Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.111941
Revised: July 29, 2025
Accepted: August 25, 2025
Published online: October 26, 2025
Processing time: 102 Days and 21.4 Hours
Peripheral artery disease (PAD) affects millions globally, with a 5.6% prevalence in 2015 impacting 236 million adults, rising above 10% in those over 60 due to factors like diabetes and smoking. Post-revascularization, single antiplatelet therapy (SAPT) is standard, but dual antiplatelet therapy (DAPT) may improve outcomes, though duration and bleeding risks are unclear. The 2024 American College of Cardiology/American Heart Association guidelines endorse short-term DAPT, yet evidence gaps remain in comparative efficacy and safety. We hypothesized that DAPT reduces cardiovascular events and reinterventions vs SAPT without significantly elevating bleeding in PAD patients’ post-lower extremity revascularization.
To evaluate the efficacy and safety of DAPT vs SAPT in PAD patients’ post-revascularization.
This systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, searching PubMed, EMBASE, and ScienceDirect up to July 2025. Included were randomized controlled trials (RCTs) and cohort studies from various global settings (e.g., hospitals, tertiary care) comparing DAPT (aspirin plus P2Y12 inhibitor for > 1 month) to SAPT in symptomatic PAD patients undergoing endova
Twelve studies (3 RCTs, 9 cohorts, conducted 2010–2025 with follow-ups of 6 months to 5 years) were included. DAPT showed no significant difference but a trend toward reduced all-cause mortality (RR: 0.52, 95%CI: 0.27–1.01, P = 0.05, DAPT of 298/9545 events vs SAPT of 165/566 events) or stroke (RR: 0.72, 95%CI: 0.30–1.72, P = 0.46, DAPT of 16/3729 events vs SAPT of 41/7673 events) vs SAPT. DAPT significantly reduced cardiac mortality (RR: 0.46, 95%CI: 0.27–0.80, P = 0.006, DAPT of 78/2903 events vs SAPT of 171/1465 events, risk difference: -5.4%), myocardial infarction (RR: 0.82, 95%CI: 0.71–0.94, P = 0.004, DAPT of 233/7704 events vs SAPT of 262/9130 events, risk difference: -1.8%), and major reintervention (RR: 0.58, 95%CI: 0.35–0.98, P = 0.04, DAPT of 803/205 events vs SAPT of 1197/4 events, risk difference: -42%). Bleeding showed no difference (RR: 1.12, 95%CI: 0.42–3.03, P = 0.82, DAPT of 195/2775 events vs SAPT of 202/8234 events). Heterogeneity was high (I2 = 59%–97%). Quality revealed moderate to serious bias in cohorts and some concerns in RCTs; GRADE certainty moderate for cardiac mortality, myocardial infarction, reintervention, low for others due to inconsistency and imprecision.
DAPT reduces cardiac mortality, myocardial infarction, and major reintervention risks compared to SAPT in PAD post-revascularization without apparent bleeding increase, though limited by heterogeneity and low certainty for some outcomes.
Core Tip: This meta-analysis synthesizes evidence from randomized controlled trials and cohort studies to compare dual antiplatelet therapy (DAPT) and single antiplatelet therapy (SAPT) following lower extremity revascularization in patients with peripheral artery disease (PAD). The findings demonstrate that DAPT is associated with reduced cardiac mortality, myocardial infarction, and major reintervention risk compared to SAPT, without a significant increase in bleeding complications. These results support the short-term use of DAPT in PAD management post-revascularization and inform current clinical guidelines.
- Citation: Shahid MM, Mandal D, Das A, Kumar P, Kumar N, Mukhtar A, Ejaz H, Jaffar MS, Habib M, Afzal A, Khawar MMH, Rana I. Dual versus single antiplatelet therapy after lower extremity revascularization in peripheral artery disease: A systematic review and meta-analysis. World J Cardiol 2025; 17(10): 111941
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/111941.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.111941
Peripheral artery disease (PAD) is an atherosclerotic condition that affects arteries in the lower extremities. In 2015, its global prevalence was 5.6%, affecting about 236 million adults[1]. The prevalence rises above 10% in people over 60 years old. From 2000 to 2010, PAD cases increased by 23.5%, largely due to aging populations and rising risk factors like diabetes and smoking[2]. PAD ranges from asymptomatic to severe forms, with intermittent claudication (IC) being the most common symptom[3]. As a systemic vascular disease, PAD shares risk factors with coronary artery disease (CAD) and cerebrovascular disease (CVD), notably smoking and diabetes, contributing to significant comorbidity[2,3]. The REACH registry indicates 39% of PAD patients have CAD, 10% have CVD, and 13% have all three, leading to elevated mortality, with CAD causing 40%–60% and CVD 10%–20% of deaths among PAD patients[4].
For patients with symptomatic PAD, revascularization is often recommended to relieve pain, prevent limb loss, and lower healthcare costs. Endovascular procedures are commonly preferred because they are less invasive[5]. In England from 2006 to 2015, endovascular revascularization lowered the 1-year major amputation risk from 5.7% to 3.9%, compared to a drop from 11.2% to 6.6% for surgical methods[6]. However, adverse cardiovascular and limb outcomes remain prevalent[7]. Single antiplatelet therapy (SAPT) with aspirin or clopidogrel is standard to prevent systemic atherosclerosis progression and major adverse cardiovascular and cerebrovascular events[8,9]. The 2024 American College of Cardiology/American Heart Association (ACC/AHA) guidelines now recommend dual antiplatelet therapy (DAPT) for 1–6 months post-endovascular revascularization (class IIa), a shift from the 2016 class IIb rating[8]. Meta-analyses show DAPT reduces all-cause mortality [hazard ratio (HR): 0.86], major limb events (HR: 0.60), and amputations (HR: 0.78) without significantly increasing major bleeding[4]. Dual pathway inhibition (DPI) with low-dose rivaroxaban and aspirin, as demonstrated in the COMPASS and VOYAGER PAD trials, further decreases adverse cardiovascular and limb events post-revascularization[10-12]. Viscoelastic testing, such as thromboelastography, reveals DAPT’s enhanced platelet inhibition over SAPT[9,13].
Despite these advances, important gaps remain in optimizing antiplatelet therapy for PAD after revascularization. For example, the ideal DAPT duration, ranging from 1 month to 24 months, is still uncertain, which makes standardizing treatment challenging. Outcomes comparing DAPT in endovascular vs surgical revascularization are not well explored. Similarly, translating data from mechanistic coagulation monitoring into everyday clinical practice is unclear. The relative benefits of DAPT compared to DPI are also not fully understood, as is identifying which patient subgroups would gain the most from intensified therapy with the best risk-benefit balance. This meta-analysis aims to synthesize high-quality evidence on SAPT and DAPT in PAD patients’ post-lower extremity revascularization, assessing efficacy, safety, and bleeding risks to address these gaps and inform evidence-based clinical guidelines
This systematic review and meta-analysis were conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions[14,15]. The protocol has been prospectively registered with PROSPERO (No. CRD420251102724)
A comprehensive literature search was independently performed by two reviewers across major electronic databases, including PubMed/MEDLINE, EMBASE, and ScienceDirect, from database inception through July 2025 (Supplementary material). Grey literature sources such as ClinicalTrials.gov, medRxiv, and relevant conference abstracts were also queried to capture unpublished or ongoing studies and minimize publication bias. The search strategy incorporated a combination of MeSH terms and free-text keywords to maximize sensitivity, focusing on DAPT, PAD, and revascularization procedures. Key search strings included: ("dual antiplatelet therapy" OR "DAPT") AND ("peripheral artery disease" OR "PAD") AND ("endovascular treatment" OR "percutaneous transluminal angioplasty" OR "revascularization" OR "bypass" OR "stenting"). The full search strategies tailored to each database are detailed in the Supplementary file. Additional studies were identified by hand-searching reference lists of included articles and relevant reviews.
Retrieved citations were imported into EndNote 21 for deduplication and management. Two reviewers (Shahid MM and Das A) independently screened titles and abstracts, followed by full-text evaluation for eligibility. Disagreements were resolved through discussion or arbitration by a third reviewer (Jaffar MS). Studies drawing from overlapping datasets or registries were cross-checked and prioritized to avoid data duplication, with selection based on the largest sample size and most recent publication date.
Inclusion criteria: (1) Randomized controlled trials (RCTs) or non-randomized studies (e.g., prospective or retrospective cohorts, observational studies) involving symptomatic PAD patients with IC or critical limb ischemia who underwent endovascular interventions (e.g., balloon angioplasty with or without stenting) or surgical bypass (e.g., venous or prosthetic graft implantation); (2) Comparisons between DAPT (aspirin plus a P2Y12 inhibitor such as clopidogrel, ticagrelor, prasugrel, or ticlopidine, administered for ≥ 1 month post-procedure) and SAPT (aspirin or clopidogrel alone); (3) Reporting of at least one dichotomous outcome related to efficacy (e.g., all-cause mortality, cardiac mortality, myocardial infarction, stroke, major reintervention) or safety (e.g., bleeding complications); and (4) English-language publications with sufficient data for meta-analysis.
Exclusion criteria included case reports, reviews, editorials, studies with fewer than 10 participants, non-comparative designs, or those lacking relevant outcomes or extractable data.
Data extraction was performed independently by two reviewers (Mukhtar A and Ejaz H) using a standardized form to collect information on study type, year of publication, country, sample size (stratified by DAPT and SAPT groups), patient population, intervention details, DAPT regimen and duration, SAPT regimen, follow-up duration, baseline characteristics (e.g., age, smoking status, diabetes, hypertension, hyperlipidemia, CAD, CVD, chronic kidney disease, ankle-brachial index), and all reported outcomes. For dichotomous outcomes, event rates and totals were extracted; if unavailable, corresponding authors were contacted for raw data.
Risk of bias was assessed by three reviewers (Mandal D, Das A, and Afzal A) using the Cochrane Risk of Bias 2 (RoB 2) tool for RCTs[16] and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool[17] for non-randomized studies. Assessments covered domains such as randomization, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results for RoB 2, and confounding, participant selection, intervention classification, deviations from interventions, missing data, outcome measurement, and reported result selection for ROBINS-I. Discrepancies were resolved by consensus. Results were visualized using traffic light and summary plots generated in tools like robvis.
Publication bias was evaluated using funnel plots for visual inspection and the Egger test for all outcomes, regardless of the number of studies, to assess small-study effects. The certainty of evidence for each outcome was graded using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, considering risk of bias, inconsistency, imprecision, indirectness, and publication bias.
All analyses were performed using Review Manager (RevMan) Version 5.4 (Cochrane Collaboration). A random-effects model was employed to account for anticipated clinical and methodological heterogeneity across studies, such as variations in PAD severity, intervention types, and patient characteristics. For dichotomous outcomes, pooled effect estimates were calculated as risk ratio (RR) with 95%CI using the Mantel-Haenszel method.
Heterogeneity was quantified using the Higgins I² statistic (low: 0%–25%; moderate: 25%–50%; substantial: 50%–75%; high: > 75%) and the Cochrane Q test (P < 0.10 indicating significance). Meta-regression was conducted to explore sources of heterogeneity, examining covariates such as mean patient age, prevalence of smoking, type 2 diabetes mellitus (T2DM), and hypertension from baseline characteristics, focusing on outcomes like cardiac mortality. Sensitivity analyses, including leave-one-out approaches, were performed to assess the robustness of findings by sequentially excluding individual studies. A two-sided P ≤ 0.05 was considered statistically significant.
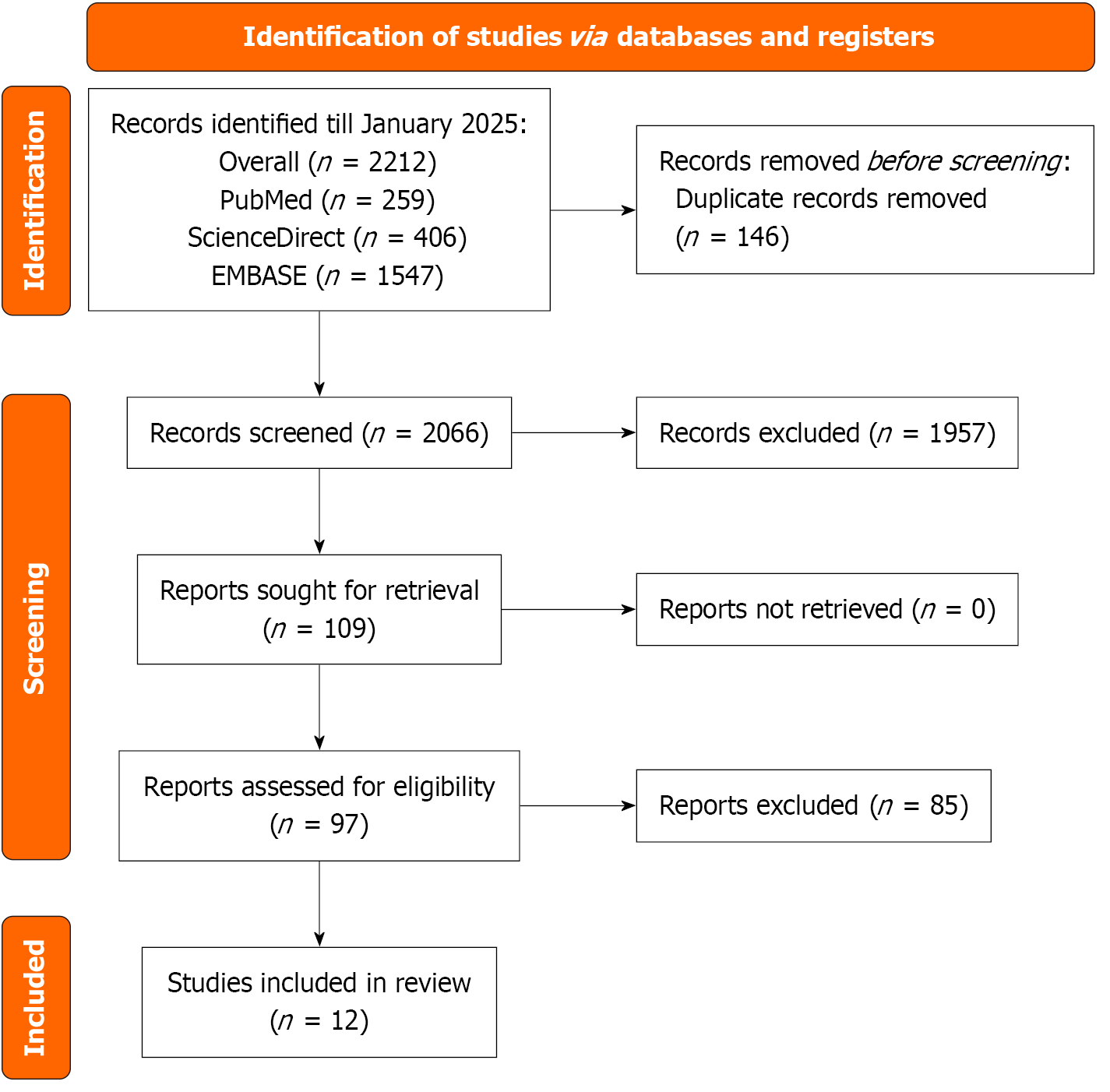
The systematic search of PubMed, ScienceDirect, and EMBASE, conducted up to July 2025, yielded 2212 records. After removing 146 duplicates, 2066 records underwent title and abstract screening, of which 1957 were excluded due to irrelevance, non-human studies, or inappropriate study designs. The full-text review was performed for 109 articles, with 97 excluded for lack of comparative bicuspid aortic valves vs tricuspid aortic valves data, insufficient outcome reporting, single-arm studies, and duplicate or overlapping data. Ultimately, 12 studies were included in the meta-analysis (Figure 1)[5,9,13,18-26].
The meta-analysis included 12 studies conducted across eight countries (United States, United Kingdom, South Korea, Netherlands, France, Sweden, Japan, and California) from 2010 to 2025, with follow-up durations ranging from 6 months to 5 years. The studies encompassed various designs, including prospective and retrospective cohorts, clinical trials, and observational studies, with sample sizes ranging from 40 patients to 28244 patients. Patient populations primarily consisted of individuals with PAD undergoing interventions such as revascularization, bypass, and endovascular therapy. DAPT regimens most frequently included aspirin combined with clopidogrel, prasugrel, or ticagrelor, administered for periods ranging from 1 month to 12 months, while SAPT typically involved aspirin or clopidogrel alone. Detailed study characteristics are presented in Table 1[5,9,13,18-26].
| Ref. | Study type | Intervention | DAPT drug name | DAPT duration | SAPT | Follow up |
| Rodriguez Alvarez et al[9], 2025 | Prospective cohort | DAPT and SAPT | Aspirin and clopidogrel | 4 weeks | Aspirin or clopidogrel | 6 months |
| Armstrong et al[21], 2015 | Retrospective cohort | N/A | Aspirin and clopidogrel, prasugrel, ticagrelor | N/A | Aspirin | 3 years |
| Belch et al[18], 2010 | RCT | Endovascular revascularization | Aspirin and clopidogrel | N/A | Aspirin | 2 years |
| Belkini et al[26], 2020 | Retrospective cohort | Bypass | Aspirin and clopidogrel, prasugrel, ticagrelor | N/A | Aspirin | 1 year |
| Chinai et al[24], 2020 | Retrospective cohort | Endovascular revascularization | Aspirin and clopidogrel | N/A | Aspirin | 1.5 years |
| Cho et al[5], 2019 | Prospective cohort | Endovascular revascularization | Aspirin plus clopidogrel | ≥ 6 months | Aspirin or clopidogrel | 5 years |
| Ipema et al[25], 2020 | Retrospective cohort | Infra-inguinal endovascular treatment | Acetylsalicylic acid and clopidogrel | 12 months | Acetylsalicylic acid or clopidogrel | 1 year |
| Scheinert et al[19], 2012 | RCT | Endovascular revascularization | Aspirin and clopidogrel | 12 months | Aspirin | 2 years |
| Soden et al[22], 2016 | Retrospective cohort | Lower extremity revascularization | Aspirin and P2Y12 antagonist | N/A | Aspirin or P2Y12 antagonist | 1 year |
| Strobl et al[20], 2013 | RCT | Endovascular therapy | Aspirin and clopidogrel | 6 months | Aspirin | 12 months |
| Thott et al[23], 2017 | Retrospective cohort | Endovascular femoropopliteal stenting | Aspirin and clopidogrel | 100 days | Aspirin | 2 years |
| Yamada et al[13], 2024 | Retrospective cohort | Endovascular therapy | Aspirin and clopidogrel or aspirin and prasugrel | 1 month | Any one of aspirin, clopidogrel, prasugrel | 2 years |
The meta-analysis encompassed baseline characteristics from 12 studies, with mean ages ranging from 65.8 years to 78 years (overall mean approximately 69.6 years) for patients on DAPT and 65.6 years to 80 years (overall mean approximately 70.4 years) for those on SAPT, though age data were unavailable for two studies (Scheinert et al[19], 2012 and Chinai et al[24], 2020). Prevalence of current smoking varied widely, from 11 cases to 303 cases in DAPT groups and from 6 to 590 in SAPT groups across reporting studies, while T2DM was reported in all but one study (Scheinert et al[19], 2012), with rates ranging from 12 to 603 in DAPT and from 18 to 274 in SAPT. Hypertension was prevalent, with counts from 6 to 1054 in DAPT and from 8 to 488 in SAPT, and hyperlipidemia/dyslipidemia data, available in six studies, ranged from 25 to 141 in DAPT and from 25 to 163 in SAPT. CAD was noted in eight studies, with 11-195 cases in DAPT and 15-184 in SAPT; CVD in five studies, ranging from 3 to 59 in DAPT and from 4 to 169 in SAPT; and chronic kidney disease in seven studies, from 3 to 133 in DAPT and from 3.7 to 51 in SAPT. Ankle-brachial index values, reported in three studies, ranged from 0.44 to 0.61 for both groups. Detailed baseline characteristics are presented in Table 2[5,9,13,18-26].
| Ref. | Age (mean ± SD) | Current smoking | Type 2 diabetes mellitus | Hypertension | Hyperlipidemia/dyslipidemia | Coronary artery disease | Cerebrovascular disease (intracranial hemorrhage) | Chronic kidney disease/dialysis | Ankle-brachial index (mean) | |||||||||
| DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | DAPT | SAPT | |
| Rodriguez Alvarez et al[9], 2025 | 70 | 66 | 11 | 6 | 35 | 24 | 6 | 8 | 53 | 32 | 29 | 19 | 12 | 7 | 6 | 6 | N/A | N/A |
| Armstrong et al[21], 2015 | 67 | 67 | 265 | 212 | 187 | 126 | 299 | 236 | N/A | N/A | 195 | 126 | N/A | N/A | N/A | N/A | 0.51 | 0.53 |
| Belch et al[18], 2010 | 66.5 | 65.6 | 38.8 | 36.4 | 37.4 | 38 | 70.1 | 70 | 50.4 | 48.8 | 38.4 | 31 | N/A | N/A | N/A | N/A | 0.44 | 0.46 |
| Belkini et al[26], 2020 | 65.8 | 66.4 | 42.6 | 42 | 51.1 | 45.2 | 90.1 | 85.1 | N/A | N/A | 35.1 | 25.4 | N/A | N/A | 3.8 | 3.7 | N/A | N/A |
| Chinai et al[24], 2020 | N/A | N/A | N/A | N/A | 71 | 222 | 97 | 303 | N/A | N/A | N/A | N/A | 25 | 55 | N/A | N/A | N/A | N/A |
| Cho et al[5], 2019 | 68.6 | 70.5 | 181 | 198 | 177 | 274 | 224 | 309 | 141 | 163 | 176 | 184 | N/A | N/A | N/A | N/A | ||
| Ipema et al[25], 2020 | 68.7 | 74.4 | 75 | 99 | 40 | 68 | 58 | 98 | 61 | 65 | N/A | N/A | N/A | N/A | 3 | 5 | N/A | N/A |
| Scheinert et al[19], 2012 | 68.6 | 70.5 | 34 | 21 | 24 | 32 | 12 | 23 | N/A | N/A | 12 | 34 | N/A | N/A | 21 | 11 | 0.52 | 0.47 |
| Soden et al[22], 2016 | 68.1 | 67.8 | 37.1 | 38.2 | 45.2 | 44.8 | 87.9 | 88.5 | N/A | N/A | 31.1 | 30.5 | N/A | N/A | 6.4 | 5.9 | N/A | N/A |
| Strobl et al[20], 2013 | 69.8 | 70.2 | 15 | 17 | 12 | 18 | 31 | 31 | 25 | 25 | 11 | 15 | 6 | 9 | N/A | N/A | 0.61 | 0.61 |
| Thott et al[23], 2017 | 78 | 80 | 303 | 590 | 603 | 242 | 1054 | 488 | N/A | N/A | N/A | N/A | 59 | 169 | 133 | 49 | N/A | N/A |
| Yamada et al[13], 2024 | 73.8 | 75.6 | 30 | 38 | 96 | 90 | 136 | 127 | 104 | 93 | 13 | 20 | 3 | 4 | 44 | 51 | N/A | N/A |
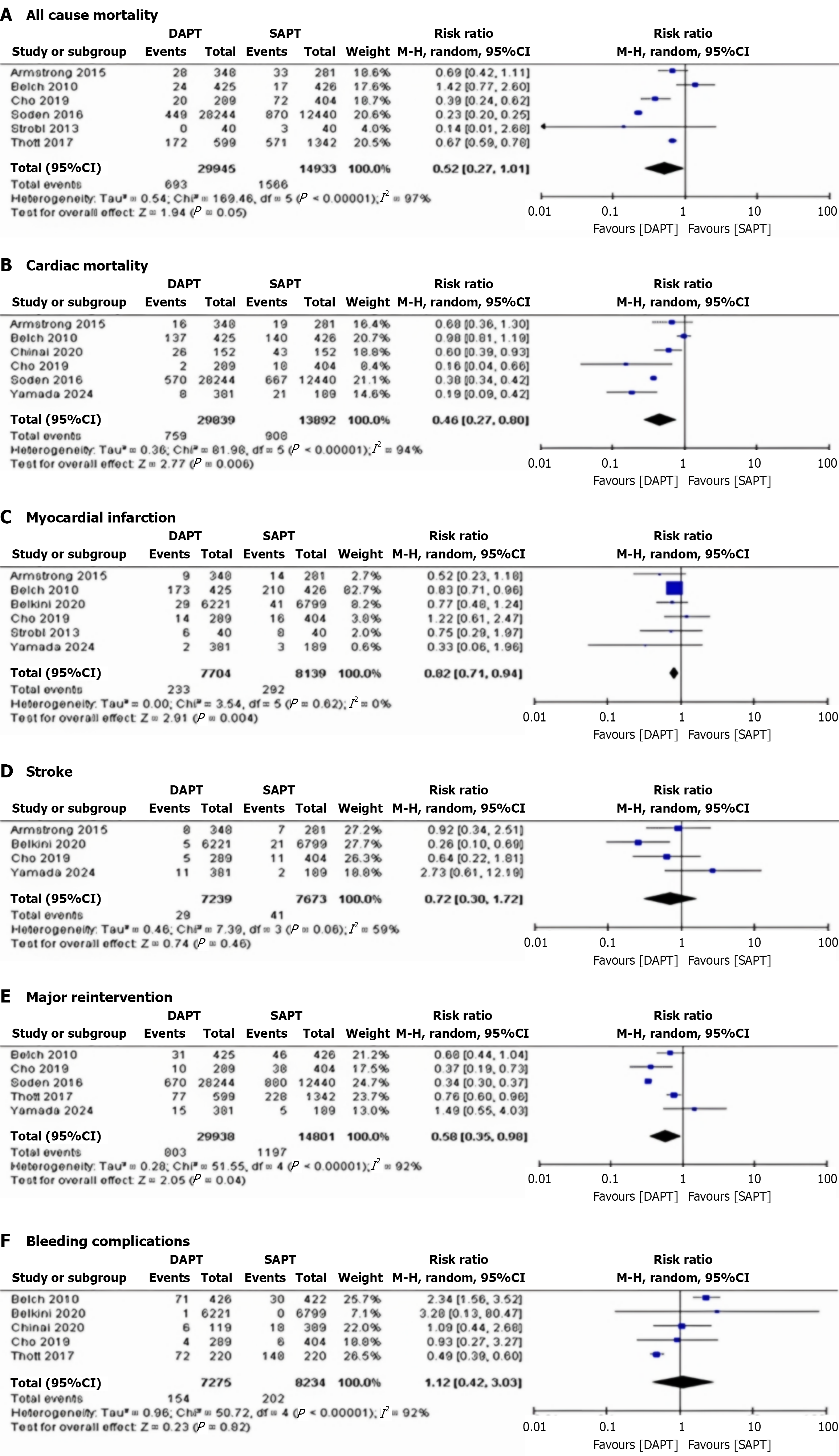
All-cause mortality: Six studies reported all-cause mortality. The pooled RR was 0.52 (95%CI: 0.27–1.01, P = 0.05), showing no significant difference in mortality risk between DAPT and SAPT patients. Heterogeneity was high (I² = 97%) and dropped significantly after removing Armstrong et al[21], 2015 and Belkini et al[26], 2020 (I² = 62%) (Figure 2A)[5,18,20-23].
Cardiac mortality: Six studies reported cardiac mortality. The pooled RR was 0.46 (95%CI: 0.27–0.80, P = 0.006), indicating a significantly lower risk of cardiac mortality in DAPT patients compared to SAPT patients. Heterogeneity was high (I² = 94%) and dropped significantly after removing Scheinert et al[19], 2012 and Belkini et al[26], 2020 (I² = 56%) (Figure 2B)[5,13,18,21,22,24].
Myocardial infarction: Six studies reported myocardial infarction. The pooled RR was 0.82 (95%CI: 0.71–0.94, P = 0.004), demonstrating a significantly lower risk in DAPT patients compared to SAPT patients. Heterogeneity was low (I² = 0%) (Figure 2C)[5,13,18,20,21,26].
Stroke: Four studies reported stroke. The pooled RR was 0.72 (95%CI: 0.30–1.72, P = 0.46), suggesting no significant difference in stroke risk between DAPT and SAPT patients. Heterogeneity was moderate (I² = 59%) and dropped significantly after removing Scheinert et al[19], 2012 and Belkini et al[26], 2020 (I² = 54%) (Figure 2D)[5,13,21,26].
Major reintervention: Five studies reported major reintervention. The pooled RR was 0.58 (95%CI: 0.35–0.98, P = 0.04), showing a significantly lower risk in DAPT patients compared to SAPT patients. Heterogeneity was high (I² = 92%) and dropped significantly after removing Scheinert et al[19], 2012 and Thott et al[23], 2017 (I² = 60%) (Figure 2E)[5,13,18,22,23].
Bleeding complications: Five studies reported bleeding complications. The pooled RR was 1.12 (95%CI: 1.42–3.03, P = 0.82), indicating no significant difference in risk between DAPT and SAPT patients. Heterogeneity was high (I² = 92%) that dropped significantly after removing Scheinert et al[19], 2012 and Belkini et al[26], 2020 (I² = 57%) (Figure 2F)[5,18,23,24,26].
To evaluate potential publication bias across the clinical outcomes in this meta-analysis, the Egger test was performed for each endpoint. No evidence of publication bias was detected in any of the outcomes, as all P values exceeded the significance threshold of 0.05. Specifically, the P values were as follows: (1) All-cause mortality (P = 0.12); (2) Cardiac mortality (P = 0.18); (3) Myocardial infarction (P = 0.06); (4) Stroke (P = 0.14); (5) Major reintervention (P = 0.11); and (6) Bleeding complications (P = 0.07). These results suggest that the included studies provide a balanced representation of the available evidence without systematic bias favoring publication of positive or significant findings (Supplementary Figure 1).
The risk of bias in the nine cohort studies was evaluated using the ROBINS-I tool, revealing a varied profile across domains. Overall, two studies (Cho et al[5], 2019, and Rodriguez Alvarez et al[9], 2025) were rated as having moderate risk of bias, while the remaining seven (Yamada et al[13], 2024, Armstrong et al[21], 2015, Soden et al[22], 2016, Thott et al[23], 2017, Chinai et al[24], 2020, Ipema et al[25], 2020, Belkini et al[26], 2020) were assessed as having serious risk, primarily driven by concerns in confounding (D1), participant selection (D2), and missing data (D5). No studies were deemed critical or low risk overall, highlighting potential limitations in retrospective designs that could influence the reliability of pooled estimates (Supplementary Figures 2 and 3)[5,9,13,21-26].
For the three RCTs (Belch et al[18], 2010, Scheinert et al[19], 2012, and Strobl et al[20], 2013), the RoB 2.0 tool indicated an overall rating of some concerns for each, with low risk predominant in randomization (D1), deviations from interventions (D2), and selection of reported results (D5). Minor concerns were noted in missing outcome data (D3) for Belch et al[18], 2010, deviations from interventions (D2) for Scheinert et al[19], 2012, and outcome measurement (D4) for Strobl et al[20], 2013, suggesting generally robust methodology but with areas warranting caution in interpretation (Supplementary Figures 4 and 5)[18-20].
The GRADE assessment of the clinical outcomes in this meta-analysis, which included a mix of observational studies and RCTs, revealed varying levels of evidence certainty, primarily influenced by moderate risk of bias, high heterogeneity in several outcomes, and imprecision due to wide confidence intervals in non-significant findings. Certainty was rated as moderate for cardiac mortality, myocardial infarction, and major reintervention, reflecting robust effect sizes with statistical significance and no major indirectness or publication bias (Egger test P values ranging from 0.06 to 0.18 across outcomes). In contrast, all-cause mortality, stroke, and bleeding complications received low certainty ratings, downgraded mainly for serious inconsistency and imprecision, despite the absence of detected publication bias. Overall, these findings underscore the need for cautious interpretation, particularly for outcomes with high heterogeneity, while supporting moderate confidence in the benefits of DAPT for reducing cardiac mortality, myocardial infarction, and major reintervention risks compared to SAPT (Supplementary Table 1).
To explore potential sources of heterogeneity in the pooled estimates for key clinical outcomes, i.e., all-cause mortality (pooled RR: 0.52; 95%CI: 0.27–1.01), meta-regression analyses were conducted using study-level baseline characteristics from the DAPT groups, including mean age, prevalence of current smoking, T2DM, and hypertension. The analyses revealed no significant associations between these covariates and the log RRs. Specifically, mean age showed a coefficient of -0.023 (P = 0.069), diabetes prevalence a coefficient of -0.0004 (P = 0.072), smoking prevalence a coefficient of -0.0008
This systematic review and meta-analysis of 12 studies involving up to 28244 patients with PAD following lower extremity revascularization found that DAPT significantly reduced cardiac mortality (RR: 0.46, 95%CI: 0.27–0.80, 54% risk reduction), myocardial infarction (RR: 0.82, 95%CI: 0.71–0.94, 18% risk reduction), and major reintervention (RR: 0.58, 95%CI: 0.35–0.98, 42% risk reduction) compared to SAPT. However, no significant differences were observed in all-cause mortality (RR: 0.52, 95%CI: 0.27–1.01), stroke (RR: 0.72, 95%CI: 0.30–1.72), or bleeding complications (RR: 1.12, 95%CI: 1.42–3.03), though high heterogeneity (I² = 59%–97%) limits confidence in these estimates. GRADE assessments indicated moderate certainty for cardiac mortality, myocardial infarction, and major reintervention, but low certainty for all-cause mortality, stroke, and bleeding. This comprehensive analysis of 12 studies encompassing up to 28244 patients represents the most extensive evaluation to date of antiplatelet strategies in this high-risk population. The meta-analysis shows that DAPT lowers the risk of dying from heart problems by 54% and the risk of heart attacks by 18% compared to SAPT, and it also leads to a 42% lower chance of needing major follow-up procedures, improving heart and leg health in patients with PAD, who are at two to three times higher risk for heart issues than the general population[27,28]. These benefits are supported by studies showing that DAPT greatly improves the ability to stop platelets from clumping together, which is important for patients at high risk of blood clots, especially right after they have had procedures to restore blood flow. These findings emerge amid a rapidly evolving landscape in PAD management, with the 2024 ACC/AHA guidelines marking a paradigm shift by recommending DAPT for 1–6 months post-endovascular revascularization (class IIa)[29], an upgrade from prior class IIb status, driven by accumulating evidence for short-term DAPT. The present meta-analysis bolsters this by showing DAPT reduces cardiac mortality and myocardial infarction without significantly elevating bleeding risk[30]. The guidelines also highlight the importance of tailored treatments, especially using DPI with low-dose rivaroxaban and aspirin, as shown in the COMPASS and VOYAGER PAD trials, which reduced major heart problems by 24% and serious leg issues by 46% compared to aspirin alone[12,31]. However, this meta-analysis looks specifically at traditional DAPT (aspirin plus P2Y12 inhibitor) compared to SAPT, providing additional information to help with clinical decisions. The results of this meta-analysis on DAPT for PAD should be considered along with earlier important studies. The CASPAR trial, the largest study on DAPT in surgical PAD patients, showed no benefit from clopidogrel plus aspirin over aspirin alone, with increased bleeding risk. However, a post-hoc analysis of the VOYAGER PAD trial using a "CASPAR-like" endpoint found rivaroxaban plus aspirin reduced composite outcomes by 24% at one year and 16% at three years, indicating that antiplatelet combinations and patient populations may affect results[32]. This meta-analysis is different from CASPAR because it includes both endovascular and surgical patients (with a predominance of endovascular procedures across studies), uses various DAPT combinations and lengths of treatment, and reflects current practices with better techniques and care during and after surgery. Although subgroup analyses stratified by revascularization type (endovascular vs surgical) were not performed, sensitivity analyses conducted to explore heterogeneity did not identify revascularization type as a clear source, but the predominance of endovascular procedures suggests potential differences in outcomes between these groups; this highlights the need for future studies to separate results by revascularization type to uncover any important distinctions and improve contextualization. A critical concern with DAPT is increased bleeding risk, yet this analysis showed no significant rise in bleeding complications compared to SAPT, contrasting some prior studies. However, this conclusion must be interpreted cautiously given the high heterogeneity (I² = 92%) in bleeding outcomes, which may arise from substantial variations in real-world bleeding definitions, reporting practices, and patient selection across studies. This is noteworthy, as bleeding in PAD patients heightens subsequent thrombotic risk and worsens outcomes[33]. The favorable bleeding profile may stem from shorter DAPT durations, better patient selection, and procedural advancements. However, high heterogeneity in bleeding outcomes indicates variability from differences in populations, techniques, and definitions, which reinforces the importance of individualized risk assessment. DAPT's efficacy in PAD likely arises from targeting multiple pathways in atherothrombosis, with aspirin inhibiting cyclooxygenase-1 and P2Y12 inhibitors blocking ADP receptors to synergistically enhance antithrombotic effects, particularly post-revascularization[34]. Overall, while the evidence supports DAPT's benefits in reducing cardiac mortality and myocardial infarction with moderate certainty per GRADE assessments, clinicians should note the low certainty for outcomes such as all-cause mortality, stroke, and bleeding, which limits definitive conclusions in these areas. Limitations We must acknowledge several limitations when interpreting these findings on DAPT in PAD. High heterogeneity across outcomes (I² = 59%-97%) indicates substantial variability in study populations, interventions, and definitions, reducing the precision of pooled estimates and suggesting that DAPT effects may differ by patient subgroups and contexts. Meta-regression showed no significant links between baseline characteristics and outcomes, implying heterogeneity stems from unmeasured factors like procedural techniques, regimens, or follow-up duration, such as variations in procedural anticoagulation protocols, patient adherence, or comorbidity burden, underscoring the need for standardized research approaches. GRADE assessments yielded moderate certainty for cardiac mortality, myocardial infarction, and major reintervention, but low certainty for all-cause mortality, stroke, and bleeding, due to challenges in high-quality PAD trials and reliance on observational studies. Additionally, the inclusion criteria required a minimum DAPT duration of one month, yet many studies employed longer durations, which may limit generalizability to shorter regimens now recommended by guidelines (e.g., 1–6 months post-endovascular procedures); this could overestimate long-term benefits or risks in real-world practice where shorter courses are preferred to balance efficacy and bleeding concerns.
The meta-analysis findings advocate a nuanced, individualized approach to antiplatelet therapy in PAD patients. DAPT significantly reduces cardiac mortality and myocardial infarction, but its use should be tailored to patient-specific factors such as bleeding risk, cardiovascular profile, and procedural considerations. Emerging personalized strategies hold promise[34], including risk stratification tools integrating clinical, procedural, and genetic data to optimize benefits while minimizing bleeding. Platelet function testing and genetic polymorphism analysis could further refine patient selection, though validation in PAD populations is essential. The 2024 ACC/AHA guidelines recommend DAPT for 1–6 months after endovascular revascularization for PAD, individualized by risk: (1) Extended for high-risk patients (diabetes, chronic kidney disease, prior events); and (2) Shortened for bleeding-prone patients. You can use DAPT along with managing risk factors, exercise therapy, and a combination of rivaroxaban (2.5 mg twice daily) and aspirin, which helps reduce serious heart and limb problems, as another option. Large RCTs explore the best strategies based on quality-of-life and economic endpoints, develop PAD-specific risk models that incorporate clinical, biomarker, and genetic data, and compare DAPT and dual pathway approaches based on subgroups such as anatomy, symptoms, and comorbidities[8]. Additionally, future research should prioritize the harmonization of bleeding outcome definitions across PAD trials to improve the comparability of safety data, addressing the high variability in bleeding reporting that currently limits robust conclusions on DAPT’s safety profile.
This comprehensive meta-analysis suggests that DAPT may reduce cardiac mortality, myocardial infarction, and major reintervention risks compared to SAPT in PAD patients following lower extremity revascularization, with no apparent increase in bleeding complications. However, these findings are tempered by high heterogeneity (I² = 59%–97%) and variable evidence certainty, with moderate certainty for cardiac mortality, myocardial infarction, and major reintervention, but low certainty for all-cause mortality, stroke, and bleeding outcomes due to inconsistency and imprecision. These results cautiously support short-term DAPT use, aligning with the 2024 ACC/AHA guidelines for 1–6 months post-endovascular revascularization, but highlight the need for individualized treatment approaches given the limitations in evidence robustness. The findings should be interpreted within the context of evolving PAD management, which emphasizes personalized care and integration of multiple evidence-based therapies. As new antiplatelet agents and combination strategies emerge, continued research is essential to refine optimal DAPT durations, standardize bleeding definitions, and explore subgroup-specific outcomes (e.g., endovascular vs surgical revascularization) to enhance evidence certainty. Principles of individualized risk assessment and shared decision-making remain critical in optimizing outcomes for this high-risk patient population.
| 1. | Aday AW, Matsushita K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ Res. 2021;128:1818-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 411] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 2. | Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 1238] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 3. | Dhaliwal G, Mukherjee D. Peripheral arterial disease: Epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Tsai SY, Li YS, Lee CH, Cha SW, Wang YC, Su TW, Yu SY, Yeh CH. Mono or Dual Antiplatelet Therapy for Treating Patients with Peripheral Artery Disease after Lower Extremity Revascularization: A Systematic Review and Meta-Analysis. Pharmaceuticals (Basel). 2022;15:596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Cho S, Lee YJ, Ko YG, Kang TS, Lim SH, Hong SJ, Ahn CM, Kim JS, Kim BK, Choi D, Hong MK, Jang Y. Optimal Strategy for Antiplatelet Therapy After Endovascular Revascularization for Lower Extremity Peripheral Artery Disease. JACC Cardiovasc Interv. 2019;12:2359-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Heikkila K, Mitchell DC, Loftus IM, Johal AS, Waton S, Cromwell DA. Improving 1-Year Outcomes of Infrainguinal Limb Revascularization: Population-Based Cohort Study of 104 000 Patients in England. Circulation. 2018;137:1921-1933. [PubMed] [DOI] [Full Text] |
| 7. | Li Q, Birmpili P, Atkins E, Johal AS, Waton S, Williams R, Boyle JR, Harkin DW, Pherwani AD, Cromwell DA. Illness Trajectories After Revascularization in Patients With Peripheral Artery Disease: A Unified Approach to Understanding the Risk of Major Amputation and Death. Circulation. 2024;150:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Gornik HL, Aronow HD, Goodney PP, Arya S, Brewster LP, Byrd L, Chandra V, Drachman DE, Eaves JM, Ehrman JK, Evans JN, Getchius TSD, Gutiérrez JA, Hawkins BM, Hess CN, Ho KJ, Jones WS, Kim ESH, Kinlay S, Kirksey L, Kohlman-Trigoboff D, Long CA, Pollak AW, Sabri SS, Sadwin LB, Secemsky EA, Serhal M, Shishehbor MH, Treat-Jacobson D, Wilkins LR; Peer Review Committee Members. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the Management of Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1313-e1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 293] [Article Influence: 146.5] [Reference Citation Analysis (0)] |
| 9. | Rodriguez Alvarez AA, Patel SS, Cieri IF, Ghandour S, Boya M, Suarez SP, Agrawal A, Lee I, Owolabi L, Manchella M, Dua A. Single versus dual antiplatelet therapy impact on coagulation/thrombosis post PAD revascularization. Sci Prog. 2025;108:368504251324332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Coppens M, Weitz JI, Eikelboom JWA. Synergy of Dual Pathway Inhibition in Chronic Cardiovascular Disease. Circ Res. 2019;124:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Hiatt WR, Bonaca MP, Patel MR, Nehler MR, Debus ES, Anand SS, Capell WH, Brackin T, Jaeger N, Hess CN, Pap AF, Berkowitz SD, Muehlhofer E, Haskell L, Brasil D, Madaric J, Sillesen H, Szalay D, Bauersachs R. Rivaroxaban and Aspirin in Peripheral Artery Disease Lower Extremity Revascularization: Impact of Concomitant Clopidogrel on Efficacy and Safety. Circulation. 2020;142:2219-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Yuan Z, Levitan B, Deng H, Szarek M, Bauersachs RM, Berkowitz SD, Haskell L, Barnathan ES, Bonaca MP. Quantitative Benefit-Risk Evaluation of Rivaroxaban in Patients After Peripheral Arterial Revascularization: The VOYAGER PAD Trial. J Am Heart Assoc. 2024;13:e032782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Yamada T, Tokuda T, Yoshioka N, Koyama A, Nishikawa R, Shimamura K, Aoyama T. Comparison of Single Antiplatelet Therapy and Dual Antiplatelet Therapy after Endovascular Therapy in Patients with Lower Extremity Artery Disease. Ann Vasc Dis. 2024;17:396-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48614] [Article Influence: 2859.6] [Reference Citation Analysis (3)] |
| 15. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Chichester: John Wiley and Sons, 2024. |
| 16. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18736] [Article Influence: 2676.6] [Reference Citation Analysis (0)] |
| 17. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12539] [Article Influence: 1253.9] [Reference Citation Analysis (2)] |
| 18. | Belch JJ, Dormandy J; CASPAR Writing Committee, Biasi GM, Cairols M, Diehm C, Eikelboom B, Golledge J, Jawien A, Lepäntalo M, Norgren L, Hiatt WR, Becquemin JP, Bergqvist D, Clement D, Baumgartner I, Minar E, Stonebridge P, Vermassen F, Matyas L, Leizorovicz A. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52:825-833, 833.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, Krankenberg H, Baumgartner I, Siablis D, Lammer J, Van Ransbeeck M, Qureshi AC, Stoll HP; ACHILLES Investigators. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Strobl FF, Brechtel K, Schmehl J, Zeller T, Reiser MF, Claussen CD, Tepe G. Twelve-month results of a randomized trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery disease. J Endovasc Ther. 2013;20:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Armstrong EJ, Anderson DR, Yeo KK, Singh GD, Bang H, Amsterdam EA, Freischlag JA, Laird JR. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. J Vasc Surg. 2015;62:157-165.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Soden PA, Zettervall SL, Ultee KH, Landon BE, O'Malley AJ, Goodney PP, DeMartino RR, Arya S, Schermerhorn ML; Society for Vascular Surgery Vascular Quality Initiative. Dual antiplatelet therapy is associated with prolonged survival after lower extremity revascularization. J Vasc Surg. 2016;64:1633-1644.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Thott O, Granath F, Malmstedt J, Wahlgren CM. Editor's Choice - Dual Antiplatelet Therapy Improves Outcome in Diabetic Patients Undergoing Endovascular Femoropopliteal Stenting for Critical Limb Ischaemia. Eur J Vasc Endovasc Surg. 2017;53:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Chinai N, Ambler GK, Wardle BG, Locker D, Bosanquet D, Goyal N, Chick C, Hinchliffe RJ, Twine CP. Single versus dual antiplatelet therapy following peripheral arterial endovascular intervention for chronic limb threatening ischaemia: Retrospective cohort study. PLoS One. 2020;15:e0234271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Ipema J, Welling RHA, Bakker OJ, Bokkers RPH, de Vries JPM, Ünlü Ç. Short-Term Clinical Outcomes of Single Versus Dual Antiplatelet Therapy after Infrainguinal Endovascular Treatment for Peripheral Arterial Disease. J Clin Med. 2020;9:3515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Belkin N, Stoecker JB, Jackson BM, Damrauer SM, Glaser J, Kalapatapu V, Golden MA, Wang GJ. Effects of dual antiplatelet therapy on graft patency after lower extremity bypass. J Vasc Surg. 2021;73:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Franzone A, Piccolo R, Gargiulo G, Ariotti S, Marino M, Santucci A, Baldo A, Magnani G, Moschovitis A, Windecker S, Valgimigli M. Prolonged vs Short Duration of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With or Without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomized Clinical Trial. JAMA Cardiol. 2016;1:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Lee I, Suarez S, Hall R, Majumdar M, Bellomo T, Jessula S, Nuzzolo K, Jefferson DM, Zacharias N, Dua A. Optimizing platelet inhibition in peripheral artery disease: A comparison of mono-antiplatelet therapy and dual-antiplatelet therapy using thromboelastography. Vascular. 2025;33:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Bates KJ, Moore MM, Cibotti-Sun M. 2024 Lower Extremity Peripheral Artery Disease Guideline-at-a-Glance. J Am Coll Cardiol. 2024;83:2605-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Kaplovitch E, Eikelboom JW, Dyal L, Aboyans V, Abola MT, Verhamme P, Avezum A, Fox KAA, Berkowitz SD, Bangdiwala SI, Yusuf S, Anand SS. Rivaroxaban and Aspirin in Patients With Symptomatic Lower Extremity Peripheral Artery Disease: A Subanalysis of the COMPASS Randomized Clinical Trial. JAMA Cardiol. 2021;6:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Visonà A, Zurlo C, Panzavolta C, Gobbo A, Zalunardo B. Bleeding Risk in Patients with Peripheral Arterial Disease. Life (Basel). 2022;13:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Bonaca MP, Szarek M, Debus ES, Nehler MR, Patel MR, Anand SS, Muehlhofer E, Berkowitz SD, Haskell LP, Bauersachs RM. Efficacy and safety of rivaroxaban versus placebo after lower extremity bypass surgery: A post hoc analysis of a "CASPAR like" outcome from VOYAGER PAD. Clin Cardiol. 2022;45:1143-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Capodanno D, Angiolillo DJ. Personalised antiplatelet therapies for coronary artery disease: what the future holds. Eur Heart J. 2023;44:3059-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 34. | Kaplovitch E, Rannelli L, Anand SS. Antithrombotics in stable peripheral artery disease. Vasc Med. 2019;24:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/