Copyright
©The Author(s) 2026.
World J Cardiol. Feb 26, 2026; 18(2): 114265
Published online Feb 26, 2026. doi: 10.4330/wjc.v18.i2.114265
Published online Feb 26, 2026. doi: 10.4330/wjc.v18.i2.114265
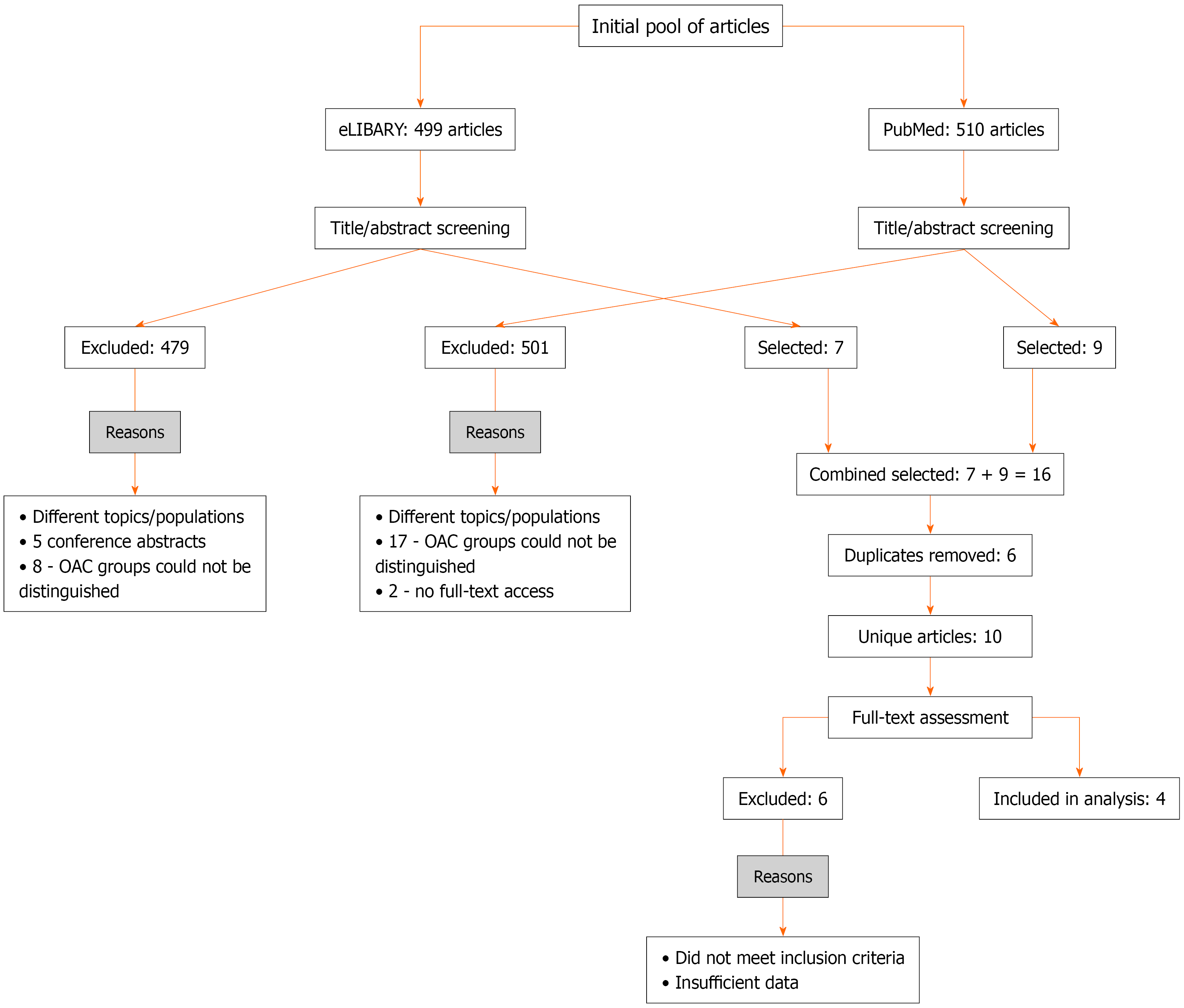
Figure 1 Flowchart of literature screening.
OAC: Oral anticoagulant.
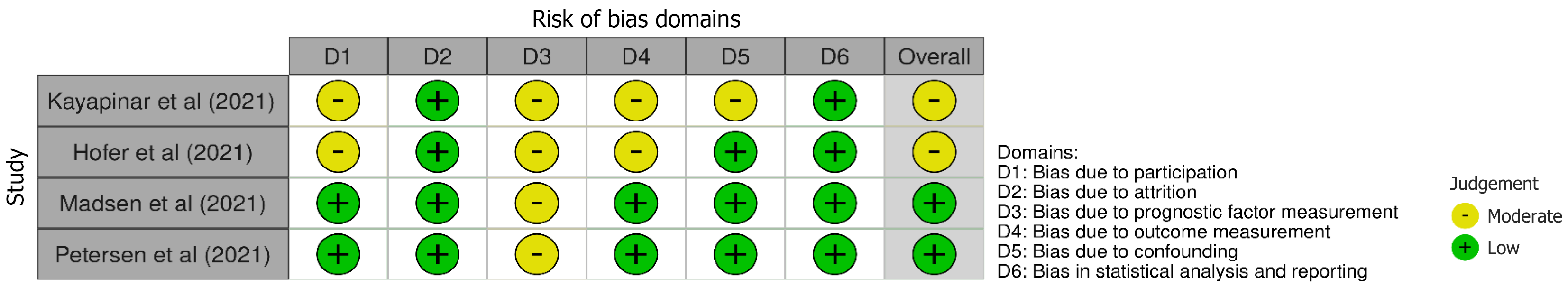
Figure 2 Risk of bias assessment in prognostic studies using the Quality in Prognosis Studies tool.
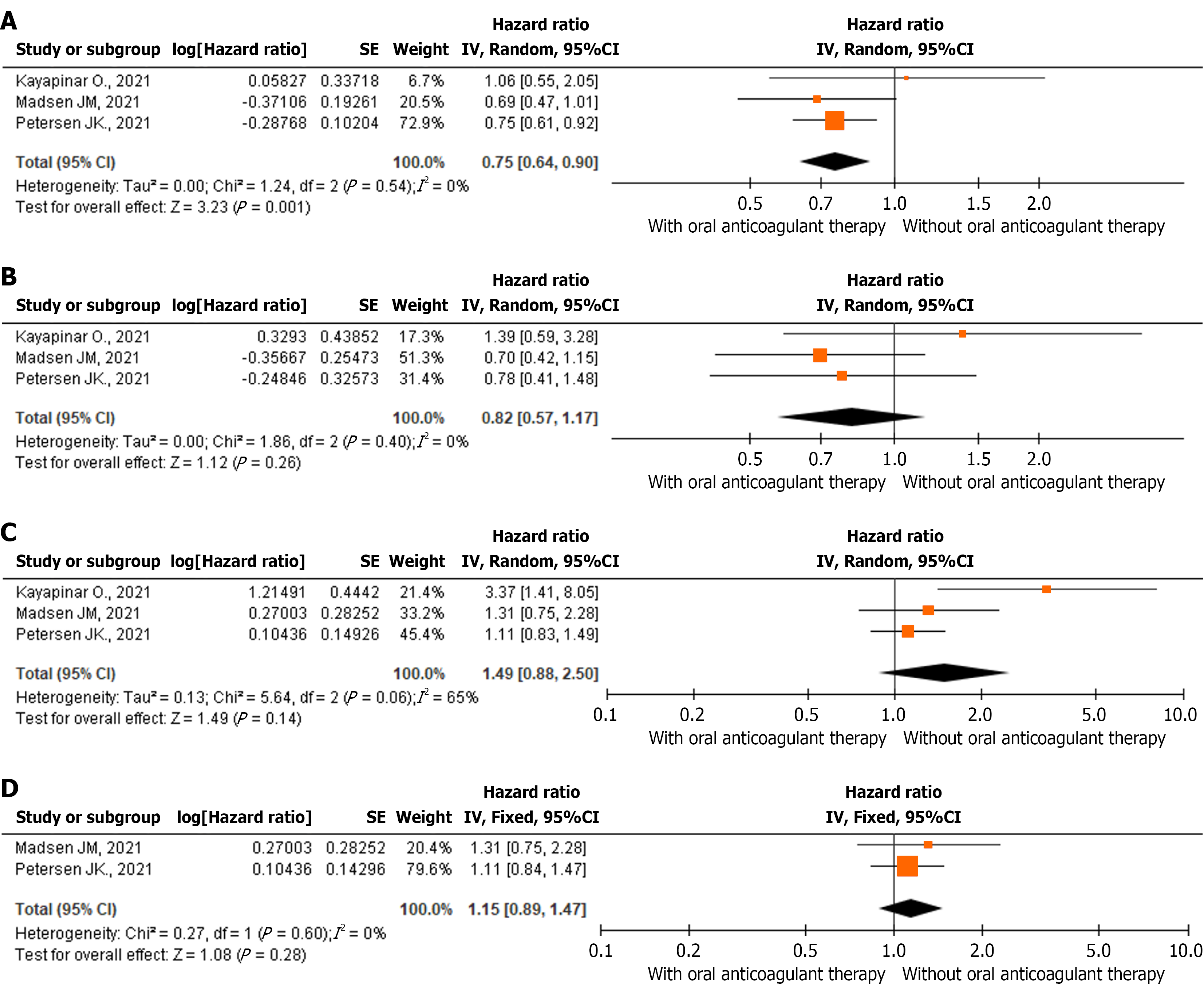
Figure 3 Results of the meta-analysis.
A: Hazard ratios (HR) for all-cause mortality in patients with new-onset atrial fibrillation (NOAF) and acute myocardial infarction (AMI); B: HR for ischemic stroke in patients with NOAF and AMI; C: HR for major bleeding and bleeding requiring hospitalization in patients with NOAF and AMI; D: HR for major bleeding and bleeding requiring hospitalization in patients with NOAF and AMI after exclusion of the study by Kayapinar et al[40]. Orange squares represent the weighted HR for each individual study (the size of the squares corresponds to the study weight); black lines represent the 95% confidence interval; the black diamond represents the pooled HR. CI: Confidence interval.
- Citation: Pereverzeva KG, Glenza A, Yakushin SS. Oral anticoagulant therapy and outcomes in new-onset atrial fibrillation during acute myocardial infarction: A systematic review and meta-analysis. World J Cardiol 2026; 18(2): 114265
- URL: https://www.wjgnet.com/1949-8462/full/v18/i2/114265.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i2.114265