Published online Nov 28, 2025. doi: 10.4329/wjr.v17.i11.113012
Revised: August 27, 2025
Accepted: November 4, 2025
Published online: November 28, 2025
Processing time: 106 Days and 19.2 Hours
Non-traumatic headache is a common presentation in both emergency and outpatient settings, where timely identification of raised intracranial pressure (ICP) is crucial to prevent severe neurological complications. Conventional diag
Core Tip: Non-traumatic headache can be the first sign of raised intracranial pressure, requiring rapid recognition to prevent neurological deterioration. Conventional diagnostics such as computed tomography and lumbar puncture have limitations. Point-of-care ultrasound measurement of optic nerve sheath diameter is a fast, non-invasive method with high sensitivity and specificity for detecting elevated intracranial pressure. Its use can expedite diagnosis, guide urgent management, and reduce unnecessary imaging, especially in outpatient and resource-limited settings. Standardized training and emerging artificial intelligence-assisted measurement promise to enhance its reliability and broaden its clinical impact.
- Citation: Tlaiss Y, Tarchichi A, Atallah K, Al Mashtoub E, Zalzali I, Chokor Z, Fassih I, Harb N, Kassas J, Hamze H. Point-of-care ultrasonography for detecting raised intracranial pressure through optic nerve sheath diameter in non-traumatic headache patients. World J Radiol 2025; 17(11): 113012
- URL: https://www.wjgnet.com/1949-8470/full/v17/i11/113012.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i11.113012
Headache is one of the most frequent neurological complaints in both emergency departments and outpatient clinics. Non-traumatic headaches account for approximately 2%-4% of all emergency visits, making it a common yet challenging presentation[1]. While the majority of headaches are benign primary disorders (e.g. migraine or tension-type), clinicians must remain vigilant for secondary causes. In particular, even in the absence of trauma, a headache can be the first sign of elevated intracranial pressure (ICP) due to an underlying pathology that may be life-threatening. Early identification of an ICP-related headache is crucial, as delayed recognition of raised ICP can lead to catastrophic neurological outcomes[2]. Elevated ICP may precipitate cerebral ischemia, brain herniation, or even death if not promptly managed[2]. Evidence from multiple studies and meta-analyses indicates that optic nerve sheath diameter (ONSD) measurements above 5.0-5.7 mm in adults strongly correlate with elevated ICP, showing pooled sensitivities and specificities approaching 90%. Therefore, timely recognition and intervention are essential to prevent catastrophic outcomes. Therefore, distinguishing high-risk headaches suggestive of intracranial hypertension from benign causes is a critical priority for emergency physicians, neurologists, and ophthalmologists.
Conventional diagnostic approaches for suspected increased ICP each have significant limitations. Neuroimaging such as computed tomography (CT) of the head is often the first step to evaluate secondary headaches, but a normal CT does not rule out elevated ICP and only reveals specific causes (e.g. mass lesions or hemorrhage) when present. Furthermore, CT involves radiation exposure and may not be immediately accessible in all settings. Direct measurement of cerebrospinal fluid pressure via lumbar puncture (LP) remains the diagnostic gold standard for confirming intracranial hypertension, but LP is invasive, time-consuming, and contraindicated if elevated ICP is strongly suspected due to the risk of precipitating herniation. These logistical and safety limitations of CT and LP can delay diagnosis in an acute headache scenario[3]. In the emergency setting, there is a pressing need for a rapid, bedside technique to screen for raised ICP that avoids the delays and risks of conventional tests[3].
Point-of-care ultrasonography (POCUS) measurement of the ONSD has emerged as a promising solution to this diagnostic gap. The optic nerve sheath is contiguous with the intracranial subarachnoid space, and it expands when ICP rises. Bedside ocular ultrasound allows clinicians to directly visualize this sheath distension. Ultrasound-based ONSD assessment is non-invasive, repeatable, and can be performed in seconds at the patient’s bedside, making it ideal for urgent evaluation. It eliminates the need to transport unstable patients for radiographic studies and avoids the radiation and procedural risks associated with CT or LP[3]. In recent years, multiple studies and meta-analyses have demonstrated that an enlarged ONSD on ultrasound correlates strongly with raised ICP, with high sensitivity and specificity reported for detecting intracranial hypertension[4]. In practice, a normal ONSD measurement has a high negative predictive value, helping clinicians confidently rule out significant ICP elevation in many cases[5]. As a result, ONSD POCUS is being increasingly adopted as a fast screening tool for elevated ICP in emergency and critical care settings.
Clinical scenarios where ONSD ultrasonography is particularly indicated include patients presenting with acute severe headache accompanied by red-flag features such as visual disturbances, altered mental status, or focal neurological deficits. It is also useful in suspected idiopathic intracranial hypertension (IIH), meningitis or intracranial infection, and in patients where CT is delayed or unavailable, or LP is contraindicated. In these situations, bedside POCUS offers a rapid and non-invasive alternative to guide urgent management decisions.
In summary, timely detection of elevated ICP in patients with headache (even without trauma) is of great clinical importance to prevent neurological deterioration. Unfortunately, traditional modalities like CT and LP have practical drawbacks in the acute setting. ONSD measurement by POCUS offers a rapid, non-invasive approach to bridge this gap. This paper explores the utility of POCUS-measured ONSD for early identification of raised ICP in non-traumatic headache patients, aiming to guide clinicians in integrating this technique into early headache evaluation for better patient outcomes.
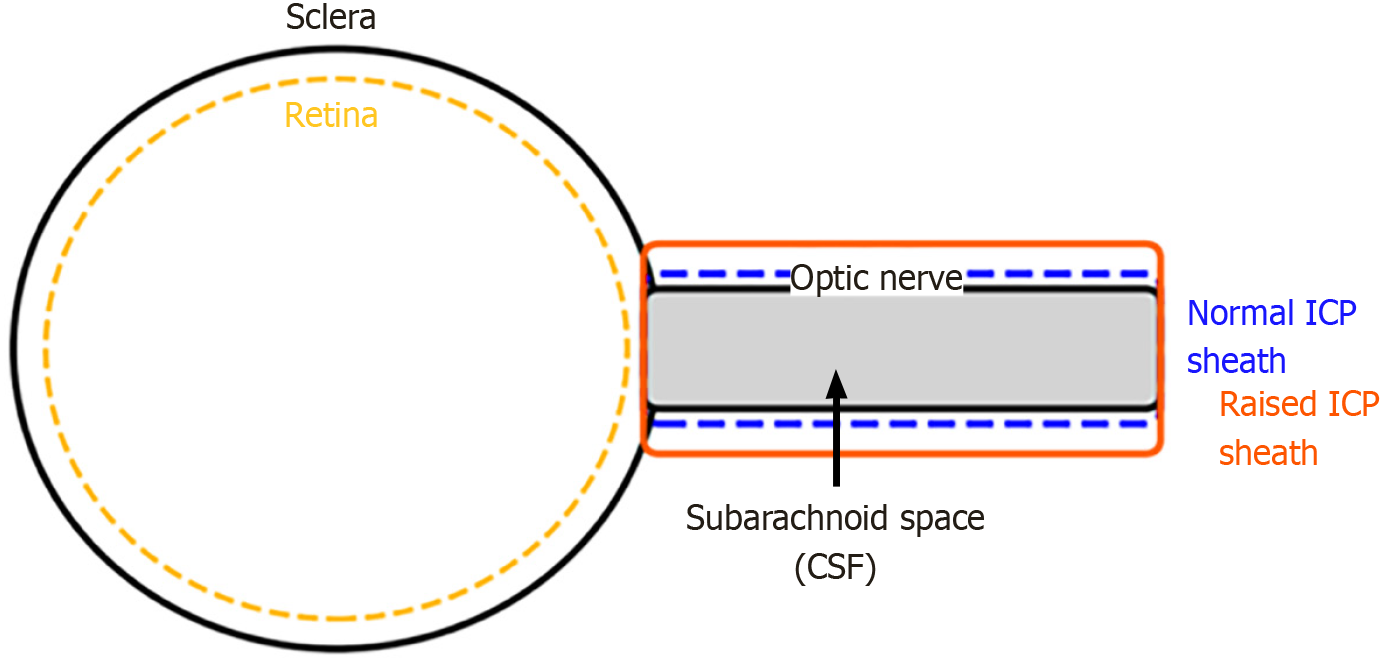
The optic nerve sheath is a continuation of the dura mater, the outermost membrane of the meninges that envelops the brain. Similar to the brain, the nerve is surrounded by the arachnoid and pia mater, with cerebrospinal fluid (CSF) filling the subarachnoid space in between[6].
Due to this anatomy, alterations in ICP are transmitted to the optic nerve sheath via the subarachnoid space, leading to its dilation as ICP rises. Due to this physiological connection, the ONSD can be used as a non-invasive substitute biomarker for elevated ICP[7]. Ultrasonographic evaluation of the ONSD has proven to be safe, rapid, and portable across diverse settings such as emergency and critical care units. This technique visualizes optic nerve sheath enlargement resulting from increased CSF pressure in the subarachnoid space as ICP rises.
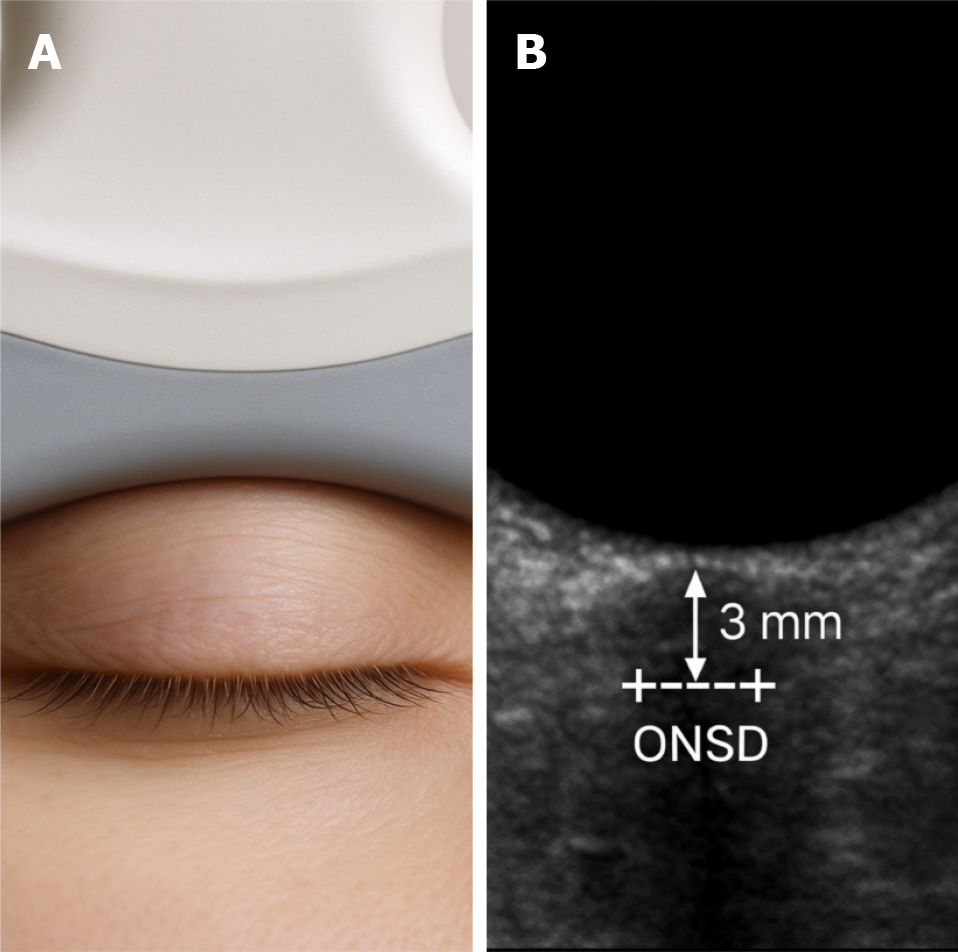
The technique requires the patient to be positioned supine and the eye in a neutral gaze. A high-frequency linear transducer (typically 7.5-15 MHz) is placed over the closed upper eyelid. To prevent any pressure on the eye, which might lead to inaccurate readings, a small amount of ultrasonic gel is utilized[8].
The ONSD is measured 3 mm posterior to the retina, along a line perpendicular to the optic nerve’s long axis, to provide the most reliable values[9]. Typically, assessments are conducted on both eyes, and an average is calculated. Several systematic reviews and studies tried to give a threshold value for abnormal ONSD. It was found that a common cutoff for elevated ICP is an ONSD measurement exceeding 5.0 mm in adults (or as high as 5.7 mm according to certain studies). However, the cutoff can differ with population, pathology, and measurement method[10]. For example, a cutoff of 5.9 mm was reported in pediatric intensive care studies[11]. A sensitivity of 90% and a specificity of 85% for detecting increased ICP by ONSD were reported in a meta-analysis[12]. Nevertheless, accuracy can be affected by patient’s position, probe orientation, and measurement site. Therefore, to ensure consistency and minimize variability between observers, this technique requires attention to probe placement, angle, and site of measurement[12]. Although ONSD ultrasound cannot replace direct ICP monitoring in critical care and neurosurgical settings, it serves as a valuable bedside instrument, especially in circumstances where invasive monitoring is either unfeasible or not recommended.
The optic nerve sheath expands in response to increased ICP due to its continuity with the intracranial subarachnoid space, which is filled with CSF. This anatomical relationship is the basis for using ONSD measurement as a surrogate marker for raised ICP (Figure 1). The technique is illustrated in Figure 2A, which shows probe positioning over the closed eyelid, and Figure 2B, which demonstrates a typical sonographic appearance of the ONSD with calipers.
Because of the anatomical continuity between the intracranial subarachnoid space and the optic nerve sheath, elevations in ICP lead to measurable sheath distension. Numerous studies have validated this correlation and assessed the diagnostic performance of ONSD ultrasonography in detecting raised ICP[13]. Research has shown that patients with elevated ICP tend to have significantly larger ONSD values compared to those with normal ICP. Analysis of 22 studies conducted over 17 years revealed that patients with elevated ICP had a mean ONSD of 5.82 mm [95% confidence interval (CI): 5.58-6.06 mm], which closely matches magnetic resonance imaging-based measurements. A subset of 15 studies found that patients with elevated ICP had an ONSD of 5.6 mm, compared to 4.1 mm in those without elevated ICP, which further supports its diagnostic utility[14].
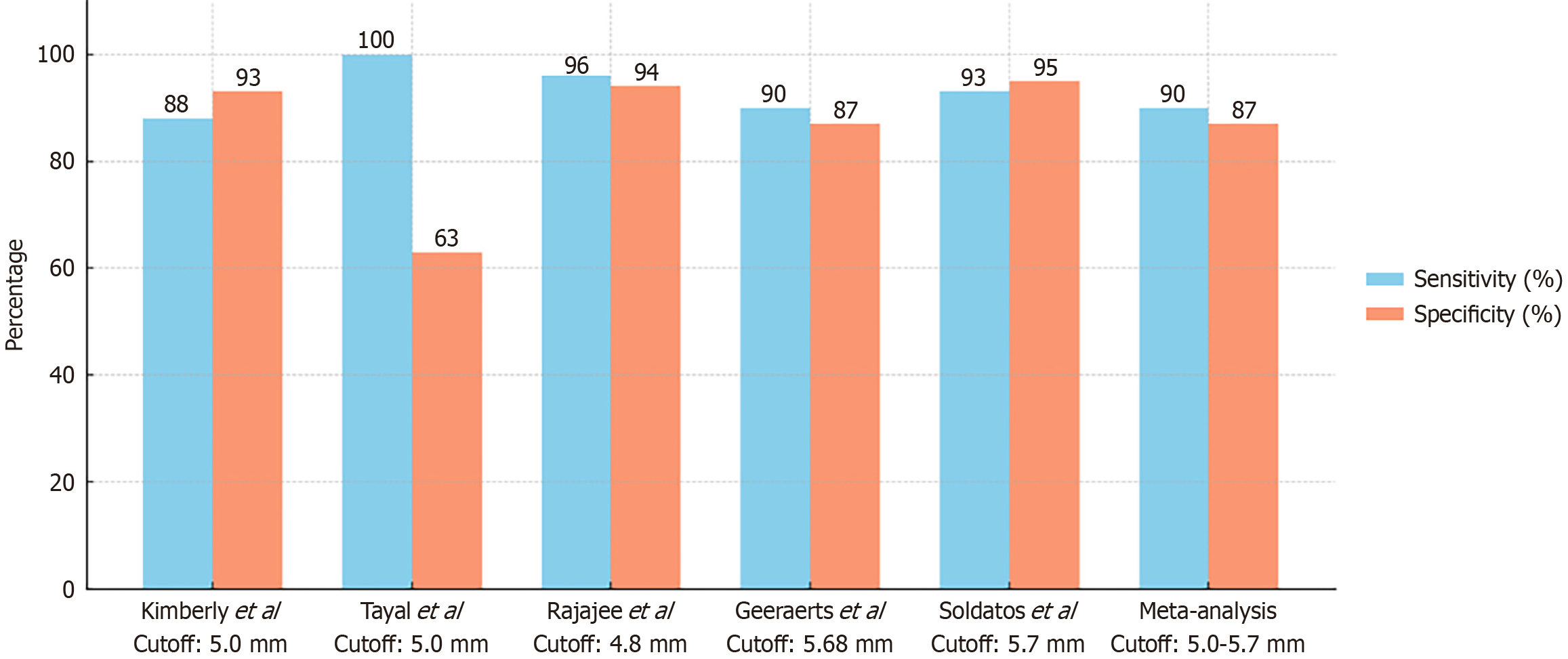
ONSD ultrasonography demonstrates robust diagnostic performance. A study found that sonographic ONSD had a 93.2% sensitivity and 91.1% specificity in detecting raised ICP when compared to CT imaging, with an overall accuracy of 92%[15]. A practical threshold of 5.0 mm has been identified in multiple studies, yielding both high negative and positive predictive values. An ONSD cutoff of 5.0 mm yielded a sensitivity of 88% and a specificity of 93%[16]. Another study at the same threshold reported 100% sensitivity and 95% specificity[17]. An optimal cutoff of at least 4.8 mm was shown to detect ICP ≥ 20 mmHg with 96% sensitivity and 94% specificity[18].
Other studies have proposed higher cut-off values. Research has shown that a cutoff of 6.1 mm yields 100% sensitivity and 83% specificity for ICP above 20 cmH2O[19]. An optimal cutoff of 5.68 mm was reported[20] while 5.7 mm was identified as the most accurate value[21].
A recent meta-analysis revealed that the pooled sensitivity was 0.90 (95%CI: 0.85-0.93) and the pooled specificity was 0.87 (95%CI: 0.80-0.91)[22]. Studies with higher cutoffs, ranging from 5.6 mm to 6.3 mm, demonstrated greater specificity (0.93) compared to 0.78, without affecting sensitivity, implying that this range may have more practical clinical applications[22].
A systematic review of 12 studies found that ONSD values below 5 mm typically indicate normal ICP, whereas values above 6 mm suggest elevated ICP exceeding 20 mmHg, with a sensitivity of nearly 90% and a specificity of around 85%. The diagnostic accuracy was highest when ONSD values were 5.85 mm or greater, particularly beyond 6 mm[23].
Reported sensitivity and specificity values vary depending on the population studied and the measurement cut-off used, with most adult thresholds ranging from 5.0 mm to 5.7 mm. A summary of key studies, including their diagnostic performance and cut-off values, is provided in Figure 3.
There is no consensus in the literature on a definitive ONSD threshold, with reported cutoffs varying from 4.1 mm to 7.2 mm. The high sensitivity of ONSD ultrasonography, despite its variability, makes it a valuable tool for screening and ruling out elevated ICP, and for guiding decisions on further invasive testing[22]. The need for standardizing ultrasound measurement techniques and cutoffs in future studies is highlighted by these findings[23].
POCUS measurement of ONSD has become a critical bedside tool in emergency departments, particularly for patients presenting with “red flag” headaches that may indicate elevated ICP. It is particularly valuable for rapid assessment of patients who present to the ED with headaches associated with symptoms that indicate increased ICP such as visual disturbances, altered level of consciousness, or neurological deficits. Rapid identification of raised ICP allows clinicians to make immediate clinical decisions, often prior to definitive imaging.
Patients with serious intracranial disorders reported increasing headaches typically demonstrate much larger ONSD measurements with the average ONSD around 6.2 mm, while the average ONSD in patients with benign headaches is about 4.5 mm. An ONSD cut-off of approximately 5.2 mm is found to accurately distinguish patients that require urgent evaluation and intervention with those with benign headaches with sensitivity near 98% and specificity at 100%[24]. In another systematic review, they concluded that ultrasound was reliable as a diagnostic tool for the detection of elevated ICP, with a pooled sensitivity of 92% and specificity of 86%[25].
Clinicians practicing in the ED use ocular ultrasound to screen patients with severe or concerning headaches rapidly. If they see an enlarged ONSD, they escalate imaging or clinical intervention. If they see a normal ONSD, they can reassure the patient while then guiding their clinical thought process towards other possible diagnosis. Ocular ultrasound in this scenario is easier and more effective than traditional fundoscopy because of the accessible, fast, and non-invasive nature of ultrasound. Fundoscopy can be highly subjective but may also alter the patient experience dramatically (and cause discomfort) in cases of urgency[26].
A good example of the potential value of POCUS is a patient with an acute neurological decline. At the bedside, ONSD ultrasound demonstrated extremely high ICP, leading to potentially lifesaving interventions such as intubation and neurosurgery before obtaining advanced imaging[27]. These examples document how bedside ultrasound can change patient care quickly by allowing pivotal time-sensitive interventions to occur.
Use of ocular POCUS in the initial evaluation of headache patients in urgent-care or emergency settings greatly impacts the speed at which the diagnosis is made and treatment initiated. A scan will facilitate the immediate evaluation of elevated ICP, allowing a clinical workup to be directed towards the most urgent imaging and management decisions for the patient. Conversely, if ONSD is normal, an immediate management decision may delay imaging and the need for invasive procedures, which would be useful in low-resource settings, emergency departments, urgent care centers, and specialist consultations where waiting for advanced imaging is a reality.
In outpatient care, POCUS can also assist to expedite patient management decisions. A recent outpatient case of a chronic headache required a very prompt ultrasound assessment. The ultrasound performed while the patient was at the bedside found an ONSD of 6 mm which was stretched out considerably; enough to facilitate a clinical referral and a subsequent definitive diagnosis of IIH and to avoid potentially unnecessary visits to the emergency department and advanced imaging[28]. This incorporation of ultrasound in outpatient care will accelerate and simplify patient management.
Serial ONSD measurements in critical care allow dynamic real time assessments and monitoring of ICP. One perioperative patient suffering from acute neurological deterioration underwent a timely ultrasound evaluation which revealed marked elevation in the ONSD allowing for timely medical interventions such as osmotic therapy[29]. Thus, a series of ultrasound assessment allows for monitoring patients' treatment response, while identifying worsening progression necessitating intervention.
Additionally, ultrasound assessments provide opportunities to eliminate unnecessary imaging and procedures. Patients with primary benign headaches demonstrated normal ONSD measurements, nearby safely ruling out the need of urgent neuroimaging and thus decrease overall costs and the threat of radiation[25]. Therefore, this type of selective approach is highly beneficial in outpatient or resource limited environments.
POCUS is also important outside of acute care and still works great in outpatient neurology and ophthalmology clinics. Patients suffering with chronic headaches or symptoms suggestive of IIH can be evaluated very fast with ultrasound, allowing for quick confirmation or exclusion of any high ICP.
Ocular ultrasound is recommended in the routine outpatient evaluation of patients with suspected IIH and gives reliable diagnostic information even before traditional signs (such as papilledema) are apparent[30]. Depending on patient’s treatment and the resolution or exacerbation of symptoms, serial ultrasound measurements provide objective indications for clinician to justify treatment effectiveness instead of relying on repeated invasive measures.
In primary care, ultrasound provides a hybrid step between their first suspicion and a definitive diagnosis, supporting their ultimate decision of whether to urgently refer or order additional tests. This reduces wasted time and resources, while also helping ensure their patients receive appropriate and timely care.
POCUS is particularly important in rural and resource-limited settings, especially because many of the higher-end imaging modalities are not available. Studies evaluating optic nerve ultrasound have shown a reasonable correlation with CT scan findings in rural clinical environments, and a cutoff of 5.2 mm for ONSD was found to be a reasonable predictor of ICP[31]. This simple diagnostic modality allows urgent transfer of patients or a quick local intervention allowing clinicians in remote areas to quickly identify the need for further intervention.
Because ultrasonography is highly portable and easy to use, particularly relevant to the practice of rural healthcare, it allows clinicians to almost immediately identify candidates for urgent neurological intervention from a substantial distance from an acute care site[3]. In particular, if rural clinicians encounter any patient with headache or with new-onset alertness-reducing neurologic issue such as suspected meningitis; they can quickly perform ultrasound without waiting for other imaging thus allowing for immediate decisions about transfer or management. A resolution of “normal” on ultrasound means these providers can confidently locally manage the patient and prevent unnecessary transfers - saving precious resources and time.
In conclusion, ocular ultrasound is a simple and effective addition to the clinical workflow of a range of healthcare providers which can improve the accuracy of diagnosis, the speed of patient management, and efficiency of resource use in an array of settings from urban emergency departments to remote rural clinics.
Despite its benefits, ONSD measurement using POCUS presents several limitations that must be acknowledged when interpreting its results. One of the primary challenges is the variability in measurements between different operators. The technique requires precise anatomical visualization and probe positioning, and without sufficient training, there is a significant risk of inter- and intra-operator variability[32]. This variability can influence diagnostic accuracy, particularly when small differences in sheath diameter may have clinical significance. In addition, a newly introduced automated frame selection method achieved an intraclass correlation coefficient of only 0.782 compared to expert clinicians, reflecting substantial operator related differences in manually selecting optimal images[33].
Proper training and continued practice are essential for clinicians to obtain reliable measurements. Nevertheless, many emergency and critical care providers lack formal training in ocular ultrasonography. A study found that even after a brief instructional session, novice users displayed considerable variation in ONSD measurements[34], emphasizing the importance of hands-on experience and quality assurance mechanisms. In a recent study, after a brief 4 hours theoretical and hands on training session, intensive care unit residents and medical students achieved measurement agreement within a 0.5 mm of expert values on Bland Altman analysis, while nursing students exhibited the highest error rates[35].
Patient-specific factors can also affect the accuracy of ONSD assessment. Chronic optic nerve conditions like glaucoma or optic neuritis can change the structure of the optic nerve sheath, making ONSD measurements less reliable. For example, patients with normal tension glaucoma have been shown to have smaller sheath diameters despite normal ICP[36]. Additionally, acquiring a clear image of the optic nerve sheath in patients with periorbital edema or obesity can be technically challenging due to increased tissue depth and poor acoustic windows[18].
Technical aspects of ultrasound imaging can significantly influence the accuracy of ONSD measurements. Factors such as gain, frequency settings, dynamic range, and common imaging artifacts (like acoustic shadowing, speckle, and beam refraction) can distort the results if not appropriately adjusted or accounted for during scanning[37]. Importantly, ONSD cutoff selection involves a sensitivity–specificity balance: Lower thresholds improve detection rates but may increase false positives. For instance, a pediatric emergency care study found that an ONSD cutoff of 4.9 mm had a sensitivity of 90% but specificity as low as 47%, leading to potential overdiagnosis[38]. Variations in equipment further complicate measurement consistency. High-frequency linear transducers are generally recommended, but variations in image resolution, presets, and device quality can lead to inconsistent results. Mean error rates in ONSD measurements differed significantly across three ultrasound systems[39], suggesting that “device choice alone can materially impact diagnostic performance”.
Contraindications to ONSD ultrasonography are limited but important to consider. Absolute contraindication includes suspected or confirmed open globe injury, where probe pressure could cause extrusion of ocular contents. Relative contraindications include recent ocular surgery, significant orbital trauma, or severe eye infections, where manipulation of the eyelid may worsen the condition. In these cases, alternative methods for ICP evaluation should be used.
POCUS assessment of the ONSD continues to evolve with new technologies and protocols that enhance its role in ICP monitoring. Ongoing research is exploring artificial intelligence (AI)-assisted interpretation and automated measurement tools. Advanced image-analysis algorithms have been developed to automatically measure ONSD on ultrasound images with high precision comparable to expert clinicians, reducing operator-dependent variability[18,40]. These AI-driven systems may enable reliable ONSD assessment even by less experienced users and in austere settings, by providing consistent measurements and identifying optimal images for analysis.
Comprehensive training programs and standardized protocols are essential to ensure safe and reliable use. Proper operator training and uniform technique are critical, as measurement inconsistencies have historically limited ONSD’s reliability. An international consensus in 2024 introduced a standardized ONSD ultrasound quality criteria checklist to improve consistency in image acquisition and measurement[37]. Studies also demonstrate that with brief dedicated training, even non-physician providers (e.g. nurses or medical students) can learn to obtain accurate ONSD mea
Future studies should aim to validate ONSD measurement in broader non-traumatic headache populations. To date, ONSD ultrasound is well-established for detecting raised ICP in traumatic brain injury - it has even been incorporated into some traumatic brain injury management guidelines[41]. However, evidence in non-traumatic neurologic conditions (such as spontaneous intracerebral hemorrhage, stroke, meningitis, or IIH) is less extensive and sometimes inconsistent[42]. Recent reviews indicate ONSD can be a useful adjunct for identifying elevated ICP in acute stroke, intracranial bleeding, and intracranial infections, but call for further research to refine diagnostic cutoffs and validate its prognostic value in these diverse, non-trauma patient groups[42]. Expanding research to headache patients without trauma (to distinguish benign vs dangerous causes) will be important for broader clinical adoption.
Integrating POCUS ONSD into clinical guidelines could improve care efficiency. Incorporating ONSD measurement into emergency and critical care algorithms may streamline the evaluation of suspected intracranial hypertension. For example, using ONSD ultrasound at the bedside could help triage which headache patients require urgent neuroimaging, thereby reducing unnecessary radiation and delays. Formal guideline endorsements and inclusion in protocols (e.g. for emergency headache workups or intensive care unit monitoring) would standardize its use and magnify its impact on care delivery. Integrating ONSD into routine practice may enable clinicians to make faster, more informed decisions in time-sensitive neurological emergencies.
Ultimately, POCUS-based ONSD measurement represents a promising tool for early ICP detection that warrants broader adoption and further study. It is a rapid, noninvasive, and repeatable bedside technique with growing evidence of diagnostic accuracy for raised ICP in both traumatic and non-traumatic contexts[43,44]. ONSD ultrasound is considered safe and low-cost, with the potential to screen patients who may need confirmatory neuroimaging or invasive monitoring[43]. Key next steps include leveraging innovations like AI to assist interpretation, ensuring practitioners are well-trained, and conducting large multicenter trials in varied patient populations. With these improvements, ONSD POCUS may be more widely adopted as an efficient early-warning tool for intracranial hypertension, improving patient triage and outcomes.
Recently, an international expert consensus introduced the ONSD POCUS Quality Criteria Checklist (ONSD POCUS QCC), providing standardized guidance for image acquisition and measurement in clinical and research settings[45].
POCUS measurement of the ONSD is a rapid, non-invasive, and reliable method for detecting raised ICP in patients presenting with non-traumatic headache. Given its high diagnostic accuracy, ease of bedside application, and utility across emergency, outpatient, and resource-limited settings, it serves as a valuable adjunct to traditional neuroimaging and invasive monitoring. While operator dependency and variability in cut-off values remain limitations, advances in standardized training and AI-assisted measurement are likely to enhance consistency. Wider adoption and further validation in diverse patient populations could significantly improve early diagnosis, triage efficiency, and patient outcomes.
| 1. | Munoz-Ceron J, Marin-Careaga V, Peña L, Mutis J, Ortiz G. Headache at the emergency room: Etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PLoS One. 2019;14:e0208728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Patel S, Maria-Rios J, Parikh A, Okorie ON. Diagnosis and management of elevated intracranial pressure in the emergency department. Int J Emerg Med. 2023;16:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 3. | Lau T, Ahn JS, Manji R, Kim DJ. A Narrative Review of Point of Care Ultrasound Assessment of the Optic Nerve in Emergency Medicine. Life (Basel). 2023;13:531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Lin JJ, Chen AE, Lin EE, Hsia SH, Chiang MC, Lin KL. Point-of-care ultrasound of optic nerve sheath diameter to detect intracranial pressure in neurocritically ill children - A narrative review. Biomed J. 2020;43:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Koziarz A, Sne N, Kegel F, Nath S, Badhiwala JH, Nassiri F, Mansouri A, Yang K, Zhou Q, Rice T, Faidi S, Passos E, Healey A, Banfield L, Mensour M, Kirkpatrick AW, Nassar A, Fehlings MG, Hawryluk GWJ, Almenawer SA. Bedside Optic Nerve Ultrasonography for Diagnosing Increased Intracranial Pressure: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Boysen SR, Pang JM, Mikler JR, Knight CG, Semple HA, Caulkett NA. Comparison of tranexamic acid plasma concentrations when administered via intraosseous and intravenous routes. Am J Emerg Med. 2017;35:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, Taccone FS, Citerio G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2018;44:1284-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 9. | Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, Shook AC, Smielewski P, Czosnyka M, Gupta AK, Menon DK. Erratum to: Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2015;41:177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Liu D, Li Z, Zhang X, Zhao L, Jia J, Sun F, Wang Y, Ma D, Wei W. Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol. 2017;17:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Aslan N, Yıldızdaş D, Horoz ÖÖ, Özsoy M, Yöntem A, Çetinalp E, Mert GG. Evaluation of ultrasonographic optic nerve sheath diameter and central retinal artery Doppler indices by point-of-care ultrasound in pediatric patients with increased intracranial pressure. Turk J Pediatr. 2021;63:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Robba C, Ball L, Battaglini D, Iannuzzi F, Brunetti I, Fiaschi P, Zona G, Taccone FS, Messina A, Mongodi S, Pelosi P. Effects of positive end-expiratory pressure on lung ultrasound patterns and their correlation with intracranial pressure in mechanically ventilated brain injured patients. Crit Care. 2022;26:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Getachew M, Abdulahi M, Muluberehan N, Hussen Z, Alemayehu A, Abebe T, Hussein A, Hutchinson P, Kolias A, Semework M, Tirsit A, Laeke T, Tadela A, Hassen GW. Optic nerve sheath diameter measurement using point-of-care ultrasound to assess intracranial pressure of adult patients with traumatic brain injury in resource-limited setting. Interdiscip Neurosurg. 2023;34:101847. [DOI] [Full Text] |
| 14. | Montorfano L, Yu Q, Bordes SJ, Sivanushanthan S, Rosenthal RJ, Montorfano M. Mean value of B-mode optic nerve sheath diameter as an indicator of increased intracranial pressure: a systematic review and meta-analysis. Ultrasound J. 2021;13:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Incecik F, Ozcan N, Ozcanyuz DG, Mert GG. Acetazolamide-Induced Agranulocytosis in a Patient with Pseudotumor Cerebri. Ann Indian Acad Neurol. 2020;23:732-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 19. | Širanović M, Turković TM, Gopčević A, Kelečić M, Kovač N, Kovač J, Rode B, Vučić M. Comparison of ultrasonographic measurement of optic nerve sheath diameter (ONSD) versus direct measurement of intracranial pressure (ICP) in traumatic brain injury patients. Signa Vitae. 2011;6:33-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J, Benhamou D. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007;33:1704-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Soldatos T, Chatzimichail K, Papathanasiou M, Gouliamos A. Optic nerve sonography: a new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Berhanu D, Ferreira JC, Abegão Pinto L, Aguiar de Sousa D, Lucas Neto L, Tavares Ferreira J. The role of optic nerve sheath ultrasonography in increased intracranial pressure: A systematic review and meta analysis. J Neurol Sci. 2023;454:120853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Martínez-Palacios K, Vásquez-García S, Fariyike OA, Robba C, Rubiano AM; noninvasive ICP monitoring international consensus group. Using Optic Nerve Sheath Diameter for Intracranial Pressure (ICP) Monitoring in Traumatic Brain Injury: A Scoping Review. Neurocrit Care. 2024;40:1193-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 24. | Bastani Viarsagh S, Agar A, Lawlor M, Fraser C, Golzan M. Non-invasive assessment of intracranial pressure through the eyes: current developments, limitations, and future directions. Front Neurol. 2024;15:1442821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | İşlek Yüksel A, Selvi F, Yüksel S, Avcı M, Çalışkan Günay G, Kozacı N. The Role of Optic Nerve Sheath Diameter in Differential Diagnosis in Patients with Headache, A Prospective, Randomized Controlled Study. Glob Emerg Crit Care. 2025;4:23-19. [DOI] [Full Text] |
| 26. | Wilson CL, Leaman SM, O'Brien C, Savage D, Hart L, Jehle D. Novice emergency physician ultrasonography of optic nerve sheath diameter compared to ophthalmologist fundoscopic evaluation for papilledema. J Am Coll Emerg Physicians Open. 2021;2:e12355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Amin D, McCormick T, Mailhot T. Elevated Intracranial Pressure Diagnosis with Emergency Department Bedside Ocular Ultrasound. Case Rep Emerg Med. 2015;2015:385970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Gupta A, Nagaraju SP, Bhojaraja MV, Swaminathan SM, Mohan PB. Hypertension in Chronic Kidney Disease: An Update on Diagnosis and Management. South Med J. 2023;116:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Naik SS, Krishnakumar M, Sundaram M, Goyal A. Saved by the bell: Point of care ocular ultrasound in raised intracranial pressure. J Neurosci Rural Pract. 2022;13:806-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | De Bernardo M, Vitiello L, De Pascale I, Capasso L, Cornetta P, Rosa N. Optic Nerve Ultrasound Evaluation in Idiopathic Intracranial Hypertension. Front Med (Lausanne). 2022;9:845554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Sujan MU, Rao MR, Kisan R, Abhishekh HA, Nalini A, Raju TR, Sathyaprabha TN. Influence of hydrotherapy on clinical and cardiac autonomic function in migraine patients. J Neurosci Rural Pract. 2016;7:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 389] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 33. | Li R, Tang W, Li P, Huang Q, She J, Li S, Xu H, Wan Y, Liu J, Fu H, Li X, Chen J. Automated measurement of optic nerve sheath diameter using ocular ultrasound video. Med Biol Eng Comput. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Maude RR, Hossain MA, Hassan MU, Osbourne S, Sayeed KL, Karim MR, Samad R, Borooah S, Dhillon B, Day NP, Dondorp AM, Maude RJ. Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in Bangladesh. PLoS One. 2013;8:e81013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Garofalo E, Neri G, Bosco V, Caroleo Z, Virdò F, Mastrangelo H, Guzzi G, Cammarota G, Robba C, Longhini F, Bruni A; ONSD study group. Efficacy of a theoretical-practical course for the ultrasound measurement of the optic nerve diameter in different healthcare operators. Ultrasound J. 2025;17:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Lee SH, Kim TW, Lee EJ, Kil H. Association between Optic Nerve Sheath Diameter and Lamina Cribrosa Morphology in Normal-Tension Glaucoma. J Clin Med. 2023;12:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Hirzallah MI, Sarwal A, Dentinger AM, Robba C, Valaikienė J, Lochner P, Schlachetzki F, Mills DM, Ertl M, Hakimi R, Bhise S, Pansell J. Ultrasonographic Optic Nerve Sheath Diameter Technical Pitfalls and Imaging Artifacts. J Ultrasound Med. 2025;44:1103-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Nagpal V, Batra P, Bhaskar V, Gupta P, Bhatt S, Sahu P. Optic Nerve Sheath Diameter by Point of Care Ultrasound to Detect Raised Intracranial Pressure in Children. Pediatr Emerg Care. 2025;41:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Johnson GGRJ, Jelic T, Derksen A, Unger B, Zeiler FA, Ziesmann MT, Gillman LM. Accuracy of Optic Nerve Sheath Diameter Measurements in Pocket-Sized Ultrasound Devices in a Simulation Model. Front Med (Lausanne). 2022;9:831778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Meiburger KM, Naldi A, Michielli N, Coppo L, Fassbender K, Molinari F, Lochner P. Automatic Optic Nerve Measurement: A New Tool to Standardize Optic Nerve Assessment in Ultrasound B-Mode Images. Ultrasound Med Biol. 2020;46:1533-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 2377] [Article Influence: 264.1] [Reference Citation Analysis (1)] |
| 42. | Bhide M, Juneja D, Singh O, Mohanty S. Optic nerve sheath diameters in nontraumatic brain injury: A scoping review and role in the intensive care unit. World J Crit Care Med. 2024;13:97205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Lochner P, Czosnyka M, Naldi A, Lyros E, Pelosi P, Mathur S, Fassbender K, Robba C. Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol Sci. 2019;40:2447-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Aletreby W, Alharthy A, Brindley PG, Kutsogiannis DJ, Faqihi F, Alzayer W, Balhahmar A, Soliman I, Hamido H, Alqahtani SA, Karakitsos D, Blaivas M. Optic Nerve Sheath Diameter Ultrasound for Raised Intracranial Pressure: A Literature Review and Meta-analysis of its Diagnostic Accuracy. J Ultrasound Med. 2022;41:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Hirzallah MI, Lochner P, Hafeez MU, Lee AG, Krogias C, Dongarwar D, Hartman ND, Ertl M, Robba C, Malojcic B, Valaikiene J, Sarwal A, Hakimi R, Schlachetzki F; Optic Nerve Sheath Diameter Point-of-Care Ultrasonography Quality Criteria Checklist (ONSD POCUS QCC) Expert Panelists. Optic Nerve Sheath Diameter Point-of-Care Ultrasonography Quality Criteria Checklist: An International Consensus Statement on Optic Nerve Sheath Diameter Imaging and Measurement. Crit Care Med. 2024;52:1543-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/