Published online Nov 28, 2025. doi: 10.4329/wjr.v17.i11.112638
Revised: August 29, 2025
Accepted: October 31, 2025
Published online: November 28, 2025
Processing time: 118 Days and 1.1 Hours
Thyroid-associated ophthalmopathy (TAO), an autoimmune disorder closely associated with thyroid dysfunction, requires timely diagnosis and ongoing accu
Core Tip: Thyroid-associated ophthalmopathy, an autoimmune disorder linked to thyroid dysfunction, needs timely dia
- Citation: Shi JF, Zhou WY, Zhang HX, Shen Y, Zhang H, Li T. Advancements and challenges of ultrasound imaging in the management of thyroid-associated ophthalmopathy. World J Radiol 2025; 17(11): 112638
- URL: https://www.wjgnet.com/1949-8470/full/v17/i11/112638.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i11.112638
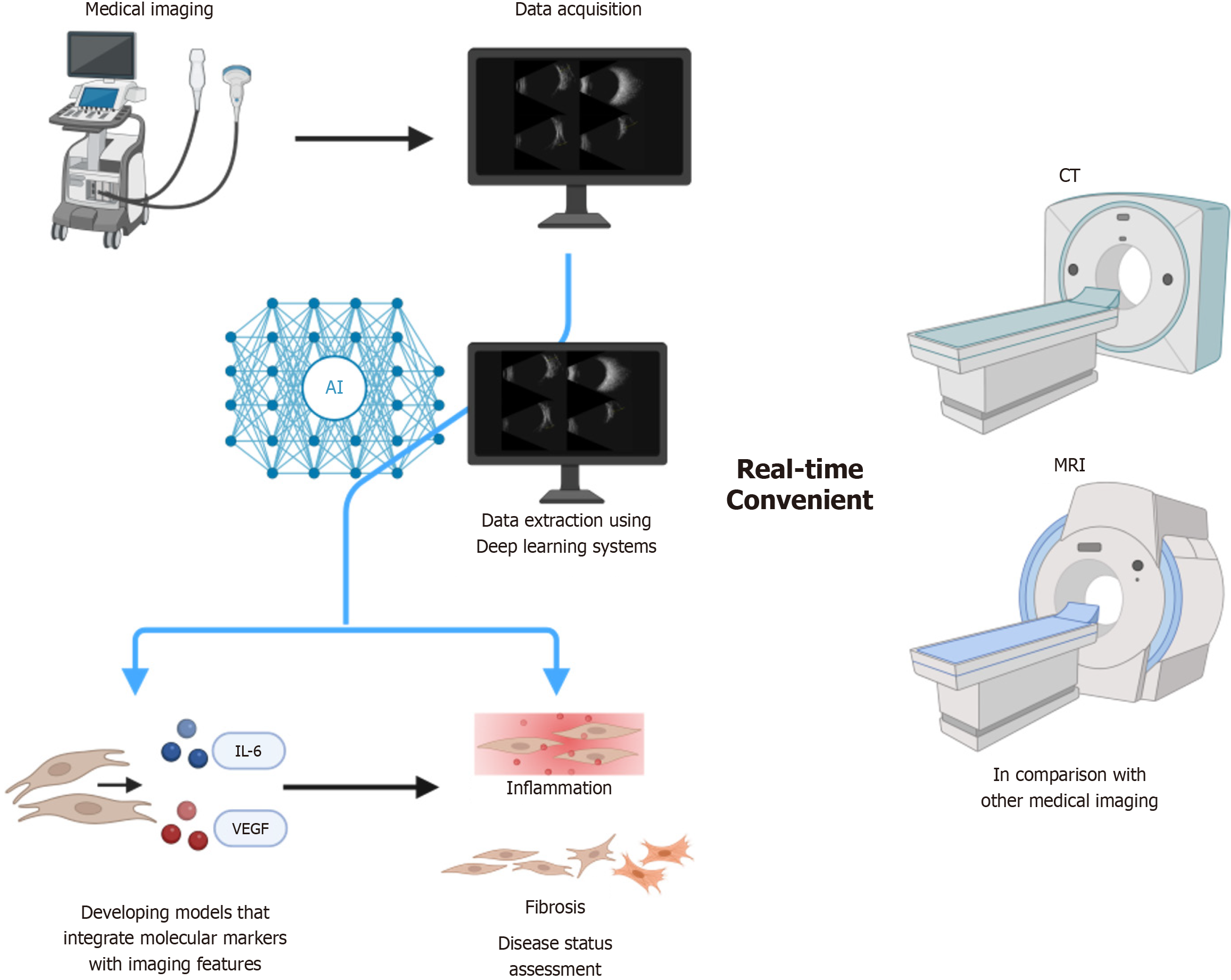
Thyroid-associated ophthalmopathy (TAO), a condition closely linked to Graves’ disease, presents significant diagnostic and management challenges. With rising incidence rates and substantial impact on quality of life, early and accurate diagnosis is critical. Current diagnostic approaches rely on clinical evaluation and imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), which have limitations, including radiation exposure and high cost. Ultrasound has emerged as a preferred alternative because of its accessibility, noninvasiveness, and real-time monitoring capabilities. However, its utility in TAO is constrained by limited resolution for deep orbital structures, a lack of standardized protocols that introduce inter-center variability, and operator dependence. In this manuscript, we examine how emerging technologies, including ultrasound elastography and artificial intelligence (AI)-driven analysis, can address these limitations. We highlight the unique advantages of ultrasound in assessing disease activity and severity, and propose strategies to standardize protocols and enhance diagnostic precision for personalized TAO management. These strategies form the basis of the diagnostic framework detailed in subsequent sections.
Ultrasound imaging is essential for the morphological evaluation of TAO, chiefly by measuring standardized parameters such as extraocular muscle thickness, the anteroposterior diameter of the lacrimal gland, and optic nerve sheath diameter (ONSD). Research has shown that extraocular muscle thickness is significantly increased in individuals diagnosed with TAO and strongly correlates with disease activity[1-3]. During the active phase of TAO, enlargement and thickening of the ocular muscles are primarily driven by inflammatory processes, particularly affecting the extraocular muscles[4]. This thickening, together with the proliferation of retro-orbital adipose tissue, is a major contributor to proptosis observed in TAO patients[4]. Evidence indicates that extraocular muscle thickness is markedly greater during the active phase, with the inferior rectus muscle exhibiting particularly pronounced thickening compared with the inactive phase[3]. Consequently, regular assessment of inferior rectus muscle thickness is crucial for monitoring disease progression and evaluating treatment effectiveness. Ultrasound, a noninvasive imaging modality, can accurately quantify inferior rectus muscle thickness, and its results correlate positively with MRI, thereby aiding early diagnosis and ongoing treatment assessment[5].
Furthermore, the establishment of standardized measurement protocols and consistent ultrasound techniques is essential to ensure comparability of findings across medical facilities. Integrating ultrasound with other imaging techniques, such as MRI, can yield a more comprehensive assessment of extraocular muscle condition in patients with TAO[6-8]. In patients with TAO, the condition of the optic nerve is crucial for preserving visual function[9-11]. Mea
Lacrimal gland volume is a key indicator for assessing tear-secretion function. In patients with TAO, increased volume may indicate an active disease phase[14]. Lacrimal gland function is affected by fluctuations in thyroid hormone levels, and swelling or dysfunction can exacerbate dry eye symptoms. Both ultrasonography and MRI are effective for quantifying lacrimal gland volume. Changes in volume correlate closely with TAO activity; patients with active disease typically have larger volumes[14-16]. Incorporating lacrimal gland volume with other clinical parameters can provide a comprehensive assessment of tear-secretion function in TAO and support timely intervention.
Three-dimensional ultrasound reconstruction is a promising advance in medical imaging. This technique collects multi-angle data from two-dimensional ultrasound images and uses computational algorithms to reconstruct three-dimensional anatomical structures, thereby improving the precision of volume measurements. The technology provides comprehensive anatomical insights that clarify lesion spatial relationships. Its noninvasive nature and real-time capability are particularly valuable for evaluating ocular diseases[17]. Compared with conventional imaging modalities, three-dimensional ultrasound offers detailed structural information without tissue damage, which is essential for early diagnosis and for recurrent preoperative evaluation of gallbladder disorders, uterine pathologies, and ocular conditions[18-20]. Moreover, this technology can facilitate foreign-body extraction from favorable positions and support decompression surgery in patients with TAO[21]. The real-time imaging feature enables clinicians to monitor dynamic lesion changes instantaneously, thereby improving diagnostic accuracy and supporting timely treatment decisions. With ongoing technological progress, three-dimensional ultrasound is expected to open new avenues for early diagnosis and management of TAO.
In functional imaging, combining the Doppler blood flow grading system with elastography stiffness thresholds provides new insights for early detection of TAO. Notably, the Adler scoring Doppler blood flow grading system, originally developed for assessing blood flow in breast cancer[22], is now widely applied across conditions, including liver cancer, cervical cancer, renal cancer, rheumatoid arthritis, white matter lesions in sickle cell disease, and carpal tunnel syndrome[23-32]. It can also evaluate microcirculatory effects in patients with coronary artery disease and chronic heart failure after surgery and endovascular myocardial revascularization[33].
Consequently, this system may effectively assess blood flow in extraocular muscles. Studies report significantly increased blood flow in extraocular muscles in patients with TAO, closely correlating with inflammatory activity[34]. Elastography stiffness thresholds also offer new opportunities for early diagnosis of TAO. Elastography is a noninvasive imaging technique that evaluates lesions by measuring tissue stiffness, with results closely linked to pathological conditions such as fibrosis and tumors[35-37]. This technique is broadly divided into two types: Strain elastography, which provides qualitative stiffness assessments[35], and shear wave elastography, which provides quantitative stiffness values[38]. Research indicates that ultrasound shear wave elastography and perfusion measurements can serve as biomarkers for tumor response[39]. For conditions requiring long-term follow-up, such as tumors, fatty liver disease, and liver cirrhosis, this technology confers notable advantages[36,39-44]. Studies show that ultrasound findings in patients with TAO may correlate positively with fibrosis, providing important evidence for assessing the degree of fibrosis in this population[45]. The primary multidisciplinary clinical applications of the Adler-Doppler blood flow grading system are detailed in Table 1.
| Classification | Application | Implications in TAO |
| Origin research | Evaluating tumor vascularity in breast cancer[43] | Basis for grading orbital inflammation |
| Cross-disease validation | Hemodynamic assessment in oncology (liver, cervical, renal cancers)[44-53] | Adapted for TAO microcirculation monitoring |
| Cardiovascular surgery | Microcirculation evaluation in CVD post-revascularization[54] | Potential for assessing TAO-related ischemia |
| TAO specific validation | Detection of inflammatory-driven blood flow changes in extraocular muscles[69] | Correlates with disease activity and fibrosis |
More recently, advances in high-frequency probes have improved resolution, enabling measurement of extraocular muscle thickness and detection of orbital changes in real time[46]. Ultrasound is increasingly recognized for its clinical utility in monitoring disease activity and guiding management decisions. Nevertheless, broader adoption of ultrasound in TAO remains limited by several intrinsic factors, including inadequate resolution for deep orbital structures[47], a lack of standardized acquisition protocols that lead to between-center variability[48], and high operator dependence that reduces reproducibility[49].
Recent technological progress offers opportunities to address these shortcomings. Ultrasound elastography enables quantitative assessment of tissue stiffness, potentially distinguishing active inflammatory changes from chronic fibrosis[35,38]. Moreover, advances in AI and machine learning have opened new avenues for automated image analysis, reducing operator dependence and improving diagnostic consistency[50-52]. These developments may expand the role of ultrasound from a supplementary tool to a central modality for diagnosis, staging, and longitudinal monitoring of TAO.
The inflammatory reaction in TAO is a core component of the pathogenic mechanism. Initially, abnormal thyroid hormone levels and autoimmune responses lead to infiltration of inflammatory cells in the orbit, consisting primarily of CD4+ T cells and monocytes/macrophages. Subsequently, thyroid-stimulating hormone receptor and insulin-like growth factor-1 synergistically promote fibroblast proliferation and differentiation into adipocytes, resulting in expansion of adipose tissue within extraocular muscles and other orbital fat and connective tissues. In addition, the ability of orbital fibroblasts to synthesize hydrophilic glycosaminoglycans, predominantly hyaluronic acid, increases, contributing to edema and further expansion of orbital tissues[53,54]. Research indicates that severe active TAO is significantly associated with infiltration of CD4+ T cells and monocytes/macrophages[55,56]. The Clinical Activity Score correlates positively with the degree of lymphocyte infiltration (including total lymphocytes, T cells, and B cells) and macrophages[57-59]. Monitoring inflammatory activity can be facilitated by imaging modalities such as ultrasound, which helps determine whether the disease is in an active phase[47]. Furthermore, ultrasound plays a crucial role in evaluating inflammatory biomarkers. Vascular endothelial growth factor (VEGF) is critical for maintaining physiological vascular homeostasis across diverse cell types and tissues. It has been implicated in the molecular mechanisms underlying tumor growth and metastasis, as well as in retinal disorders linked to several blinding conditions, such as age-related macular degeneration and diabetic and hypertensive retinopathy. Pathogenic effects associated with VEGF are primarily due to its role in modulating vascular permeability and promoting neovascularization[60]. Research indicates increased vascular density in acute TAO compared with chronic TAO and control orbital fat, suggesting an angiogenic response. This pro-angiogenic and pro-lymphangiogenic microenvironment may result from heightened expression of VEGF receptors, including VEGFR-2, VEGF-A, VEGF-C, and VEGF-D. These observations suggest that periorbital edema in acute TAO may be partially driven by immature blood vessels and lymphatic capillaries with insufficient functional capacity to adequately drain the stroma[61]. Numerous studies also indicate that ultrasound can be used to evaluate abnormal blood flow. Using techniques such as color Doppler imaging and pulsed Doppler mode, together with advances in nanoparticle technology, ultrasound can quantitatively assess hemodynamic alterations in ocular tissues, thereby providing insights into VEGF expression levels and facilitating evaluation of the inflammatory response[61-63].
The advantages of ultrasound for evaluating inflammation and associated biomarkers derive from its noninvasive nature, real-time capability, and cost-effectiveness. In monitoring patients with Crohn’s disease, ultrasound can assess structural alterations within the intestine while simultaneously evaluating disease activity and treatment effectiveness by comparing dynamic changes in inflammatory markers[62]. This multifaceted approach establishes ultrasound as a valuable tool that offers immediate feedback in the management of inflammatory conditions such as TAO. Dynamic monitoring is also a major application of ultrasound in assessing inflammatory response. By performing routine ul
During the inactive phase of TAO, common pathological alterations include adipose tissue hyperplasia, increased synthesis of hyaluronic acid, and conversion of fibroblasts into myofibroblasts, which ultimately leads to tissue fibrosis. These changes are primarily driven by interactions among T cells, B cells, and orbital fibroblasts[53,54,65]. The fibrotic stage is characterized by irreversible structural damage, with symptoms frequently associated with mechanical compression and increased tissue stiffness. Timely identification of the transition from the active to the fibrotic phase is essential for mitigating long-term complications, enhancing prognosis, and improving quality of life for patients. Research indicates that applying color Doppler to assess blood flow parameters in the ophthalmic artery, superior ophthalmic vein, and central retinal artery may aid in differentiating between the active and inactive phases of TAO. Patients with a Clinical Activity Score of 3 or higher (indicative of the active phase) demonstrate significantly elevated arterial Doppler parameters, including peak systolic velocity and end diastolic velocity of the ophthalmic artery, as well as peak systolic velocity of the central retinal artery, whereas the maximum velocity of the superior ophthalmic vein shows a significant decrease, suggesting ongoing inflammatory activity within the orbit[66]. However, the observed increase in arterial blood flow velocity may also reflect a secondary response to intraorbital inflammation from diverse etiologies[67]. As noted above, strain elastography qualitatively assesses tissue stiffness[35], whereas shear wave elastography quantitatively evaluates fibrosis status[38]. In patients with chronic liver disease, routine ultrasound examinations facilitate monitoring of liver fibrosis progression and treatment efficacy[68]. Evidence suggests that regular ultrasound assessments significantly improve early detection of liver fibrosis progression and inform treatment decisions. Clinicians can more thoroughly evaluate hepatic changes by combining elastography (for stiffness) with B-mode ultrasound (for anatomy)[69]. At the molecular level, transforming growth factor beta 1 (TGF-β1) is a key biomarker related to fibrosis. It plays a critical role in its development[70,71].
Numerous studies have established a significant correlation between elevated serum TGF-β1 levels and fibrosis severity, indicating its potential utility in fibrosis assessment[72,73]. Therefore, combining elastography with TGF-β1 detection may provide more precise information for early diagnosis and evaluation of TAO-related fibrosis. In addition, long-term follow-up should incorporate clinical manifestations of patients and biochemical indicators to formulate personalized management plans, thereby improving outcomes and enhancing quality of life for patients. Establishing a multidisciplinary collaborative team that integrates ultrasound technology with other imaging modalities, such as MRI, can facilitate dynamic monitoring and assessment of fibrosis progression, ensuring optimal treatment and care[74].
Ultrasound imaging provides real-time guidance for muscle monitoring, injections, and treatment assessment. However, challenges remain in visualizing deep orbital structures and in standardizing protocols, which limit diagnostic consistency. Future progress will require enhanced resolution, unified criteria, and integration of nanotechnology and AI to transform ultrasound into a dynamic platform for stratified therapies. This evolution will bridge precision medicine with the complexity of TAO, paving the way for personalized management and improved outcomes. This roadmap (Figure 1) positions ultrasound as a central platform for precision management of TAO and may reduce healthcare costs by 30%-40% compared with current imaging paradigms.
Current diagnostic approaches rely primarily on clinical evaluation and radiologic imaging. CT was widely adopted for delineating extraocular muscle enlargement and bony anatomy; however, concerns about radiation exposure limit repeated use[75]. MRI subsequently emerged as a superior tool for soft-tissue assessment, enabling visualization of orbital inflammation and disease activity[76-78]. Despite these advantages, CT and MRI are costly, less accessible for routine follow-up, involve radiation exposure (for CT), and are impractical for dynamic monitoring. Drawing on research evidence for imaging techniques in TAO, this article analyzes key characteristics of three modalities, ultrasound, CT, and MRI, from the perspectives of technical parameters and comparative medicine. Evidence derived from nine core papers (data as of 2023) focuses on six dimensions: Spatial resolution, hemodynamic evaluation, disease activity staging, bone structure evaluation, cost and accessibility, and safety. Key comparisons are outlined in Table 2.
| Technical dimension | Ultrasound | CT/MRI | Ref. |
| Spatial resolution | Moderate (can distinguish muscle/fat layers) | High (MRI provides superior muscle texture detail) | [1,76] |
| Hemodynamic assessment | Superior (real-time Doppler quantification) | Limited (requires contrast-enhanced scanning) | [46,77] |
| Disease activity staging | Based on hemodynamic + structural dynamics | MRI T2 signal indicates edema | [1] |
| Bony structure assessment | Cannot visualize bony walls | Essential (preoperative surgical decompression planning) | [76] |
| Cost and accessibility | Superior (portable equipment) | Higher (especially MRI) | [46,76] |
| Safety | Superior (non-ionizing, repeatable) | CT radiation risk; MRI metallic contraindications | [46,76] |
In the future, ultrasound-guided orbital decompression surgery may reduce surgical complications and shorten recovery times. Ultrasound for real-time monitoring of localized drug injections also enhances procedural precision and effectiveness[79-81]. This monitoring increases treatment safety and yields data to inform personalized strategies, thereby improving overall therapeutic efficacy. As a cost-effective and efficient imaging modality, ultrasound provides real-time imaging and is suitable for repeated use to support ongoing assessment of the condition[82]. Weekly ultrasound evaluations can monitor changes in extraocular muscle thickness, guiding adjustments to hormone dosages and ensuring the safety and effectiveness of treatment regimens. This approach promotes greater personalization and accuracy in treatment and contributes to improved patient outcomes.
In ultrasound medicine, the integration of targeted therapy and nanotechnology is a pivotal research focus, offering innovative strategies for precise management of TAO. Sonodynamic therapy, a leading technology in this field, activates chemical agents (sonosensitizers) through nonthermal ultrasound effects, enabling superior tissue penetration and spatial selectivity. This noninvasive modality has shown significant advantages in oncology[83-85]. Advances in multifunctional nanoparticles have further expanded the potential applications of this technology. These nanoparticles have been designed as targeted drug carriers for tumors, long-acting ultrasound contrast agents, and enhancers of ultrasound-mediated drug delivery, warranting further investigation as potential anticancer agents[86]. In the future, such nanoparticles may serve as targeted carriers for TAO-related inflammatory factors, such as insulin-like growth factor-1R and interleukin-6, facilitate real-time visualization and monitoring of treatment processes as long-acting ultrasound contrast agents, and improve drug permeation across biological barriers through ultrasound-mediated cavitation. Although research on localized nanoparticle drug delivery systems for TAO remains nascent, ongoing advances in ultrasound molecular imaging and responsive nanomaterials offer promising opportunities for developing an ultrasound-guided, nanoparticle-targeted therapy paradigm for TAO.
Intelligent technologies have markedly improved the quality and efficiency of medical care for the diagnosis and treatment of TAO[50-52]. The integration of AI, particularly in imaging, is progressively reshaping conventional medical paradigms. Research demonstrates that deep learning systems can distinguish papilledema, normal optic discs, and various disc abnormalities by analyzing fundus photographs obtained with pharmacological mydriasis. One system achieved an area under the curve of 0.96 [95% confidence interval (CI): 0.95-0.97], with a sensitivity of 96.4% (95%CI: 93.9-98.3) and a specificity of 84.7% (95%CI: 82.3-87.1)[87]. By harnessing deep learning and big data analytics, AI can extract relevant information from extensive imaging datasets, thereby aiding healthcare professionals in making more precise diagnoses of conditions such as coronavirus disease 2019 and solid cancers, as well as in evaluating coronary artery disease[88-94]. The incorporation of AI into ultrasound imaging analysis has significantly enhanced diagnostic accuracy, enabling the detection of subtle lesions with greater precision than traditional methods and thereby reducing risks of misdiagnosis and missed diagnoses. However, variability among models may lead to errors, and a high false-positive rate could necessitate substantial changes in clinical applications[94]. AI also supports the development of personalized treatment plans through data mining, which can improve treatment outcomes and enhance quality of life for patients. Nonetheless, implementation faces challenges related to data quality and quantity; limited case numbers may adversely affect model performance, and difficulties persist in the architectural design of deep learning algorithms for medical imaging. In addition, the “black box” nature of AI reduces transparency in the decision-making process, potentially fostering skepticism among patients and healthcare providers regarding the results. Consequently, improving the interpretability of AI models represents a critical direction for future research[95,96].
Ultrasound offers advantages for TAO assessment, but key limitations remain, including resolution, operator dependence, and protocol variability. Recent technological progress offers opportunities to address these shortcomings. Ultrasound elastography enables quantitative assessment of tissue stiffness, potentially distinguishing active inflammatory changes from chronic fibrosis. Advances in AI and machine learning have opened avenues for automated image analysis, reducing operator dependence and improving diagnostic consistency. These developments may expand the role of ultrasound from a supplementary tool to a central modality for the diagnosis, staging, and longitudinal monitoring of TAO. To advance the field, combining 3D ultrasound with shear-wave elastography and microvascular Doppler, developing validated algorithms using multicenter datasets, and establishing imaging-VEGF/interleukin-6 stratification models are essential and promising steps. This tripartite approach positions ultrasound as a cost-effective, radiation-free cornerstone for TAO management, with the potential to reduce healthcare costs in future medicine.
| 1. | Lennerstrand G, Tian S, Isberg B, Landau Högbeck I, Bolzani R, Tallstedt L, Schworm H. Magnetic resonance imaging and ultrasound measurements of extraocular muscles in thyroid-associated ophthalmopathy at different stages of the disease. Acta Ophthalmol Scand. 2007;85:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Han JS, Seo HS, Lee YH, Lee H, Suh SI, Jeong EK, Sapkota N, Kim KJ. Fractional anisotropy and diffusivity changes in thyroid-associated orbitopathy. Neuroradiology. 2016;58:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Stoyanova NS, Konareva-Kostianeva M, Mitkova-Hristova V, Angelova I. Correlation between Intraocular Pressure and Thickness of Extraocular Muscles, the Severity and Activity of Thyroid-associated Orbitopathy. Folia Med (Plovdiv). 2019;61:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Yang M, Du BX, Wang YJ, He WM. [Clinical Analysis of 2 170 Cases of Thyroid-Associated Ophthalmopathy Involving Extraocular Muscles]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021;52:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Karhanova M, Kovar R, Frysak Z, Sin M, Zapletalova J, Rehak J, Herman M. Correlation between magnetic resonance imaging and ultrasound measurements of eye muscle thickness in thyroid-associated orbitopathy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Rodríguez-González N, Pérez-Rico C, López-Para Giménez R, Arévalo-Serrano J, Del Amo García B, Calzada Domingo L, Flores Ruiz L, Blanco R. [Short-tau inversion-recovery (STIR) sequence magnetic resonance imaging evaluation of orbital structures in Graves' orbitopathy]. Arch Soc Esp Oftalmol. 2011;86:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nagy EV, Toth J, Kaldi I, Damjanovich J, Mezosi E, Lenkey A, Toth L, Szabo J, Karanyi Z, Leovey A. Graves' ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000;142:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, Tasan E. Graves' ophthalmopathy: the role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. Br J Radiol. 2015;88:20140677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Zhang T, Xiao W, Ye H, Chen R, Mao Y, Yang H. Peripapillary and Macular Vessel Density in Dysthyroid Optic Neuropathy: An Optical Coherence Tomography Angiography Study. Invest Ophthalmol Vis Sci. 2019;60:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Fan X, Zha X, Wang N, Han Y. Preliminary Study on the Correlation Between Episcleral Venous Pressure and Intraocular Pressure in TAO Patients and Its Clinical Value. Altern Ther Health Med. 2024;30:522-527. [PubMed] |
| 11. | Zeng P, Wang J, Tian P, Peng YY, Liang JQ, Wang M, Zhou SY. Macular and peripapillary optical coherence tomography angiography metrics in thyroid-associated ophthalmopathy with chorioretinal folds. Photodiagnosis Photodyn Ther. 2023;42:103146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Elkady ZIR, Elwan S, Said AMA, Farweez YAT, Mahdy MMM. Optical Coherence TomographyStudy of peripapillary Nerve Fiber layer ThicknessinThyroid Associated Ophthalmopathy. QJM, Int J Med. 2020;113:hcaa058.007. [DOI] [Full Text] |
| 13. | Sallam A, Abdelaal Ahmed Mahmoud M Alkhatip A, Kamel MG, Hamza MK, Yassin HM, Hosny H, Younis MI, Ramadan E, Algameel HZ, Abdelhaq M, Abdelkader M, Mills KE, Mohamed H. The Diagnostic Accuracy of Noninvasive Methods to Measure the Intracranial Pressure: A Systematic Review and Meta-analysis. Anesth Analg. 2021;132:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Byun JS, Moon NJ, Lee JK. Quantitative analysis of orbital soft tissues on computed tomography to assess the activity of thyroid-associated orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Caltabiano C, Rana K, Beecher MB, Selva D. Radiological measurements of lacrimal gland in thyroid eye disease. Int Ophthalmol. 2024;44:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Zhao RX, Shi TT, Luo S, Liu YF, Xin Z, Yang JK. The value of SPECT/CT imaging of lacrimal glands as a means of assessing the activity of Graves' orbitopathy. Endocr Connect. 2022;11:e210590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kharlap SI. [Modern ultrasound methods of examination in clinical ophthalmology. Background problems and future prospects]. Vestn Ross Akad Med Nauk. 2003;32-38. [PubMed] |
| 18. | Pacheco GHAS, Castro PT, Tonni G, Werner H, Araujo Júnior E. Prenatal diagnosis of duplicated gallbladder: two-dimensional and three-dimensional ultrasound imaging and reconstruction. Ultrasound Obstet Gynecol. 2025;66:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Alonso L, Carugno J. Chronic Endometritis: Three-dimensional Ultrasound and Hysteroscopy Correlation. J Minim Invasive Gynecol. 2020;27:993-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Behrendt S, Rochels R. [Perspectives in diagnosis and therapy of orbital diseases]. Klin Monbl Augenheilkd. 1994;204:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Li Y, Wei XL, Pang KK, Ni PJ, Wu M, Xiao J, Zhang LL, Zhang FX. A comparative study on the features of breast sclerosing adenosis and invasive ductal carcinoma via ultrasound and establishment of a predictive nomogram. Front Oncol. 2023;13:1276524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Ferreira De Matos C, Cougoul P, Zaharie OM, Kermorgant M, Pavy-Le Traon A, Gales C, Senard JM, Strumia M, Bonneville F, Nasr N. Cerebrovascular and cardiovascular autonomic regulation in sickle cell patients with white matter lesions. Eur J Neurol. 2024;31:e16183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Kuang X, Wang H, Chai W, Yuan H, He T, Shi M, Jiang T. Clinical diagnostic value of contrast-enhanced ultrasound combined with microflow imaging in benign and malignant renal tumors: A retrospective cohort study. Biomol Biomed. 2024;24:1370-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Zhang D, Wang Y, Yang F, Mao Y, Mu J, Zhao L, Xu W. Diagnostic Value of Multi-Mode Ultrasonic Flow Imaging Examination in Solid Renal Tumors of Different Sizes. J Clin Med. 2023;12:566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Chen M, Fu X, Shen Y. Evaluation of Multimode Color Doppler Flow Imaging in the Diagnosis of Solid Renal Tumor. Contrast Media Mol Imaging. 2021;2021:6656877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Anand AC, Nandi B, Acharya SK, Arora A, Babu S, Batra Y, Chawla YK, Chowdhury A, Chaoudhuri A, Eapen EC, Devarbhavi H, Dhiman R, Datta Gupta S, Duseja A, Jothimani D, Kapoor D, Kar P, Khuroo MS, Kumar A, Madan K, Mallick B, Maiwall R, Mohan N, Nagral A, Nath P, Panigrahi SC, Pawar A, Philips CA, Prahraj D, Puri P, Rastogi A, Saraswat VA, Saigal S, Shalimar, Shukla A, Singh SP, Verghese T, Wadhawan M; INASL Task-Force on Acute Liver Failure. Indian National Association for the Study of the Liver Consensus Statement on Acute Liver Failure (Part 1): Epidemiology, Pathogenesis, Presentation and Prognosis. J Clin Exp Hepatol. 2020;10:339-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Han H, Ding H, Ji Z, Zhang W, Wang Q, Wang W. Primary Application of Micro-Flow Imaging Technology in the Diagnosis of Hepatic Tumors. Ultrasound Med Biol. 2019;45:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Karahan AY, Arslan S, Ordahan B, Bakdik S, Ekiz T. Superb Microvascular Imaging of the Median Nerve in Carpal Tunnel Syndrome: An Electrodiagnostic and Ultrasonographic Study. J Ultrasound Med. 2018;37:2855-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Yang F, Zhao J, Liu C, Mao Y, Mu J, Wei X, Jia J, Zhang S, Xin X, Tan J. Superb microvascular imaging technique in depicting vascularity in focal liver lesions: more hypervascular supply patterns were depicted in hepatocellular carcinoma. Cancer Imaging. 2019;19:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Che D, Yang Z, Wei H, Wang X, Gao J. The Adler grade by Doppler ultrasound is associated with clinical pathology of cervical cancer: Implication for clinical management. PLoS One. 2020;15:e0236725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Kurtulus Ozturk E, Ozturk S, Kelesoglu Dincer AB. The reliability and validity of superb microvascular imaging as a potential disease activity marker in rheumatoid arthritis. Ultraschall Med. 2025;46:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Kulchitskaya DB, Shovkun TV, Yarnykh EV, Konchugova TV, Knyazeva TA, Gushchina NV, Chernyakhovsky OB, Kolbakhova SN. [Impact of external counterpulsation on microcirculation in patients with coronary heart disease complicated by chronic heart failure after surgical and endovascular myocardial revascularization]. Vopr Kurortol Fizioter Lech Fiz Kult. 2019;96:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Kurioka Y, Inaba M, Kawagishi T, Emoto M, Kumeda Y, Inoue Y, Morii H, Nishizawa Y. Increased retinal blood flow in patients with Graves' disease: influence of thyroid function and ophthalmopathy. Eur J Endocrinol. 2001;144:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Balleyguier C, Canale S, Ben Hassen W, Vielh P, Bayou EH, Mathieu MC, Uzan C, Bourgier C, Dromain C. Breast elasticity: principles, technique, results: an update and overview of commercially available software. Eur J Radiol. 2013;82:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Yu JH, Lee HA, Kim SU. Noninvasive imaging biomarkers for liver fibrosis in nonalcoholic fatty liver disease: current and future. Clin Mol Hepatol. 2023;29:S136-S149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 36. | Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 1, Principles and Techniques. AJR Am J Roentgenol. 2015;205:22-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 37. | Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 943] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 38. | Voutouri C, Mpekris F, Panagi M, Krolak C, Michael C, Martin JD, Averkiou MA, Stylianopoulos T. Ultrasound stiffness and perfusion markers correlate with tumor volume responses to immunotherapy. Acta Biomater. 2023;167:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 39. | Huang JX, Liu FT, Sun L, Ma C, Fu J, Wang XY, Huang GL, Zhang YT, Pei XQ. Comparing shear wave elastography of breast tumors and axillary nodes in the axillary assessment after neoadjuvant chemotherapy in patients with node-positive breast cancer. Radiol Med. 2024;129:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Taiji R, Cortes AC, Zaske AM, Williams M, Dupuis C, Tanaka T, Nishiofuku H, Chintalapani G, Peterson CB, Avritscher R. Liver Cancer Vascularity Driven by Extracellular Matrix Stiffness: Implications for Imaging Research. Invest Radiol. 2023;58:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Ozturk A, Kumar V, Pierce TT, Li Q, Baikpour M, Rosado-Mendez I, Wang M, Guo P, Schoen S Jr, Gu Y, Dayavansha S, Grajo JR, Samir AE. The Future Is Beyond Bright: The Evolving Role of Quantitative US for Fatty Liver Disease. Radiology. 2023;309:e223146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Chen CM, Tang YC, Huang SH, Pan KT, Lui KW, Lai YH, Tsui PH. Ultrasound tissue scatterer distribution imaging: An adjunctive diagnostic tool for shear wave elastography in characterizing focal liver lesions. Ultrason Sonochem. 2023;101:106716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053-6062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (8)] |
| 44. | Rajabi MT, Papageorgiou K, Taban M, Hwang CJ, Hosseini SS, Rajabi MB, Goldberg RA. Ultrasonographic motion analysis of lower eyelid compartments in patients with chronic thyroid associated ophthalmopathy. J Curr Ophthalmol. 2017;29:310-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Kim MG, Yoon C, Lim HG. Recent Advancements in High-Frequency Ultrasound Applications from Imaging to Microbeam Stimulation. Sensors (Basel). 2024;24:6471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Karhanová M, Čivrný J, Kalitová J, Schovánek J, Malušková M, Hrevuš M, Schreiberová Z. [Ultrasound Examination of the Orbit in Patients With Thyroid- Associated Orbitopathy – Examination Guide and Recommendations for Everyday Practice. A Review]. Cesk Slov Oftalmol. 2024;80:3-9. [DOI] [Full Text] |
| 47. | Stegemann E, Larbig J, Portig I, Weiske N, Bürger T, Stegemann B. Reliability of a Standardized Ultrasound Protocol for the Diagnosis of Thoracic Outlet Syndrome. Ultraschall Med. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Alyami J, Almutairi FF, Aldoassary S, Albeshry A, Almontashri A, Abounassif M, Alamri M. Interobserver variability in ultrasound assessment of thyroid nodules. Medicine (Baltimore). 2022;101:e31106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 49. | Liu Z, Tan K, Zhang H, Sun J, Li Y, Fang S, Li J, Song X, Zhou H, Zhai G. CT-based artificial intelligence prediction model for ocular motility score of thyroid eye disease. Endocrine. 2024;86:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 50. | Lin LY, Zhou P, Shi M, Lu JE, Jeon S, Kim D, Liu JM, Wang M, Do S, Lee NG. A Deep Learning Model for Screening Computed Tomography Imaging for Thyroid Eye Disease and Compressive Optic Neuropathy. Ophthalmol Sci. 2024;4:100412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Ji Y, Chen N, Liu S, Yan Z, Qian H, Zhu S, Zhang J, Wang M, Jiang Q, Yang W. Research Progress of Artificial Intelligence Image Analysis in Systemic Disease-Related Ophthalmopathy. Dis Markers. 2022;2022:3406890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1150] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 53. | Lee ACH, Kahaly GJ. Pathophysiology of thyroid-associated orbitopathy. Best Pract Res Clin Endocrinol Metab. 2023;37:101620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 54. | Eckstein AK, Quadbeck B, Tews S, Mann K, Krüger C, Mohr CH, Steuhl KP, Esser J, Gieseler RK. Thyroid associated ophthalmopathy: evidence for CD4(+) gammadelta T cells; de novo differentiation of RFD7(+) macrophages, but not of RFD1(+) dendritic cells; and loss of gammadelta and alphabeta T cell receptor expression. Br J Ophthalmol. 2004;88:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Pawlowski P, Wawrusiewicz-Kurylonek N, Eckstein A, Reszec J, Luczynski W, Johnson K, Kretowski A, Bakunowicz-Lazarczyk A, Gorska M, Szamatowicz J, Chyczewski L, Mysliwiec J. Disturbances of modulating molecules (FOXP3, CTLA-4/CD28/B7, and CD40/CD40L) mRNA expressions in the orbital tissue from patients with severe graves' ophthalmopathy. Mediators Inflamm. 2015;2015:340934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Rotondo Dottore G, Torregrossa L, Caturegli P, Ionni I, Sframeli A, Sabini E, Menconi F, Piaggi P, Sellari-Franceschini S, Nardi M, Latrofa F, Vitti P, Marcocci C, Basolo F, Marinò M. Association of T and B Cells Infiltrating Orbital Tissues With Clinical Features of Graves Orbitopathy. JAMA Ophthalmol. 2018;136:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 57. | Rotondo Dottore G, Torregrossa L, Lanzolla G, Mariotti S, Menconi F, Piaggi P, Cristofani Mencacci L, Posarelli C, Maglionico MN, Dallan I, Figus M, Nardi M, Marcocci C, Basolo F, Marinò M. Role of the mononuclear cell infiltrate in Graves' orbitopathy (GO): results of a large cohort study. J Endocrinol Invest. 2022;45:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Pawlowski P, Reszec J, Eckstein A, Johnson K, Grzybowski A, Chyczewski L, Mysliwiec J. Markers of inflammation and fibrosis in the orbital fat/connective tissue of patients with Graves' orbitopathy: clinical implications. Mediators Inflamm. 2014;2014:412158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1985] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 60. | Wong LL, Lee NG, Amarnani D, Choi CJ, Bielenberg DR, Freitag SK, D'Amore PA, Kim LA. Orbital Angiogenesis and Lymphangiogenesis in Thyroid Eye Disease: An Analysis of Vascular Growth Factors with Clinical Correlation. Ophthalmology. 2016;123:2028-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Barchi A, Dal Buono A, D'Amico F, Furfaro F, Zilli A, Fiorino G, Parigi TL, Peyrin-Biroulet L, Danese S, Allocca M. Leaving behind the Mucosa: Advances and Future Directions of Intestinal Ultrasound in Ulcerative Colitis. J Clin Med. 2023;12:7569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Allocca M, Furfaro F, Fiorino G, Peyrin-Biroulet L, Danese S. Point-of-Care Ultrasound in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 63. | Randhawa A, Guzowski T. Intestinal Ultrasound: Envisioning a New Future for Crohn's Disease Management. ACG Case Rep J. 2024;11:e01511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Buonfiglio F, Ponto KA, Pfeiffer N, Kahaly GJ, Gericke A. Redox mechanisms in autoimmune thyroid eye disease. Autoimmun Rev. 2024;23:103534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 65. | Yanik B, Conkbayir I, Acaroglu G, Hekimoglu B. Graves' ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound. 2005;33:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Jianu DC, Jianu SN, Petrica L, Motoc AG, Dan TF, Lăzureanu DC, Munteanu M. Clinical and color Doppler imaging features of one patient with occult giant cell arteritis presenting arteritic anterior ischemic optic neuropathy. Rom J Morphol Embryol. 2016;57:579-583. [PubMed] |
| 67. | Poon C, Pellow C, Hynynen K. Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments. Theranostics. 2021;11:1655-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Choi HJ, Han M, Seo H, Park CY, Lee EH, Park J. The new insight into the inflammatory response following focused ultrasound-mediated blood-brain barrier disruption. Fluids Barriers CNS. 2022;19:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 69. | Hess A, Gentile SD, Ben Saad A, Rahman RU, Habboub T, Pratt DS, Mullen AC. Single-cell transcriptomics stratifies organoid models of metabolic dysfunction-associated steatotic liver disease. EMBO J. 2023;42:e113898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Moreau JM, Velegraki M, Bolyard C, Rosenblum MD, Li Z. Transforming growth factor-β1 in regulatory T cell biology. Sci Immunol. 2022;7:eabi4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 71. | Xu MY, Hu JJ, Shen J, Wang ML, Zhang QQ, Qu Y, Lu LG. Stat3 signaling activation crosslinking of TGF-β1 in hepatic stellate cell exacerbates liver injury and fibrosis. Biochim Biophys Acta. 2014;1842:2237-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Song X, Shi J, Liu J, Liu Y, Yu Y, Qiu Y, Cao Z, Pan Y, Yuan X, Chu Y, Wu D. Recombinant truncated latency-associated peptide alleviates liver fibrosis in vitro and in vivo via inhibition of TGF-β/Smad pathway. Mol Med. 2022;28:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 73. | Biris AI, Karamatzanis I, Biri D, Biris IA, Maravegias N. Non-Invasive Ultrasound Diagnostic Techniques for Steatotic Liver Disease and Focal Liver Lesions: 2D, Colour Doppler, 3D, Two-Dimensional Shear Wave Elastography (2D-SWE), and Ultrasound-Guided Attenuation Parameter (UGAP). Cureus. 2024;16:e72087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Lee KH, Jang SY, Lee SY, Yoon JS. Graded decompression of orbital fat and wall in patients with Graves' orbitopathy. Korean J Ophthalmol. 2014;28:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Song Y, Li T, Tang W, Lv Q, Zhang XX, Zhou WY, Shi YQ. Application progress of magnetic resonance imaging in thyroid associated ophthalmopathy. Front Endocrinol (Lausanne). 2025;16:1537957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Karhanová M, Čivrný J, Kalitová J, Schovánek J, Pašková B, Schreiberová Z, Hübnerová P. Computed tomography and magnetic resonance imaging of the orbit in the diagnosis and treatment of thyroid-associated orbitopathy - experience from practice. A Review. Cesk Slov Oftalmol. 2023;79:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Yu Y, Feng T, Qiu H, Gu Y, Chen Q, Zuo C, Ma H. Simultaneous photoacoustic and ultrasound imaging: A review. Ultrasonics. 2024;139:107277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 78. | Cai L, Hu M, Lin L, Zheng T, Liu J, Li Z. Evaluation of the efficacy of triamcinolone acetonide in the treatment of keloids by high-frequency ultrasound. Skin Res Technol. 2020;26:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Cool SK, Geers B, Roels S, Stremersch S, Vanderperren K, Saunders JH, De Smedt SC, Demeester J, Sanders NN. Coupling of drug containing liposomes to microbubbles improves ultrasound triggered drug delivery in mice. J Control Release. 2013;172:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Mlosek RK, Malinowska S, Woźniak W. Lipoma removal using a high-frequency ultrasound-guided injection of a Class III CE-marked device-Empirical findings. J Cosmet Dermatol. 2019;18:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 81. | Chee C, Hibbert EJ, Lam P, Nanan R, Liu A. Sonographic and other nonglycemic factors can predict large-for-gestational-age infants in diet-managed gestational diabetes mellitus: A retrospective cohort study. J Diabetes. 2020;12:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Bernal A, Calcagno C, Mulder WJM, Pérez-Medina C. Imaging-guided nanomedicine development. Curr Opin Chem Biol. 2021;63:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Canaparo R, Foglietta F, Barbero N, Serpe L. The promising interplay between sonodynamic therapy and nanomedicine. Adv Drug Deliv Rev. 2022;189:114495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 84. | Ovais M, Mukherjee S, Pramanik A, Das D, Mukherjee A, Raza A, Chen C. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv Mater. 2020;32:e2000055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 85. | Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 86. | Milea D, Najjar RP, Zhubo J, Ting D, Vasseneix C, Xu X, Aghsaei Fard M, Fonseca P, Vanikieti K, Lagrèze WA, La Morgia C, Cheung CY, Hamann S, Chiquet C, Sanda N, Yang H, Mejico LJ, Rougier M-B, Kho R, Thi Ha Chau T, Singhal S, Gohier P, Clermont-Vignal C, Cheng C-Y, Jonas JB, Yu-Wai-Man P, Fraser CL, Chen JJ, Ambika S, Miller NR, Liu Y, Newman NJ, Wong TY, Biousse V; BONSAI Group. Artificial Intelligence to Detect Papilledema from Ocular Fundus Photographs. N Engl J Med. 2020;382:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 87. | Rajpurkar P, Lungren MP. The Current and Future State of AI Interpretation of Medical Images. N Engl J Med. 2023;388:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 234] [Reference Citation Analysis (0)] |
| 88. | Freeman K, Geppert J, Stinton C, Todkill D, Johnson S, Clarke A, Taylor-Phillips S. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. BMJ. 2021;374:n1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 89. | Forrest IS, Petrazzini BO, Duffy Á, Park JK, Marquez-Luna C, Jordan DM, Rocheleau G, Cho JH, Rosenson RS, Narula J, Nadkarni GN, Do R. Machine learning-based marker for coronary artery disease: derivation and validation in two longitudinal cohorts. Lancet. 2023;401:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 90. | Batra P, Khera AV. Machine learning to assess coronary artery disease status-is it helpful? Lancet. 2023;401:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 91. | Adams SJ, Stone E, Baldwin DR, Vliegenthart R, Lee P, Fintelmann FJ. Lung cancer screening. Lancet. 2023;401:390-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 252] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 92. | Abbasi J. Artificial Intelligence in COVID-19 Imaging Mismatched to the Clinic. JAMA. 2021;326:124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 904] [Article Influence: 129.1] [Reference Citation Analysis (6)] |
| 94. | Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit Health. 2021;3:e745-e750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 526] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 95. | Nagaraju RM, Gurushankar G, Bhimarao, Kadakola B. Efficacy of High Frequency Ultrasound in Localization and Characterization of Orbital Lesions. J Clin Diagn Res. 2015;9:TC01-TC06. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Silverman RH. High-resolution ultrasound imaging of the eye - a review. Clin Exp Ophthalmol. 2009;37:54-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/