Published online Dec 22, 2025. doi: 10.4291/wjgp.v16.i4.111865
Revised: August 30, 2025
Accepted: November 27, 2025
Published online: December 22, 2025
Processing time: 164 Days and 3 Hours
Colorectal cancer remains as one of the most common cancers that are diagnosed and remains as a significant contributor to morbidity and mortality. Despite advances in techniques, improving access to diagnostic modalities and increasing awareness, it often presents at a later stage and can recur despite treatment. Recurrence can be variable and can occur years after treatment. Liver is the most common location for metastasis to occur followed by lungs. However, atypical sites of metastasis can occur although unusual and colorectal cancer can spread to the spleen, hilum of the liver, adrenals, bone, skeletal muscles, skin, prostate, brain, parotid gland, thyroid gland and even the cardiac muscle. It is crucial to recognize the metachronous nature of the metastasis and to only present at a single site as within this lies the rarity of the case. The mass itself mimicked a cholangiocarcinoma or a Klatskin’s tumor initially and only through pathology was the diagnosis established. We present an unusual case of recurrent colorectal cancer that occurred several years post treatment and presented as an isolated metastasis to the hilum of the liver leading to biliary obstruction without any other identifiable lesions including in the colon itself.
A 68-year-old male with history of colon cancer presented with obstructive jaundice to the hospital. After evaluation with imaging studies was diagnosed with mass at the hilum of the liver that was leading to obstruction. With percutaneous biopsies obtained by interventional radiology, the diagnosis of metastatic adenocarcinoma originating from the colon was established. He was deemed not to be a surgical candidate and is currently pursuing chemotherapy.
A metastatic adenocarcinoma of the colon that presents as a hilar mass and mimics cholangiocarcinoma is very rare. The metachronous nature along with the isolated metastasis involving the hilum of the liver makes this case unique. Diagnosis can be challenging and needs a tissue specimen along with immunostaining to achieve an accurate diagnosis and provide appropriate treatment. Biliary decompression is performed either endoscopically or percutaneously and is part of the multidisciplinary approach involving medical and surgical oncology teams.
Core Tip: Colorectal cancer is a commonly encountered malignancy and has potential to metastasize to distant organs. We present an atypical case of an isolated metastatic site that involved only the hilum of the liver and was a metachronous lesion that occurred several years after the initial diagnosis. Presentation in this manner can mimic cholangiocarcinoma and diagnosis is established through pathology.
- Citation: Amin N, Daglilar E, Chela HK. Isolated hilar mass mimicking cholangiocarcinoma as a rare metastatic manifestation of recurrent colorectal cancer: A case report. World J Gastrointest Pathophysiol 2025; 16(4): 111865
- URL: https://www.wjgnet.com/2150-5330/full/v16/i4/111865.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v16.i4.111865
Colorectal cancer is the third most common cause of cancer and the second most common cause of cancer-related death worldwide[1]. Despite advances in endoscopy and attempts at improvement in access, it remains a challenging malig
A 68-year-old male was referred from Oncology Clinic to the Emergency Department for evaluation of new onset elevation of liver chemistries.
A recent high-quality colonoscopy 3 months prior to the most recent hospitalization in September 2024 had revealed three diminutive tubular adenomas. These pre-cancerous growths were extremely small in size and did not show evidence of any high-grade dysplasia or other concerning features and all were resected completely. Upon presentation, he had scleral icterus, appeared frail and cachectic with vital signs remarkable for bradycardia. Abdominal exam elicited ten
He had a remote history of invasive mucinous adenocarcinoma of rectosigmoid in 2017 (pT3pN1bM0, periregional lymph node involvement) for which he underwent adjuvant chemotherapy and rectosigmoidectomy. The chemotherapy regimen he received was XELOX for a total duration of 8 cycles. Subsequently he had done well clinically and was in remission but in 2021, he was unfortunately diagnosed with invasive mucinous adenocarcinoma of cecum/appendix (infiltrating to visceral peritoneum) with stage pT4ap N0cM0 for which he initially underwent cecal segmental colectomy and appendectomy and this was followed shortly by completion right hemicolectomy and lymphadenectomy (0/18 noted were positive). Following this, he underwent adjuvant chemotherapy with 5-FU and leucovorin which was completed in April 2022.
There was no known family history of malignancy and he had never undergone genetic testing.
Upon presentation, he had scleral icterus, appeared frail and cachectic with vital signs remarkable for bradycardia. Abdominal exam elicited tenderness in RUQ without any obvious palpable masses.
Labs were pertinent for normal hemoglobin and showed elevated bilirubin of 3.4 gm/dL, alkaline phosphatase of 230 U/L, alanine transaminase of 215 U/L, aspartate transaminase of 95 U/L. Acute hepatitis panel was unremarkable. Of note, liver chemistries a few months prior were normal.
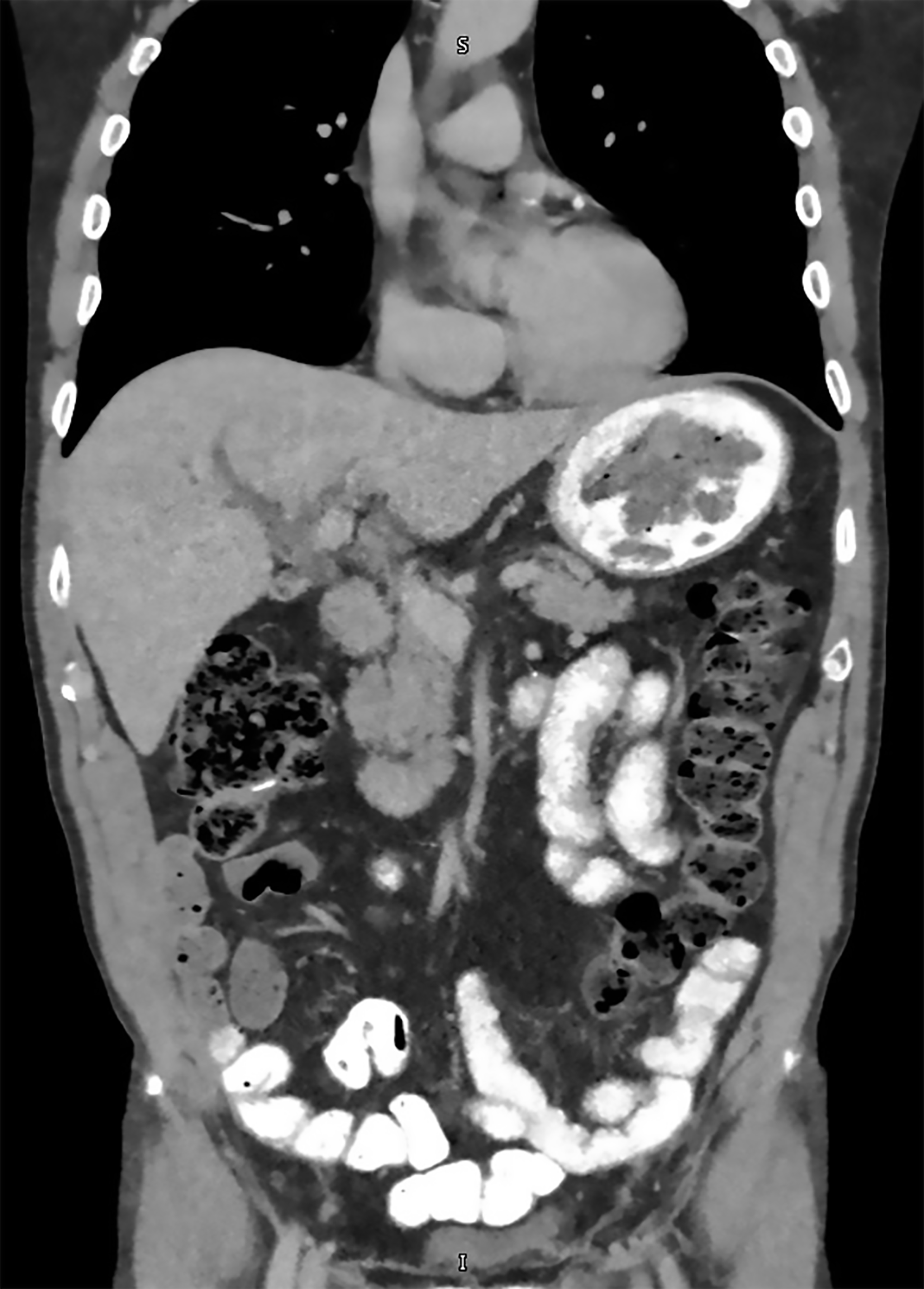
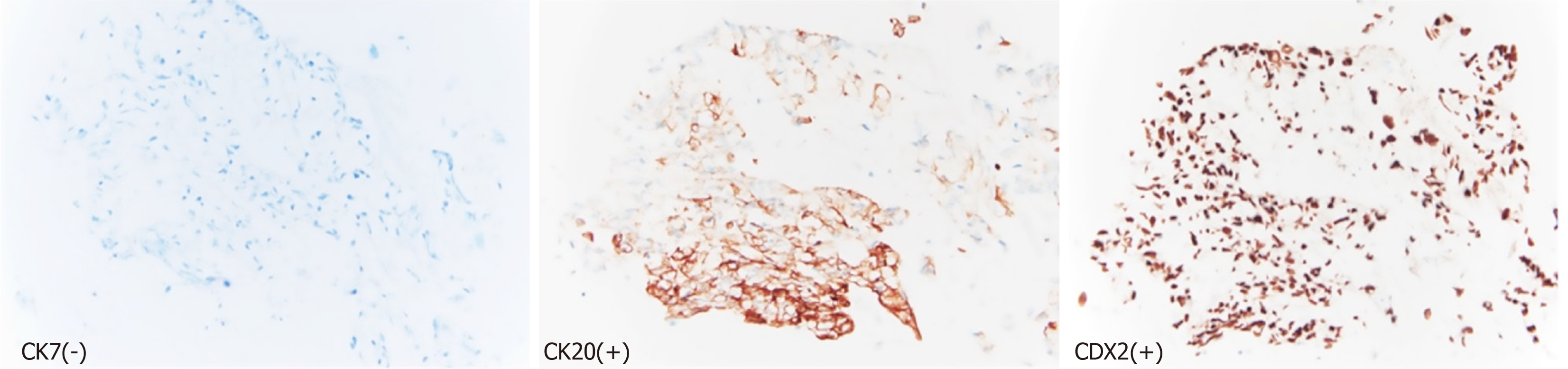
Imaging with computed tomography (CT) abdomen revealed severe intrahepatic bile duct dilatation with suspected abnormality at the level of the common hepatic duct (Figure 1). The distal common bile duct was normal in caliber. Magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) showed intrahepatic biliary duct dilatation with a soft tissue lesion seen at the hilum branch point between the right and left biliary system that measured at least 2.6 cm in length (Figure 2). No focal liver lesions were noted. About 6 months prior to this scam, CT scans of the chest, abdomen and pelvis revealed only a tiny 4 mm left lung base subpleural nodule which was unchanged compared to previous scans. The nature of the biliary obstruction was intraluminal in nature and for further evaluation and biliary decompression, options were discussed as to the route for decompression. An endoscopic retrograde cholangiopancreatography (ERCP) was discussed vs options such as external drainage approach and patient opted for an external drain with interventional radiology as he feared potential complications of ERCP. Interventional radiology team placed an external biliary drain and obtained cytology samples simultaneously. The pathology results showed adenocarcinoma that favored a colorectal source of origin. Immunohistochemical studies demonstrated the tumor cells to be positive for cytokeratin (CK) 20 and caudal-type homeobox 2 (CDX2), and negative for CK7 (Figure 3).
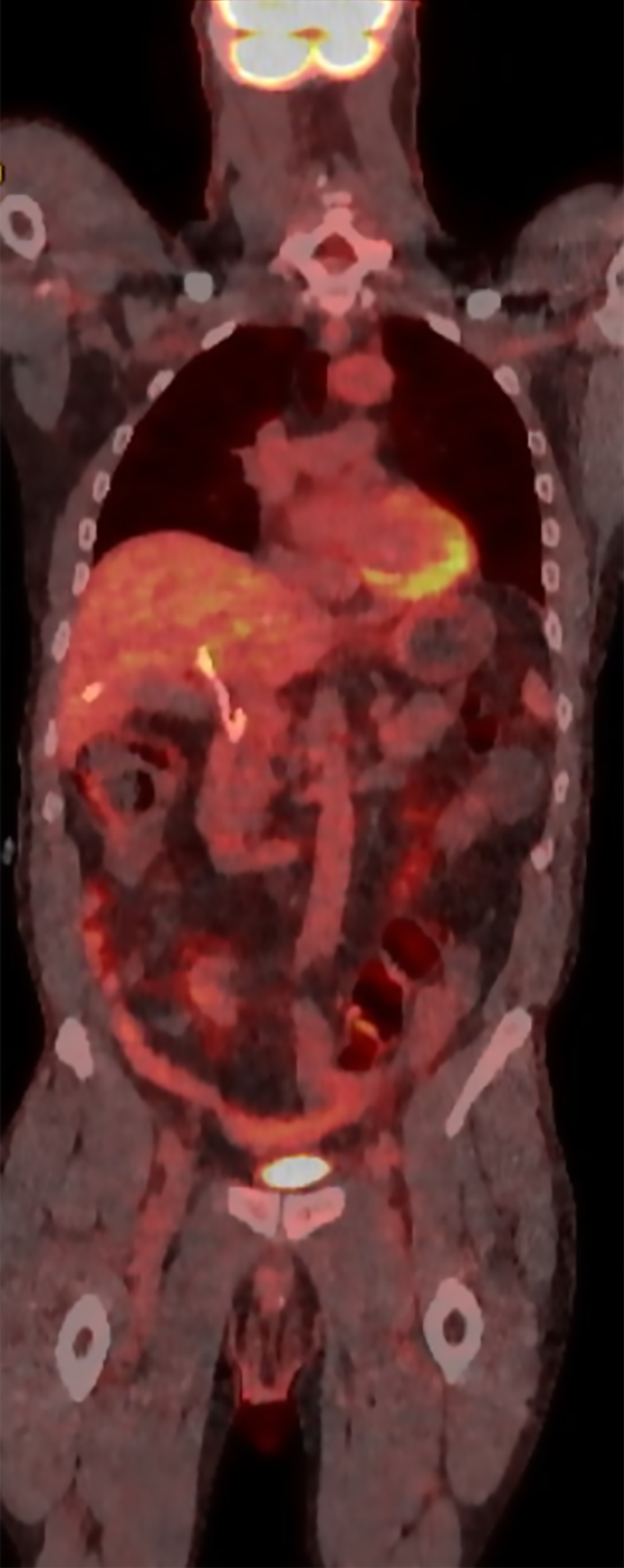
A positron emission tomography-CT (PET-CT) scan was performed after discharge that revealed no definitive fluorodeoxyglucose PET/CT evidence of malignancy. There was no abnormal activity elsewhere including the bowel and prior surgical sites and no lymphadenopathy was noted (Figure 4). Mucinous implants to the hilum from colorectal malignancy could have been a possible differential in this case given the presentation. However, there was no evidence of any ascites or features of peritoneal carcinomatosis on any of the imaging studies he underwent.
Medical and surgical oncology teams were consulted and he was deemed not to be a surgical candidate due to presence of circulating tumor DNA as well as overall functional status.
Recurrent colorectal cancer presenting as isolated liver hilar metastasis with pathology showing adenocarcinoma consistent with colorectal primary.
Neoadjuvant chemotherapy was discussed with the patient and he wished to proceed with this. He received FOLFIRINOX with bevacizumab for 8 cycles and is currently undergoing treatment with mFOLFIRI.
Following up closely with oncology as outpatient. Periodic monitoring with labs including CEA levels as well as cross sectional imaging of the abdomen is being performed with the last MRI abdomen without any obvious lesions reported.
Colorectal cancer is a common malignancy that is encountered all across the world. Although efforts to increase awareness and colorectal screening continue to be underway, colorectal cancer is sometimes diagnosed at an advanced stage when it has already spread to other organs and sites in the body. The sites of metastasis that are usually encoun
The diagnosis requires tissue sampling in order to differentiate from a cholangiocarcinoma. Histopathology would reveal adenocarcinoma and further testing with immunohistology would be needed to evaluate origin. In the case presented by Ofuchi et al[7], they emphasized that colorectal cancer can mimic a cholangiocarcinoma and imaging studies as well as histopathology stains may not be adequate enough to yield the correct diagnosis. The role of immunostaining is important to prevent errors in diagnosis and management of the patient[7]. In the case presented by Ofuchi et al[7], the patient underwent extensive surgery with right hemihepatectomy and bile duct resection based on the imaging findings appearing to be classic for cholangiocarcinoma. Onishi et al[11], also reveal a similar encounter where a patient presented with cholangitis and obstructive jaundice due to tumor infiltrating within the bile duct leading to occlusion and immunostains aided in the diagnosis. The patient was also noted to have a tumor in the descending colon for which resection was performed[11]. In both of these cases, there was no other hepatic involvement which is similar to our case as well. Stains such as CK7, CK20, CDX2 are utilized to aid in the diagnosis with CK7-negative, CK20-positive, CDX2-positive results pointing towards adenocarcinoma originating from the colon. In our case, there was a suspicion of initially a de novo diagnosis of cholangiocarcinoma. However further evaluation with a tissue diagnosis confirmed the diagnosis of metastatic adenocarcinoma arising from the colon. The key to arriving at the diagnosis was through the use of pathology with immunostaining and this was confirmed by second review by expert pathologists as well. In our patient, it is unclear as to how the metastasis occurred as there was no lymph node involvement on the last colon cancer that was diagnosed in 2021 and not any lymphadenopathy on the recent scans either. However, at the time of the initial diagnosis there was regional lymph node involvement and if this indeed stay dormant and manifest so many years later in the form of a recurrence, then it is unusual. There was no peritoneal involvement even though the last two colon cancers were mucinous adenocarcinomas, this one was only reported by pathologists as adenocarcinoma but did not report mucinous features. There was no evidence of ascites or peritoneal carcinomatosis on imaging. Mucinous implants can be seen in pseudomyxoma peritonei and is characterized by the presence of ascites that is ’gelatinous’ in consistency given the mucinous content[12].
Use of imaging modalities is certainly challenging including PET-CT scan. There can be limitations in the use of PET-CT as the uptake of cholangiocarcinoma is reported to be variable and challenging to determine abnormal uptake due to physiologic excretion of tracer in the normal hepatic parenchyma. The drain which was already present in the case of our patient also further complicated the evaluation in this region. PET-CT itself can have varying sensitivity for evaluating liver metastasis which can be significantly reduced for lesions < 1.5 cm[13]. PET-CT can face other challenges as well as compared to modalities such as PET-MR when evaluating the hepatobiliary system due to the high glucose metabolism of the liver, the variable activity of tumors and quality of images[13,14].
Management of the malignant biliary obstruction can also be challenging especially when due to a metastasis. Colorectal cancer can involve the liver itself and this can lead to obstruction which may not be amenable to endoscopic drainage techniques. Involvement of the pancreas or lymph nodes in the region and extra hepatic bile duct as well as even the peritoneum can contribute to biliary obstruction[15]. Biliary decompression can be performed either with extensive surgical procedures or more often with either endoscopic or interventional radiology guided routes. Percuta
A metastatic adenocarcinoma of the colon that presents as a hilar mass and mimics cholangiocarcinoma is very rare. The metachronous nature along with the isolated metastasis involving the hilum of the liver makes this case unique. Diagnosis can be challenging and needs a tissue specimen along with immunostaining to achieve an accurate diagnosis and provide appropriate treatment. Biliary decompression is performed either endoscopically or percutaneously and is part of the multidisciplinary approach involving medical and surgical oncology teams.
| 1. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 421] [Reference Citation Analysis (0)] |
| 2. | Francoa PIG, Pascual-Panganiban A. The parotid gland, an unusual site of colorectal cancer metastasis. Ecancermedicalscience. 2023;17:1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Coelho MI, Albano MN, Costa Almeida CE, Reis LS, Moreira N, Almeida CMC. Colon cancer metastasis to the thyroid gland: A case report. Int J Surg Case Rep. 2017;37:221-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Choi PW, Kim CN, Chang SH, Chang WI, Kim CY, Choi HM. Cardiac metastasis from colorectal cancer: a case report. World J Gastroenterol. 2009;15:2675-2678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Qiu M, Hu J, Yang D, Cosgrove DP, Xu R. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6:38658-38666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Shalata W, Abu Jama A, Abu Salman A, Golosky M, Solomon A, Abu Saleh O, Michlin R, Shalata S, Agbarya A, Yakobson A. Unexpected and Rare Sites of Metastasis in Oncologic Patients. J Clin Med. 2023;12:6447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Ofuchi T, Hayashi H, Yamao T, Higashi T, Takematsu T, Nakao Y, Yamamura K, Imai K, Yamashita YI, Baba H. Colon cancer metastasis mimicking a hilar cholangiocarcinoma: a case report and literature review. Surg Case Rep. 2020;6:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1740] [Article Influence: 348.0] [Reference Citation Analysis (1)] |
| 9. | Rumpold H, Niedersüß-Beke D, Heiler C, Falch D, Wundsam HV, Metz-Gercek S, Piringer G, Thaler J. Prediction of mortality in metastatic colorectal cancer in a real-life population: a multicenter explorative analysis. BMC Cancer. 2020;20:1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Pretzsch E, Bösch F, Neumann J, Ganschow P, Bazhin A, Guba M, Werner J, Angele M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J Oncol. 2019;2019:7407190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 11. | Onishi I, Kayahara M, Takei R, Makita N, Munemoto M, Yagi Y, Kawashima A. Recurrent biliary dissemination of colon cancer liver metastasis: a case report. J Med Case Rep. 2018;12:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Southey MV, Webster AR. Pseudomyxoma peritonei. Med J Australia. 1926;1:703-704. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Tahtabasi M, Erturk SM, Basak M. Comparison of MRI and 18F-FDG PET/CT in the Liver Metastases of Gastrointestinal and Pancreaticobiliary Tumors. Sisli Etfal Hastan Tip Bul. 2021;55:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Zhou N, Meng X, Zhang Y, Yu B, Yuan J, Yu J, Zhu H, Yang Z. Diagnostic Value of Delayed PET/MR in Liver Metastasis in Comparison With PET/CT. Front Oncol. 2021;11:717687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Okamoto T. Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer. World J Gastroenterol. 2022;28:985-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (4)] |
| 16. | Cortese F, Acquafredda F, Mardighian A, Zurlo MT, Ferraro V, Memeo R, Spiliopoulos S, Inchingolo R. Percutaneous insertion of a novel dedicated metal stent to treat malignant hilar biliary obstruction. World J Gastrointest Oncol. 2022;14:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/