Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.112001
Revised: July 31, 2025
Accepted: September 10, 2025
Published online: October 26, 2025
Processing time: 101 Days and 11.9 Hours
Heart failure (HF), especially in patients with preserved ejection fraction and mid-range ejection fraction, remains a significant global health burden. Interatrial shunt devices (IASDs), which allow blood flow from the left to the right atrium, offer a novel treatment approach by reducing left atrial pressure and alleviating symptoms.
To evaluate the efficacy and safety of IASDs in patients with HF through a sys
We performed a systematic review and meta-analysis following Preferred Re
Nine studies involving 1689 patients were included. IASDs significantly improved cardiac output [mean difference (MD): 0.72, 95%CI: 0.13-1.32, P = 0.02], right atrial pressure (RAP) (MD: 0.70, 95%CI: 0.14-1.26, P = 0.01), and 6-minute walk distance (MD: 71.63, 95%CI: 24.13-119.13, P = 0.003). There were no significant differences in major adverse cardiac events, myocardial infarction, ischemic stroke, or new-onset atrial fibrillation. However, all-cause mortality [risk ratio (RR): 1.49, 95%CI: 1.02-2.18, P = 0.04] and cardiovascular death (RR: 1.66, 95%CI: 1.01-2.74, P = 0.05) were significantly higher in the shunt group.
IASDs offer significant short-term improvements in cardiac output, RAP, and exercise capacity in HF patients. However, long-term safety concerns, particularly regarding mortality, necessitate further research and careful patient selection.
Core Tip: Interatrial shunt devices represent a novel, device-based approach for managing heart failure, particularly in patients with preserved and mid-range ejection fraction. This systematic review and meta-analysis evaluated their efficacy and safety across multiple clinical outcomes. While short-term benefits were observed in exercise capacity, cardiac output, and right atrial pressure, increased all-cause and cardiovascular mortality raise concerns about long-term safety. These findings highlight the need for cautious patient selection and further high-quality randomized trials.
- Citation: Khan A, Rath S, Fatima N, Hayat U, Dawer P, Khan H, Ullah W, Ud Din Z, Sehar A, Hassan IN. Efficacy and safety of interatrial shunt treatment for heart failure: A systematic review and meta-analysis. World J Cardiol 2025; 17(10): 112001
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/112001.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.112001
With more than 64 million cases worldwide and high rates of morbidity, mortality, and medical expenses, heart failure (HF) remains a major global health burden[1]. Despite advances in device-based therapies and pharmaceutical therapy, a significant proportion of patients, particularly those with preserved ejection fraction (HFpEF) and mid-range ejection fraction (HFmrEF), nonetheless experience chronic symptoms and recurrent hospitalizations[2]. The urgent need for novel treatment approaches is highlighted by the inadequate therapeutic benefits of existing therapy strategies in these groups[3].
One novel approach to intervention is the creation of an interatrial shunt, which permits blood to go from the left to the right atrium in an effort to decompress the left atrium. The symptoms of dyspnea and pulmonary congestion may be lessened by this reduction in left atrial pressure (LAP)[4]. In HFpEF, this physiological hypothesis is particularly appealing, as elevated left-sided filling pressures during exercise are a significant contributor to symptomatology[5]. The atrial flow regulator (AFR), V-wave device, and interatrial shunt device (IASD) are among the IASDs that have been explored during the last 10 years. Each of these devices has unique haemodynamic profiles and design features[6-8].
Haemodynamic improvements, exercise capacity, and quality of life have all showed promising results in pilot trials and early-phase research[4,9]. For instance, the REDUCE LAP-HF I and II trials found that interatrial shunting signi
Additionally, a recent 2-year follow up from the ALT-FLOW Early Feasibility Study evaluating the APTURE shunt a novel left atrial to coronary sinus shunt-demonstrated sustained improvements in symptoms, functional capacity, and quality of life in patients with HFpEF and HFmrEF, without adverse effects on right heart function[12].
Larger randomized controlled trials (RCTs), like the REDUCE LAP-HF II study, have also shown conflicting results, failing to meet their primary goal and casting doubt on the constancy of effect across various patient populations. There is a strong need to compile the most recent data in order to assess the overall efficacy and safety profile of interatrial shunt therapy, given the expanding body of research and increased clinical interest in this treatment. Although analogous themes have been covered in previous reviews, they frequently concentrated on device-specific results or lacked tho
The clinical impact of IASDs has not yet been comprehensively evaluated throughout the whole range of HF pheno
Our meta-analysis was conducted in accordance with the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. Our protocol is registered with PROSPERO with reference number (CRD420251012144)[14-16]
A Systematic literature search was undertaken on PubMed, Cochrane, EMBASE, and Clinical Trials.gov from their inception to April 2025. Additional relevant studies were found by screening the reference lists of included studies and similar systematic reviews.
The following terms ("heart failure") AND ("interatrial septum" OR “interatrial septal device”) were used as either medical subject heading terms or keywords. Detailed search strategy is available in Supplementary Table 1.
All observational studies and RCTs that compared the safety and efficacy of interatrial shunt treatment in HF patients to a control group, were included in this meta-analysis. Studies conducted on animals were also excluded. No language or date restrictions were applied.
Rayyan was used to specify and remove duplicates of all the studies generated by our literature search. Two authors (Khan H and Ullah W) independently screened the abstracts and titles to exclude all irrelevant studies. The remaining studies that met our eligibility requirements underwent full-text screening. Any conflicts regarding the studies' selection were settled by a third author (Khan A).
Relevant data was extracted into a pre-piloted Excel spreadsheet which included author name, publication year, sample size, study design, mean age, gender, body mass index, hypertension, diabetes, coronary artery disease, atrial fibrillation/flutter, New York Heart Association Class, Left Ventricular Ejection Fraction, Device and outcomes.
The outcomes of our study included both continuous and dichotomous variables. Continuous variables were cardiac output, mean right atrial pressure (RAP), mean pulmonary artery pressure, and 6MWD, while the dichotomous outcomes included all-cause mortality, cardiovascular death, Ischemic stroke, all-cause HF events, major adverse cardiac events (MACE), cardiac death, myocardial infarction and new-onset atrial fibrillation.
The revised Cochrane Risk of Bias tool (RoB 2.0) was used to evaluate the risk of bias in the included randomized trials. This tool assesses bias in five domains which consist of (1) Bias due to the randomization process; (2) Bias caused by deviations from intended interventions; (3) Bias resulting from missing outcome data; (4) Bias in the outcome measure
The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used for non-randomized studies. This method assesses bias in seven domains, which include confounding, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement, and reported result selection.
Observational cohort studies were evaluated using the Newcastle-Ottawa Scale (NOS), which is based on three main factors: (1) Study group selection; (2) Group comparability; and (3) Exposure or outcome determination.
We used Review Manager (version 5.4.1) software to conduct our meta-analyses. The Mantel-Haenszel method was used for dichotomous outcomes and inverse variance was used for continuous outcomes. Risk ratio (RR) and corresponding 95%CI were extracted. A random-effects model was used to carry out the meta-analyses. Pooled estimates were presented as a forest plot and the Higgins I2 statistic was calculated to evaluate the statistical heterogeneity. A leave-one-out sensitivity analysis was conducted to evaluate the sustainability of the pooled estimates by reanalyzing the results after sequentially eliminating each study.
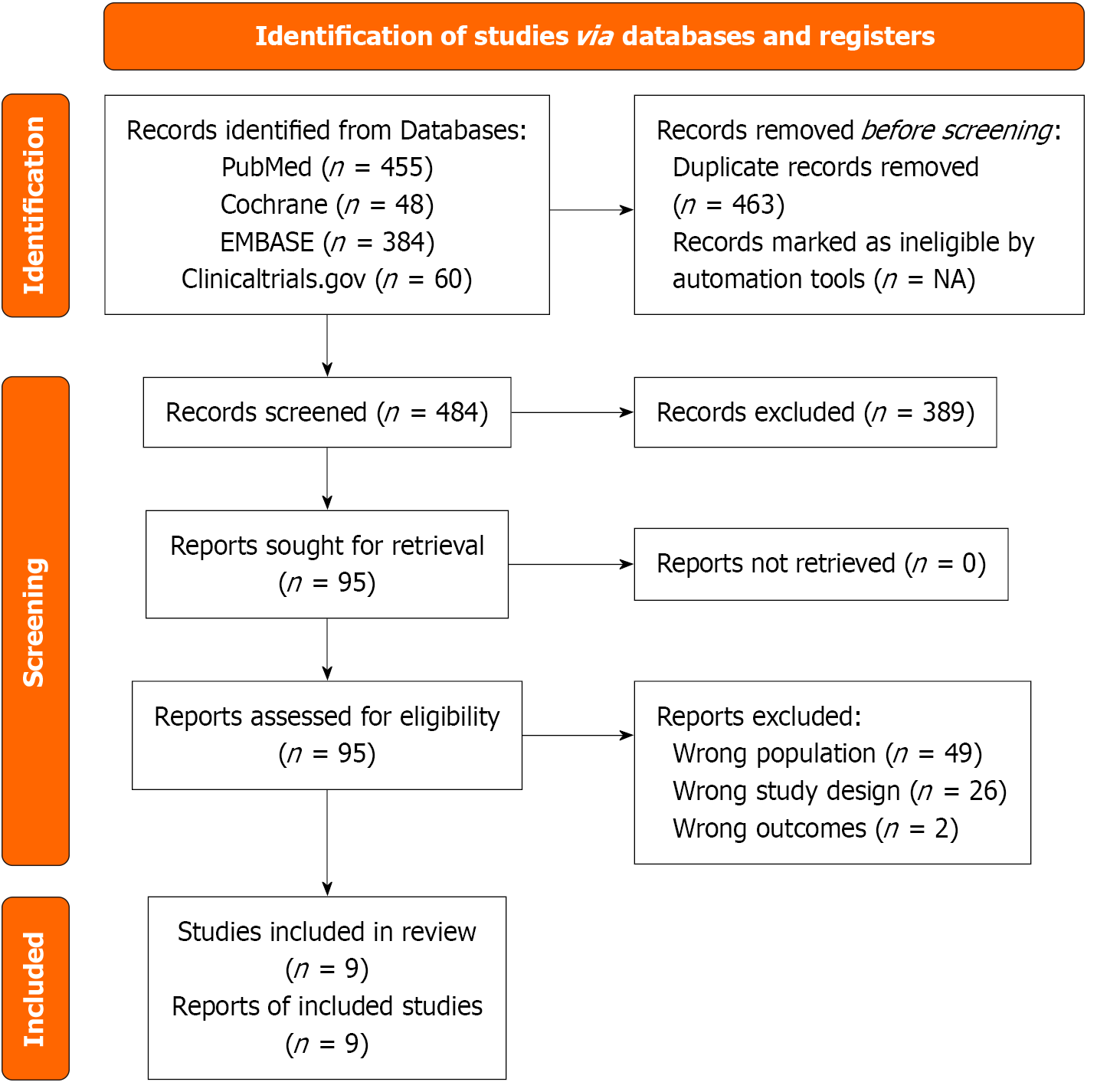
The initial search gathered about 947 articles. After removing 463 duplicates, 484 articles remain for screening. Title and abstract screening resulted in the exclusion of 389 articles, and 77 out of the remaining 95 studies were excluded after full-text screening (49 due to wrong population, 2 due to having the wrong outcome and 26 due to having the wrong study design). Eventually, 9 articles were included in the final review (Figure 1).
This meta-analysis included 9 studies that met the eligibility criteria[7,10,11,13,18-22]. It included a total of 1689 patients who underwent HF. Of these, 832 patients (49.25%) utilized the IASD, while 857 patients (50.74%) opted for the placebo/sham approach as the control group. Three studies[10,11,18] used the IASD (DC Devices Inc, Tewksbury, MA, United States), three studies[19-21] used the V-wave IASD (V-wave, V-wave Ltd, Or Akiva, Israel), one study[7] used AFR (Occlutech, Istanbul, Türkiye), one study[22] used IASD system II (Corvia Medical) and one study[13] used APTURE transcatheter shunt. The publication years ranged from 2014 to 2025. The characteristics of the studies are detailed in Tables 1 and 2[4,7,10,12,17-21].
| Ref. | Device | Study design | Device | Left ventricular ejection fraction | New York Heart Association class | Participants control | Participants IASD | Follow-up |
| Søndergaard et al[17], 2014 | IASD | Pilot trial | IASD | ≥ 45% | III/IV | 11 | 11 | 1 month |
| Kaye et al[4], 2016 | IASD | Single-arm study | IASD | ≥ 40% | II/IV | 64 | 64 | 12 months |
| Del Trigo et al[18], 2016 | V-wave | Cohort study | V-wave | ≤ 40% | III/IV | 10 | 10 | 3 months |
| Feldman et al[10], 2018 | IASD | Phase 2, randomized control trial | IASD | ≥ 40% | III/IV | 22 | 21 | 1 months |
| Rodés-Cabau et al[19], 2024 | V-wave | Non-randomized control trial (single-arm open-label study) | V-wave | > 15% | III/IV | 38 | 36 | 12 months |
| Paitazoglou et al[7], 2021 | AFR device | Prospective, multicentre, open-label, non-randomised pilot | AFR device | ≥ 15% | III/IV | 36 | 36 | 3 months |
| Stone et al[20], 2024 | Ventura shunt | Randomized, double-blind, placebo procedure–controlled, multicenter trial | Ventura shunt | 30.2% (≤ 40%) in 206 patients and preserved 55.8% (> 40%) in 302 patients | II–IVa | 258 | 250 | 2 years |
| Gustafsson et al[21], 2024 | IASD system II (Corvia Medical) | Multicenter, randomized, double blind, sham-controlled trial | IASD system II (Corvia Medical) | ≥ 50% | II/III | 312 | 309 | 2 years |
| Fioretti et al[12], 2025 | APTURE shunt | Open-label, single-arm, nonrandomized trial | APTURE shunt | > 40% | II–IV | 106 | 95 | 2 years |
| Ref. | Age, mean ± SD (years) | Male sex (%) | Body mass index (kg/m2) | Coronary artery disease (%) | Hypertension (%) | Diabetes mellitus (%) | Atrial fibrillation/flutter (%) | Left ventricular ejection fraction, mean ± SD (%) |
| Søndergaard et al[17], 2014 | 70 ± 11.9 | 45 | Not reported | 36 | 91 | 45 | 36 | 57 ± 9 |
| Kaye et al[4], 2016 | 69 ± 8 | 35 | 33 ± 6 | 36 | 81 | 33 | 36 | 47 ± 7 |
| Del Trigo et al[18], 2016 | 62 ± 8 | 90 | 31 ± 5 | 90 | 70 | 70 | 70 | 25 ± 8 |
| Feldman et al[10], 2018 | 69.6 ± 8.3/70.0 ± 9.2 (treatment/control) | 63.6/36.4 | 35.2 ± 6.4/35.1 ± 9.1 | 22.7% (5/22) | 81.8/90.9 | 54.5/54.4 | 59.1/54.5 | 59.9 ± 9.0/58.5 ± 6.9 |
| Rodés-Cabau et al[19], 2024 | 66 ± 9 | 92 | 30 ± 6 | 79 | 84 | 68 | 53 | 50 ± 9 (HFpEF), 26 ± 7 (HFrEF) |
| Paitazoglou et al[7], 2021 | 67.3 ± 8.6 | 58.3 | 30.7 ± 6.7 | 55.6 | 66.7 | 52.8 | 47.2 | 51.5 ± 6 (HFpEF), 31.9 ± 7 (HFrEF) |
| Stone et al[20], 2024 | 73 ± 7.5 | 62.8 | 30.2 ± 5.6 | 64.7 | 83.6 | 49 | 64.7 | 45.4 ± 18.9 vs 45.3 ± 17.9 |
| Gustafsson et al[21], 2024 | 71.67 ± 8.17 | 38.5 | 31.9 ± 2.3 | 16.1 | 88.2 | 36.9 | 62.1 | HFrEF (7.1) vs HFpEF (92.9) |
| Fioretti et al[12], 2025 | 70.9 ± 8.47 | 49.5% (47/95) | 33.7 ± 7.0 | 56.8% (54/95) | 87.4 % (83/95) | 40.0% (38/95) | 58.9% (56/95) | 62.50 ± 8.00 |
The risk of bias in included studies was assessed using Cochrane ROB 2.0 for RCTs (Supplementary Figure 1)[10,20,21], ROBINS-I for non-randomized studies (Supplementary Figure 2)[4,7,12,17,19] and the NOS for observational studies (Supplementary Table 2)[18].
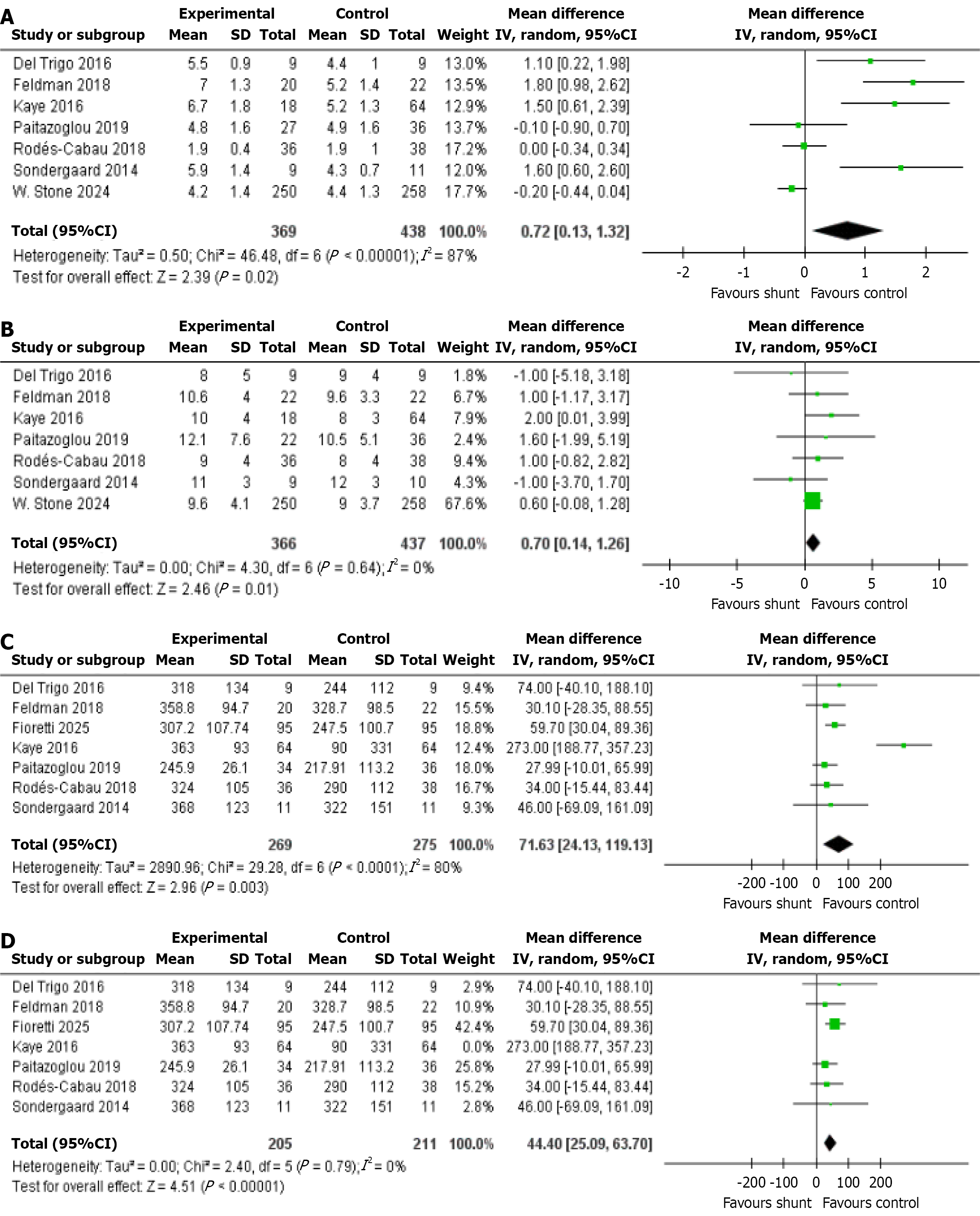
Cardiac output: This outcome was reported across seven studies, encompassing a total of 807 patients, 369 of whom received the IASD, while 438 were assigned to the placebo/sham group as controls. The statistical analysis showed a significant difference in cardiac output [mean difference (MD): 0.72, 95%CI: 0.13-1.32, P = 0.02, I2 = 87%; Figure 2A][4,7,10,17-20].
Mean RAP: Seven studies reported this outcome, consisting of a total of 803 patients. Of these, 366 underwent treatment with the IASD, while 437 were placed in the placebo/sham control group. The statistical analysis highlighted a significant difference in the mean RAP (MD: 0.70, 95%CI: 0.14-1.26, P = 0.01, I2 = 0%; Figure 2B)[4,7,10,17-20].
6MWD: This outcome was documented by 7 studies, involving a total of 544 patients. A total of 269 patients received treatment with the IASD, while 275 were allocated to the placebo/sham control group. Statistical analysis indicated a significant difference in the 6MWD between patients who received the IASD and those in the placebo/sham control group (MD: 71.63, 95%CI: 24.13-119.13, P = 0.003, I2 = 80%; Figure 2C)[4,7,10,12,17-19].
6MWD (LOO): LOO analysis was conducted due to high heterogeneity (I2 = 80%). The omission of Kaye et al[4], 2016, resulted in a clear reduction in heterogeneity, confirming its significant contribution to variability. Despite this, the overall effect estimate remained consistent (MD: 44.40, 95%CI: 25.09-63.70, P < 0.00001; Figure 2D)[4,7,10,12,17-19].
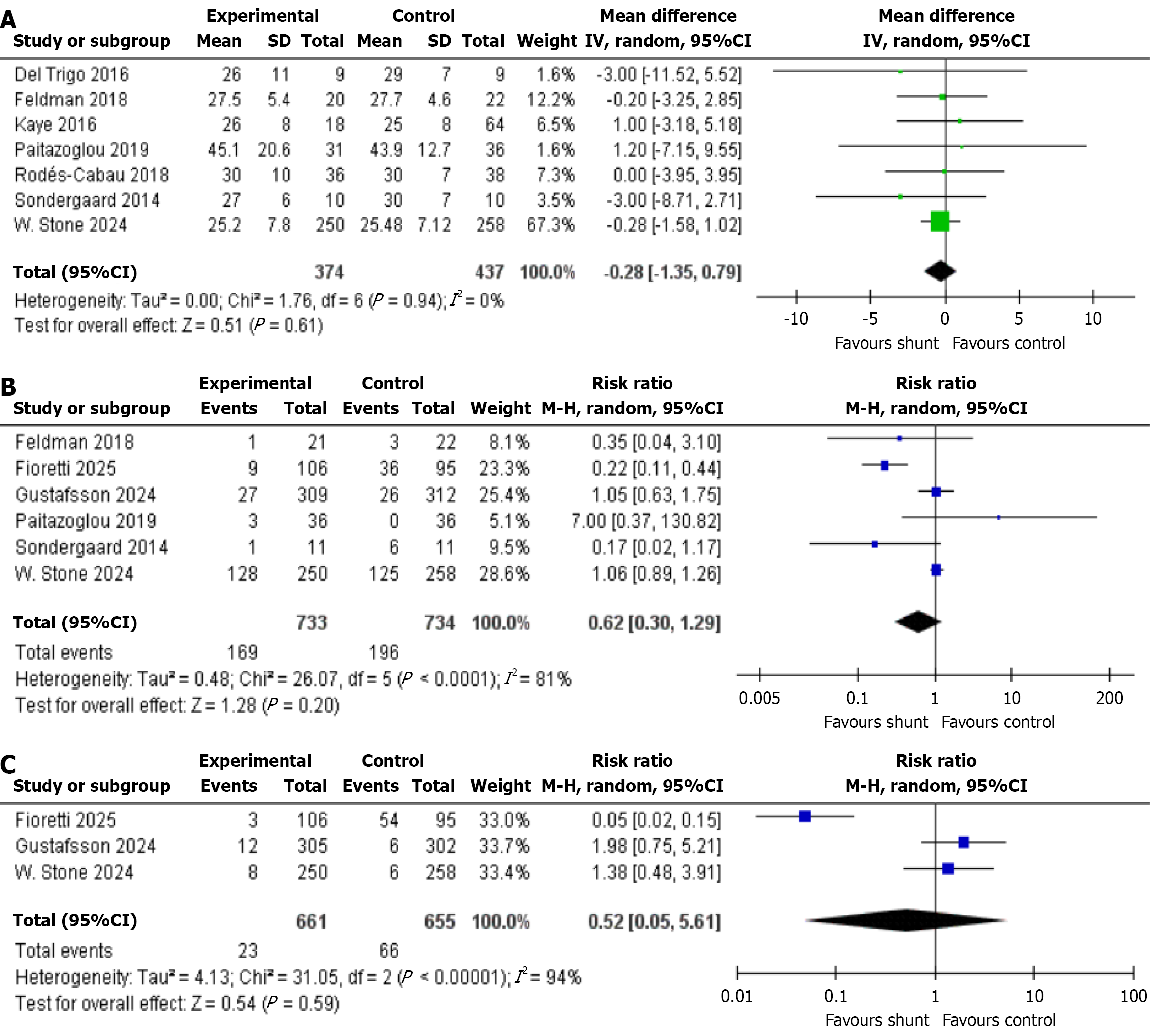
Mean pulmonary artery pressure: This outcome is reported by seven studies involving 811 patients in total-374 with IASD and 437 with placebo/sham group. The analysis found no statistically significant difference in medial plantar artery perforator between the two groups (MD: -0.28, 95%CI: -1.35 to 0.79, P = 0.61, I2 = 0%; Figure 3A)[4,7,10,17-20].
All-cause HF events: Six studies reported this outcome, involving a total of 1467 patients. Among them, 733 received treatment with the IASD, while 734 were assigned to the placebo/sham control group. The analysis revealed no statistically significant difference in the occurrence of all-cause HF events between the two methods (RR: 0.62, 95%CI: 0.30-1.29, P = 0.20, I2 = 81%; Figure 3B)[7,10,12,17,20,21].
Cardiac death: Cardiac death was reported by three studies, comprising 1316 patients (IASD was 661 vs control was 655). The statistical analysis indicated no significant difference in the risk of cardiac death (RR: 0.52, 95%CI: 0.05-5.61, P = 0.59, I2 = 94%; Figure 3C)[12,20,21].
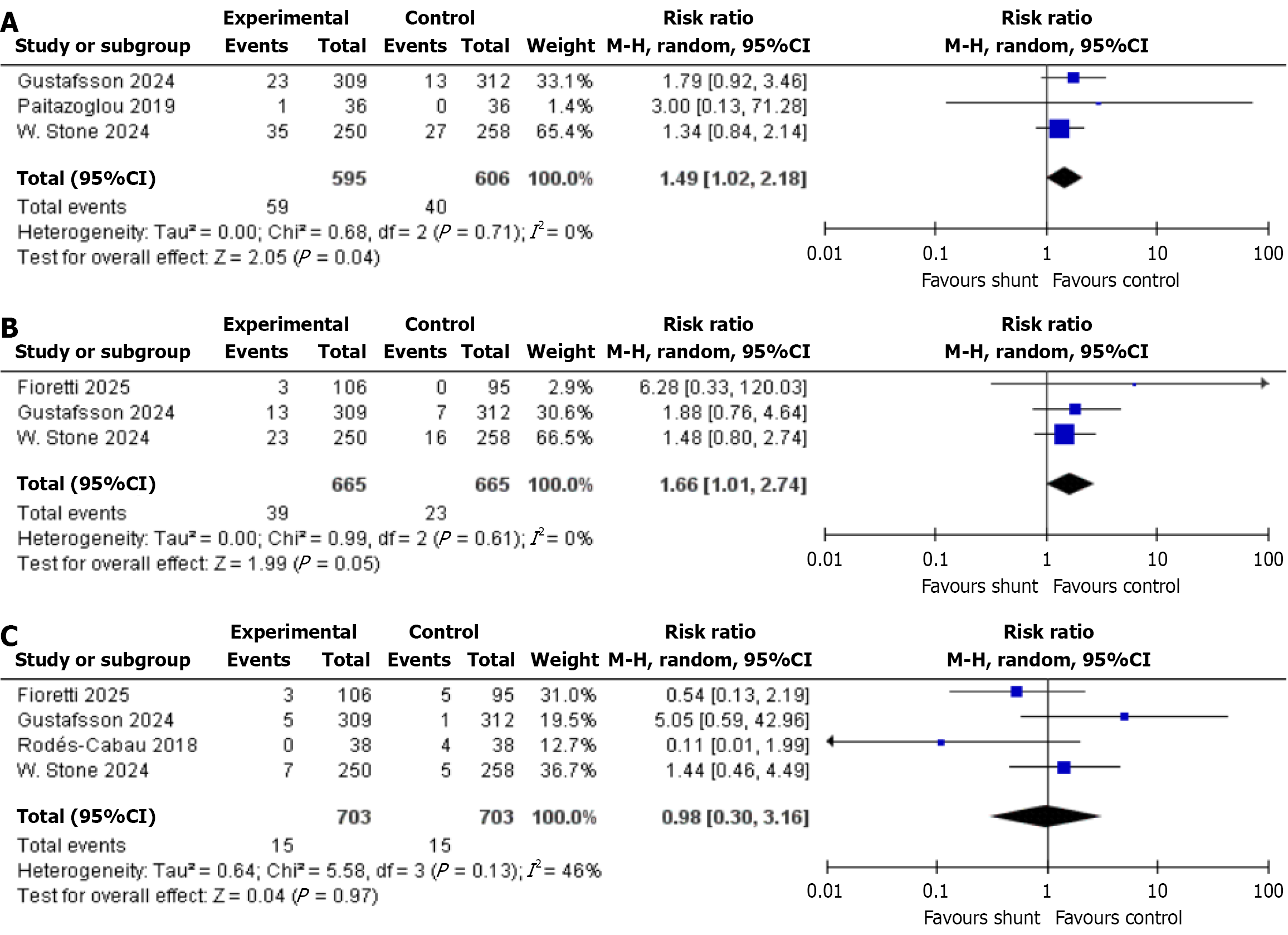
All-cause mortality: This outcome was reported by 3 studies, which together included a total of 1201 patients-595 of whom received the IASD, while 606 were assigned to the placebo/sham group as controls. The statistical analysis showed a significant drop in the risk of all-cause mortality (RR: 1.49, 95%CI: 1.02-2.18, P = 0.04, I2 = 0%; Figure 4A)[7,20,21].
Cardiovascular death: A total of three studies with a total sample size of 1330 patients (IASD was 665 and control was 665) reported the cardiovascular death outcome among patients in both groups. A significant difference was observed in the incidence of cardiovascular death between IASD and placebo group (RR: 1.66, 95%CI: 1.01-2.74, P = 0.05, I2 = 0%; Figure 4B)[12,20,21].
Ischemic stroke: According to four studies, which included a total of 1406 patients (703 with IASD and 703 with placebo/sham as control group). The analysis found no statistically significant difference in risk of ischemic stroke between the two groups (RR: 0.98, 95%CI: 0.30-3.16, P = 0.97, I2 = 46%; Figure 4C)[12,19-21].
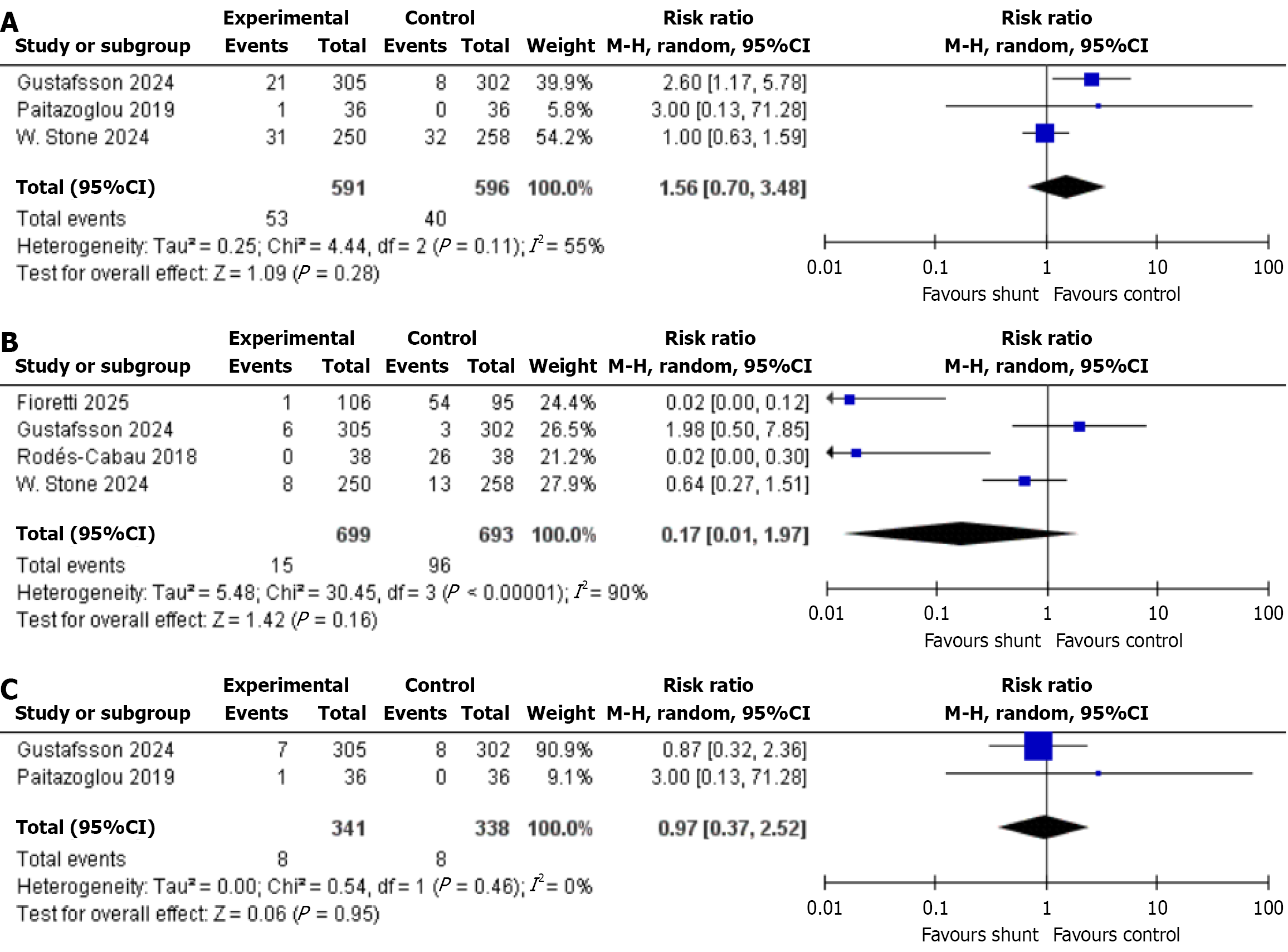
MACE: Three of the included studies assessed this outcome. In a total of 1187 patients (IASD was 591 vs was control 596), statistical analysis revealed no significant difference between the two approaches (RR: 1.56, 95%CI: 0.70-3.48, P = 0.28, I2 = 55%; Figure 5A)[7,20,21].
Myocardial infarction: Only four out of nine studies with 1392 patients (IASD was 699 vs placebo was 693) reported the incidence of myocardial infarction. Our analysis concluded no significant difference (RR: 0.17, 95%CI: 0.01-1.97, P = 0.16, I2 = 90%; Figure 5B)[12,19-21].
New onset atrial fibrillation: The new onset of AF was examined across two studies, encompassing a total of 679 patients-341 treated with IASD and 338 with Placebo. The results of the analysis revealed no significant statistical diffe
Our meta-analysis presented a comprehensive analysis of cardiac and patient outcomes following intervention with IASDs vs control for management of HF. We recorded a significantly higher RAP on using shunt devices. The shunt arm also noted significantly higher 6MWD times. On the other hand, long term outcomes like development of new-onset AF, myocardial infarction, ischemic stroke, cardiovascular death, cardiac tamponade, and HF were similar between both groups. Cardiac output was significantly higher on use of shunt devices. Risk of all-cause mortality and cardiovascular death was significantly elevated in the shunt arm.
Interatrial shunt therapy has gained attention over the past decade as a novel therapeutic approach for symptomatic HF patients, particularly those with elevated LAP and preserved EF. Early-phase trials such as REDUCE LAP-HF I demonstrated promising hemodynamic and symptomatic improvements following shunt placement[10]. Mechanistically, the shunt acts as a passive pressure-relief valve, enabling autoregulatory blood flow across the atrial septum, especially during exercise or volume overload[22]. The therapeutic rationale is particularly relevant in HFpEF, where elevated LAP and pulmonary congestion are often disproportionate to declines in systolic function[23]. However, subsequent studies, including REDUCE LAP-HF II and RELIEVE-HF, have yielded mixed results, with some failing to meet primary end
Atrial shunts function mainly to lower LAP in patients with HF[24]. This acts through an autoregulatory mechanism, maintaining low atrial pressures[21]. Additionally, pulmonary wedge pressure is reduced, enhancing exercise tolerance in patients with HF[25]. However, recent studies have shown significant differences in their findings, with certain evidence suggesting that the efficacy of these shunts may be limited to patients with HFpEF[21], with higher incidence of adverse events in those with lower EFs.
Across all types of HF, we noted significantly higher RAPs on use of shunt devices. These findings are consistently reported across a majority of the included studies (Kaye et al[4], 2016, Stone et al[20], 2024). This is mainly due to the device functioning as a left to right shunt, reducing the elevated LAP, and diverting blood flow to RAP. Despite this, the increase in RAP was not considerably high, suggesting that while RAP increases, it may not impair daily functioning. Elevated RAP results in lung congestion, thus causing dyspnea on exertion. The device may be suitable in reducing risks of pulmonary hypertension. While the rise in RAP may be unavoidable due to the nature of the device, this increase must be as minimal as possible to preserve exercise tolerance. Certain included studies, including Fioretti et al[12] have shown insignificant rise in RAP[26]. This was noted in patients with mildly reduced or preserved EF. This may suggest a higher safety of the device in those with lower risk of decompensation owing to poor EF.
A major concern with the use of IASDs were the adverse events. However, the majority of the recent studies have noted no obvious increase in incidence of adverse events[6,27,28]. Safety outcomes across studies have been followed up to 1 year and show similar incidences of adverse events on use of shunt and placebo, suggesting long-term efficacy of these devices. However, our study noted a significantly higher risk of all-cause death and cardiovascular mortality. The RELIEVE-HF trial suggests poorer safety in those with preserved EF than those with mildly reduced EF. However, the trial failed to achieve its primary endpoint, thus requiring a longer follow-up period to assess the efficacy of shunt devices across all types of HF. A reason for this difference may lie in the right ventricular reserve and compliance diffe
Additionally, we noted significantly higher 6MWD in the shunt cohort, suggesting improvement in exercise tolerance. This was consistently reported across included studies[4], both at 6-month and 1 year of follow-up. Improvement in 6MWD holds significance in improving patient condition, both physically and helping in rehabilitation in the elderly[30]. Concurrently, shunts have shown reduction in pulmonary capillary wedge pressures, thus resulting in better exercise performance[6].
We also recorded a significantly improved cardiac output on use of IASDs vs placebo. Interatrial shunting lowers LAP by decompressing the left atrium, which reduces backward pressure into the pulmonary veins. This improves pulmonary venous return, facilitating more effective left ventricular (LV) filling and increasing stroke volume and cardiac output[8]. Elevated LAP in HFpEF reflects impaired LV relaxation and compliance. By reducing LAP, an interatrial shunt allows the LV to fill more effectively during diastole, improving preload and cardiac output through the Frank-Starling mechanism[4].
This meta-analysis is constrained by several limitations. First, the number of eligible studies and total patient sample was relatively small, limiting statistical power. Second, we did not perform subgroup analyses by HF phenotype (HFpEF, HFmrEF, HF with preserved ejection fraction), which may have obscured differential responses. Third, heterogeneity in device types, procedural expertise, and follow-up protocols may have influenced outcome variability. Moreover, we could not consistently account for important confounding factors such as age, frailty, comorbidities like pulmonary hy
Future trials should focus on stratified patient selection using hemodynamic or biomarker-guided criteria and stan
IASDs may represent a promising therapeutic option for selected patients with HF, particularly those with elevated LAP and preserved or mildly reduced EF. These devices have the potential to improve quality of life and functional capacity without significantly increasing adverse event risk. However, patient selection is crucial. Identifying individuals with appropriate right heart function and atrial compliance is essential to minimize RAP-related complications. The favorable safety and functional outcomes reported in the short to intermediate term suggest that interatrial shunts may comple
| 1. | Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:387-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 563] [Article Influence: 112.6] [Reference Citation Analysis (1)] |
| 2. | Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 3. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1415] [Article Influence: 157.2] [Reference Citation Analysis (0)] |
| 4. | Kaye DM, Hasenfuß G, Neuzil P, Post MC, Doughty R, Trochu JN, Kolodziej A, Westenfeld R, Penicka M, Rosenberg M, Walton A, Muller D, Walters D, Hausleiter J, Raake P, Petrie MC, Bergmann M, Jondeau G, Feldman T, Veldhuisen DJ, Ponikowski P, Silvestry FE, Burkhoff D, Hayward C. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e003662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 886] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 6. | Yi T, Li M, Fan F, Qiu L, Wang Z, Weng H, Shang X, Zhang C, Ma W, Zhang Y, Huo Y. Haemodynamic changes of interatrial shunting devices for heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2022;9:1987-1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Paitazoglou C, Bergmann MW, Özdemir R, Pfister R, Bartunek J, Kilic T, Lauten A, Schmeisser A, Zoghi M, Anker SD, Sievert H, Mahfoud F; AFR-PRELIEVE Investigators. One-year results of the first-in-man study investigating the Atrial Flow Regulator for left atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail. 2021;23:800-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Hasenfuß G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, Malek F, Van der Heyden J, Lang I, Petrie MC, Cleland JG, Leon M, Kaye DM; REDUCE LAP-HF study investigators. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Shah SJ, Feldman T, Ricciardi MJ, Kahwash R, Lilly S, Litwin S, Nielsen CD, van der Harst P, Hoendermis E, Penicka M, Bartunek J, Fail PS, Kaye DM, Walton A, Petrie MC, Walker N, Basuray A, Yakubov S, Hummel SL, Chetcuti S, Forde-McLean R, Herrmann HC, Burkhoff D, Massaro JM, Cleland JGF, Mauri L. One-Year Safety and Clinical Outcomes of a Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018;3:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, Basuray A, Hummel SL, Forde-McLean R, Nielsen CD, Lilly S, Massaro JM, Burkhoff D, Shah SJ; REDUCE LAP-HF I Investigators and Study Coordinators. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation. 2018;137:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Griffin JM, Borlaug BA, Komtebedde J, Litwin SE, Shah SJ, Kaye DM, Hoendermis E, Hasenfuß G, Gustafsson F, Wolsk E, Uriel N, Burkhoff D. Impact of Interatrial Shunts on Invasive Hemodynamics and Exercise Tolerance in Patients With Heart Failure. J Am Heart Assoc. 2020;9:e016760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Fioretti F, Hibbert B, Eckman PM, Simard T, Labinaz M, Nazer B, Wiley M, Gupta B, Sauer AJ, Shah H, Sorajja P, Pineda AM, Missov E, Aldaia L, Koulogiannis K, Gray WA, Zahr F, Butler J. Left Atrial-to-Coronary Sinus Shunting in Heart Failure With Mildly Reduced or Preserved Ejection Fraction: The ALT-FLOW Trial (Early Feasibility Study) 2-Year Results. JACC Heart Fail. 2025;13:987-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 806] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 14. | Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf). 2022;44:e588-e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48623] [Article Influence: 2860.2] [Reference Citation Analysis (3)] |
| 16. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26276] [Article Influence: 1751.7] [Reference Citation Analysis (4)] |
| 17. | Søndergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Del Trigo M, Bergeron S, Bernier M, Amat-Santos IJ, Puri R, Campelo-Parada F, Altisent OA, Regueiro A, Eigler N, Rozenfeld E, Pibarot P, Abraham WT, Rodés-Cabau J. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Rodés-Cabau J, Lindenfeld J, Abraham WT, Zile MR, Kar S, Bayés-Genís A, Eigler N, Holcomb R, Núñez J, Lee E, Perl ML, Moravsky G, Pfeiffer M, Boehmer J, Gorcsan J, Bax JJ, Anker S, Stone GW. Interatrial shunt therapy in advanced heart failure: Outcomes from the open-label cohort of the RELIEVE-HF trial. Eur J Heart Fail. 2024;26:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Stone GW, Lindenfeld J, Rodés-Cabau J, Anker SD, Zile MR, Kar S, Holcomb R, Pfeiffer MP, Bayes-Genis A, Bax JJ, Bank AJ, Costanzo MR, Verheye S, Roguin A, Filippatos G, Núñez J, Lee EC, Laufer-Perl M, Moravsky G, Litwin SE, Prihadi E, Gada H, Chung ES, Price MJ, Thohan V, Schewel D, Kumar S, Kische S, Shah KS, Donovan DJ, Zhang Y, Eigler NL, Abraham WT; RELIEVE-HF Investigators. Interatrial Shunt Treatment for Heart Failure: The Randomized RELIEVE-HF Trial. Circulation. 2024;150:1931-1943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 21. | Gustafsson F, Petrie MC, Komtebedde J, Swarup V, Winkler S, Hasenfuß G, Borlaug BA, Mohan RC, Flaherty JD, Sverdlov AL, Fail PS, Chung ES, Lurz P, Lilly S, Kaye DM, Cleland JGF, Cikes M, Leon MB, Cutlip DE, van Veldhuisen DJ, Solomon SD, Shah SJ. 2-Year Outcomes of an Atrial Shunt Device in HFpEF/HFmrEF: Results From REDUCE LAP-HF II. JACC Heart Fail. 2024;12:1425-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Brida M, Chessa M, Celermajer D, Li W, Geva T, Khairy P, Griselli M, Baumgartner H, Gatzoulis MA. Atrial septal defect in adulthood: a new paradigm for congenital heart disease. Eur Heart J. 2022;43:2660-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 23. | Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81:1810-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 394] [Reference Citation Analysis (0)] |
| 24. | Jagadeesan V, Gray WA, Shah SJ. Atrial Shunt Therapy for Heart Failure: An Update. J Soc Cardiovasc Angiogr Interv. 2023;2:101203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, Gao Q, Hasenfuß G, Kahwash R, Kaye DM, Litwin SE, Lurz P, Massaro JM, Mohan RC, Ricciardi MJ, Solomon SD, Sverdlov AL, Swarup V, van Veldhuisen DJ, Winkler S, Leon MB; REDUCE LAP-HF II investigators. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 26. | Lichtblau M, Bader PR, Saxer S, Berlier C, Schwarz EI, Hasler ED, Furian M, Grünig E, Bloch KE, Ulrich S. Right Atrial Pressure During Exercise Predicts Survival in Patients With Pulmonary Hypertension. J Am Heart Assoc. 2020;9:e018123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Udelson JE, Barker CM, Wilkins G, Wilkins B, Gooley R, Lockwood S, Potter BJ, Meduri CU, Fail PS, Solet DJ, Feldt K, Kriegel JM, Shaburishvili T. No-Implant Interatrial Shunt for HFpEF: 6-Month Outcomes From Multicenter Pilot Feasibility Studies. JACC Heart Fail. 2023;11:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Guimaraes L, Lindenfeld J, Sandoval J, Bayés-Genis A, Bernier M, Provencher S, Rodés-Cabau J. Interatrial shunting for heart failure: current evidence and future perspectives. EuroIntervention. 2019;15:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Nagueh SF. Noninvasive Measurement of Left Atrial Stiffness in Patients With Heart Failure and Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2023;16:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Zhu J, Liu Y, Jiang H, Liu Q, Yao Z, He Y, Xia L, Wu J. Analysis of factors associated with 6MWD among older patients with chronic heart failure. J Int Med Res. 2023;51:3000605231166275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/