Published online Nov 28, 2025. doi: 10.4329/wjr.v17.i11.111651
Revised: September 15, 2025
Accepted: November 6, 2025
Published online: November 28, 2025
Processing time: 142 Days and 11.4 Hours
Shear wave elastography (SWE) is a non-invasive ultrasound-based technique used to assess tissue stiffness, which reflects underlying pathological changes. While SWE has been widely applied for liver fibrosis evaluation, its application to other abdominal organs, such as the spleen and pancreas, is gaining interest. However, normal stiffness values and inter-system agreement remain poorly defined.
To assess the feasibility and agreement of liver, spleen, and pancreas stiffness using three SWE methods.
This single-center observational study enrolled 50 healthy adult volunteers. Liver, spleen, and pancreas stiffness were assessed using three SWE methods: Point-SWE (p-QElaXto) and 2-Dimensional-SWE (2D-QElaXto) with Esaote MyLab 9, and 2D-SWE with SuperSonic Imagine. Feasibility, inter-operator reproducibility, and concordance among systems were evaluated. Stiffness was expressed as median kPa values, and technical reliability was assessed using the interquartile range/median ratio and stability index thresholds.
Liver and spleen stiffness assessment was feasible in > 98% of patients, while pancreas stiffness was measurable in 84%-88% depending on the SWE technique. Mean liver stiffness ranged between 3.9-4.7 kPa across techniques, spleen stiffness ranged from 19.4-23.0 kPa, and pancreas stiffness from 5.2-7.6 kPa. Inter-operator agreement was excellent for liver (intraclass correlation coefficient > 0.90) and good to moderate for spleen and pancreas (intraclass correlation coefficient from 0.43 to 0.90). Bland-Altman analysis confirmed good correlation but also systematic differences among devices, especially in pancreas measurements.
This is the first study to establish normal liver, spleen, and pancreas stiffness using MyLab 9 SWE integrated methods as compared to SuperSonic Imagine, with acceptable inter-technique agreement. Liver and spleen values matched existing guidelines; pancreas SWE showed more variability and reduced reproducibility.
Core Tip: In the literature, normal stiffness values for liver, spleen, and pancreas in healthy individuals are not well established. This is partly due to differences in equipment and cut-off values used, and partly due to inter-operator variability. In this study, we evaluated these parameters in 50 healthy individuals using three different methods (two of which were integrated into the same scan) to assess their correlation with clinical and technical features.
- Citation: Viceconti N, Paratore M, Del Zompo F, Zocco MA, Ainora ME, Esposto G, Gasbarrini A, Pompili M, Riccardi L, Garcovich M. Shear wave elastography in healthy patients: Pancreatic stiffness is less reliable than liver and spleen measurements. World J Radiol 2025; 17(11): 111651
- URL: https://www.wjgnet.com/1949-8470/full/v17/i11/111651.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i11.111651
Shear wave elastography (SWE) is a non-invasive ultrasound (US) technique that quantifies tissue stiffness and is increasingly applied to abdominal organs[1,2]. Establishing reference values in healthy individuals is crucial, since abnormal stiffness is associated with clinically relevant conditions such as liver fibrosis, portal hypertension, and pancreatic disease. In chronic liver disease, SWE-derived liver stiffness strongly correlates with fibrosis stage and disease progression[3-5]. Spleen stiffness has also emerged as a promising surrogate marker of portal hypertension[2,6,7]. However, reliable cut-offs still require robust normal ranges in healthy subjects and reproducible measurements across different systems. In the literature, liver and spleen normal stiffness values are not well established. There is a great variability of normal stiffness values, partly due to the availability of different equipment and the different cut-off em
The use of SWE is steadily increasing, also engaging other organs such as the pancreas. In fact, pancreatic stiffness has been linked to steatosis, metabolic syndrome, acute and chronic pancreatitis, and post-surgical outcomes[10]. Never
Esaote MyLab 9 is one of the latest equipment introduced in 2017 by Esaote (Genova, Italy), and it is embedded with point-SWE (p-QElaXto) and 2-Dimensional-SWE (2D-QElaXto). To date, no published studies have established normal reference values for abdominal organs using this platform to the best of our knowledge. The aim of the present study was to assess the feasibility and agreement of liver, spleen, and pancreas stiffness measurements obtained with p-QElaXto and 2D-QElaXto on Esaote MyLab 9, compared with 2D-SWE on the SuperSonic Imagine Aixplorer (SSI). We sought to define normal stiffness ranges in healthy individuals and to evaluate their relationship with clinical and technical factors. Additionally, we examined inter-operator variability across the different SWE modalities.
The study was designed as an observational single-center study according to the principles of the Declaration of Helsinki and the ethical standards of the local institutional research committee (Comitato Etico, Fondazione Policlinico Uni
The inclusion criteria were: (1) Age ≥ 18 years; no history of focal and/or diffuse disease of the analyzed organs; (2) No hematological disorders or other pathological conditions; (3) Negative viral serology for hepatitis B, hepatitis C and human immunodeficiency virus; (4) Laboratory tests within the normal range (in the previous 6 months), alcohol consumption not exceeding 30 g/daily for men and 20 g/daily for women; and (5) Suitable visualization and normal morphology of examined organs in US imaging. The exclusion criteria were: (1) Body mass index (BMI) > 30 kg/m2; (2) Moderate/severe steatosis at standard B-mode; and (3) Ongoing or previous infections, recent or past injury, important recent or past bleeding, immune system disorders, and pregnant or breastfeeding women. Subjects with mild hepatic steatosis or slightly overweight were retained, as low-grade steatosis is common in the general population and does not substantially affect shear-wave elastography. Moreover, standard B-mode US reliably detects moderate-to-severe, but not necessarily mild steatosis[14].
The examination consisted of stiffness evaluation through p-QElaXto, 2D-QElaXto, and SSI by an experienced operator (> 5 years of experience with different elastography techniques). A standardized protocol was used to minimize pre-compression, enforce breath-hold at end-expiration, and ensure uniform region of interest (ROI) placement. Moreover, to test the reproducibility of the SWE techniques, the first 10 patients enrolled in the study underwent additional elastography examination performed by a second operator, and median organ stiffness values were compared. To minimize measurement bias, operators performed SWE examinations independently and were blinded to prior results and to each other’s measurements when assessing interobserver reproducibility.
Liver, spleen, and pancreas stiffness were measured with the patient in the supine position and during breath-hold in neutral respiration, using standardized acoustic windows for each organ. For liver and spleen, intercostal approaches were used; for pancreas, the best available window (head, body, or tail) was selected according to individual anatomy. For each organ, up to 10 valid measurements were obtained in accordance with the European Federation of Societies for Ultrasound in Medicine and Biology and the World Federation for Ultrasound in Medicine and Biology (WFUMB recommendations, which indicate that a minimum of 5-10 acquisitions improves measurement reliability and reproducibility, especially in liver elastography. Full details of probe placement and acquisition protocols are provided in the Supplementary material.
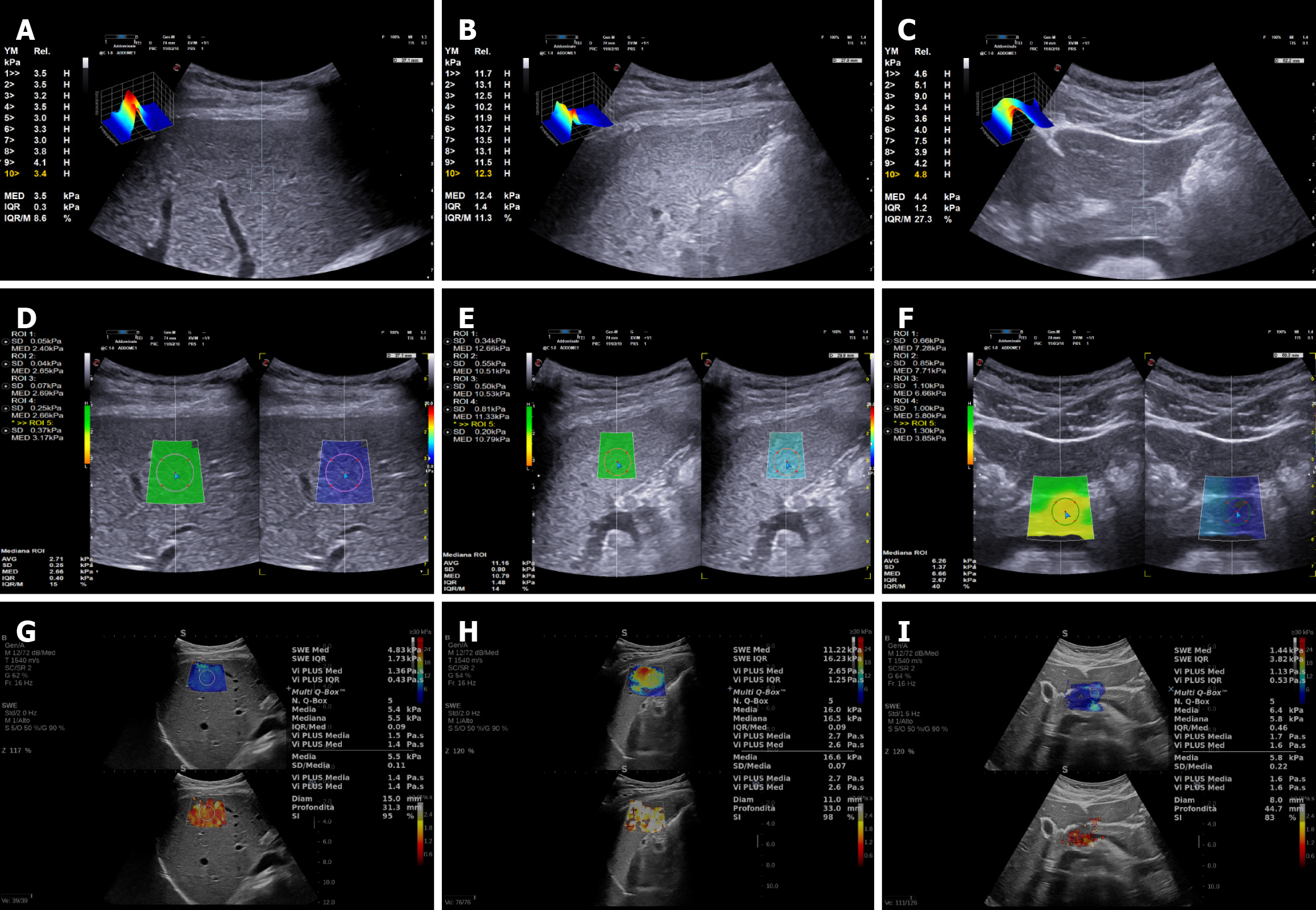
SWE with Esaote was performed with a MyLab 9 equipment with convex broadband abdominal probe C1-8 MHz, obtaining up to 10 valid measurements with p-QElaXto and 5 valid measurements with 2D-QElaXto for every organ, considering median values in kPa and the ratio between interquartile range (IQR) and the median value (M) (directly provided by the software) for the analysis (Figure 1A-F, respectively). To further investigate whether a reduced number of acquisitions could still provide reliable results for p-QElaXto, we compared the mean values obtained from 10 measurements with those derived from the first 5 acquisitions. 2D-SSI was performed with Mach 30 Aixplorer (Aix-en-Provence, France) with a convex probe XC6-1 MHz, obtaining 5 valid measurements for every organ, recording median values in kPa and stability index (SI) (Figure 1G-I). For 2D-SWE systems, liver stiffness was measured in an ROI of 10 mm and 15 mm in a central area of the colorimetric map showing the best signal homogeneity. Moreover, for pancreas stiffness measurement, the ROI dimension was adjusted according to organ anatomy when using 2D-SWE techniques. All the SWE measurements for every single patient lasted no longer than 10 minutes for both elastography techniques.
Technical feasibility was calculated after the exclusion of technical failures and unreliable measurements. Based on current literature, “technical failures” were defined as no successful measurement after 10 attempts for p-QElaXto and as the inability to measure stiffness in a homogeneous ROI of at least 10 mm for 2D-SWE techniques. “Unreliable mea
Individual patient measurements derived from multiple samples (e.g., liver stiffness derived from five or ten mea
We enrolled a total of 50 patients in the study period. Organ stiffness measurements were obtained by the three different SWE techniques (2D-QElaXto, p-QElaXto, and SSI). Descriptive statistics of the “healthy” study population are reported in Table 1. Among the whole cohort, the mean BMI was 23 kg/m2, with only two subjects showing a hyperechoic liver (both with mild liver steatosis and BMI > 25 kg/m2) and one subject with a hyperechoic pancreas (mild fatty infiltration) on US scanning. In two other patients, pancreas visualization on US was suboptimal due to intestinal gas, and therefore, pancreatic stiffness was not performed.
| Variables | Total | Males | Females |
| Age (years) | 37 ± 10 | 39 ± 11 | 35 ± 9 |
| BMI (kg/m2) | 23 ± 3 | 24 ± 2 | 21 ± 2 |
| Spleen bipolar diam (cm) | 10.8 ± 1.3 | 11.6 ± 0.9 | 9.8 ± 1.0 |
| Spleen area (cm2) | 33.3 ± 8.6 | 37 ± 8 | 29 ± 7 |
| Pancreas body thick (cm) | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.2 |
The feasibility of the three SWE techniques was excellent, and successful measurements were obtained in all 50 subjects for liver and spleen stiffness assessment. In only one patient, IQR/M ratio was > 30% when performing liver stiffness with 2D-QElaXto with a ROI of 10 mm (but not with a ROI of 15 mm). In another single subject, spleen stiffness measurements were unreliable (IQR/M ratio > 30% or SI < 80%) with all three techniques. These unreliable mea
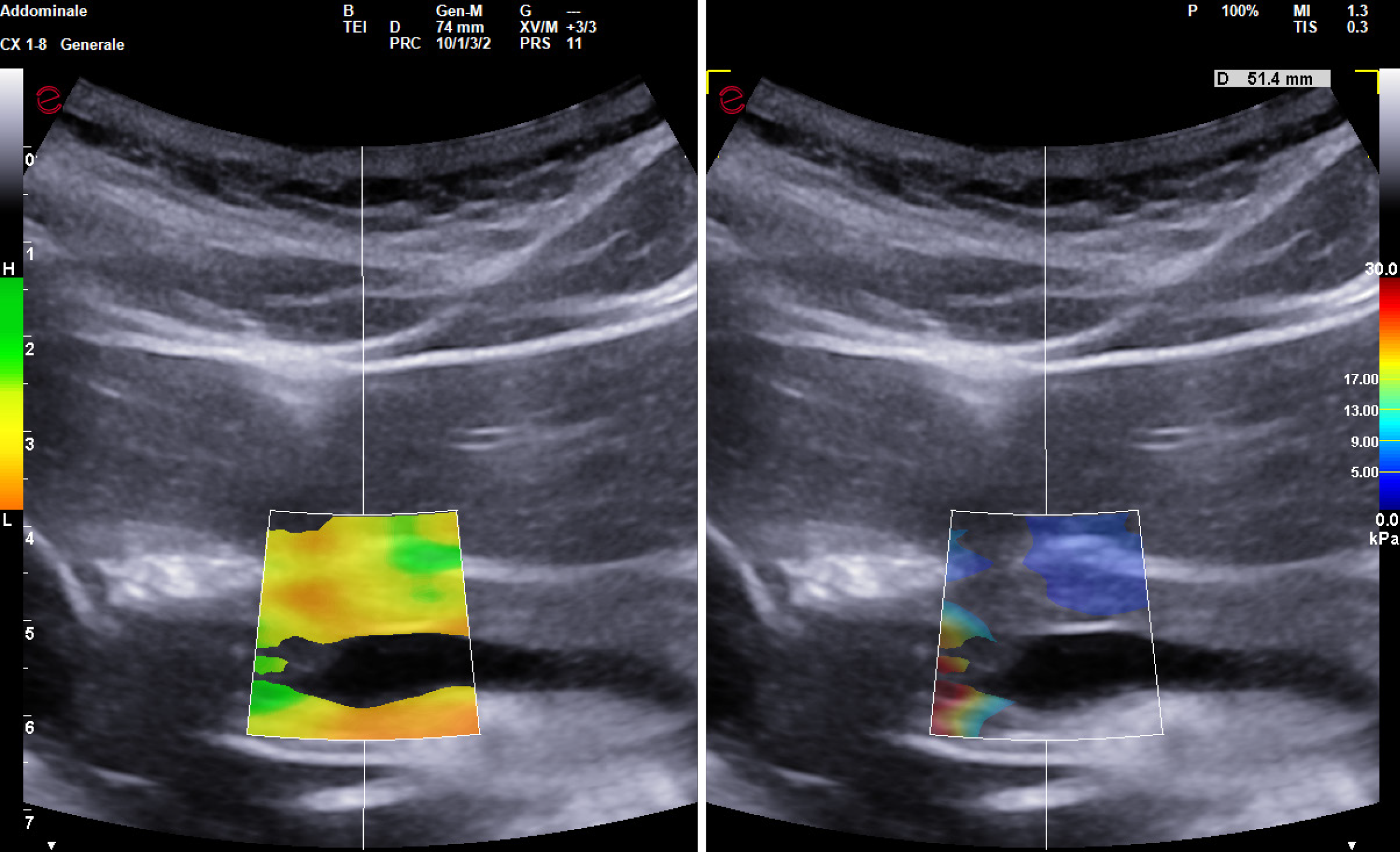
When performing SWE of the pancreas, the feasibility was not quite as excellent as for the liver and spleen. Specifically, in six subjects undergoing pancreas evaluation with 2D-QElaXto, measurements were either unreliable or showed inhomogeneous filling of the colorimetric map (Figure 2). Assessment with p-QElaXto was unreliable in ten patients when only the first 5 measurements were considered, but if the other five measurements were added, IQR/M ratio was > 30% only in four patients. Pancreas stiffness with SSI was not feasible in four patients due to SI < 80% (three patients) or inhomogeneous filling of the colorimetric map (one patient). Eventually, if the two failed visualizations of the organ are included, the overall feasibility of pancreas stiffness was 84%, 88% and 88% for 2D-QElaXto, p-QElaxto with 10 measurements, and SSI, respectively.
The ICC for liver stiffness measurement evaluated on 10 subjects was excellent for all three SWE techniques: 0.963 (95%CI: 0.850.99) for 2D-QElaXto (with a ROI of 15 mm), 0.906 (95%CI: 0.62-0.98) for p-QElaXto (10 measurements), and 0.928 (95%CI: 0.70-0.98) for SSI (with a ROI of 15 mm). Interoperator agreement was also very good for spleen stiffness measurements, but only for p-QElaXto and SSI, with a reliability of 0.929 (95%CI: 0.56-0.98) and 0.835 (95%CI: 0.38-0.96), respectively. 2D-QElaXto showed only a moderate agreement with an agreement of 0.519 (95%CI: -0.85 to 0.87). A similar trend was also observed for pancreas stiffness interobserver reproducibility: 0.831 (95%CI: 0.28-0.96) for p-QElaXto, 0.844 (95%CI: 0.41-0.96) for SSI, but only 0.434 (95%CI: -1.00 to 0.86) for 2D-QElaXto.
The overall values of the median liver stiffness in the 50 healthy subjects ranged from 2.4 kPa to 8.1 kPa across the different SWE techniques (Table 2). Specifically, considering the whole study group, the mean liver stiffness value for 2D-QElaXto was 3.9 kPa regardless of whether a 10 mm or 15 mm ROI was used. For p-QElaX the mean liver stiffness values were 4.4 kPa for both 5 and 10 measurements. Mean liver stiffness obtained with SSI was 4.6 kPa and 4.7 kPa using a 10 mm and 15 mm ROI, respectively.
| SWE technique | 2D-QElaXto | 2D-QElaXto | p-QElaXto | p-QElaXto | SSI | SSI |
| Mean liver stiffness (kPa) | 3.9 | 3.9 | 4.4 | 4.4 | 4.6 | 4.7 |
| Range (kPa) | 2.4-6.5 | 2.7-6.8 | 3-0-8.1 | 3.0-8.0 | 3.5-7.3 | 3.5-7.1 |
| SD | 0.8 | 0.8 | 0.9 | 0.9 | 0.9 | 0.9 |
| 95%CI | 3.66-4.11 | 3.69-4.15 | 4.13-4.66 | 4.12-4.65 | 4.41-4.89 | 4.44-4.93 |
Bland-Altman analysis showed that 2D-QElaXto average values were 0.8 kPa (CI: -1.9 kPa to 0.4 kPa) lower than those obtained with SSI, even in the presence of a significant correlation (r = 0.75; P < 0.001). A similar trend was observed for mean p-QElaXto values, which were only 0.3 kPa (CI: -1.6 kPa to 1.0 kPa) lower than those obtained with SSI (r = 0.71; P < 0.001) (Supplementary material). For the liver stiffness measurements, concordance was good (CCC = 0.67-0.80; CV < 12%), confirming the robustness of measurements across systems.
The mean value of spleen bipolar diameter was 10.8 ± 1.3 cm, while the splenic area and spleen width mean values were 33.3 ± 8.6 cm2 and 3.5 ± 0.6 cm, respectively. The overall values of the median spleen stiffness in the whole group ranged from 9.8 kPa to 46.9 kPa for the various SWE techniques (Table 3). Specifically, the mean spleen stiffness value for 2D-QElaXto was 19.4 kPa with an ROI of 10 mm and 20.0 kPa with an ROI of 15 mm. For p-QElaX, the mean spleen stiffness values were 23.0 kPa and 22.9 kPa when performing 5 or 10 measurements, respectively. Mean spleen stiffness values obtained with SSI were 21.9 kPa using a 10 mm ROI and 21.7 kPa with a 15 mm ROI.
| SWE technique | 2D-QElaXto | 2D-QElaXto | p-QElaXto | p-QElaXto | SSI | SSI |
| Mean spleen stiffness (kPa) | 19.4 | 20.0 | 23.0 | 22.9 | 21.9 | 21.7 |
| Range (kPa) | 9.8-26.7 | 11.7-32.6 | 13.5-46.9 | 13.1-36.1 | 13.4-35.9 | 13.5-33.8 |
| SD | 3.9 | 4.3 | 6.1 | 5.0 | 4.8 | 4.6 |
| 95%CI | 18.27-20.60 | 18.72-21.20 | 21.10-24.86 | 21.45-24.34 | 20.57-23.33 | 20.37-23.04 |
Bland-Altman analysis showed that 2D-QElaXto average values were 1.7 kPa (CI: -7.4 kPa to 3.9 kPa) lower than those obtained with SSI, even in the presence of a significant good correlation (r = 0.79; P < 0.001). Regarding p-QElaXto, mean values were 1.2 kPa (CI: -6.4 kPa to 8.8 kPa) higher than those obtained with SSI (r = 0.68; P < 0.001) (Supplementary material). For the spleen stiffness measurements, CCC ranged from 0.61 to 0.73 (CV < 13%), consistent with moderate-to-good agreement.
Pancreas stiffness evaluation was strongly influenced by organ visualization in every single subject. Consequently, a complete US assessment of the pancreas - including head, body, and tail - was achieved only in 23 subjects (46% of the whole population). SWE was therefore performed in the organ section with the best acoustic window. For 2D-SWE techniques (2D-QElaXto and SSI), the ROI dimension was adjusted according to organ anatomy (mean ROI size was 8.0 mm). The median pancreas stiffness in the whole group ranged from 2.7 kPa to 18.0 kPa for the various SWE tec
| SWE technique | 2D-QElaXto | p-QElaXto (5 meas) | p-QElaXto (10 meas) | SSI |
| Mean pancreas stiffness (kPa) | 5.2 | 5.9 | 6.1 | 7.6 |
| Range (kPa) | 2.9-15.0 | 2.8-17.4 | 3.1-18.0 | 2.7-17.2 |
| SD | 1.9 | 2.5 | 2.4 | 2.6 |
| 95%CI | 4.63-5.80 | 5.03-6.70 | 5.40-6.83 | 6.86-8.41 |
Bland-Altman analysis showed that 2D-QElaXto average values were 2.2 kPa (CI: -6.3 kPa to 1.9 kPa) lower than those obtained with SSI, with only a moderate correlation (r = 0.56; P < 0.001). A similar trend was observed for mean p-QElaXto values, which were 1.5 kPa (CI: -6.4 kPa to 3.4 kPa) lower than those obtained with SSI (r = 0.49; P = 0.01) (Supplementary material). However, the correlation was stronger when comparing 2D-QElaXto with p-QElaXto average values (r = 0.75; P < 0.05). As opposed to liver and spleen, pancreatic stiffness measurements showed lower concordance (CCC = 0.35-0.66; CV 22%-32%), in line with the higher variability already observed in the Bland-Altman analysis.
Over recent years, ultrasonography and elastography techniques have garnered growing interest due to their capability for both qualitative and quantitative analysis of tissue stiffness, offering additional data compared to traditional sem
The different SWE techniques demonstrated excellent feasibility for the assessment of liver and spleen stiffness, whereas the feasibility for pancreatic evaluation was moderately lower. This limitation is primarily attributable to various anatomical and functional factors. Assessing pancreatic stiffness remains a technically demanding and clinically intr
Concerning stiffness measurements, our findings aligned with the well-established reference values for liver ela
Regarding the spleen stiffness, values ranged from 19.4 kPa (2D-QElaXto, 10 mm ROI) to 23.0 kPa (p-QElaXto, 5 measurements). Few studies have investigated spleen stiffness in healthy adults, and their reported values are consistent with our results[1,9,26]. For example, Leung et al[26] reported a mean spleen stiffness value of 17.3 ± 2.6 kPa in a cohort of healthy volunteers, with no statistically significant difference observed between male and female subjects. In recent years, efforts have focused on defining universal cut-off values for spleen elastography. Similar to liver elastography, these thresholds aim to rule out clinically significant portal hypertension (CSPH) or high-risk esophageal varices in patients with compensated advanced chronic liver disease (cACLD)[7,27,28]. Recently, the role of spleen stiffness in identifying or ruling out CSPH in cACLD patients was clarified by the 2024 WFUMB guidelines[13], which introduced a “Baveno VII−SSM dual-cutoff model”[13,29]. This model incorporates LSM, SSM and platelet count to improve diagnostic accuracy, establishing 21 kPa as the upper SSM threshold for excluding CSPH - slightly lower than the threshold proposed in Baveno VII[13]. It should be noted, however, that these thresholds refer to values obtained by transient elastography in cACLD patients, and no guideline-based cut-offs are currently available for SWE. The spleen stiffness values obtained in our cohort, together with the high reproducibility of liver and spleen measurements, support the clinical use of SWE for non-invasive assessment of liver fibrosis and for spleen-based evaluation of portal hypertension. Future studies should clarify whether spleen stiffness values in healthy individuals overlap with those of cACLD patients without CSPH.
Lastly, regarding pancreatic elastography, the mean stiffness values in healthy subjects in our cohort ranged from 5.2 kPa with 2D-QElaX to 6.1 kPa with SSI. These findings are in line with previously reported normal ranges. For example, Kaya et al[20] found median values around 4-5 kPa during free breathing, while Nosakova et al[21] reported a mean of approximately 6.1 kPa using 2D-SWE. Similarly, Gallotti et al[12] described mean velocities of 1.40 m/second (approximately 6 kPa) with ARFI in healthy volunteers, and Choudhury et al[30] reported mean values of 8.7-9.0 kPa in an Indian cohort, highlighting possible variability related to population and methodology. Other studies confirmed normal pancreas stiffness around 6-7 kPa in controls[31], whereas markedly higher values (23.8 ± 6.7 kPa) were reported by Durmaz et al[32]. Taken together, our data fall within the lower-to-mid portion of the spectrum of published reference values, supporting their reliability while underlining the considerable heterogeneity observed across studies depending on technique, respiratory phase, and anthropometric factors.
Although inter-system variability currently limits routine pancreatic SWE, emerging evidence indicates that stiffness measurements may provide additional value in detecting pancreatic steatosis, in correlation with metabolic syndrome and cardiovascular risk stratification[33,34], and in the evaluation of inflammatory changes in acute and chronic pancreatitis[11,33]. Latest evidence reports also a significant correlation between concomitant increase of pancreatic and splenic stiffness in patients with portal hypertension[35]. We acknowledge some potential limitations of our study. Firstly, the limited number of participants in our study may have affected the statistical power, potentially increasing the margin of error. Also, the recruitment of hospital staff and medical students may introduce a selection bias, as this group is relatively homogeneous in terms of age, health status, and BMI. To strengthen the validity of our findings, future multicenter studies should include a more demographically diverse cohort to improve generalizability. Secondly, it is important to note that our investigation was conducted within a single institution. Thirdly, measurements in our study were performed using a generation-specific device, and the normal values generated may be representative of this model and generation. However, it would be advisable to verify the concordance between different models and generations of the same manufacturer because the lack of harmonization across US platforms restricts comparability of SWE values, highlighting the importance of multicenter studies and standardized acquisition protocols for clinical adoption.
Our study provides preliminary reference stiffness values in healthy adults using three SWE techniques, with robust liver and spleen reproducibility that supports integration of SWE into multiparametric US assessment of chronic liver disease and portal hypertension. In contrast, pancreatic stiffness measurements remain more variable, highlighting the need for optimized acquisition protocols and standardization before SWE can be reliably applied in pancreatic routine clinical practice. Future multicenter studies with larger cohorts should focus on harmonizing reference values and technical parameters across different systems and manufacturers, enabling SWE to evolve into a truly reproducible, quantitative, and cross-platform non-invasive tool for abdominal organ evaluation.
Thanks to Fondazione Roma for its commitment to support our research.
| 1. | Pawluś A, Inglot MS, Szymańska K, Kaczorowski K, Markiewicz BD, Kaczorowska A, Gąsiorowski J, Szymczak A, Inglot M, Bladowska J, Zaleska-Dorobisz U. Shear wave elastography of the spleen: evaluation of spleen stiffness in healthy volunteers. Abdom Radiol (NY). 2016;41:2169-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 2. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Bernuzzi S, Salvaneschi L, Filice C; Elastography Study Group. Ultrasound point shear wave elastography assessment of liver and spleen stiffness: effect of training on repeatability of measurements. Eur Radiol. 2014;24:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (1)] |
| 4. | Wu M, Wu L, Jin J, Wang J, Li S, Zeng J, Guo H, Zheng J, Chen S, Zheng R. Liver Stiffness Measured with Two-dimensional Shear-Wave Elastography Is Predictive of Liver-related Events in Patients with Chronic Liver Disease Due to Hepatis B Viral Infection. Radiology. 2020;295:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 6. | Gibiino G, Garcovich M, Ainora ME, Zocco MA. Spleen ultrasound elastography: state of the art and future directions - a systematic review. Eur Rev Med Pharmacol Sci. 2019;23:4368-4381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Reiberger T. The Value of Liver and Spleen Stiffness for Evaluation of Portal Hypertension in Compensated Cirrhosis. Hepatol Commun. 2022;6:950-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Dong Y, Sirli R, Ferraioli G, Sporea I, Chiorean L, Cui X, Fan M, Wang WP, Gilja OH, Sidhu PS, Dietrich CF. Shear wave elastography of the liver - review on normal values. Z Gastroenterol. 2017;55:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Giuffrè M, Macor D, Masutti F, Abazia C, Tinè F, Patti R, Buonocore MR, Colombo A, Visintin A, Campigotto M, Crocè LS. Evaluation of spleen stiffness in healthy volunteers using point shear wave elastography. Ann Hepatol. 2019;18:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Sezgin O, Yaraş S, Özdoğan O. Pancreatic Steatosis Is Associated with Both Metabolic Syndrome and Pancreatic Stiffness Detected by Ultrasound Elastography. Dig Dis Sci. 2022;67:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Dietrich CF, Hocke M. Elastography of the Pancreas, Current View. Clin Endosc. 2019;52:533-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Gallotti A, D'Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Ferraioli G, Barr RG, Berzigotti A, Sporea I, Wong VW, Reiberger T, Karlas T, Thiele M, Cardoso AC, Ayonrinde OT, Castera L, Dietrich CF, Iijima H, Lee DH, Kemp W, Oliveira CP, Sarin SK. WFUMB Guideline/Guidance on Liver Multiparametric Ultrasound: Part 1. Update to 2018 Guidelines on Liver Ultrasound Elastography. Ultrasound Med Biol. 2024;50:1071-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 14. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1171] [Article Influence: 78.1] [Reference Citation Analysis (2)] |
| 15. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 606] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 16. | Dietrich CF, Abramowicz JS, Chammas MC, Chou YH, Condous G, Kim SH, Nolsøe CP, Vinayak S, Jenssen C. World Federation for Ultrasound in Medicine and Biology (WFUMB) Policy Document Development Strategy - Clinical Practice Guidelines, Position Statements and Technological Reviews (on behalf of the WFUMB publication committee and Executive Bureau). Ultrasound Med Biol. 2021;47:2779-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Bartko JJ. Measures of agreement: a single procedure. Stat Med. 1994;13:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Huang J, Peng J, Long H, Ruan S, Yao L, Xie X, Lin M, Zhang X. Feasibility and Measurement Value of Pancreatic 2-D Shear Wave Elastography in Healthy Adults: Evaluation, Influencing Factors, Reference Range, Measurement Stability and Reproducibility. Ultrasound Med Biol. 2024;50:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Marasco G, Ricci C, Grasso V, Alvisi M, Serra C, Ravaioli F, Casadei R, Colecchia A. Pancreatic ultrasound elastography is not useful to predict the risk of pancreatic fistulas after pancreatic resection. Updates Surg. 2020;72:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kaya M, Gürün E. Do deep inspiration breath-holds and free-breathing affect pancreatic tissue stiffness in shear wave elastography? Abdom Radiol (NY). 2022;47:2390-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 21. | Nosakova L, Uhrik P, Pindura M, Vojtko M, Hoferica J, Cmarkova K, Miklusica J, Banovcin P. Pancreatic stiffness and anthropometric parameters in healthy volunteers. Bratisl Lek Listy. 2024;125:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Stumpf S, Jaeger H, Graeter T, Oeztuerk S, Schmidberger J, Haenle MM, Kratzer W; Elasto-Study Group Ulm. Influence of age, sex, body mass index, alcohol, and smoking on shear wave velocity (p-SWE) of the pancreas. Abdom Radiol (NY). 2016;41:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Abu-Yousef MM, El-Zein Y. Improved US visualization of the pancreatic tail with simethicone, water, and patient rotation. Radiology. 2000;217:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (1)] |
| 25. | Mulabecirovic A, Mjelle AB, Gilja OH, Vesterhus M, Havre RF. Liver elasticity in healthy individuals by two novel shear-wave elastography systems-Comparison by age, gender, BMI and number of measurements. PLoS One. 2018;13:e0203486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT, Chan HL, Chu WC. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 27. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1063] [Article Influence: 212.6] [Reference Citation Analysis (2)] |
| 28. | Song J, Huang J, Huang H, Liu S, Luo Y. Performance of spleen stiffness measurement in prediction of clinical significant portal hypertension: A meta-analysis. Clin Res Hepatol Gastroenterol. 2018;42:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1848] [Article Influence: 462.0] [Reference Citation Analysis (2)] |
| 30. | Choudhury SR, Verma M, Gupta P, Singh H, Sharma V, Kochhar R. Ultrasound Shear Wave Elastography of Normal Pancreas in Adult Subjects. J Gastrointestinal Abdominal Radiol. 2023;06:148-153. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Suzuki H, Ishikawa T, Ohno E, Iida T, Uetsuki K, Yashika J, Yamada K, Yoshikawa M, Furukawa K, Nakamura M, Honda T, Ishigami M, Kawashima H, Fujishiro M. An initial trial of quantitative evaluation of autoimmune pancreatitis using shear wave elastography and shear wave dispersion in transabdominal ultrasound. Pancreatology. 2021;21:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Durmaz MS, Arslan S, Özbakır B, Güngör G, Tolu İ, Arslan FZ, Sivri M, Koplay M. Effectiveness of Shear Wave Elastography in the diagnosis of acute pancreatitis on admission. Med Ultrason. 2018;20:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Paratore M, Garcovich M, Ainora ME, Del Vecchio LE, Cuccia G, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives. Diagnostics (Basel). 2023;13:2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 34. | Zhang Y, Liu Y, Petrov MS. Relationship of fat in the pancreas with cardiovascular disease: A systematic review and meta-analysis. Obes Rev. 2025;26:e13914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Wekerle M, Murillo K, vonBoscamp M, Hauber V, Ebert MP, Antoni C, Hirth M. Point-shear wave elastography generated by acoustic radiation force impulse in chronic pancreatitis. United European Gastroenterol J. 2024;12:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/