Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.111870
Revised: August 6, 2025
Accepted: September 17, 2025
Published online: October 26, 2025
Processing time: 105 Days and 16.5 Hours
Non-ST-elevation myocardial infarction (NSTEMI) is a prevalent acute coronary syndrome among the elderly, a population often underrepresented in clinical trials. Frailty, a marker of physiologic vulnerability, may influence the risks and benefits of percutaneous coronary intervention (PCI) in these patients.
To evaluate the impact of frailty status on in-hospital outcomes among patients aged ≥ 75 years with NSTEMI undergoing PCI.
We conducted a retrospective cohort study using the 2021-2022 National Inpatient Sample to evaluate the impact of frailty on in-hospital outcomes among NSTEMI patients aged ≥ 75 years undergoing PCI. Patients were stratified into three frailty categories using the Hospital Frailty Risk Score. Multivariable logistic and generalized linear models with interaction terms assessed the association between frailty and clinical outcomes.
Among 456690 NSTEMI admissions, 37.95%, 50.71%, and 11.34% were categorized as low, intermediate, and high frailty, respectively. PCI use declined with increasing frailty (35.0% in low vs 7.5% in high; P < 0.001). Adjusted mortality was lower with PCI across all frailty levels [odds ratios (OR): 0.27 (low), 0.37 (intermediate), 0.43 (high); all P < 0.001]. However, the mortality benefit was attenuated with increasing frailty (interaction OR: 1.56 and 1.83 for intermediate and high vs low frailty; P < 0.001). Frailty was independently associated with higher odds of complications, including acute kidney injury, respiratory failure, delirium, and bleeding. PCI was associated with shorter hospital stays in low (-0.90 days) but longer in the high-frail category (+2.47 days; P < 0.001), and increasing frailty correlated with significantly higher hospital charges.
In elderly NSTEMI patients, PCI conferred a survival benefit across all frailty strata, although with a diminishing magnitude as frailty increased. Frailty correlated with increased complications and healthcare resource utilization.
Core Tip: This study evaluates how frailty affects the outcomes of percutaneous coronary intervention (PCI) in patients aged ≥ 75 years with non-ST-elevation myocardial infarction. Using the Hospital Frailty Risk Score, we found that PCI is associated with a reduction in in-hospital mortality across all frailty categories. However, the benefits of PCI decrease as frailty increases, while complications and healthcare utilization significantly rise. These findings emphasize the need for individualized treatment strategies. While frailty should not prevent PCI, it should be considered in shared decision-making to balance the survival benefits against the procedural risks and resource demands in this high-risk population.
- Citation: Popat A, Vempati R, Kodali LSM, Sai Santhosha Mrudula A, Haddad F, Jain A, Krishnamoorthy G, Sharma P. Frailty status and outcomes of percutaneous coronary intervention in elderly patients with non-ST-elevation myocardial infarction. World J Cardiol 2025; 17(10): 111870
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/111870.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.111870
One of the leading causes of death globally has been ischemic heart disease (IHD)[1]. As people age, the death rate rises even more. However, there are limited guidelines on managing the elderly due to the underrepresentation of this population. In the United States alone, IHD resulted in 223067 deaths among people aged 75 and over[2].
Non-ST-elevation myocardial infarction (NSTEMI) is a more common Acute Coronary Syndrome in elderly patients[3]. The National Institute for Health and Care Excellence guideline and the European Society of Cardiology recommend that NSTEMI patients receive coronary angiography followed by percutaneous coronary intervention (PCI), regardless of age and characteristics like frailty, cognitive decline, functional decline, and comorbidities[4,5]. Similarly, the 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes endorses consideration of multivessel PCI in appropriately selected elderly patients with NSTEMI, informed by recent evidence from FIRE (complete vs culprit-only), SMILE (single-staged vs multi-staged), and BIOVASC (immediate vs staged) trials showing improved outcomes with complete revascularization, even when performed in a single setting[6-9].
Frailty, which is characterised by a progressive decline in physiological function across multiple organ systems, leads to heightened vulnerability to stressors and is associated with an elevated risk of adverse outcomes such as functional deterioration, institutionalization, and mortality[10]. Frailty highly influences the management of NSTEMI since it is associated with high mortality, revascularization, and bleeding risks compared to non-frail patients[11]. It is a common geriatric condition characterized by decreased physiologic reserve and increased susceptibility to stress, which can lead to adverse health outcomes[11]. Frailty has also been found to be an independent factor influencing the length of hospital stay in NSTEMI patients undergoing PCI. Frail patients had an increased duration of hospital stay when compared to non-frail patients[12]. Studies have also reported an increased mortality, including cardiovascular and non-cardio
Frailty, present in nearly 1 in 4 patients ≥ 65 years undergoing PCI, is associated with diminished physiological reserve and worsened tolerance to procedural stressors. Yet, many clinical risk models omit frailty despite its known prognostic significance[14]. Despite increased complications in the elderly and frail, there are limited guidelines on managing NSTEMI in this population. This retrospective study using the Nationwide Inpatient Sample (NIS) database aims to analyse the influence of frailty on various clinical outcomes in NSTEMI patients following PCI. By stratifying patients based on frailty status, the study enables clinicians to determine the appropriateness of PCI across different frailty categories and helps identify specific complications within each group.
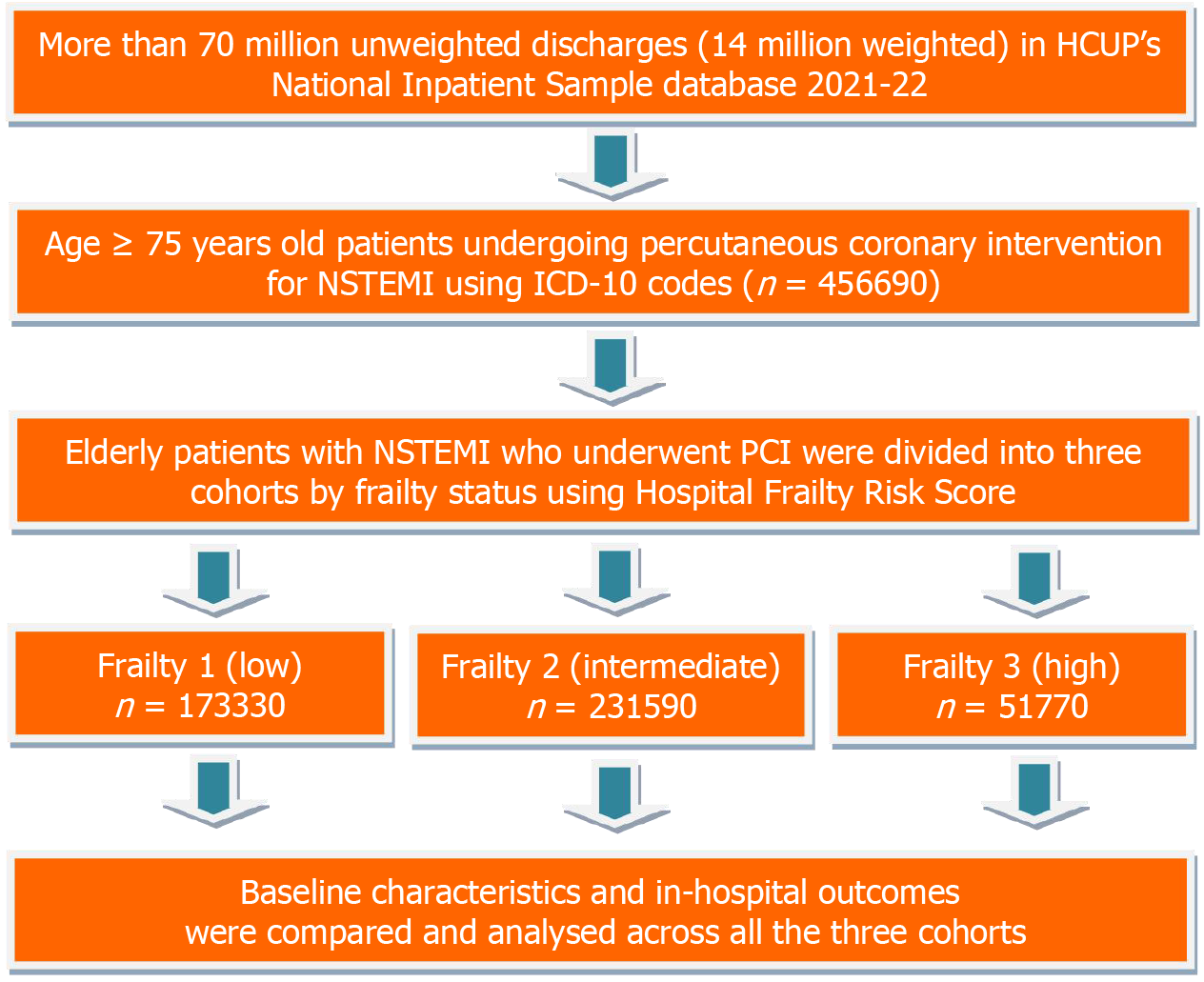
This study utilized the 2021 and 2022 NIS database from the Healthcare Cost and Utilization Project, which were the most recent databases available at the time of the study's initiation. It is the most extensive United States all-payer inpatient healthcare dataset that is available to the public. The data includes the discharge information of 20% of hospitals from over 47 states in the United States. On average, there are 7 million unweighted discharges each year, which amounts to more than 35 million weighted discharges nationwide. A primary diagnosis and up to 39 secondary discharge diagnoses are present in every NIS inpatient admission. As the NIS has de-identified national data, our Institutional Review Board exempted the study from a formal review and approval requirement. For more information about the database, please visit the Healthcare Cost and Utilization Project website[15] (Figure 1).
We identified hospitalizations of adult patients aged ≥ 75 years with a primary diagnosis of NSTEMI using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes (Supplementary Table 1). Frailty was assessed using the Hospital Frailty Risk Score (HFRS), a validated claims-based tool developed by Gilbert et al[16] and Teo et al[17], which uses weighted ICD-10-CM diagnosis codes (Supplementary Table 2) to identify patients with characteristics consistent with frailty. Each patient’s score was calculated based on the presence of these codes in the discharge record. We then categorized patients into three frailty groups: Low frailty (HFRS < 5), intermediate frailty (HFRS 5-15), and high frailty (HFRS > 15), as suggested in the original validation study. This stratification allowed us to examine how increasing levels of frailty influenced clinical outcomes and the effectiveness of PCI in elderly NSTEMI patients. The final analytical sample was generated after applying discharge weights to produce national estimates (Figure 1).
Patient-level demographic variables, including age, sex, race/ethnicity, and median household income quartile by ZIP code, were obtained directly from the NIS core dataset. Hospital-level characteristics-such as geographic region, teaching status, bed size, and urban-rural classification-were also derived from the NIS and are defined according to HCUP data element specifications. Comorbidity burden was assessed using the Charlson Comorbidity Index (CCI), originally developed by Charlson et al[18] to predict mortality risk based on weighted comorbid conditions. In this study, the CCI was derived from ICD-10-CM diagnosis codes available in the NIS 2021–2022 dataset[15]. The index was calculated using a validated STATA module based on the enhanced ICD-10 adaptation by Quan et al[19], which maps 17 comorbidities with assigned weights. This approach was selected to align with the ICD-10-CM coding structure of the NIS (Supplementary Table 3).
The primary outcome, in-hospital mortality, was identified using the HCUP variable DIED, which indicates whether the patient died during the hospitalization. Secondary outcomes-including acute kidney injury (AKI), AKI requiring dialysis, acute respiratory failure (ARF), ARF requiring mechanical ventilation, pulmonary edema, stroke, transient ischemic attack (TIA), cardiogenic shock, mechanical circulatory support (MCS), cardiac arrest, hypotension, pulmonary embolism, delirium, bleeding complications, and major bleeding requiring transfusion-were identified using ICD-10-CM diagnosis and procedure codes (Supplementary Table 1). These codes were applied to the diagnosis and procedure fields in the NIS dataset, following HCUP guidelines for coding and classification. Continuous outcomes included hospital length of stay (LOS), measured in days, and total hospital charges (TOTCHG), reported in United States dollars. Both variables are standard data elements in the NIS core file and were used as provided, without additional transformation.
All analyses incorporated the complex survey design of the NIS using the svyset command in STATA/MP 17.0 (StataCorp LLC, College Station, TX), which accounted for strata, primary sampling units (PSUs), and discharge-level weights. Descriptive statistics were used to summarize patient- and hospital-level variables. Group comparisons for categorical variables were conducted using the Pearson χ2 test. Continuous variables were assessed using the independent-sample t-test or the Mann-Whitney U test based on distributional assumptions. Multivariable logistic regression models were developed to estimate adjusted odds ratios (OR) and 95% confidence intervals (CI) for binary outcomes. Generalized linear models were used to evaluate associations with continuous outcomes, applying log or identity link functions as appropriate. All models were adjusted for relevant confounders and included interaction terms to assess whether the association between PCI and outcomes varied by frailty category. Statistical significance was defined as a two-sided P value of less than 0.05.
A total of 456690 patients aged ≥ 75 years admitted with NSTEMI were included in the study. Among them, 37.95%
| Variable | Low frailty | Intermediate frailty | High frailty | P value |
| Frailty category (distribution) | 173330 (37.95) | 231590 (50.71) | 51770 (11.34) | |
| Female | 78657 (45.38) | 111047 (47.95) | 26439 (51.07) | < 0.001 |
| Male | 94673 (54.62) | 120543 (52.05) | 25331 (48.93) | < 0.001 |
| Race | < 0.001 | |||
| White | 138716 (80.03) | 177282 (76.55) | 37430 (72.30) | |
| Black | 12393 (7.15) | 20334 (8.78) | 5394 (10.42) | |
| Hispanic | 12532 (7.23) | 19129 (8.26) | 5042 (9.74) | |
| Asian/Pacific islander | 4998 (2.88) | 8522 (3.68) | 2362 (4.56) | |
| Native American | 797 (0.46) | 810 (0.35) | 129 (0.25) | |
| Race: Other | 3911 (2.26) | 5513 (2.38) | 1413 (2.74) | |
| Income quartile | < 0.001 | |||
| USD 1-USD 47999 (Q1) | 46698 (26.96) | 65692 (28.36) | 14653 (28.30) | |
| USD 48000-USD 60999 (Q2) | 47884 (27.63) | 61937 (26.74) | 13093 (25.30) | |
| USD 61000-81999 (Q3) | 42807 (24.69) | 55572 (24.01) | 12408 (23.97) | |
| ≥ USD 82000 (Q4) | 35941 (20.73) | 48090 (20.89) | 11616 (22.43) | |
| Hospital region | < 0.001 | |||
| Northeast | 32565 (18.78) | 40319 (17.42) | 8196 (15.82) | |
| Midwest | 37023 (21.37) | 51032 (22.03) | 12217 (23.59) | |
| South | 69335 (40.03) | 92377 (39.87) | 20973 (40.50) | |
| West | 34399 (19.82) | 47862 (20.69) | 10384 (20.09) | |
| Hospital location | < 0.001 | |||
| Rural | 17350 (10.01) | 22559 (9.74) | 4297 (8.30) | |
| Urban non-teaching | 33846 (19.54) | 45161 (19.49) | 9847 (19.02) | |
| Urban teaching | 122134 (70.45) | 163870 (70.76) | 37626 (72.69) | |
| Hospital bed size | 0.4382 | |||
| Small | 41278 (23.82) | 54223 (23.41) | 12309 (23.77) | |
| Medium | 53416 (30.82) | 71741 (30.98) | 16472 (31.83) | |
| Large | 78636 (45.35) | 105626 (45.61) | 22989 (44.40) | |
| Charlson comorbidity index | < 0.001 | |||
| Index: 1 | 29286 (16.90) | 10362 (4.47) | 1403 (2.71) | |
| Index: 2 | 41525 (23.97) | 29847 (12.89) | 4859 (9.39) | |
| Index: 3 | 102519 (59.12) | 191381 (82.64) | 45497 (87.90) | |
| Comorbidity | ||||
| Hypertension | 154965 (89.40) | 208460 (90.01) | 44895 (86.72) | < 0.001 |
| Dyslipidemia | 129420 (74.70) | 153680 (66.36) | 29790 (57.54) | < 0.001 |
| Diabetes | 68680 (39.60) | 106650 (46.05) | 22445 (43.36) | < 0.001 |
| Atrial fibrillation | 54655 (31.50) | 91210 (39.38) | 22065 (42.62) | < 0.001 |
| Atrial flutter | 6360 (3.67) | 10130 (4.37) | 2210 (4.27) | < 0.001 |
| Chronic kidney disease | 45745 (26.39) | 116275 (50.21) | 27225 (52.59) | < 0.001 |
| HFrEF | 32635 (18.83) | 59320 (25.61) | 11965 (23.11) | < 0.001 |
| HFpEF | 23630 (13.63) | 46060 (19.89) | 9905 (19.13) | < 0.001 |
| Obesity | 22765 (13.13) | 29365 (12.68) | 5260 (10.16) | < 0.001 |
| Smoking | 58485 (33.74) | 80570 (34.79) | 13520 (26.12) | < 0.001 |
| Cannabis use | 350 (0.20) | 480 (0.21) | 70 (0.14) | < 0.001 |
| Peripheral vascular disease | 16150 (9.32) | 25230 (10.89) | 4675 (9.03) | < 0.001 |
| Obstructive sleep apnea | 15325 (8.84) | 20245 (8.74) | 3115 (6.02) | < 0.001 |
| Cerebral infarct | 980 (0.57) | 8325 (3.59) | 6735 (13.01) | < 0.001 |
| Hemorrhagic stroke | 370 (0.21) | 1770 (0.76) | 1235 (2.39) | < 0.001 |
| COPD | 29870 (17.23) | 57500 (24.83) | 11700 (22.60) | < 0.001 |
| Depression | 11630 (6.71) | 24685 (10.66) | 6150 (11.88) | < 0.001 |
| Outcomes | ||||
| PCI | 60682 (35.03) | 38926 (16.79) | 3894 (7.51) | < 0.001 |
| AKI | 22178 (12.81) | 116223 (50.17) | 37091 (71.66) | < 0.001 |
| AKI requiring dialysis | 173 (0.10) | 5740 (2.44) | 2855 (5.40) | < 0.001 |
| ARF | 19737 (11.37) | 100938 (43.54) | 29270 (56.54) | < 0.001 |
| ARF requiring mechanical ventilation | 1851 (1.03) | 22222 (9.59) | 8107 (19.49) | < 0.001 |
| Pulmonary edema | 1858 (1.02) | 4913 (2.12) | 1219 (2.26) | < 0.001 |
| Transient ischemic attack | 584 (0.34) | 1542 (0.66) | 420 (0.79) | < 0.001 |
| Stroke | 983 (0.57) | 8521 (3.59) | 6774 (13.01) | < 0.001 |
| Cardiogenic shock | 4492 (2.58) | 22898 (9.88) | 6578 (12.79) | < 0.001 |
| Cardiogenic shock requiring MCS | 1085 (0.63) | 3995 (1.73) | 675 (1.3) | < 0.001 |
| Cardiac arrest | 2729 (1.57) | 13061 (5.73) | 4083 (7.86) | < 0.001 |
| Hypotension | 6852 (3.91) | 27516 (11.95) | 8725 (16.81) | < 0.001 |
| Pulmonary embolism | 2064 (1.19) | 5402 (2.31) | 1434 (2.73) | < 0.001 |
| Delirium | 293 (0.17) | 6670 (2.93) | 4799 (9.24) | < 0.001 |
| Bleed complication | 11992 (6.90) | 32802 (14.17) | 10421 (20.13) | < 0.001 |
| Major bleeding | 2826 (1.65) | 9548 (4.09) | 3259 (6.26) | < 0.001 |
The proportion of female patients increased across frailty categories, with 45.38% (n = 78657) in low frailty, 47.95%
White patients represented the majority in each group but decreased with higher frailty: 80.03% (n = 138716) in low, 76.55% (n = 177282) in intermediate, and 72.30% (n = 37430) in the high frailty category (P < 0.001). The proportions of Black (7.15%, 8.78%, and 10.42%), Hispanic (7.23%, 8.26%, and 9.74%), and Asian/Pacific Islander (2.88%, 3.68%, and 4.56%) patients increased with frailty (P < 0.001 for each). Native American (0.46%, 0.35%, and 0.25%) and other race (2.26%, 2.38%, and 2.74%) categories also differed significantly by frailty (P < 0.001).
Regarding income quartiles, patients in the lowest quartile (Q1) comprised 26.96% (n = 46698) of low, 28.36%
Patients in low, intermediate, and high frailty category were most frequently admitted in the South (40.03%, 39.87%, and 40.50%, respectively), followed by the West (19.82%, 20.69%, and 20.09%), Midwest (21.37%, 22.03%, and 23.59%), and Northeast (18.78%, 17.42%, and 15.82%) regions (P < 0.001).
Hospital location varied significantly across frailty categories: 10.01%, 9.74%, and 8.30% of patients were admitted to rural hospitals; 19.54%, 19.49%, and 19.02% to urban non-teaching hospitals; and 70.45%, 70.76%, and 72.69% to urban teaching hospitals (P = 0.0008).
Hospital bed size was similar across frailty groups: Small hospitals accounted for 23.82%, 23.41%, and 23.77% of admissions; medium-sized hospitals for 30.82%, 30.98%, and 31.83%; and large hospitals for 45.35%, 45.61%, and 44.40% of patients in low, intermediate, and high frailty category, respectively (P = 0.4382) (Table 1).
Among patients stratified by frailty, the prevalence of hypertension was 89.40% (n = 154965) in low, 90.01% (n = 208460) in intermediate, and 86.72% (n = 44895) in high frailty category (P < 0.001). Dyslipidemia was observed in 74.70%
A CCI of 3 or greater was observed in 59.12% (n = 102519) of low, 82.64% (n = 191381) of intermediate, and 87.90%
The proportion of patients undergoing PCI decreased with increasing frailty: 35.03% (n = 60682) in low, 16.79%
| Outcome | Low frailty (OR, 95%CI, P value) | Intermediate frailty (OR, 95%CI, P value) | High frailty (OR, 95%CI, P value) | Interaction | |
| Intermediate vs low frailty (OR, 95%CI, | High vs low frailty (OR, 95%CI, | ||||
| Mortality | 0.27 (0.22-0.32), | 0.37 (0.33-0.40), < 0.001 | 0.43 (0.34-0.54), | 1.56 (1.27-1.93), < 0.001 | 1.83 (1.37-2.44), |
| Acute kidney injury | 0.79 (0.75-0.82), | 0.83 (0.78-0.87), < 0.001 | 1.09 (0.91-1.30), 0.35 | 1.06 (0.97-1.15), 0.21 | 1.36 (1.12-1.64), 0.001 |
| Acute kidney injury requiring dialysis | 0.92 (0.78-1.09), 0.35 | 0.84 (0.38-1.88), 0.67 | 1.83 (1.42-2.36), | 0.91 (0.40-2.08), 0.82 | 1.99 (1.46-2.72), |
| Acute respiratory failure | 0.45 (0.42-0.49), | 0.69 (0.66-0.73), < 0.001 | 0.89 (0.76-1.03), 0.12 | 1.65 (1.50-1.82), < 0.001 | 2.13 (1.79-2.52), |
| Acute respiratory failure with mechanical ventilation | 0.35 (0.27-0.47), | 0.63 (0.57-0.69), < 0.001 | 1.00 (0.83-1.22), 0.99 | 1.78 (1.33-2.39), < 0.001 | 2.79 (1.98-3.93), |
| Pulmonary edema | 0.64 (0.50-0.82), | 1.13 (0.96-1.33), 0.13 | 1.73 (1.16-2.56), 0.007 | 1.98 (1.48-2.65), < 0.001 | 3.00 (1.89-4.76), |
| Transient ischemic attack | 0.70 (0.45-1.08), 0.11 | 1.04 (0.76-1.43), 0.79 | 2.45 (1.29-4.69), 0.006 | 1.55 (0.90-2.66), 0.11 | 3.04 (1.42-6.51), 0.004 |
| Stroke | 0.38 (0.26-0.57), | 0.57 (0.48-0.67), < 0.001 | 0.86 (0.68-1.08), 0.20 | 1.60 (1.04-2.44), 0.03 | 2.48 (1.57-3.92), |
| Cardiogenic shock | 0.53 (0.45-0.62), | 1.06 (0.98-1.16), 0.15 | 1.79 (1.48-2.17), | 2.03 (1.70-2.43), < 0.001 | 3.35 (2.61-4.30), |
| Shock with mechanical circulatory support | 1.06 (0.79-1.44), 0.66 | 4.52 (3.86-5.29), < 0.001 | 12.09 (8.23-17.76), | 4.37 (3.17-6.03), < 0.001 | 11.99 (7.50-19.16), |
| Cardiac arrest | 0.54 (0.44-0.67), | 0.78 (0.69-0.87), < 0.001 | 1.24 (0.96-1.61), 0.09 | 1.46 (1.15-1.85), 0.002 | 2.28 (1.64-3.18), |
| Hypotension | 0.83 (0.73-0.95), 0.006 | 1.18 (1.09-1.27), < 0.001 | 1.31 (1.09-1.58), 0.005 | 1.41 (1.22-1.62), < 0.001 | 1.54 (1.24-1.92), |
| Pulmonary embolism | 0.29 (0.22-0.39), | 0.29 (0.22-0.37), < 0.001 | 0.45 (0.24-0.85), 0.01 | 0.99 (0.66-1.46), 0.96 | 1.55 (0.77-3.12), 0.22 |
| Delirium | 0.70 (0.38-1.29), 0.25 | 0.96 (0.83-1.11), 0.57 | 1.60 (1.28-2.00), | 1.28 (0.71-2.32), 0.41 | 2.20 (1.18-4.09), 0.01 |
| Bleeding | 0.43 (0.39-0.48), | 0.80 (0.74-0.86), < 0.001 | 1.27 (0.98-1.67), 0.07 | 1.86 (1.64-2.11), < 0.001 | 2.82 (2.30-3.46), |
| Bleeding with transfusion | 0.27 (0.20-0.35), | 0.69 (0.60-0.79), < 0.001 | 1.28 (0.98-1.67), 0.07 | 2.63 (1.97-3.51), < 0.001 | 4.62 (3.18-6.74), |
| Length of stay (days) | −0.90 (−0.98 to −0.82), < 0.001 | −0.16 (−0.34 to 0.01), 0.07 | +2.47 (+1.68 to +3.26), < 0.001 | +0.82 (+0.64 to +0.99), | +3.63 (+2.83 to +4.43), < 0.001 |
| Total charges (in USD) | +$46247 (43651 to 48844), < 0.001 | +$69008 (64291 to 73724), < 0.001 | +$111550 (96015 to 127084), < 0.001 | +$27665 (22945 to 32386), | +$72538 (57002 to 88074), < 0.001 |
The adjusted odds of in-hospital mortality with PCI compared to no PCI were 0.27 (95%CI: 0.22-0.32, P < 0.001) in low, 0.37 (95%CI: 0.33-0.40, P < 0.001) in intermediate, and 0.43 (95%CI: 0.34-0.54, P < 0.001) in high frailty category. The interaction terms were 1.56 (95%CI: 1.27-1.93, P < 0.001) for intermediate vs low frailty and 1.83 (95%CI: 1.37-2.44,
In this adjusted multivariable analysis, frailty was a strong independent predictor of in-hospital mortality among NSTEMI patients not undergoing PCI. Compared to the low frailty category, the odds of mortality were significantly higher in intermediate (OR 4.72, 95%CI: 4.39-5.08, P < 0.001) and high frailty categories (OR 7.71, 95%CI: 7.09-8.39,
Age was associated with increased mortality in low (OR 1.07, P < 0.001) and intermediate (OR 1.01, P < 0.001), but not in high frailty category (OR 1.00, P = 0.918). Female sex was associated with lower mortality in intermediate (OR 0.78,
Among racial groups, the Black race was associated with increased mortality in the high frailty category (OR 1.18,
Higher income was consistently associated with lower mortality. Compared to the lowest income quartile, Q2-Q4 were protective in the intermediate frailty category (ORs 0.86, 0.84, and 0.83, all P < 0.001), and this association persisted in the interaction model for Q2 (OR 0.89, P < 0.001), Q3 (OR 0.86, P < 0.001), and Q4 (OR 0.84, P < 0.001) (Table 3).
| Variable | Low frailty | Intermediate frailty | High frailty | Interaction |
| OR (95%CI, P value) | OR (95%CI, P value) | OR (95%CI, P value) | OR (95%CI, P value) | |
| Intermediate vs low frailty without PCI | - | - | - | 4.72 (4.39-5.08), 0.001 |
| High vs low frailty without PCI | - | - | - | 7.71 (7.09-8.39), 0.001 |
| PCI effect in intermediate vs low frailty | - | - | - | 1.56 (1.27-1.93), 0.001 |
| PCI effect in high vs low frailty | - | - | - | 1.83 (1.37-2.44), 0.001 |
| Age | 1.07 (1.06-1.08), < 0.001 | 1.01 (1.01-1.02), 0.001 | 1.00 (0.99-1.01), 0.918 | 1.02 (1.01-1.02), 0.001 |
| Female (vs male) | 0.93 (0.82-1.07), 0.310 | 0.78 (0.74-0.83), 0.001 | 0.82 (0.74-0.90), 0.001 | 0.81 (0.77-0.84), 0.001 |
| Race ref: White | ||||
| Black | 0.93 (0.72-1.19), 0.556 | 0.94 (0.84-1.04), 0.201 | 1.18 (1.01-1.38), 0.032 | 0.99 (0.91-1.07), 0.804 |
| Hispanic | 0.85 (0.66-1.09), 0.202 | 0.91 (0.82-1.01), 0.066 | 0.93 (0.79-1.10), 0.389 | 0.90 (0.83-0.98), 0.017 |
| Asian/Pacific Islander | 1.05 (0.71-1.55), 0.801 | 1.14 (1.00-1.30), 0.052 | 1.21 (0.97-1.51), 0.085 | 1.15 (1.03-1.29), 0.013 |
| Native American | 0.49 (0.12-2.03), 0.327 | 1.09 (0.71-1.68), 0.691 | 1.29 (0.54-3.09), 0.572 | 1.05 (0.72-1.52), 0.799 |
| Race: Other | 0.91 (0.59-1.39), 0.652 | 1.09 (0.92-1.29), 0.333 | 1.35 (1.03-1.75), 0.028 | 1.13 (0.98-1.29), 0.084 |
| Income quartile ref: USD 47999 (Q1) | ||||
| USD 48000-USD 60999 (Q2) | 0.91 (0.77-1.08), 0.286 | 0.86 (0.80-0.92), 0.001 | 1.00 (0.88-1.14), 0.974 | 0.89 (0.84-0.94), 0.001 |
| USD 61000-81999 (Q3) | 0.83 (0.69-1.00), 0.055 | 0.84 (0.78-0.91), 0.001 | 0.94 (0.82-1.08), 0.365 | 0.86 (0.81-0.91), 0.001 |
| ≥ USD 82000 (Q4) | 0.75 (0.61-0.92), 0.007 | 0.83 (0.76-0.91), 0.001 | 0.90 (0.78-1.04), 0.161 | 0.84 (0.78-0.90), 0.001 |
| Hospital region ref: Northeast | ||||
| Midwest | 1.03 (0.83-1.26), 0.809 | 0.85 (0.78-0.93), 0.001 | 0.74 (0.63-0.87), 0.001 | 0.85 (0.79-0.91), 0.001 |
| South | 1.12 (0.93-1.34), 0.242 | 0.89 (0.82-0.97), 0.005 | 0.69 (0.60-0.80), 0.001 | 0.87 (0.81-0.93), 0.001 |
| West | 1.15 (0.93-1.43), 0.190 | 1.00 (0.91-1.09), 0.973 | 0.90 (0.76-1.05), 0.183 | 0.99 (0.92-1.07), 0.841 |
| Hospital location Ref: Non-teaching | ||||
| Teaching | 0.97 (0.88-1.07), 0.531 | 1.04 (0.99-1.08), 0.107 | 1.04 (0.96-1.12), 0.384 | 1.03 (0.99-1.06), 0.153 |
| Hospital bed size ref: Small | ||||
| Medium | 1.07 (0.90-1.26), 0.443 | 1.15 (1.06-1.24), 0.001 | 1.05 (0.93-1.19), 0.449 | 1.12 (1.05-1.19), 0.001 |
| Large | 0.90 (0.77-1.06), 0.215 | 1.18 (1.10-1.27), 0.001 | 1.13 (1.00-1.28), 0.049 | 1.13 (1.07-1.20), 0.001 |
| Charlson comorbidity index Ref: 1 | ||||
| 2 | 1.25 (1.00-1.56), 0.046 | 1.12 (0.97-1.29), 0.123 | 0.97 (0.72-1.31), 0.856 | 1.15 (1.03-1.28), 0.011 |
| 3 | 1.28 (1.05-1.55), 0.013 | 0.97 (0.85-1.10), 0.621 | 0.84 (0.65-1.10), 0.209 | 1.02 (0.93-1.13), 0.679 |
Geographic region influenced mortality risk, with patients in the Midwest and South showing significantly lower odds of death in intermediate and high frailty compared to the Northeast. In the high frailty category, the Midwest (OR 0.74,
Hospital bed size was a significant predictor. Admission to medium or large hospitals, compared to small hospitals, was associated with higher mortality in intermediate frailty (medium OR 1.15, P < 0.001; large OR 1.18, P < 0.001) and in high frailty (large OR 1.13, P = 0.049). This association was consistent in the interaction terms (medium OR 1.12, P = 0.001; large OR 1.13, P < 0.001).
Regarding comorbidity burden, a Charlson Index of 2 was associated with increased mortality in low frailty (OR 1.25,
The adjusted odds of AKI for PCI vs no PCI were 0.79 (95%CI: 0.75-0.82, P < 0.001), 0.83 (95%CI: 0.78-0.87, P < 0.001), and 1.09 (95%CI: 0.91-1.30, P = 0.35) in low, intermediate, and high frailty category, respectively. For AKI requiring dialysis, the ORs were 0.92 (P = 0.35), 0.84 (P = 0.67), and 1.83 (P < 0.001). For ARF, ORs were 0.45 (P < 0.001), 0.69 (P < 0.001), and 0.89 (P = 0.12); and for ARF requiring mechanical ventilation, 0.35 (P < 0.001), 0.63 (P < 0.001), and 1.00 (P = 0.99).
The odds for pulmonary edema were 0.64 (P < 0.001), 1.13 (P = 0.13), and 1.73 (P = 0.007); for TIA, 0.70 (P = 0.11), 1.04 (P = 0.79), and 2.45 (P = 0.006); and for stroke, 0.38 (P < 0.001), 0.57 (P < 0.001), and 0.86 (P = 0.20). Cardiogenic shock showed ORs of 0.53 (P < 0.001), 1.06 (P = 0.15), and 1.79 (P < 0.001), while use of MCS was associated with ORs of 1.06
Cardiac arrest ORs were 0.54 (P < 0.001), 0.78 (P < 0.001), and 1.24 (P = 0.09); hypotension ORs were 0.83 (P = 0.006), 1.18 (P < 0.001), and 1.31 (P = 0.005); pulmonary embolism ORs were 0.29, 0.29, and 0.45 (P < 0.01); and delirium ORs were 0.70 (P = 0.25), 0.96 (P = 0.57), and 1.60 (P < 0.001). Bleeding complication ORs were 0.43 (P < 0.001), 0.80 (P < 0.001), and 1.27 (P = 0.07); and transfusion-requiring bleeding ORs were 0.27 (P < 0.001), 0.69 (P < 0.001), and 1.28 (P = 0.07) (Table 2).
Among patients undergoing PCI, the mean LOS was shorter by −0.90 days (95%CI: -0.98 to -0.82, P < 0.001) in the low and by -0.16 days (95%CI: -0.34 to 0.01, P = 0.07) in the intermediate, but longer by +2.47 days (95%CI: +1.68 to +3.26,
PCI was associated with higher TOTCHGs of $46247 (95%CI: $43651 to $48844, P < 0.001) in low, $69008 (95%CI: $64291 to $73724, P < 0.001) in intermediate, and $111550 (95%CI: $96015 to $127084, P < 0.001) in high frailty. The interaction terms indicated incremental charges of $27665 (P < 0.001) for intermediate vs low and $72538 (P < 0.001) for high vs low frailty (Table 2).
In our study, we compared NSTEMI patients aged 75 years or older undergoing PCI with those who did not and found a significant correlation between frailty status and PCI outcomes. Our primary findings include that: (1) PCI was associated with reduced in-hospital mortality in all frail groups of NSTEMI patients; (2) Beneficial effect of PCI was diminished with increased frailty status; (3) Higher frailty was associated with higher odds of in-hospital mortality and post-PCI complications; and (4) High frail group exhibited an increase in healthcare resource utilization. To the best of our knowledge, this is the largest retrospective study reporting PCI outcomes in elderly NSTEMI patients, stratified by frailty status.
Among the older NSTEMI patients, only 38% were classified as the low frailty group, highlighting the increased vulnerability in this cohort. Demographically, higher frailty status was associated with female sex, all racial groups except white, and lower-income populations. These findings highly align with previous studies reporting the correlation between frailty and various sociodemographic factors[20,21]. Specifically, higher income was a protective factor against frailty in elderly NSTEMI patients. Several mechanisms can contribute to the correlation between socioeconomic status (SES) and frailty. A study by Szanton et al[22] summarized mechanisms depicting a possible association of SES and frailty status. Lower SES was associated with decreased physical activity[23], resulting in sarcopenia[24], a key feature of frailty. Moreover, lower-income groups have reduced access to adequate nutrition[25], making them more prone to frailty[26].
Additionally, the comorbidity burden rose with advancing frailty status, as evidenced by over 88% of patients classified in the high frail group exhibiting a CCI of 3 or higher. Singh et al[27] reported comparable results, particularly among cardiovascular patients, and indicated that the presence of various comorbidities should prompt physicians to suspect frailty, which might be masked by the comorbidities.
Our study found a significant reduction in in-hospital mortality following PCI in all frailty groups, and higher frailty was associated with higher odds of in-hospital mortality, suggesting it as a strong predictor of mortality. In addition, adverse outcomes following PCI, such as ARF, cardiogenic shock, and major bleeding complications, had a higher incidence with increased frailty. On the other hand, PCI was associated with reduced odds of AKI in low and intermediate frail groups, but not in the high frail group.
A recent meta-analysis of twenty-one studies reported similar findings that elderly frail patients undergoing PCI had significantly higher risks of in-hospital mortality (RR: 3.45, 95%CI: 1.90-6.25, P < 0.0001) and major bleeding complications compared to non-frail patients[28]. Another study by Mele et al[29] found that frailty was associated with poor outcomes in older NSTEMI patients treated with PCI, despite its survival benefit. Consistent with a recent study based on the National Cardiovascular Data Registry Cath PCI registry of over 1.3 million patients[14], our findings affirm that increasing frailty is associated with a graded rise in in-hospital mortality and procedural complications, independent of traditional bedside mortality risk scores.
James et al[30] concluded that frailty and cardiovascular disease (CVD) have a bidirectional relationship, which might explain their increased proportionality and adverse outcomes. For instance, biological changes such as chronic inflammation, immune activation, cellular changes, metabolic dysregulation, comorbidities, and environmental factors were associated with both frailty and CVD[30]. Another retrospective study using the Acute Coronary Treatment and Intervention Outcomes Network Registry reported that frailty, being more prevalent in NSTEMI patients, can help physicians and patients in shared decision-making to prevent adverse outcomes following invasive management[31].
Furthermore, the high frail group was associated with lower PCI utilization and reduced relative benefit of PCI, which coincides with previous studies[29]. This might be due to several factors that impact clinicians' decisions to opt for conservative management in these patients. Higher risk of post-operative complications[30], including bleeding[31], the presence of multimorbidity[32], and evaluation of risk vs benefit ratio[29], and also that frail adults with NSTEMI exhibit more complex and high-risk angiographic features-such as severe calcification, high SYNTAX scores, and vulnerable plaque morphology-independent of age, and face significantly higher risks of adverse cardiovascular and bleeding events following angiography[6,14], were a few reasons that might be responsible for the underutilization of invasive management in them.
Frail individuals often possess a reduced renal reserve, a decline primarily attributable to age-related nephrosclerosis, concurrent CKD, or volume depletion[33]. The link between age, frailty, and kidney disease is not surprising, and the kidneys undergo normal aging processes that involve both anatomical and physiological changes[33]. These age-related changes in the kidneys differ from those seen in kidney diseases, which are relatively common among older adults[33]. During PCI, the use of iodinated contrast agents carries the risk of inducing contrast-induced nephropathy, particularly in patients who already show signs of impaired baseline renal function, diminished renal reserve, or hemodynamic instability, which are notably more common among the frail population[33-35]. Moreover, frail patients are more likely to experience significant fluctuations in blood pressure and cardiac output throughout and following the procedure[35]. Such variability can compromise renal perfusion, consequently heightening the risk of ischemic tubular injury[35]. Other contributing factors that are more frequently encountered in elderly and frail patients include polypharmacy[33,36], which increases vulnerability to nephrotoxic drugs, along with longer procedural durations associated with anatomically complex or calcified lesions[14].
While PCI was associated with a survival benefit across all frailty categories, the magnitude of benefit was attenuated in patients with higher frailty. Compared to those with low frailty, patients in the high-frailty group had significantly greater odds of AKI (OR 1.36; P = 0.001), AKI requiring dialysis (OR 1.99; P < 0.001), bleeding (OR 2.82; P < 0.001), and bleeding requiring transfusion (OR 4.62; P < 0.001). Similar trends were observed in the intermediate-frailty group when compared to low frailty. These findings highlight an important clinical trade-off: While PCI improves survival, it is also associated with a higher burden of complications among frail patients, particularly those with high frailty (Table 3). These results reinforce the importance of individualized, frailty-informed decision-making in elderly NSTEMI patients[14]. Frailty should not be considered a contraindication to PCI, but rather a key factor that modifies procedural risk and should inform risk-benefit discussions[6]. Incorporating frailty into pre-procedural stratification models may guide more nuanced therapeutic strategies, including contrast minimization, use of radial access[6,14], tailored antithrombotic regimens, and careful peri-procedural hemodynamic management[37]. A multidisciplinary heart team approach is essential to balance the potential for survival benefit against the risk of complications and to optimize outcomes in this high-risk population[6].
Elderly NSTEMI patients who underwent PCI experienced varying LOS, with a reduction of 0.9 days in the low frailty group and a significant increase of 2.47 days in the high frailty group. On the other hand, frail patients receiving PCI experienced more healthcare costs that significantly increased with their frailty status. Multimorbidity, higher odds of complex lesions, higher rates of procedural complications, and slower recovery rates due to frailty might be the reason for the increased utilization of healthcare resources[14,32].
Frail patients face an elevated risk of functional deterioration during and after hospitalization, making early implementation of physical therapy and structured cardiac rehabilitation with gradual mobilization a critical aspect of inpatient care[10,38]. In the post-discharge setting, cardiac rehabilitation and tailored nutritional support remain integral to optimizing long-term recovery and reducing the risk of adverse cardiovascular outcomes in patients with acute coronary syndrome[10].
Firstly, our study represented a large, nationally representative cohort of elderly patients with NSTEMI, enhancing the generalizability to real-world practice in the country. Second, utilising the HFRS allowed robust subgroup analysis by physiologic reserve, not just by age or the comorbidity count. Third, our study provided a comprehensive outcome assessment of the in-hospital outcomes, including mortality, complications, LOS, and total costs incurred, which offers a full-spectrum risk-benefit profile of the PCI. In addition, methodological rigor is achieved through the use of survey-weighted multivariable regression models with interaction terms, which strengthens causal inference and highlights differential effects across frailty strata.
Our study has several limitations. A key limitation of this study is the absence of angiographic and detailed procedural data, such as the extent of coronary artery disease, the number of stents placed, and procedural complexity or success. These clinical details are critical in understanding in-hospital outcomes following PCI, particularly among frail patients who may face distinct technical challenges or risks. As this information is not captured in the NIS database, it limits our ability to contextualize the observed differences in outcomes across frailty categories fully. Second, our analysis was based on NIS, leading to misclassification or underestimation of frailty status and a lack of post-discharge follow-up to report long-term outcomes. Moreover, using ICD-10-CM codes for diagnoses might include documentation variability and coding errors from different hospitals. Additionally, multiple admissions from a single patient weren’t linked in NIS due to a lack of unique patient identifiers, leading to a potential duplication of readmissions. Third, there was a possibility of confounding from cognitive impairment, medication adherence, or patient preferences, which were highly relevant in frail patients. Fourth, as it was a retrospective study, we couldn’t account for the causal association between PCI and its outcomes. Furthermore, there might be a possibility of selection bias, along with a lack of information on the context of clinical decision-making, which might mask the reason for reduced PCI rates in the high-frail group.
Our findings indicate that frailty status significantly influences PCI outcomes, despite limitations. This study supports the 2025 ACC/AHA/ACEP/NAEMSP/SCAI guidelines' recommendations for individualized PCI decision-making in elderly patients and provides empirical evidence for the value of frailty stratification in guiding treatment strategies, addressing an evidence gap, as elderly and frail populations are often underrepresented in clinical trials; thus, it offers much-needed insights into this high-risk subgroup. Although PCI is underutilized, the high-frail group demonstrated a survival benefit, highlighting the importance of considering frailty as an assessment tool instead of an exclusion criterion in clinical decision-making. PCI use in select elderly patients is supported by evidence showing that even among the most frail, PCI was associated with reduced in-hospital mortality, reinforcing its potential benefit. The findings highlight the need for individualized care; the diminishing benefit and increasing complication rates with higher frailty highlight the importance of frailty-guided clinical decision-making. Our study informs risk–benefit discussions with patients, as these findings can guide shared decision-making conversations, particularly with frail older adults. Increased hospital charges and longer stays among frail PCI recipients may influence hospital planning and policy decisions; our study raises awareness of resource utilization.
Future studies should aim to conduct larger prospective research to investigate the long-term outcomes linked to frailty and identify which subgroup of frail patients could benefit the most from invasive management. In addition, frailty can be incorporated into existing NSTEMI risk prediction models, like the Thrombolysis in Myocardial Ischemia, Global Registry of Acute Coronary Events scores, to enhance the clinical utility.
In conclusion, our study found that frailty strongly predicted in-hospital mortality in elderly NSTEMI patients under
| 1. | Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2466] [Cited by in RCA: 2361] [Article Influence: 81.4] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. Deaths by sex and age group for a selected country or area and year. Ischaemic heart disease. [Cited 14 June 2025] Available from: https://platform.who.int/mortality/themes/theme-details/topics/indicator-groups/indicator-group-details/MDB/ischaemic-heart-disease. |
| 3. | Kunadian V, Mossop H, Shields C, Bardgett M, Watts P, Teare MD, Pritchard J, Adams-Hall J, Runnett C, Ripley DP, Carter J, Quigley J, Cooke J, Austin D, Murphy J, Kelly D, McGowan J, Veerasamy M, Felmeden D, Contractor H, Mutgi S, Irving J, Lindsay S, Galasko G, Lee K, Sultan A, Dastidar AG, Hussain S, Haq IU, de Belder M, Denvir M, Flather M, Storey RF, Newby DE, Pocock SJ, Fox KAA; British Heart Foundation SENIOR-RITA Trial Team and Investigators. Invasive Treatment Strategy for Older Patients with Myocardial Infarction. N Engl J Med. 2024;391:1673-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Acute coronary syndromes. NICE. 2020. Available from: https://www.nice.org.uk/guidance/ng185/chapter/Recommendations#nstemi-and-unstable-angina-early-management. |
| 5. | Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 2814] [Article Influence: 938.0] [Reference Citation Analysis (0)] |
| 6. | Rao SV, O'Donoghue ML, Ruel M, Rab T, Tamis-Holland JE, Alexander JH, Baber U, Baker H, Cohen MG, Cruz-Ruiz M, Davis LL, de Lemos JA, DeWald TA, Elgendy IY, Feldman DN, Goyal A, Isiadinso I, Menon V, Morrow DA, Mukherjee D, Platz E, Promes SB, Sandner S, Sandoval Y, Schunder R, Shah B, Stopyra JP, Talbot AW, Taub PR, Williams MS; Peer Review Committee Members. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2025;85:2135-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 7. | Biscaglia S, Guiducci V, Escaned J, Moreno R, Lanzilotti V, Santarelli A, Cerrato E, Sacchetta G, Jurado-Roman A, Menozzi A, Amat Santos I, Díez Gil JL, Ruozzi M, Barbierato M, Fileti L, Picchi A, Lodolini V, Biondi-Zoccai G, Maietti E, Pavasini R, Cimaglia P, Tumscitz C, Erriquez A, Penzo C, Colaiori I, Pignatelli G, Casella G, Iannopollo G, Menozzi M, Varbella F, Caretta G, Dudek D, Barbato E, Tebaldi M, Campo G; FIRE Trial Investigators. Complete or Culprit-Only PCI in Older Patients with Myocardial Infarction. N Engl J Med. 2023;389:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 8. | Diletti R, den Dekker WK, Bennett J, Schotborgh CE, van der Schaaf R, Sabaté M, Moreno R, Ameloot K, van Bommel R, Forlani D, van Reet B, Esposito G, Dirksen MT, Ruifrok WPT, Everaert BRC, Van Mieghem C, Elscot JJ, Cummins P, Lenzen M, Brugaletta S, Boersma E, Van Mieghem NM; BIOVASC Investigators. Immediate versus staged complete revascularisation in patients presenting with acute coronary syndrome and multivessel coronary disease (BIOVASC): a prospective, open-label, non-inferiority, randomised trial. Lancet. 2023;401:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 9. | Sardella G, Lucisano L, Garbo R, Pennacchi M, Cavallo E, Stio RE, Calcagno S, Ugo F, Boccuzzi G, Fedele F, Mancone M. Single-Staged Compared With Multi-Staged PCI in Multivessel NSTEMI Patients: The SMILE Trial. J Am Coll Cardiol. 2016;67:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL 2nd, DeVon HA, Alexander KP; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 214] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 11. | Dou Q, Wang W, Wang H, Ma Y, Hai S, Lin X, Liu Y, Zhang X, Wu J, Dong B. Prognostic value of frailty in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. BMC Geriatr. 2019;19:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Murali-Krishnan R, Iqbal J, Rowe R, Hatem E, Parviz Y, Richardson J, Sultan A, Gunn J. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Shimono H, Tokushige A, Kanda D, Ohno A, Hayashi M, Fukuyado M, Akao M, Kawasoe M, Arikawa R, Otsuji H, Chaen H, Okui H, Oketani N, Ohishi M. Association of preoperative clinical frailty and clinical outcomes in elderly patients with stable coronary artery disease after percutaneous coronary intervention. Heart Vessels. 2023;38:1205-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Peterson B, Kochar A, Young R, Senman B, Rymer J, Wojdyla D, Orkaby AR, Nanna M, Damluji AA, Campbell G, Swaminathan RV, Afilalo J, Alexander KP, Sutton NR, Yeh RW, Bhatt DL. Effect of Frailty on In-Hospital Mortality and Complications of PCI: An NCDR Registry Report. J Am Coll Cardiol. 2025;85:2416-2420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | HCUP-US NIS Overview. Available from: https://hcup-us.ahrq.gov/nisoverview.jsp. |
| 16. | Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, Bardsley M, Conroy S. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1161] [Article Influence: 145.1] [Reference Citation Analysis (8)] |
| 17. | Teo Z, Oh YZ, Huang W, Lee S, Chang TY, Lim A, Sim LE, Espelata WDV, Conroy S, Rosario BH. Validation of Hospital Frailty Risk Score in Heart Failure. J Asian Pac Soc Cardiol. 2024;3:e16. [DOI] [Full Text] |
| 18. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 39669] [Article Influence: 1017.2] [Reference Citation Analysis (0)] |
| 19. | Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6122] [Cited by in RCA: 8747] [Article Influence: 416.5] [Reference Citation Analysis (0)] |
| 20. | Singhal S, Singh S, Dewangan GC, Dey S, Banerjee J, Lee J, Upadhyaya AD, Hu P, Dey AB. The prevalence of frailty and its relationship with sociodemographic factors, regional healthcare disparities, and healthcare utilization in the aging population across India. Aging Med (Milton). 2023;6:212-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Biritwum RB, Minicuci N, Yawson AE, Theou O, Mensah GP, Naidoo N, Wu F, Guo Y, Zheng Y, Jiang Y, Maximova T, Kalula S, Arokiasamy P, Salinas-Rodríguez A, Manrique-Espinoza B, Snodgrass JJ, Sterner KN, Eick G, Liebert MA, Schrock J, Afshar S, Thiele E, Vollmer S, Harttgen K, Strulik H, Byles JE, Rockwood K, Mitnitski A, Chatterji S, Kowal P; WHO SAGE Collaboration. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas. 2016;91:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Szanton SL, Seplaki CL, Thorpe RJ Jr, Allen JK, Fried LP. Socioeconomic status is associated with frailty: the Women's Health and Aging Studies. J Epidemiol Community Health. 2010;64:63-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Feinglass J, Lin S, Thompson J, Sudano J, Dunlop D, Song J, Baker DW. Baseline health, socioeconomic status, and 10-year mortality among older middle-aged Americans: findings from the Health and Retirement Study, 1992 2002. J Gerontol B Psychol Sci Soc Sci. 2007;62:S209-S217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Franco M, Diez Roux AV, Glass TA, Caballero B, Brancati FL. Neighborhood characteristics and availability of healthy foods in Baltimore. Am J Prev Med. 2008;35:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 296] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Semba RD, Bartali B, Zhou J, Blaum C, Ko CW, Fried LP. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Singh M, Alexander K, Roger VL, Rihal CS, Whitson HE, Lerman A, Jahangir A, Nair KS. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc. 2008;83:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Wang SS, Liu WH. Impact of frailty on outcomes of elderly patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis. World J Clin Cases. 2024;12:107-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 29. | Mele M, Ragnatela I, Romano M, Tabella E, Rossi LU, Mautone F, Mele A, Liantonio A, Imbrici P, Correale M, Santoro F, Brunetti ND. Impact of Frailty on Outcome of Older Patients With Non-ST Elevation Acute Myocardial Infarction Who Undergo Percutaneous Coronary Intervention. Am J Cardiol. 2024;230:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | James K, Jamil Y, Kumar M, Kwak MJ, Nanna MG, Qazi S, Troy AL, Butt JH, Damluji AA, Forman DE, Orkaby AR. Frailty and Cardiovascular Health. J Am Heart Assoc. 2024;13:e031736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 31. | Dodson JA, Hochman JS, Roe MT, Chen AY, Chaudhry SI, Katz S, Zhong H, Radford MJ, Udell JA, Bagai A, Fonarow GC, Gulati M, Enriquez JR, Garratt KN, Alexander KP. The Association of Frailty With In-Hospital Bleeding Among Older Adults With Acute Myocardial Infarction: Insights From the ACTION Registry. JACC Cardiovasc Interv. 2018;11:2287-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Ekerstad N, Swahn E, Janzon M, Alfredsson J, Löfmark R, Lindenberger M, Andersson D, Carlsson P. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur J Prev Cardiol. 2014;21:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Vanmassenhove J, Van Biesen W, Lameire N. The interplay and interaction between frailty and acute kidney injury. Nephrol Dial Transplant. 2020;35:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Coca SG, Cho KC, Hsu CY. Acute kidney injury in the elderly: predisposition to chronic kidney disease and vice versa. Nephron Clin Pract. 2011;119 Suppl 1:c19-c24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Davenport MS, Perazella MA, Nallamothu BK. Contrast-Induced Acute Kidney Injury and Cardiovascular Imaging: Danger or Distraction? Circulation. 2023;147:847-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Docherty NG, Delles C, D'Haese P, Layton AT, Martínez-Salgado C, Vervaet BA, López-Hernández FJ. Haemodynamic frailty - A risk factor for acute kidney injury in the elderly. Ageing Res Rev. 2021;70:101408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4826] [Article Influence: 804.3] [Reference Citation Analysis (0)] |
| 38. | Ekerstad N, Pettersson S, Alexander K, Andersson D, Eriksson S, Janzon M, Lindenberger M, Swahn E, Alfredsson J. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: A follow-up after more than 5 years. Eur J Prev Cardiol. 2018;25:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/