TO THE EDITOR
We commend Li et al[1] for their recent publication in the World Journal of Diabetes, which reported an observational study on comparing the inflammatory response after dental implant surgery in patients with type 2 diabetes mellitus (T2DM, n = 146, age: 56 ± 5 years, 77 men and 69 women) and patients without T2DM (n = 60, age: 56 ± 4 years; 36 men and 24 women). This study provides relevant information for reducing the risk of peri-implantitis after dental implant surgery in patients with T2DM, which implies the need for further studies to bridge the gap between risk identification and effective clinical management in this vulnerable patient group.
In accordance with previous studies[2,3], the present study contributes to existing literature with data supporting the notion that inflammatory response markers in the gingival crevicular fluid gradually decreased over time in patients with normal blood glucose levels, while poor glycemic control may cause an increased inflammatory response in patients with T2DM, which may accelerate the process of peri-implant inflammation after implantation surgeries in edentulous or partially edentulous patients. However, it is essential to note that the complex, multifactorial etiopathogenetic mechanisms underlying the association between elevated inflammatory markers and marginal bone alterations after dental implant surgeries in patients with T2DM remain insufficiently elucidated, warranting further discussion.
In this manuscript, we describe additional etiopathogenetic underpinnings that may contribute to the association between elevated inflammatory markers and marginal bone loss after dental implant surgery in patients with T2DM.
Confounding effects of perirenal adipose tissue on the association between elevated inflammatory markers and peri-implant bone alterations in patients with T2DM
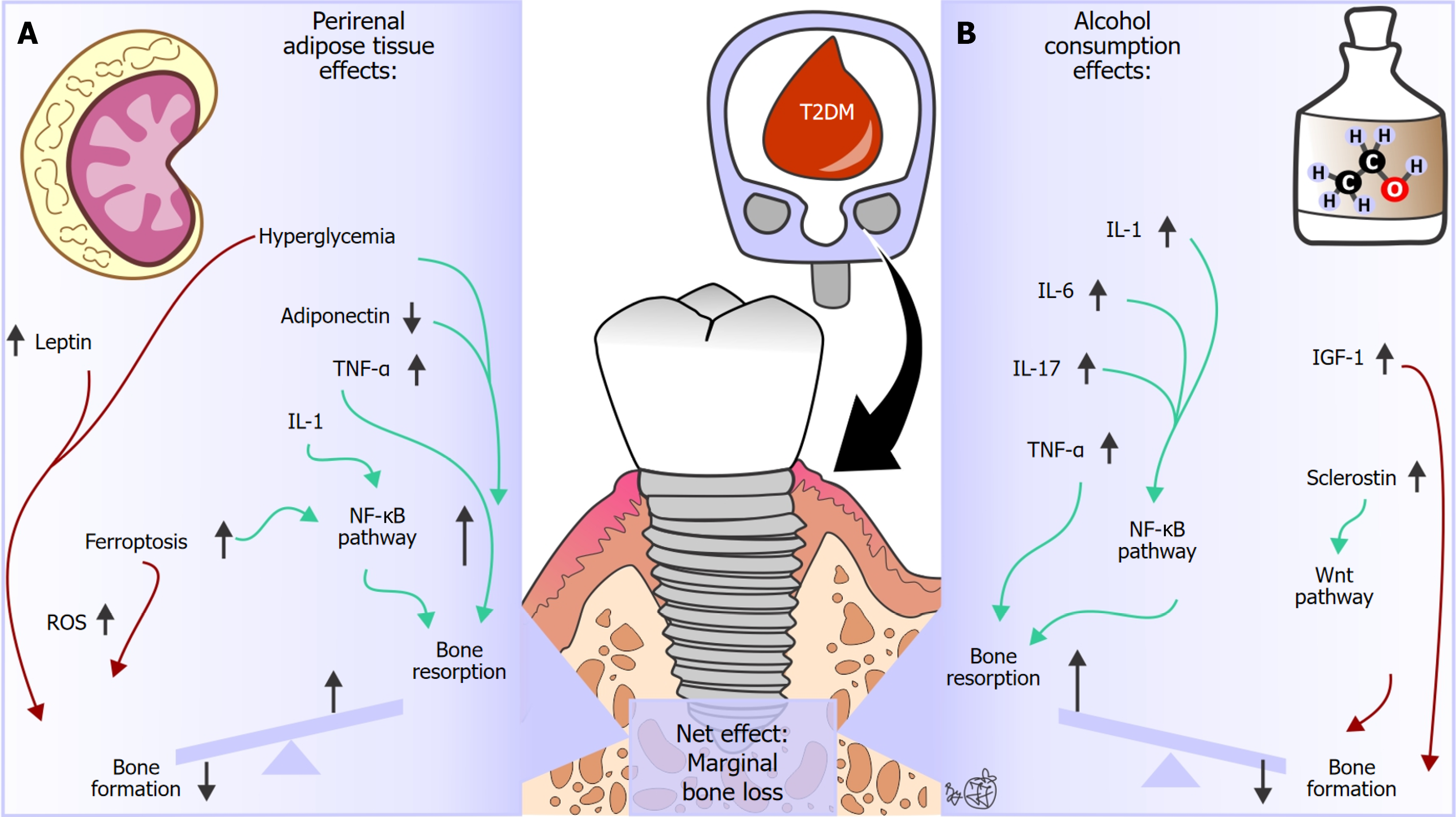
Previous studies have indicated that perirenal adipose tissue plays a crucial role in regulating bone metabolism by secreting adipokines (e.g., adiponectin, leptin, tumor necrosis factor alpha [TNF-α], interleukin 6 [IL-6]), by activating the nuclear factor kappa B (NF-κB) signaling pathway (Figure 1)[4,5]. In osteoclasts, NF-κB signaling is crucial for their differentiation from precursors and the function of bone resorption, especially during inflammation[6]. In osteoblasts, NF-κB signaling acts as a brake on bone formation through mechanisms that include interfering with bone morphogenetic protein signaling[6]. The altered systemic concentrations of inflammation markers have been associated with peri-implantitis[7], and it has been suggested that this may disrupt physiological functioning in the peri-implant tissue of patients with T2DM[3,8,9].
Figure 1 Hypothetical summary of additional etiopathogenetic underpinnings that may contribute to the association between elevated inflammatory markers and marginal bone loss in patients with type 2 diabetes mellitus.
A: Perirenal adipose tissue; B: Alcohol consumption. Green arrows indicate a subsequent excitatory effect, while red arrows indicate a subsequent inhibitory effect on bone metabolism processes. IGF-1: Insulin-like growth factor 1; IL: Interleukin; NF-κB: Nuclear factor kappa B; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor alpha.
Moreover, ferroptosis, a distinct form of cell death characterized by iron-dependent lipid peroxidation and oxidative stress, has been identified as an etiopathogenetic factor that activates the NF-κB signaling pathway[4,10,11], which may have a contributing covariant effect on marginal bone loss after dental implant surgery in patients with T2DM (Figure 1). Higher expression of ferritin and transferrin receptors, accompanied by lower expression of ferroportin[12], reportedly leads to T2DM-induced iron accumulation in bone cells. Increased iron concentrations and accumulation of reactive oxygen species trigger membrane damage and bone cell death through lipid peroxidation and glutathione depletion[4,10,12]. Moreover, previous studies have indicated that the increased number of macrophages in perirenal adipose tissue can exacerbate the inflammatory response and that perirenal adipose tissue may indirectly affect bone metabolism by enhancing insulin resistance and hyperglycemia[4,13]. Importantly, the present study describes the increased mean hemoglobin A1c values in patients with T2DM[1], revealing that uncontrolled disease and poor glycemic control contribute to marginal bone loss in these patients. Furthermore, previous studies have reported that thicker perirenal adipose tissue may affect the local metabolic environment, thereby hampering the bone tissue’s response to hormones and growth factors[8,14]. Taken together, these data indicate that perirenal adipose tissue may contribute to the observed marginal bone loss after dental implant surgery in patients with T2DM (Figure 1). However, it is essential to note that the scarce contemporary literature restricts the clinical applicability of these observations. Furthermore, the study design of the referred study (observational study, lack of adipokine serum concentration measurement, and other confounding variables that were not taken into account) limits the estimation of the potential causal relationship between perirenal adipose tissue and observed marginal bone loss after dental implant surgeries in patients with T2DM, highlighting its hypothetical nature.
Confounding effects of alcohol consumption on the association between elevated inflammatory markers and peri-implant bone alterations in patients with T2DM
It is important to highlight increasing evidence of the substantial adverse effect of alcohol consumption on hierarchical structural bone tissue organization in various skeletal sites, regardless of the presence of end-stage liver disease[15,16]. A substantial number of individuals included in the study done by Li et al[1] had a positive history of alcohol consumption (58 individuals with T2DM vs 19 individuals without T2DM), while individuals with severe liver dysfunction were excluded. Contemporary data suggest that high alcohol consumption is associated with an increase in the prevalence of peri-implantitis and marginal bone loss after dental implant surgeries[17,18]. Previous studies have noted that the dose-dependent toxic effects of alcohol on the function, differentiation, proliferation, and maturation of osteoblastic cell lines can lead to decreased bone formation[15]. Furthermore, besides reduced levels of testosterone and vitamin D, it has been reported that systemic production of pro-inflammatory cytokines (e.g., IL-1, IL-6, IL-17, TNF-α) contributes to bone loss in patients prone to alcohol consumption[15-19]. Since a detailed analysis of alcohol consumption (e.g., frequency, amount of alcohol consumption, type of alcoholic beverages) was not conducted in the present study, the stated mechanisms induced by alcohol consumption may contribute to the observed marginal bone loss after dental implant surgery in patients with T2DM (Figure 1). These observations are in accordance with a previous study done by Rodic et al[20], revealing that alcohol abuse may contribute to mandibular bone quality changes in individuals with end-stage alcohol-associated liver disease. However, it is important to note that the combined effect of alcohol consumption and T2DM on marginal bone loss after dental implant surgeries has not been investigated to date, highlighting the hypothetical nature of the causal association between alcohol consumption and observed marginal bone loss after dental implant surgery in patients with T2DM.
Conclusion
The study by Li et al[1] stimulates valuable discussion on the inflammatory response-mediated bone loss after dental implant surgery in patients with T2DM. The etiopathogenetic mechanisms underlying the association between elevated inflammatory markers and peri-implant bone alterations in patients with T2DM remain insufficiently understood. Further studies are needed to enhance our understanding of the potential covariant effects of perirenal adipose tissue and alcohol consumption on the inflammatory response-mediated bone loss after dental implant surgery in patients with T2DM. Considering the hypothetical nature of the proposed etiopathogenetic mechanisms, future studies should focus on investigating whether interventions targeting the reduction of perirenal adipose tissue, modulation of adipokine secretion, or alcohol cessation could affect dental implant outcomes in patients with T2DM. If the causal relationship is confirmed, clinical risk assessment and perioperative management in patients with T2DM undergoing dental implantation may be revised.