as the 16S rRNA[110] and 23S rRNA[111] genes.
Non-invasive methods: The Urea Breath Test (UBT) is frequently regarded as the gold standard for diagnosing H. pylori infection[112]. To conduct the UBT, the patient ingests urea labeled with either 13C or 14C isotopes. If H. pylori is present, its urease enzyme will hydrolyze the urea in the stomach, releasing labeled carbon dioxide, which can be detected in the patient's exhaled breath using either isotope ratio mass or infrared-spectrometry[85]. A recent meta-analysis demon
Detection of H. pylori antigen in stool specimens is also a form of diagnosis with high sensitivity and specificity (94.1% and 91.8%)[117], as well as an effective tool to evaluate eradication therapy. However, results may be affected due to de
Serology can also be employed in specific cases, including patients with bleeding ulcers, gastric atrophy, mucosa-associated lymphoid tissue lymphoma, gastric carcinoma, or those who have recently used antibiotics or proton pump inhibitors (PPIs), as these conditions can be related to low bacterial load that might result in a false negative in other tests[117]. Conversely, a major limitation for serology is that it cannot distinguish between active or past infection, as antibodies may persist long after bacterial clearance[119]. This limits its utility in monitoring eradication or in regions with high background seroprevalence.
Eradication therapy of H. pylori by antibiotics has been increasingly influenced by changes in the resistance profile around the world. Current resistance rates and their demographic variability point to local susceptibility testing and knowledge of the epidemiological profile of resistance as fundamental tools for successful and effective prescribing[120].
The most widely used therapeutic regimen is PPI-clarithromycin triple therapy. However, the elevated rates of resistance to clarithromycin[121,122] and frequent allergies to penicillin[123], often require alternatives, such as replacing clarithromycin with levofloxacin (250 mg-500 mg once a day), or changing to bismuth quadruple therapy (BQT). Interes
| Regimen | Drugs | Dosage | Recomendations |
| PPI-clarithromycin triple therapy | Clarithromycin; Amoxicillin; Omeprazole | 500 mg 12-hourly; 1 g 12-hourly; 20 mg 12-hourly | First line of treatment in situations of low local resistance to clarithromycin (< 15%); people without penicillin allergy; favorable individual susceptibility test for this regimen |
| Bismuth quadruple therapy | Metronidazole; Tetracycline; PPI; Bismuth subcitrate | 500 mg 8-hourly; 500 mg 6-hourly; 120-300 mg | First line of treatment in situations of high local resistance to clarithromycin (> 15%); people with penicillin allergy; when the individual susceptibility testing isn’t available |
| Rifabutin triple therapy | Rifabutin; Amoxicillin; Omeprazole | 50 mg 8-hourly; 1 g 8-hourly; 40 mg 8-hourly | Failure of other lines of treatment; recurrent Helicobacter pylori infections |
Few changes in therapeutic strategies can be seen in other regional treaties, as they adapt, for example, to the context of pharmacological availability and the resistance profile. Coelho et al[128] recommends PPI-clarithromycin triple therapy as the first line of treatment, despite increasing resistance rates, and rifabutin should only be used in the event of availability and lack of therapeutic response to other lines of treatment, following antimicrobial susceptibility testing. As for the Chinese consensus on H. pylori infection, modified dual therapy (Amoxicillin + Potent acid suppression) is considered the most recommended empirical therapeutic strategy and there is no division into therapeutic lines, as the therapy chosen will be based on local resistance, adverse effects, costs and efficacy[129]. Fallone et al[130] recommends BQT as the first line of treatment, except when resistance rates to clarithromycin are < 15% or the eradication rate is > 85%, and the use of rifabutin triple therapy is restricted to cases of failure of the other three previous therapeutic alternatives.
Although test-and-treat has been the standard method of prevention of H. pylori associated diseases, it has significant impact in terms of antibiotic consumption and resistance[74]. In this context, tailored therapy (therapy guided by antibiotic susceptibility tests) emerges as a way to minimize these issues, while also increasing effectiveness. However, there are still many obstacles to that practice. Culture based methods are extremely specific but require extensive and spe
Another limitation to tailored therapy is the dependency to an invasive procedure like an endoscopy, but recent studies have demonstrated promising results for the implying of molecular based tests using stool samples[133,134]. As
P-CABs are drugs that have been on the rise in gastroenterology as an alternative to PPIs. Its mechanism of action consists of reversible H+/K+-ATPase inhibition during the secretory stage of gastric parietal cells, achieving satisfactory effects within a few hours of administration[10]. Comparative studies of H. pylori eradication with P-CAB-based triple therapy and PPI-based triple therapy point to superior or non-inferior efficacy of the use of P-CABs, considering them as a successful first-line therapeutic alternative[139-141]. The pharmacokinetic properties of vonoprazan (one of the most studied P-CABs), such as speed of action, prolonged effect and reversibility of H+/K+-ATPase binding are also favorable characteristics for its clinical application[142]. Triple therapy could include clarithromycin, amoxicillin and 20 mg of vo
The use of probiotics in association with the therapeutic regimens for eradication of H. pylori has also been a topic of extensive research, as both the bacteria and the antibiotics cause an impact on the gut microbiota. Yet, the data regarding this topic remains conflicting. For instance, some studies propose probiotics are only beneficial in alleviating side effects[11,144], while others suggest its use also increases eradication rates[145]. The recommendations for prescription are also unclear: While a meta-analysis by Zhang et al[146] found that using a single probiotic agent for 10 or more day retrieved better results, another meta-analysis, by Wang et al[147], demonstrated that the use of different probiotics combined, for more than 14 days, is more effective. In regard of the specific agents, the ones from the genus Lactobacilli and Saccharomyces seem to be the most promising[148,149]. Notably, Saccharomyces is the only recommended for pediatric use by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition[150]. Even with the uncertain evidence, the prescription of probiotics has shown to be significantly beneficial, but more clinical studies investigating the potentiality of specific agents and strains are needed in order to formulate more specific guidelines for their use.
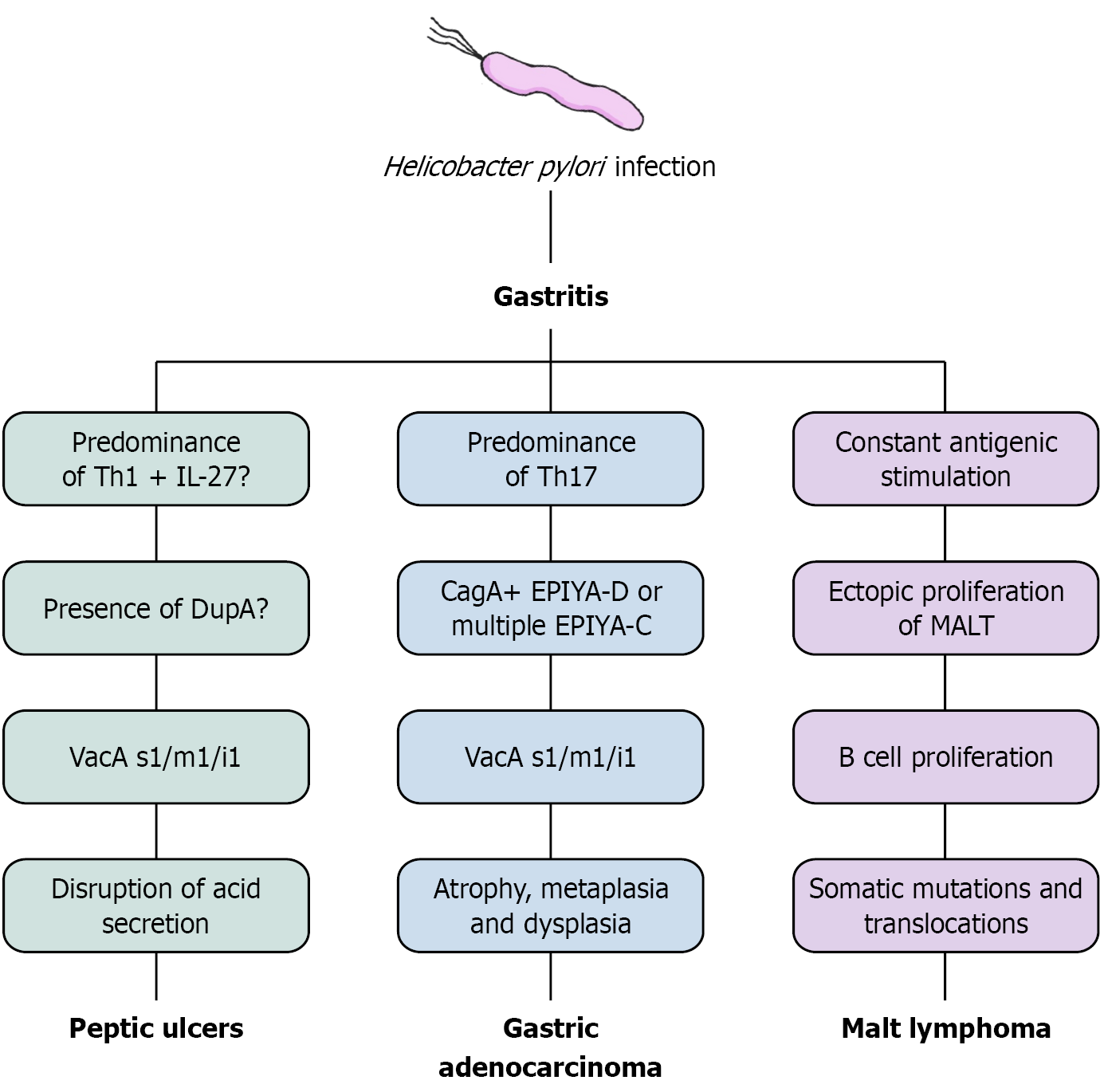
H. pylori infection and chronic gastritis can evolve to different gastric and non-gastric conditions, associated with bacterial virulence, immunological responses and environmental factors. Figure 2 summarizes some of the proposed mechanisms involved in the development of gastric diseases associated to the infection, which will be further discussed.
H. pylori infection is the prevailing cause of gastric and duodenal peptic ulcers led by chronic gastritis[151], all of which fall under the larger category of peptic ulcer disease (PUD). While duodenal ulcers are clinically presented as a disruption to the mucosal surface of the duodenum that extends beyond the pre-epithelial, epithelial, and subepithelial layers[152], gastric ulcers consist of damage in the mucosal part of the stomach breaching the muscularis mucosa and exceeding 5 mm in diameter[153].
Knowing H. pylori has tropism to gastric type epithelium, the difference on the site of ulceration is deeply linked with the gastritis extent and distribution[6]. The duodenum is normally a hostile environment to H. pylori, but its induced inflammation and dysregulation of acid secretion is capable of lowering the pH in the duodenal bulb, precipitating the glycine conjugated bile acids and allowing the bacteria to infect mostly its metaplasic gastric mucosa[154]. Similarly, when the gastritis pattern predominates in the stomach’s antrum, H. pylori-induced inflammation disrupts the secretions of gastric acid-regulating hormones such as somatostatin, stimulating gastrin secretion[155] and leading to increased acid production directly via parietal cells and indirectly via histamine release from enterochromaffin-like cells[156] resulting in a hyperacidic gastric environment and a damaged tissue, prone to ulceration[157].
Several virulence factors may have a role in increasing the risk of peptic ulceration, such as the s1 and i1 types of VacA[24,158], and strains positive for the duodenal ulcer promoting gene A (dupA), due to more severe gastritis and increased expression of IL-8 in the mucosal lining. However, while some studies have linked dupA to a higher risk of developing duodenal ulcers and GC, others have failed to find consistent evidence supporting these associations[159-161]. Other de
Gastric and duodenal ulcers, within the spectrum of PUD, have distinct and non-specific symptoms. Thus, patients with gastric ulcers tend to have postprandial abdominal pain, nausea, vomiting, and weight loss due to aversion to food, a result of the intensification of pain after eating, while duodenal ulcers commonly present as nocturnal abdominal pain, hunger followed by bloating, and nausea[168]. The main complications of peptic ulcer disease are bleeding, presented as melena or hematemesis, and perforation, presented as a sudden onset of intense pain in the upper abdomen, and gastric outlet obstruction[168]. In untreated peptic ulcer disease, symptoms often recur due to a cycle of spontaneous healing followed by relapse, driven by the continued presence of underlying causes such as H. pylori infection or NSAID usage[168].
There is robust evidence through meta-analyses that eradicating H. pylori has positive outcomes in peptic ulcer disease of both stomach and duodenum, with or without complications[169-172]. More recent evidence suggests that in H. pylori-positive duodenal ulcers eradication therapy is superior to other medical treatments, but the same has not yet been proven for H. pylori-positive gastric ulcers[171]. Eradication of H. pylori is also related to lower recurrence of gastric and duodenal ulcers in patients who were not put on anti secretory therapy for management of the disease[169].
As shown, H. pylori is a significant risk factor for gastric adenocarcinoma (GAC), driven by complex mechanisms involving both bacterial factors and host characteristics[173]. Based on epidemiological observations, Pelayo Correa proposed a model describing the cascade of events leading to intestinal-type gastric carcinogenesis: Chronic active gastritis and progresses to multifocal gastric atrophy, IM, low-grade dysplasia, high-grade dysplasia, and eventually gastric adenocarcinoma[174]. Although H. pylori infection is widely acknowledged as the most significant cause of GAC[175], metabolic, environmental, genomic and epigenetic factors all contribute to carcinogenesis[7], including family history, lifestyle factors (dietary habits, smoking, or alcohol consumption), and even Epstein-Barr virus infection[176], which explain why not all individuals infected with H. pylori develop GC.
Another determinant to whether or not a H. pylori-positive individual could evolve to GAC is an infection by more or less virulent strains. CagA-positive strains are highly correlated to GC, due to its capability of by altering host cellular signaling and creating a pro-oncogenic environment[177], and their infections result in more severe clinical outcomes and significantly elevate the risk of GC beyond that observed with H. pylori infection alone[178]. VacA is another key viru
Gastric cancer is often asymptomatic in its early stages. When symptoms do appear, they typically include dysphagia, asthenia, indigestion, vomiting, weight loss, early satiety, and/or iron-deficiency anemia[185]. These highly unspecific symptoms frequently lead to delayed diagnosis, resulting in approximately 60% of GC patients being ineligible for curative treatment due to late-stage presentation or comorbidities[186].
Eradicating H. pylori is currently regarded as the most effective strategy for preventing gastric adenocarcinoma, including proximal GC[74], especially among those without premalignant gastric lesions at the start of treatment[187], but the limited impact on overall mortality highlights the need for further research into the broader benefits of eradi
A low-grade B-cell neoplasia, the gastric mucosa-associated lymphoid tissue lymphoma (GML) is evidently correlated with H. pylori infection[189]. Its pathogenesis relies on formation of mucosa-associated lymphoid tissue (MALT) through chronic inflammation, persistent antigenic stimulation and cytokine liberation[190,191]. T helper cells also play a crucial role in lymphoid tissue formation by supporting polyclonal B cells[192,193], which are capable of recognizing auto antigens from the gastric mucosa due to cross-reactivity with bacterial epitopes. As a result, polyclonal B cells increase in number in the region, heightening the risk of translocations and double-strand DNA mutations, culminating in the emergence of antigen-dependent marginal zone lymphomas[191,194,195].
In this context, the translocation t (11;18)(q21;q21) is observed in 10% to 50% of GML cases[196-198] and leads to the activation of NF-κB mediated by the disruption of a signalosome complex involving BCL10, CARD11, and MALT1. The latter, a fusion protein, is associated with advanced stages of MALT lymphoma[199-201]. Furthermore, chronic exposure to antigens induces the release of reactive oxygen species through immune responses, which contribute to the deve
When evaluating the clinical features of GML, a wide variability of symptoms is associated with the condition, which may include indigestion, nausea, vomiting, abdominal pain and distension, as well as weight loss[204]. Its diagnosis is primarily achieved through analyses conducted via biopsy of the affected tissue[18-20]. Treatment, according to clinical guidelines, focuses on eradicating H. pylori, which results in lymphoma cure in over 75% of early-stage GML patients with H. pylori infection, and up to 29.3% of patients without H. pylori infection, who still achieve complete remission following bacterial eradication therapy despite the absence of an infection diagnosis[205-208].
These findings are conflicting, as it was anticipated that H. pylori eradication therapy would have no effect on remis
Besides the usually associated gastric diseases, H. pylori has shown correlation to extra-gastric conditions. As previously reviewed by Santos et al[216] the non-gastric manifestations found in literature can range from hematological and autoimmune to affecting different organ systems, although some still lack robust evidence to their correlations.
Iron deficiency anemia (IDA) and vitamin B12 deficiency are two of the most well-established extra-gastric manifestations of H. pylori infection, as chronic inflammation can lead to hypochlorhydria and reduced secretion of intrinsic factor, impairing iron and B12 absorption, respectively. Although these conditions are mostly correlated to cases of atrophic gastritis, they have also been observed in patients with milder inflammation[217]. Clinical trials and meta-analyses have confirmed that eradication therapy significantly improves serological parameters in H. pylori-positive individuals with IDA[218,219] and B12 deficiency[220], encouraging clinicians to test for H. pylori infection when presented to unexplained cases of these conditions.
Another condition correlated to H. pylori infection is immune thrombocytopenic purpura (ITP): An autoimmune disease associated with low platelet serum levels, allowing the occurrence of hemorrhagic lesions throughout the body. The mechanism of which H. pylori infections induces ITP is not completely elucidated yet, but the fact that H. pylori eradication in patients with ITP leads to satisfactory improvement in platelet serum levels strengthens the hypothesis that the bacterium influences the pathogenesis of this disease[221-223]. One of the theories regarding H. pylori influence is a molecular mimicry of CagA, that being, anti-CagA antibodies cross-reacting with platelet glycoprotein IIb/IIIa complex[224], opsonizing and facilitating phagocytosis by Kupffer cells and macrophages in the spleen, while also impairing megakaryopoiesis[225,226].
Due to the high prevalence of H. pylori infection[227] and its association with the development of severe diseases[206,228,229], as well as other clinically significant pathologies, including extra-gastric conditions[216], the ineffective immune response in eradicating the bacterium in a substantial proportion of patients[230] and the frequent inefficacy of antibiotic therapy due to increasing bacterial resistance[231], there is an urgent need for the development of prophylactic and therapeutic vaccines against H. pylori.
Several bacterial antigens have been identified as promising targets for vaccine development, such as urease[12], CagA[232-235], and VacA[232,236], singled out as some of the most important virulence factors for H. pylori infection and colonization, as well as catalase, BabA, HspA, GGT, FliD, and others[12]. However, due to the challenges in achieving the desired protective effects, the development of a multi-epitope and recombinant vaccines is under consideration[237,238]. One study reported promising results, demonstrating immunoprotective effects and reduced inflammation associated with infection by using the cholera toxin B subunit combined with tandem copies of T- and B-cell epitopes from the UreA and UreB subunits of urease (CTB-UE), administered in a Mongolian gerbil model against H. pylori[237].
Another study involving 80 mice and employing the EpiVax/H. pylori vaccine-a multi-epitope approach combining DNA vaccination followed by a peptide-liposome formulation-showed efficacy and high interferon-γ production[239].
Helicovaxor utilized the recombinant expression of the HpaA adhesion antigen, either alone or in combination with colonization factor antigens from enterotoxigenic Escherichia coli, enhancing the immune response against HpaA[240]. Meanwhile, another vaccine, developed by the Murdoch Children's Research Institute and currently in preclinical stages, does not aim to eradicate the infection but rather to reduce inflammation. This vaccine employs HtrA, the sole serine protease produced by H. pylori, and has demonstrated inflammation inhibition in mice[12].
Some promising vaccines are administered orally, such as the probiotic vaccine that employs Lactococcus lactis containing the UreB-IL-2 protein. This vaccine elicits an immune response with the production of anti-urease antibodies and has demonstrated the ability to reduce H. pylori colonization, confirming UreB-IL-2 as a potent candidate for oral vaccines[241].
Several other published clinical studies have not achieved the anticipated success, as immunity was not effectively developed. Nevertheless, these studies remain of significant importance for guiding future research[12].
Currently, the website https://clinicaltrials.gov lists four completed studies on both prophylactic and therapeutic vaccines for H. pylori infections. Among these is a Phase I clinical trial by Imevax evaluating the safety of the therapeutic vaccine IMX101 in both infected patients and H. pylori-negative individuals. This vaccine incorporates the GGT antigen, an undisclosed H. pylori outer membrane protein, and a mucosal adjuvant. Another completed study, a Phase III trial conducted by the Jiangsu Province Centers for Disease Control and Prevention, investigates the safety of an oral recombinant H. pylori vaccine in Chinese children. The trials identified are summarized in Table 2.
| Vaccine classification | Intervention/treatment | ClinicalTrials.gov ID | Phase | Status | Sponsor |
| Therapeutic vaccines | Biological: CTA control/biological: IMX101 vaccine | NCT03270800 | 1 | Completed | ImevaX |
| Prophylactic vaccines | Biological: H. pylori vacines/biological: Placebo Vaccine | NCT00736476 | 1 | Completed | Novartis Vaccines |
| Biological: H. pylori vaccine/biological: Placebo | NCT02302170 | 3 | Completed | Jiangsu Province Centers for Disease Control and Prevention | |
| Biological: H. pylori vaccine/biological: Placebo | NCT00613665 | 1 | Completed | Novartis Vaccines |
Important advances have been made in understanding the complex interplay between H. pylori, its virulence mecha
| 1. | Yang H, Hu B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediators Inflamm. 2022;2022:2944156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Duan M, Li Y, Liu J, Zhang W, Dong Y, Han Z, Wan M, Lin M, Lin B, Kong Q, Ding Y, Yang X, Zuo X, Li Y. Transmission routes and patterns of helicobacter pylori. Helicobacter. 2023;28:e12945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 3. | Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22:Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | McCallion WA, Murray LJ, Bailie AG, Dalzell AM, O'Reilly DP, Bamford KB. Helicobacter pylori infection in children: relation with current household living conditions. Gut. 1996;39:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 290] [Reference Citation Analysis (0)] |
| 6. | Shah SAR, Rahman H, Qasim M, Akram MS, Saygideger Y, Puspita N, Saygıdeğer Demir B, Alzahrani KJ, Rehman MFU, Alzahrani FM, Alblihd MA. Differential Proteomics of Helicobacter pylori Isolates from Gastritis, Ulcer, and Cancer Patients: First Study from Northwest Pakistan. Medicina (Kaunas). 2022;58:1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Toh JWT, Wilson RB. Pathways of Gastric Carcinogenesis, Helicobacter pylori Virulence and Interactions with Antioxidant Systems, Vitamin C and Phytochemicals. Int J Mol Sci. 2020;21:6451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Eisenbart SK, Alzheimer M, Pernitzsch SR, Dietrich S, Stahl S, Sharma CM. A Repeat-Associated Small RNA Controls the Major Virulence Factors of Helicobacter pylori. Mol Cell. 2020;80:210-226.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil. 2018;24:334-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 11. | He C, Xie Y, Zhu Y, Zhuang K, Huo L, Yu Y, Guo Q, Shu X, Xiong Z, Zhang Z, Lyu B, Lu N. Probiotics modulate gastrointestinal microbiota after Helicobacter pylori eradication: A multicenter randomized double-blind placebo-controlled trial. Front Immunol. 2022;13:1033063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Dos Santos Viana I, Cordeiro Santos ML, Santos Marques H, Lima de Souza Gonçalves V, Bittencourt de Brito B, França da Silva FA, Oliveira E Silva N, Dantas Pinheiro F, Fernandes Teixeira A, Tanajura Costa D, Oliveira Souza B, Lima Souza C, Vasconcelos Oliveira M, Freire de Melo F. Vaccine development against Helicobacter pylori: from ideal antigens to the current landscape. Expert Rev Vaccines. 2021;20:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Ali A, AlHussaini KI. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms. 2024;12:222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 14. | Igarashi M, Kitada Y, Yoshiyama H, Takagi A, Miwa T, Koga Y. Ammonia as an accelerator of tumor necrosis factor alpha-induced apoptosis of gastric epithelial cells in Helicobacter pylori infection. Infect Immun. 2001;69:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Foegeding NJ, Raghunathan K, Campbell AM, Kim SW, Lau KS, Kenworthy AK, Cover TL, Ohi MD. Intracellular Degradation of Helicobacter pylori VacA Toxin as a Determinant of Gastric Epithelial Cell Viability. Infect Immun. 2019;87:e00783-e00718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Johnson KS, Ottemann KM. Colonization, localization, and inflammation: the roles of H. pylori chemotaxis in vivo. Curr Opin Microbiol. 2018;41:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Doohan D, Rezkitha YAA, Waskito LA, Yamaoka Y, Miftahussurur M. Helicobacter pylori BabA-SabA Key Roles in the Adherence Phase: The Synergic Mechanism for Successful Colonization and Disease Development. Toxins (Basel). 2021;13:485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Xu C, Soyfoo DM, Wu Y, Xu S. Virulence of Helicobacter pylori outer membrane proteins: an updated review. Eur J Clin Microbiol Infect Dis. 2020;39:1821-1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Abdullah SK, Bakir WA, Alsikafi MI. Analysis of Correlation between the Important Helicobacter pylori Virulence Genes (CagA, SabA and Oip) and Gastric Epithelial Stem Cells (LGR5) in Patients with Gastric Disease. MMJ. 2023;22:98-105. [DOI] [Full Text] |
| 20. | Maubach G, Sokolova O, Täger C, Naumann M. CEACAMs interaction with Helicobacter pylori HopQ supports the type 4 secretion system-dependent activation of non-canonical NF-κB. Int J Med Microbiol. 2020;310:151444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Nguyen QA, Schmitt L, Mejías-Luque R, Gerhard M. Effects of Helicobacter pylori adhesin HopQ binding to CEACAM receptors in the human stomach. Front Immunol. 2023;14:1113478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 22. | Jouimyi MR, Bounder G, Boura H, Essaidi I, Bendahmane A, Benomar H, Zerouali K, Lebrazi H, Kettani A, Gbonon VC, Fatima M. The EPIYA-ABCC motif of Helicobacter pylori cagA gene and gastric carcinogenesis in Casablanca population. Afr Health Sci. 2022;22:573-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Queiroz DM, Silva CI, Goncalves MH, Braga-Neto MB, Fialho AB, Fialho AM, Rocha GA, Rocha AM, Batista SA, Guerrant RL, Lima AA, Braga LL. Higher frequency of cagA EPIYA-C phosphorylation sites in H. pylori strains from first-degree relatives of gastric cancer patients. BMC Gastroenterol. 2012;12:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D, Rugge M, Plebani M, Atherton JC. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 25. | Yamaoka Y, Osato MS, Sepulveda AR, Gutierrez O, Figura N, Kim JG, Kodama T, Kashima K, Graham DY. Molecular epidemiology of Helicobacter pylori: separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect. 2000;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Kanada R, Uchida T, Tsukamoto Y, Nguyen LT, Hijiya N, Matsuura K, Kodama M, Okimoto T, Murakami K, Fujioka T, Yanagisawa S, Moriyama M. Genotyping of the cagA gene of Helicobacter pylori on immunohistochemistry with East Asian CagA-specific antibody. Pathol Int. 2008;58:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci. 2005;96:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Naing C, Aung HH, Aye SN, Poovorawan Y, Whittaker MA. CagA toxin and risk of Helicobacter pylori-infected gastric phenotype: A meta-analysis of observational studies. PLoS One. 2024;19:e0307172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Canzian F, Rizzato C, Obazee O, Stein A, Flores-Luna L, Camorlinga-Ponce M, Mendez-Tenorio A, Vivas J, Trujillo E, Jang H, Chen W, Kasamatsu E, Bravo MM, Torres J, Muñoz N, Kato I. Genetic polymorphisms in the cag pathogenicity island of Helicobacter pylori and risk of stomach cancer and high-grade premalignant gastric lesions. Int J Cancer. 2020;147:2437-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Tegtmeyer N, Harrer A, Rottner K, Backert S. Helicobacter pylori CagA Induces Cortactin Y-470 Phosphorylation-Dependent Gastric Epithelial Cell Scattering via Abl, Vav2 and Rac1 Activation. Cancers (Basel). 2021;13:4241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM Jr, Azuma T, Hatakeyama M. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 32. | Nesić D, Miller MC, Quinkert ZT, Stein M, Chait BT, Stebbins CE. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat Struct Mol Biol. 2010;17:130-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 34. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, Carneiro F, Sobrinho-Simões M. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 473] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 35. | Atherton JC, Peek RM Jr, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 408] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 36. | Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 37. | McClain MS, Beckett AC, Cover TL. Helicobacter pylori Vacuolating Toxin and Gastric Cancer. Toxins (Basel). 2017;9:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Sewald X, Gebert-Vogl B, Prassl S, Barwig I, Weiss E, Fabbri M, Osicka R, Schiemann M, Busch DH, Semmrich M, Holzmann B, Sebo P, Haas R. Integrin subunit CD18 Is the T-lymphocyte receptor for the Helicobacter pylori vacuolating cytotoxin. Cell Host Microbe. 2008;3:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol. 2011;3:a003798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 573] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 40. | Chivers PT, Sauer RT. Regulation of high affinity nickel uptake in bacteria. Ni2+-Dependent interaction of NikR with wild-type and mutant operator sites. J Biol Chem. 2000;275:19735-19741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Freire de Melo F, Marques HS, Fellipe Bueno Lemos F, Silva Luz M, Rocha Pinheiro SL, de Carvalho LS, Souza CL, Oliveira MV. Role of nickel-regulated small RNA in modulation of Helicobacter pylori virulence factors. World J Clin Cases. 2022;10:11283-11291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Kinoshita-Daitoku R, Kiga K, Miyakoshi M, Otsubo R, Ogura Y, Sanada T, Bo Z, Phuoc TV, Okano T, Iida T, Yokomori R, Kuroda E, Hirukawa S, Tanaka M, Sood A, Subsomwong P, Ashida H, Binh TT, Nguyen LT, Van KV, Ho DQD, Nakai K, Suzuki T, Yamaoka Y, Hayashi T, Mimuro H. A bacterial small RNA regulates the adaptation of Helicobacter pylori to the host environment. Nat Commun. 2021;12:2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Mucito-Varela E, Castillo-Rojas G, Calva JJ, López-Vidal Y. Integrative and Conjugative Elements of Helicobacter pylori Are Hypothetical Virulence Factors Associated With Gastric Cancer. Front Cell Infect Microbiol. 2020;10:525335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Silva B, Nunes A, Vale FF, Rocha R, Gomes JP, Dias R, Oleastro M. The expression of Helicobacter pylori tfs plasticity zone cluster is regulated by pH and adherence, and its composition is associated with differential gastric IL-8 secretion. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Waskito LA, Miftahussurur M, Lusida MI, Syam AF, Suzuki R, Subsomwong P, Uchida T, Hamdan M, Nasronudin, Yamaoka Y. Distribution and clinical associations of integrating conjugative elements and cag pathogenicity islands of Helicobacter pylori in Indonesia. Sci Rep. 2018;8:6073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Sharafutdinov I, Tegtmeyer N, Linz B, Rohde M, Vieth M, Tay AC, Lamichhane B, Tuan VP, Fauzia KA, Sticht H, Yamaoka Y, Marshall BJ, Backert S. A single-nucleotide polymorphism in Helicobacter pylori promotes gastric cancer development. Cell Host Microbe. 2023;31:1345-1358.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 47. | Cheok YY, Tan GMY, Lee CYQ, Abdullah S, Looi CY, Wong WF. Innate Immunity Crosstalk with Helicobacter pylori: Pattern Recognition Receptors and Cellular Responses. Int J Mol Sci. 2022;23:7561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Castaño-Rodríguez N, Kaakoush NO, Mitchell HM. Pattern-recognition receptors and gastric cancer. Front Immunol. 2014;5:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1385] [Cited by in RCA: 1348] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 50. | Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1286] [Cited by in RCA: 1298] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 51. | Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 52. | Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446-6454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM Jr. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis. 2004;189:1914-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Faass L, Hauke M, Stein SC, Josenhans C. Innate immune activation and modulatory factors of Helicobacter pylori towards phagocytic and nonphagocytic cells. Curr Opin Immunol. 2023;82:102301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 55. | Coletta S, Battaggia G, Della Bella C, Furlani M, Hauke M, Faass L, D'Elios MM, Josenhans C, de Bernard M. ADP-heptose enables Helicobacter pylori to exploit macrophages as a survival niche by suppressing antigen-presenting HLA-II expression. FEBS Lett. 2021;595:2160-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Marzhoseyni Z, Mousavi MJ, Ghotloo S. Helicobacter pylori antigens as immunomodulators of immune system. Helicobacter. 2024;29:e13058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 57. | Maubach G, Vieth M, Boccellato F, Naumann M. Helicobacter pylori-induced NF-κB: trailblazer for gastric pathophysiology. Trends Mol Med. 2022;28:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Dang Y, Zhang Y, Xu L, Zhou X, Gu Y, Yu J, Jin S, Ji H, Shu Y, Zhang G, Cui S, Sun J. PUMA-mediated epithelial cell apoptosis promotes Helicobacter pylori infection-mediated gastritis. Cell Death Dis. 2020;11:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Lim MCC, Maubach G, Sokolova O, Feige MH, Diezko R, Buchbinder J, Backert S, Schlüter D, Lavrik IN, Naumann M. Pathogen-induced ubiquitin-editing enzyme A20 bifunctionally shuts off NF-κB and caspase-8-dependent apoptotic cell death. Cell Death Differ. 2017;24:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Liu AQ, Xie Z, Chen XN, Feng J, Chen JW, Qin FJ, Ge LY. Fas-associated factor 1 inhibits tumor growth by suppressing Helicobacter pylori-induced activation of NF-κB signaling in human gastric carcinoma. Oncotarget. 2017;8:7999-8009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Zhu S, Soutto M, Chen Z, Peng D, Romero-Gallo J, Krishna US, Belkhiri A, Washington MK, Peek R, El-Rifai W. Helicobacter pylori-induced cell death is counteracted by NF-κB-mediated transcription of DARPP-32. Gut. 2017;66:761-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Liu M, Hu Z, Wang C, Zhang Y. The TLR/MyD88 signalling cascade in inflammation and gastric cancer: the immune regulatory network of Helicobacter pylori. J Mol Med (Berl). 2023;101:767-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Frauenlob T, Neuper T, Regl C, Schaepertoens V, Unger MS, Oswald AL, Dang HH, Huber CG, Aberger F, Wessler S, Horejs-Hoeck J. Helicobacter pylori induces a novel form of innate immune memory via accumulation of NF-кB proteins. Front Immunol. 2023;14:1290833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Arachchi PS, Fernando N, Weerasekera MM, Senevirathna B, Weerasekera DD, Gunasekara CP. Proinflammatory Cytokine IL-17 Shows a Significant Association with Helicobacter pylori Infection and Disease Severity. Gastroenterol Res Pract. 2017;2017:6265150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Dewayani A, Fauzia KA, Alfaray RI, Waskito LA, Doohan D, Rezkitha YAA, Abdurachman A, Kobayashi T, I'tishom R, Yamaoka Y, Miftahussurur M. The Roles of IL-17, IL-21, and IL-23 in the Helicobacter pylori Infection and Gastrointestinal Inflammation: A Review. Toxins (Basel). 2021;13:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Rahimian G, Shahini Shams Abadi M, Mirzaei Y, Hussein Mer A, Ahmadi R, Azadegan-Dehkordi F. Relationship between mucosal TNF-α expression and Th1, Th17, Th22 and Treg responses in Helicobacter pylori infection. AMB Express. 2022;12:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 67. | Talayev V, Svetlova M, Zaichenko I, Voronina E, Babaykina O, Neumoina N, Perfilova K. CCR6(+) T helper cells and regulatory T cells in the blood and gastric mucosa during Helicobacter pylori infection. Helicobacter. 2024;29:e13097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 68. | Roma E, Miele E. Helicobacter pylori Infection in Pediatrics. Helicobacter. 2015;20 Suppl 1:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Freire de Melo F, Rocha AM, Rocha GA, Pedroso SH, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R, Magalhães Queiroz DM. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect. 2012;14:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | León MA, Palma C, Hernández C, Sandoval M, Cofre C, Perez-Mateluna G, Borzutzky A, Harris PR, Serrano CA. Helicobacter pylori pediatric infection changes FcεRI expression in dendritic cells and Treg profile in vivo and in vitro. Microbes Infect. 2019;21:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Rocha GA, de Melo FF, Cabral MMDA, de Brito BB, da Silva FAF, Queiroz DMM. Interleukin-27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2020;25:e12667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Yu B, Xiang L, Peppelenbosch MP, Fuhler GM. Overlapping cytokines in H. pylori infection and gastric cancer: A tandem meta-analysis. Front Immunol. 2023;14:1125658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Sutton P, Chionh YT. Why can't we make an effective vaccine against Helicobacter pylori? Expert Rev Vaccines. 2013;12:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 844] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 75. | Flores-Luna L, Camorlinga-Ponce M, Hernandez-Suarez G, Kasamatsu E, Martínez ME, Murillo R, Lazcano E, Torres J. The utility of serologic tests as biomarkers for Helicobacter pylori-associated precancerous lesions and gastric cancer varies between Latin American countries. Cancer Causes Control. 2013;24:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Miftahussurur M, Doohan D, Syam AF, Nusi IA, Waskito LA, Fauzia KA, Rezkitha YAA, Dewayani A, I'tishom R, Maulahela H, Uchida T, Yamaoka Y. The validation of the Helicobacter pylori CagA typing by immunohistochemistry: nationwide application in Indonesia. Acta Histochem. 2020;122:151594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 77. | Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, Wang C, Liu Y, Sha W, Wang B, Li Y, Zhang G, Li Y, Shi R, Xu J, Li Y, Huang M, Han S, Liu J, Ren X, Xie P, Wang Z, Cui L, Sheng J, Luo H, Wang Z, Zhao X, Dai N, Nie Y, Zou Y, Xia B, Fan Z, Chen Z, Lin S, Li ZS; Chinese Chronic Gastritis Research group. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Bertz S, Angeloni M, Drgac J, Falkeis C, Lang-Schwarz C, Sterlacci W, Veits L, Hartmann A, Vieth M. Helicobacter Infection and Gastric Adenoma. Microorganisms. 2021;9:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Waldum H, Fossmark R. Gastritis, Gastric Polyps and Gastric Cancer. Int J Mol Sci. 2021;22:6548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 80. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 722] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 81. | Jia J, Zhao H, Li F, Zheng Q, Wang G, Li D, Liu Y. Research on drug treatment and the novel signaling pathway of chronic atrophic gastritis. Biomed Pharmacother. 2024;176:116912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 82. | Annibale B, Esposito G, Lahner E. A current clinical overview of atrophic gastritis. Expert Rev Gastroenterol Hepatol. 2020;14:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 84. | Fischbach W, Bornschein J, Hoffmann JC, Koletzko S, Link A, Macke L, Malfertheiner P, Schütte K, Selgrad DM, Suerbaum S, Schulz C; Collaborators. Update S2k-Guideline Helicobacter pylori and gastroduodenal ulcer disease of the German Society of Gastroenterology, Digestive and Metabolic Diseases (DGVS). Z Gastroenterol. 2024;62:261-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20:12847-12859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (4)] |
| 86. | Glupczynski Y. Microbiological and serological diagnostic tests for Helicobacter pylori: an overview. Br Med Bull. 1998;54:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Yuan C, Lin XM, Ou Y, Cai L, Cheng Q, Zhou P, Liao J. Association between regular arrangement of collecting venules and Helicobacter pylori status in routine endoscopy. BMC Gastroenterol. 2021;21:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 88. | Hojo M, Nagahara A, Kudo T, Takeda T, Ikuse T, Matsumoto K, Ueda K, Ueyama H, Matsumoto K, Asaoka D, Shimizu T. Endoscopic findings of Helicobacter pylori gastritis in children and young adults based on the Kyoto classification of gastritis and age-associated changes. JGH Open. 2021;5:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Garcés-Durán R, García-Rodríguez A, Córdova H, Cuatrecasas M, Ginès À, González-Suárez B, Araujo I, Llach J, Fernández-Esparrach G. Association between a regular arrangement of collecting venules and absence of Helicobacter pylori infection in a European population. Gastrointest Endosc. 2019;90:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Seo JY, Ahn JY, Kim S, Na HK, Lee JH, Jung KW, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. Predicting Helicobacter pylori infection from endoscopic features. Korean J Intern Med. 2024;39:439-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 91. | Toyoshima O, Nishizawa T. Kyoto classification of gastritis: Advances and future perspectives in endoscopic diagnosis of gastritis. World J Gastroenterol. 2022;28:6078-6089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (4)] |
| 92. | Zhao J, Xu S, Gao Y, Lei Y, Zou B, Zhou M, Chang D, Dong L, Qin B. Accuracy of Endoscopic Diagnosis of Helicobacter pylori Based on the Kyoto Classification of Gastritis: A Multicenter Study. Front Oncol. 2020;10:599218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Ebigbo A, Marienhagen J, Messmann H. Regular arrangement of collecting venules and the Kimura-Takemoto classification for the endoscopic diagnosis of Helicobacter pylori infection: Evaluation in a Western setting. Dig Endosc. 2021;33:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Toyoshima O, Nishizawa T, Arita M, Kataoka Y, Sakitani K, Yoshida S, Yamashita H, Hata K, Watanabe H, Suzuki H. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol. 2018;24:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 95. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 96. | Fiuza F, Maluf-Filho F, Ide E, Furuya CK Jr, Fylyk SN, Ruas JN, Stabach L, Araujo GA, Matuguma SE, Uemura RS, Sakai CM, Yamazaki K, Ueda SS, Sakai P, Martins BC. Association between mucosal surface pattern under near focus technology and Helicobacter pylori infection. World J Gastrointest Endosc. 2021;13:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 97. | Khan H, Rauf F, Muhammad N, Javaid M, Alam S, Nasir S. Comparison of special stains (Giemsa stain and Modified Toluidine Blue stain) with immunohistochemistry as gold standard for the detection of H. pylori in gastric biopsies. Arab J Gastroenterol. 2022;23:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Sudraba A, Daugule I, Rudzite D, Funka K, Tolmanis I, Engstrand L, Janciauskas D, Jonaitis L, Kiudelis G, Kupcinskas L, Ivanauskas A, Leja M. Performance of routine Helicobacter pylori tests in patients with atrophic gastritis. J Gastrointestin Liver Dis. 2011;20:349-354. [PubMed] |
| 99. | Craanen ME, Blok P, Dekker W, Ferwerda J, Tytgat GN. Subtypes of intestinal metaplasia and Helicobacter pylori. Gut. 1992;33:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Stolte M, Müller H, Talley NJ, O'morain C, Bolling-Sternevald E, Sundin M, Eriksson S, Blum A. In patients with Helicobacter pylori gastritis and functional dyspepsia, a biopsy from the incisura angularis provides useful diagnostic information. Pathol Res Pract. 2006;202:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Parihar V, Holleran G, Hall B, Brennan D, Crotty P, McNamara D. A combined antral and corpus rapid urease testing protocol can increase diagnostic accuracy despite a low prevalence of Helicobacter pylori infection in patients undergoing routine gastroscopy. United European Gastroenterol J. 2015;3:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Puetz T, Vakil N, Phadnis S, Dunn B, Robinson J. The Pyloritek test and the CLO test: accuracy and incremental cost analysis. Am J Gastroenterol. 1997;92:254-257. [PubMed] |
| 105. | Nishikawa K, Sugiyama T, Kato M, Ishizuka J, Kagaya H, Hokari K, Asaka M. A prospective evaluation of new rapid urease tests before and after eradication treatment of Helicobacter pylori, in comparison with histology, culture and 13C-urea breath test. Gastrointest Endosc. 2000;51:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Chou CH, Sheu BS, Yang HB, Cheng PN, Shin JS, Chen CY, Lin XZ. Clinical assessment of the bacterial load of Helicobacter pylori on gastric mucosa by a new multi-scaled rapid urease test. J Gastroenterol Hepatol. 1997;12:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Bénéjat L, Ducournau A, Lehours P, Mégraud F. Real-time PCR for Helicobacter pylori diagnosis. The best tools available. Helicobacter. 2018;23:e12512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Saez J, Belda S, Santibáñez M, Rodríguez JC, Sola-Vera J, Galiana A, Ruiz-García M, Brotons A, López-Girona E, Girona E, Sillero C, Royo G. Real-time PCR for diagnosing Helicobacter pylori infection in patients with upper gastrointestinal bleeding: comparison with other classical diagnostic methods. J Clin Microbiol. 2012;50:3233-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Ramírez-Lázaro MJ, Lario S, Casalots A, Sanfeliu E, Boix L, García-Iglesias P, Sánchez-Delgado J, Montserrat A, Bella-Cueto MR, Gallach M, Sanfeliu I, Segura F, Calvet X. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS One. 2011;6:e20009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Ho SA, Hoyle JA, Lewis FA, Secker AD, Cross D, Mapstone NP, Dixon MF, Wyatt JI, Tompkins DS, Taylor GR. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol. 1991;29:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Rimbara E, Sasatsu M, Graham DY. PCR detection of Helicobacter pylori in clinical samples. Methods Mol Biol. 2013;943:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Parente F, Bianchi Porro G. The (13)C-urea breath test for non-invasive diagnosis of Helicobacter pylori infection: which procedure and which measuring equipment? Eur J Gastroenterol Hepatol. 2001;13:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Lemos FFB, de Castro CT, Silva Luz M, Rocha GR, Correa Santos GL, de Oliveira Silva LG, Calmon MS, Souza CL, Zarpelon-Schutz AC, Teixeira KN, Queiroz DMM, Freire de Melo F. Urea breath test for Helicobacter pylori infection in adult dyspeptic patients: A meta-analysis of diagnostic test accuracy. World J Gastroenterol. 2024;30:579-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (7)] |
| 114. | Makristathis A, Hirschl AM, Mégraud F, Bessède E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter. 2019;24 Suppl 1:e12641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Monk I, Benjaram S, Sahni N, Naylor P, May E, Ehrinpreis MN. 1212 H. pylori Breath Testing in an Academic Medical Center: Utilization and Treatment Outcomes. Am J Gastroenterol. 2019;114:S679-S680. [DOI] [Full Text] |
| 116. | Coelho LG, Sant'Ana CR, Oliveira RB, Cezar RCE, Araujo ACC, Silva RCTD, Trindade OR, Coelho MC, Ferrioli E, Bendassolli JA. Performance of the 13C-urea breath test for the diagnosis of H. pylori infection using a substrate synthesized in Brazil: A preliminary study. Clinics (Sao Paulo). 2018;73:e16553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Vaira D, Malfertheiner P, Mégraud F, Axon AT, Deltenre M, Hirschl AM, Gasbarrini G, O'Morain C, Garcia JM, Quina M, Tytgat GN. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet. 1999;354:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 118. | Demirtürk L, Yazgan Y, Tarçin O, Ozel M, Diler M, Oncül O, Yildirim S. Does N-acetyl cystein affect the sensitivity and specificity of Helicobacter pylori stool antigen test? Helicobacter. 2003;8:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Khorshed SA. A Comparative Study of Serological and Stool Antigen Tests for Helicobacter pylori Infection Diagnosis. J Angiotherapy. 2024;8:1-6. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 894] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 121. | Mégraud F, Graham DY, Howden CW, Trevino E, Weissfeld A, Hunt B, Smith N, Leifke E, Chey WD. Rates of Antimicrobial Resistance in Helicobacter pylori Isolates From Clinical Trial Patients Across the US and Europe. Am J Gastroenterol. 2023;118:269-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 122. | Ho JJC, Navarro M, Sawyer K, Elfanagely Y, Moss SF. Helicobacter pylori Antibiotic Resistance in the United States Between 2011 and 2021: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2022;117:1221-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 123. | Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep. 2014;14:476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 124. | Rokkas T, Gisbert JP, Malfertheiner P, Niv Y, Gasbarrini A, Leja M, Megraud F, O'Morain C, Graham DY. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-analysis. Gastroenterology. 2021;161:495-507.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 125. | Alsamman MA, Vecchio EC, Shawwa K, Acosta-Gonzales G, Resnick MB, Moss SF. Retrospective Analysis Confirms Tetracycline Quadruple as Best Helicobacter pylori Regimen in the USA. Dig Dis Sci. 2019;64:2893-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 126. | Chey WD, Howden CW, Moss SF, Morgan DR, Greer KB, Grover S, Shah SC. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2024;119:1730-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 145] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 127. | Homan M, Jones NL, Bontems P, Carroll MW, Czinn SJ, Gold BD, Goodman K, Harris PR, Jerris R, Kalach N, Kori M, Megraud F, Rowland M, Tavares M; on behalf of ESPGHAN/NASPGHAN. Updated joint ESPGHAN/NASPGHAN guidelines for management of Helicobacter pylori infection in children and adolescents (2023). J Pediatr Gastroenterol Nutr. 2024;79:758-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 128. | Coelho LGV, Marinho JR, Genta R, Ribeiro LT, Passos MDCF, Zaterka S, Assumpção PP, Barbosa AJA, Barbuti R, Braga LL, Breyer H, Carvalhaes A, Chinzon D, Cury M, Domingues G, Jorge JL, Maguilnik I, Marinho FP, Moraes-Filho JP, Parente JML, Paula-E-Silva CM, Pedrazzoli-Júnior J, Ramos AFP, Seidler H, Spinelli JN, Zir JV. IVth Brazilian Consensus Conference on Helicobacter pylori Infection. Arq Gastroenterol. 2018;55:97-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 129. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 130. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 672] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 131. | Ishibashi F, Suzuki S, Nagai M, Mochida K, Morishita T. Optimizing Helicobacter pylori Treatment: An Updated Review of Empirical and Susceptibility Test-Based Treatments. Gut Liver. 2023;17:684-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 132. | Li H, Shen Y, Song X, Tang X, Hu R, Marshall BJ, Tang H, Benghezal M. Need for standardization and harmonization of Helicobacter pylori antimicrobial susceptibility testing. Helicobacter. 2022;27:e12873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 133. | Ren X, Shi Y, Suo B, Yao X, Lu H, Li C, Zhang Y, Zhou L, Tian X, Song Z. Individualized diagnosis and eradication therapy for Helicobacter pylori infection based on gene detection of clarithromycin resistance in stool specimens: A systematic review and meta-analysis. Helicobacter. 2023;28:e12958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 134. | Bonilla S, Goldsmith J, Mitchell P, Bousvaros A. Helicobacter pylori Antimicrobial Resistance Using Next-Generation Sequencing in Stool Samples in a Pediatric Population. J Pediatr Gastroenterol Nutr. 2023;77:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 135. | Hulten KG, Genta RM, Kalfus IN, Zhou Y, Zhang H, Graham DY. Comparison of Culture With Antibiogram to Next-Generation Sequencing Using Bacterial Isolates and Formalin-Fixed, Paraffin-Embedded Gastric Biopsies. Gastroenterology. 2021;161:1433-1442.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 136. | Sun L, Liu M, Gong Y, Zhai K, Lv F, He L, Xue X, Liu X, Wang H, Fan D, You Y, Fang M, Sun L, Xu J, Zhang J. Rapid Antimicrobial Susceptibility Test of Helicobacter pylori to Metronidazole via Single-Cell Raman Spectrometry. Helicobacter. 2024;29:e13136. [PubMed] [DOI] [Full Text] |
| 137. | Li M, Wang X, Meng W, Dai Y, Wang W. Empirical versus tailored therapy based on genotypic resistance detection for Helicobacter pylori eradication: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231196357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Rokkas T, Ekmektzoglou K, Graham DY. Current role of tailored therapy in treating Helicobacter pylori infections. A systematic review, meta-analysis and critical analysis. Helicobacter. 2023;28:e12936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 139. | Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology. 2022;163:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (2)] |
| 140. | Jung YS, Kim S, Kim HY, Noh SJ, Park JH, Sohn CI, Park CH. Efficacy and Tolerability of 14-Day Tegoprazan- versus Rabeprazole-Based Triple Therapy for Eradication of Helicobacter pylori: A Real-World Evidence Study. Gut Liver. 2023;17:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 141. | Zhang M, Pang M, Zhang M. Efficacy and safety of potassium-competitive acid blockers versus proton pump inhibitors as Helicobacter pylori eradication therapy: a meta-analysis of randomized clinical trials. Clinics (Sao Paulo). 2022;77:100058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 142. | Laine L, Sharma P, Mulford DJ, Hunt B, Leifke E, Smith N, Howden CW. Pharmacodynamics and Pharmacokinetics of the Potassium-Competitive Acid Blocker Vonoprazan and the Proton Pump Inhibitor Lansoprazole in US Subjects. Am J Gastroenterol. 2022;117:1158-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 143. | Zhang WL, Lin BS, Li YY, Ding YM, Han ZX, Ji R. Efficacy and Safety of Vonoprazan and Amoxicillin Dual Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis. Digestion. 2023;104:249-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 144. | Han Z, Li Y, Nan X, Zhou T, Li L, Li Y. Effect of probiotic supplementation combined with bismuth-containing quadruple therapy on gut microbiota during Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Front Nutr. 2024;11:1484646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 145. | Viazis N, Argyriou K, Kotzampassi K, Christodoulou DK, Apostolopoulos P, Georgopoulos SD, Liatsos C, Giouleme O, Koustenis K, Veretanos C, Stogiannou D, Moutzoukis M, Poutakidis C, Mylonas II, Tseti I, Mantzaris GJ. A Four-Probiotics Regimen Combined with A Standard Helicobacter pylori-Eradication Treatment Reduces Side Effects and Increases Eradication Rates. Nutrients. 2022;14:632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 146. | Zhang M, Zhang C, Zhao J, Zhang H, Zhai Q, Chen W. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microb Pathog. 2020;147:104403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Wang Y, Wang X, Cao XY, Zhu HL, Miao L. Comparative effectiveness of different probiotics supplements for triple helicobacter pylori eradication: a network meta-analysis. Front Cell Infect Microbiol. 2023;13:1120789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One. 2014;9:e111030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 149. | Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24:e12651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 150. | Szajewska H, Berni Canani R, Domellöf M, Guarino A, Hojsak I, Indrio F, Lo Vecchio A, Mihatsch WA, Mosca A, Orel R, Salvatore S, Shamir R, van den Akker CHP, van Goudoever JB, Vandenplas Y, Weizman Z; ESPGHAN Special Interest Group on Gut Microbiota and Modifications. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J Pediatr Gastroenterol Nutr. 2023;76:232-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 151. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 569] [Article Influence: 189.7] [Reference Citation Analysis (1)] |
| 152. | Pan Y, Jiao FY. Helicobacter pylori infection and gastric microbiota: Insights into gastric and duodenal ulcer development. World J Gastroenterol. 2025;31:100044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 153. | Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191-5204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (3)] |
| 154. | Shiotani A, Graham DY. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med Clin North Am. 2002;86:1447-1466, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, Low MJ, Merchant JL. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci USA. 2003;100:12944-12949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Zavros Y, Merchant JL. Modulating the cytokine response to treat Helicobacter gastritis. Biochem Pharmacol. 2005;69:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Robinson K, Atherton JC. The Spectrum of Helicobacter-Mediated Diseases. Annu Rev Pathol. 2021;16:123-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 158. | Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 159. | Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 160. | Hussein NR, Argent RH, Marx CK, Patel SR, Robinson K, Atherton JC. Helicobacter pylori dupA is polymorphic, and its active form induces proinflammatory cytokine secretion by mononuclear cells. J Infect Dis. 2010;202:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 161. | Shiota S, Matsunari O, Watada M, Hanada K, Yamaoka Y. Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut Pathog. 2010;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 162. | Cheung AN, Ng IO. Cytomegalovirus infection of the gastrointestinal tract in non-AIDS patients. Am J Gastroenterol. 1993;88:1882-1886. [PubMed] |
| 163. | Michel A, Pawlita M, Boeing H, Gissmann L, Waterboer T. Helicobacter pylori antibody patterns in Germany: a cross-sectional population study. Gut Pathog. 2014;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 164. | Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 165. | Katschinski BD, Logan RF, Edmond M, Langman MJ. Physical activity at work and duodenal ulcer risk. Gut. 1991;32:983-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 166. | Levenstein S. Stress and peptic ulcer: life beyond Helicobacter. BMJ. 1998;316:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 167. | Oktedalen O, Guldvog I, Opstad PK, Berstad A, Gedde-Dahl D, Jorde R. The effect of physical stress on gastric secretion and pancreatic polypeptide levels in man. Scand J Gastroenterol. 1984;19:770-778. [PubMed] |
| 168. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 606] [Article Influence: 67.3] [Reference Citation Analysis (37)] |
| 169. | Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori-associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 170. | Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 171. | Ford AC, Gurusamy KS, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016;4:CD003840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 172. | Liu CC, Lee CL, Chan CC, Tu TC, Liao CC, Wu CH, Chen TK. Maintenance treatment is not necessary after Helicobacter pylori eradication and healing of bleeding peptic ulcer: a 5-year prospective, randomized, controlled study. Arch Intern Med. 2003;163:2020-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 173. | Zavros Y, Merchant JL. The immune microenvironment in gastric adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2022;19:451-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 174. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 175. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3247] [Article Influence: 129.9] [Reference Citation Analysis (1)] |
| 176. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 177. | Freire de Melo F, Marques HS, Rocha Pinheiro SL, Lemos FFB, Silva Luz M, Nayara Teixeira K, Souza CL, Oliveira MV. Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis. World J Clin Oncol. 2022;13:866-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 178. | Yong X, Tang B, Li BS, Xie R, Hu CJ, Luo G, Qin Y, Dong H, Yang SM. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Commun Signal. 2015;13:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 179. | Zhang J, Wang W, Yan S, Li J, Wei H, Zhao W. [CagA and VacA inhibit gastric mucosal epithelial cell autophagy and promote the progression of gastric precancerous lesions]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 180. | Matos JI, de Sousa HA, Marcos-Pinto R, Dinis-Ribeiro M. Helicobacter pylori CagA and VacA genotypes and gastric phenotype: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 181. | Schmidt TP, Perna AM, Fugmann T, Böhm M, Jan Hiss, Haller S, Götz C, Tegtmeyer N, Hoy B, Rau TT, Neri D, Backert S, Schneider G, Wessler S. Identification of E-cadherin signature motifs functioning as cleavage sites for Helicobacter pylori HtrA. Sci Rep. 2016;6:23264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 182. | Moonens K, Gideonsson P, Subedi S, Bugaytsova J, Romaõ E, Mendez M, Nordén J, Fallah M, Rakhimova L, Shevtsova A, Lahmann M, Castaldo G, Brännström K, Coppens F, Lo AW, Ny T, Solnick JV, Vandenbussche G, Oscarson S, Hammarström L, Arnqvist A, Berg DE, Muyldermans S, Borén T, Remaut H. Structural Insights into Polymorphic ABO Glycan Binding by Helicobacter pylori. Cell Host Microbe. 2016;19:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 183. | Yamaoka Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries. 2008;2:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 184. | Al-Maleki AR, Loke MF, Lui SY, Ramli NSK, Khosravi Y, Ng CG, Venkatraman G, Goh KL, Ho B, Vadivelu J. Helicobacter pylori outer inflammatory protein A (OipA) suppresses apoptosis of AGS gastric cells in vitro. Cell Microbiol. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 185. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 904] [Article Influence: 226.0] [Reference Citation Analysis (0)] |
| 186. | Allum W, Lordick F, Alsina M, Andritsch E, Ba-Ssalamah A, Beishon M, Braga M, Caballero C, Carneiro F, Cassinello F, Dekker JW, Delgado-Bolton R, Haustermans K, Henning G, Hutter B, Lövey J, Netíková IŠ, Obermannová R, Oberst S, Rostoft S, Saarto T, Seufferlein T, Sheth S, Wynter-Blyth V, Costa A, Naredi P. ECCO essential requirements for quality cancer care: Oesophageal and gastric cancer. Crit Rev Oncol Hematol. 2018;122:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 187. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 188. | Hatakeyama M. Impact of the Helicobacter pylori Oncoprotein CagA in Gastric Carcinogenesis. Curr Top Microbiol Immunol. 2023;444:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 189. | Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbințeanu-Braticevici C. Helicobacter pylori infection: old and new. J Med Life. 2017;10:112-117. [PubMed] |
| 190. | Rossi D, Bertoni F, Zucca E. Marginal-Zone Lymphomas. N Engl J Med. 2022;386:568-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 191. | Troppan K, Wenzl K, Neumeister P, Deutsch A. Molecular Pathogenesis of MALT Lymphoma. Gastroenterol Res Pract. 2015;2015:102656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 192. | Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107:3034-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 193. | Guindi M. Role of activated host T cells in the promotion of MALT lymphoma growth. Semin Cancer Biol. 2000;10:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 194. | Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 195. | Nakamura S, Aoyagi K, Furuse M, Suekane H, Matsumoto T, Yao T, Sakai Y, Fuchigami T, Yamamoto I, Tsuneyoshi M, Fujishima M. B-cell monoclonality precedes the development of gastric MALT lymphoma in Helicobacter pylori-associated chronic gastritis. Am J Pathol. 1998;152:1271-1279. [PubMed] |
| 196. | Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas J, Püspök A, Formanek M, Assanasen T, Müller-Hermelink HK, Cerroni L, Raderer M, Chott A. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 269] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 197. | Lima KS, Albuquerque W, Arantes VN, Drummond-Lage AP, Coelho LG. Helicobacter pylori and t(11;18)(q21;q21) translocation in gastric malt lymphoma. Arq Gastroenterol. 2014;51:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 198. | Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A, Martin-Subero JI, Siebert R, Dogan A, Isaacson PG, Du MQ. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 217] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 199. | Rosebeck S, Rehman AO, Lucas PC, McAllister-Lucas LM. From MALT lymphoma to the CBM signalosome: three decades of discovery. Cell Cycle. 2011;10:2485-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 200. | Sagaert X, Laurent M, Baens M, Wlodarska I, De Wolf-Peeters C. MALT1 and BCL10 aberrations in MALT lymphomas and their effect on the expression of BCL10 in the tumour cells. Mod Pathol. 2006;19:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 201. | Knies N, Alankus B, Weilemann A, Tzankov A, Brunner K, Ruff T, Kremer M, Keller UB, Lenz G, Ruland J. Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell proliferation via cooperative NF-κB and JNK activation. Proc Natl Acad Sci USA. 2015;112:E7230-E7238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 202. | Sagaert X, Van Cutsem E, De Hertogh G, Geboes K, Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 203. | Lemos FFB, Silva Luz M, Rocha Pinheiro SL, Teixeira KN, Freire de Melo F. Role of non-Helicobacter pylori gastric Helicobacters in helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol. 2023;29:4851-4859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 204. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 205. | Takigawa H, Yuge R, Masaki S, Otani R, Kadota H, Naito T, Hayashi R, Urabe Y, Oka S, Tanaka S, Chayama K, Kitadai Y. Involvement of non-Helicobacter pylori helicobacter infections in Helicobacter pylori-negative gastric MALT lymphoma pathogenesis and efficacy of eradication therapy. Gastric Cancer. 2021;24:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 206. | Nakamura S, Hojo M. Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. J Clin Med. 2022;12:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 207. | Jung K, Kim DH, Seo HI, Gong EJ, Bang CS. Efficacy of eradication therapy in Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A meta-analysis. Helicobacter. 2021;26:e12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 208. | Lemos FFB, de Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes Dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol. 2023;29:2202-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 209. | Joosten M, Lindén S, Rossi M, Tay AC, Skoog E, Padra M, Peters F, Perkins T, Vandamme P, Van Nieuwerburgh F, D'Herde K, Van den Broeck W, Flahou B, Deforce D, Ducatelle R, Marshall B, Haesebrouck F, Smet A. Divergence between the Highly Virulent Zoonotic Pathogen Helicobacter heilmannii and Its Closest Relative, the Low-Virulence "Helicobacter ailurogastricus" sp. nov. Infect Immun. 2016;84:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 210. | Goji S, Tamura Y, Sasaki M, Nakamura M, Matsui H, Murayama SY, Ebi M, Ogasawara N, Funaki Y, Kasugai K. Helicobacter suis-Infected Nodular Gastritis and a Review of Diagnostic Sensitivity for Helicobacter heilmannii-Like Organisms. Case Rep Gastroenterol. 2015;9:179-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 211. | Nobutani K, Yoshida M, Nishiumi S, Nishitani Y, Takagawa T, Tanaka H, Yamamoto K, Mimura T, Bensuleiman Y, Ota H, Takahashi S, Matsui H, Nakamura M, Azuma T. Helicobacter heilmannii can induce gastric lymphoid follicles in mice via a Peyer's patch-independent pathway. FEMS Immunol Med Microbiol. 2010;60:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 212. | Mimura T, Yoshida M, Nishiumi S, Tanaka H, Nobutani K, Takenaka M, Suleiman YB, Yamamoto K, Ota H, Takahashi S, Matsui H, Nakamura M, Miki I, Azuma T. IFN-γ plays an essential role in the pathogenesis of gastric lymphoid follicles formation caused by Helicobacter suis infection. FEMS Immunol Med Microbiol. 2011;63:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 213. | Yamamoto K, Tanaka H, Nishitani Y, Nishiumi S, Miki I, Takenaka M, Nobutani K, Mimura T, Ben Suleiman Y, Mizuno S, Kawai M, Uchiyama I, Yoshida M, Azuma T. Helicobacter suis KB1 derived from pig gastric lymphoid follicles induces the formation of gastric lymphoid follicles in mice through the activation of B cells and CD4 positive cells. Microbes Infect. 2011;13:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 214. | Zhao Y, Lu F, Ye J, Ji M, Pang Y, Wang Y, Wang L, Li G, Sun T, Li J, Ma D, Ji C. Myeloid-Derived Suppressor Cells and γδT17 Cells Contribute to the Development of Gastric MALT Lymphoma in H. felis-Infected Mice. Front Immunol. 2019;10:3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 215. | Lee A, O'Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations--mouse models of gastric lymphoma. Recent Results Cancer Res. 2000;156:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 216. | Santos MLC, de Brito BB, da Silva FAF, Sampaio MM, Marques HS, Oliveira E Silva N, de Magalhães Queiroz DM, de Melo FF. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol. 2020;26:4076-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 217. | Serin E, Gümürdülü Y, Ozer B, Kayaselçuk F, Yilmaz U, Koçak R. Impact of Helicobacter pylori on the development of vitamin B12 deficiency in the absence of gastric atrophy. Helicobacter. 2002;7:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 218. | Fayyaz A, Ali A, Wajid R, Javed S. Improvement in Refractory Iron Deficiency Anemia after Successful Eradication of H-Pylori. Pak J Med Health Sci. 2022;16:270-272. [DOI] [Full Text] |
| 219. | Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 220. | Cai X, Li X, Jin Y, Zhang M, Xu Y, Liang C, Weng Y, Yu W, Li X. Vitamins and Helicobacter pylori: An Updated Comprehensive Meta-Analysis and Systematic Review. Front Nutr. 2021;8:781333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 221. | Suzuki T, Matsushima M, Masui A, Watanabe K, Takagi A, Ogawa Y, Shirai T, Mine T. Effect of Helicobacter pylori eradication in patients with chronic idiopathic thrombocytopenic purpura-a randomized controlled trial. Am J Gastroenterol. 2005;100:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 222. | Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 223. | Stasi R, Rossi Z, Stipa E, Amadori S, Newland AC, Provan D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. Am J Med. 2005;118:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 224. | Zain MA, Zafar F, Ashfaq A, Jamil AR, Ahmad A. Helicobacter pylori: An Underrated Cause of Immune Thrombocytopenic Purpura. A Comprehensive Review. Cureus. 2019;11:e5551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 225. | Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 226. | Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 918] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 227. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2211] [Article Influence: 245.7] [Reference Citation Analysis (3)] |
| 228. | Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461-5473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (6)] |
| 229. | Ahmed S, Belayneh YM. Helicobacter pylori And Duodenal Ulcer: Systematic Review Of Controversies In Causation. Clin Exp Gastroenterol. 2019;12:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 230. | Lina TT, Alzahrani S, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Immune evasion strategies used by Helicobacter pylori. World J Gastroenterol. 2014;20:12753-12766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 231. | Federico A, Gravina AG, Miranda A, Loguercio C, Romano M. Eradication of Helicobacter pylori infection: which regimen first? World J Gastroenterol. 2014;20:665-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 232. | Liu KY, Shi Y, Luo P, Yu S, Chen L, Zhao Z, Mao XH, Guo G, Wu C, Zou QM. Therapeutic efficacy of oral immunization with attenuated Salmonella typhimurium expressing Helicobacter pylori CagA, VacA and UreB fusion proteins in mice model. Vaccine. 2011;29:6679-6685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 233. | Abadi AH, Mahdavi M, Khaledi A, Esmaeili SA, Esmaeili D, Sahebkar A. Study of serum bactericidal and splenic activity of Total-OMP- CagA combination from Brucella abortus and Helicobacter pylori in BALB/c mouse model. Microb Pathog. 2018;121:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 234. | Keikha M, Eslami M, Yousefi B, Ghasemian A, Karbalaei M. Potential antigen candidates for subunit vaccine development against Helicobacter pylori infection. J Cell Physiol. 2019;234:21460-21470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 235. | El Khadir M, Alaoui Boukhris S, Benajah DA, El Rhazi K, Ibrahimi SA, El Abkari M, Harmouch T, Nejjari C, Mahmoud M, Benlemlih M, Bennani B. VacA and CagA Status as Biomarker of Two Opposite End Outcomes of Helicobacter pylori Infection (Gastric Cancer and Duodenal Ulcer) in a Moroccan Population. PLoS One. 2017;12:e0170616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 236. | Malfertheiner P, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, Norelli F, Contorni M, Peppoloni S, Berti D, Tornese D, Ganju J, Palla E, Rappuoli R, Scharschmidt BF, Del Giudice G. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 237. | Li X, Xing Y, Guo L, Lv X, Song H, Xi T. Oral immunization with recombinant Lactococcus lactis delivering a multi-epitope antigen CTB-UE attenuates Helicobacter pylori infection in mice. Pathog Dis. 2014;72:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 238. | Loguercio S, Dian C, Flagiello A, Scannella A, Pucci P, Terradot L, Zagari A. In HspA from Helicobacter pylori vicinal disulfide bridges are a key determinant of domain B structure. FEBS Lett. 2008;582:3537-3541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 239. | Moss SF, Moise L, Lee DS, Kim W, Zhang S, Lee J, Rogers AB, Martin W, De Groot AS. HelicoVax: epitope-based therapeutic Helicobacter pylori vaccination in a mouse model. Vaccine. 2011;29:2085-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 240. | Tobias J, Lebens M, Wai SN, Holmgren J, Svennerholm AM. Surface expression of Helicobacter pylori HpaA adhesion antigen on Vibrio cholerae, enhanced by co-expressed enterotoxigenic Escherichia coli fimbrial antigens. Microb Pathog. 2017;105:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 241. | Zhang HX, Qiu YY, Zhao YH, Liu XT, Liu M, Yu AL. Immunogenicity of oral vaccination with Lactococcus lactis derived vaccine candidate antigen (UreB) of Helicobacter pylori fused with the human interleukin 2 as adjuvant. Mol Cell Probes. 2014;28:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/