INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a long-standing metabolic condition that predisposes patients to a spectrum of complications, particularly those affecting the microvasculature. Diabetic retinopathy (DR), diabetic nephropathy (DN), and diabetic peripheral neuropathy (DPN) are among the most prevalent microvascular complications, collectively contributing to significant morbidity and reduced quality of life. Diabetic microvascular complications (retinopathy, nephropathy, and neuropathy) are major causes of morbidity and reduced quality of life in diabetes. They share overlapping pathogenic pathways but affect different organ systems, and early detection using biomarkers may help in predicting progression and guiding interventions. Increasing evidence highlights the pivotal role of inflammation in driving the onset and progression of these complications[1]. In this context, serum C-reactive protein (CRP), a well-established acute-phase inflammatory marker, has been extensively examined as a promising biomarker for monitoring disease severity and progression in T2DM[2]. Elevated CRP levels have been associated with endothelial dysfunction, oxidative stress, and chronic inflammation, all of which contribute to microvascular damage in diabetes[3].
CRP-derived inflammation markers, such as high-sensitivity CRP (hs-CRP) and CRP-to-albumin ratio (CAR), and CRP-to-lymphocyte count ratio (CLR) provide valuable insights into the underlying inflammatory processes that contribute to microvascular damage in diabetes[4-7]. For example, CRP and hs-CRP reflects low-grade systemic inflammation, CAR integrates both inflammatory and nutritional status, and CLR captures the balance between inflammation and immune response. These markers are linked to oxidative stress, endothelial dysfunction, and pro-inflammatory cytokine activation, which accelerate disease progression.
This review aims to explore the role of serum CRP and CRP derived inflammation markers in diabetic microvascular complications, highlighting its potential as a predictive and diagnostic marker, as well as its implications for disease management and therapeutic interventions.
CRP IN T2DM
As a key biomarker of systemic inflammation, CRP has been the focus of extensive research in T2DM. Elevated CRP concentrations are consistently associated with a greater likelihood of developing T2DM and with the presence of diabetic microvascular complications, including retinopathy, nephropathy, and neuropathy. Several studies have demonstrated a strong association between elevated CRP levels and the incidence of T2DM. For instance, a large population-based Korean cohort study found that higher CRP levels were significantly associated with an increased risk of developing T2DM, particularly among individuals with obesity and hypertension[4]. In addition to predicting the onset of T2DM, elevated CRP levels are associated with both the occurrence and severity of microvascular complications in individuals with diabetes. Chronic low-grade inflammation, as indicated by increased CRP levels, contributes to endothelial dysfunction and vascular damage, which are key factors in the development of complications such as retinopathy, nephropathy, and neuropathy[2]. Furthermore, lifestyle and clinical factors have been linked to elevated CRP in individuals with T2DM. According to a cross-sectional study within the Danish Centre for Strategic Research in Type 2 Diabetes cohort, elevated CRP levels (> 3.0 mg/L) were detected in 40% of patients with newly diagnosed T2DM, with a significantly higher prevalence observed among female patients. Factors such as higher body mass index (BMI) and poor glycemic control were significantly associated with elevated CRP levels in these patients[8]. Genetic factors also play a role in CRP levels and T2DM. Research has identified specific CRP gene polymorphisms that are associated with elevated CRP levels and an increased risk of early-onset T2DM. These genetic variants may influence the inflammatory response, thereby contributing to the pathogenesis of T2DM[9]. In addition, the Mexico City Diabetes Study showed that high CRP was attributed to the development of T2DM[10]. Moreover, a study from United States involving more than 11000 participants reported that high CRP levels were associated with complications and mortality in patients with T2DM[11]. A subsequent observational study from Denmark found that elevated serum CRP levels in patients with T2DM could be markers of diabetic vascular complications[12]. These evidences suggest that CRP serves as a valuable biomarker in T2DM, reflecting underlying inflammation that contributes to disease development and progression. Monitoring CRP levels in individuals at risk for or diagnosed with T2DM can aid in identifying those at higher risk for developing microvascular complications, allowing for targeted management strategies to reduce inflammation and improve clinical outcomes.
CRP IN DN
CRP has been involved in the pathogenesis of DN, a common microvascular complication of diabetes mellitus. Increased CRP concentrations have been correlated with the advancement of DN, suggesting that CRP may play a mechanistic role in promoting renal inflammation and fibrotic processes. Experimental studies have provided valuable data regarding the pathogenic role of CRP in DN. For instance, research using human CRP transgenic mice with streptozotocin-induced diabetes demonstrated that these mice developed more serious kidney injury than the wild-type mice. These findings were supported by markers of renal injury, including enhanced urinary albumin excretion, increased expression of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α, and excessive accumulation of extracellular matrix components, particularly collagen types I, III, and IV. These findings suggest that CRP exacerbates renal inflammation and fibrosis in the context of diabetic kidney disease (DKD)[13]. Further mechanistic studies have identified specific signaling pathways through which CRP may promote DN. In a study involving db/db mice expressing human CRP, it was found that CRP exacerbated DKD via the CD32b-Smad3-mTOR signaling pathway. Activation of this pathway led to increased renal inflammation and fibrosis, highlighting a potential target for therapeutic intervention[14]. Moreover, a mendelian randomization study involving 2332 subjects revealed that CRP was casually related to DN[15]. Moreover, a meta-analysis which studied CRP levels in 1331 DN patients and 1779 control subjects, revealed that CRP levels were significantly higher in DN patients compared to controls[16]. CRP promotes epithelial mesenchymal transition and thus accelerates the development of DN[17]. These data indicate that CRP plays a contributory role in the development and progression of DN by promoting renal inflammation and fibrosis. Targeting CRP-mediated pathways may offer potential therapeutic strategies for managing this debilitating complication of diabetes.
CRP IN DR
CRP may also have a role in DR, a common microvascular complication of diabetes mellitus. Increased CRP concentrations have been associated with the occurrence and progression of DR, suggesting that inflammatory processes may play a key role in its underlying pathogenesis. Animal studies have provided insights into the pathogenic role of CRP in DR. For instance, research using human CRP transgenic rats demonstrated that elevated CRP levels exacerbated retinal damage in diabetic conditions. These rats exhibited increased retinal neovascularization, oxidative stress, and apoptosis, leading to functional impairments such as declined electroretinography responses and reduced retinal thickness. These findings suggest that elevated CRP contributes to retinal inflammation and vascular abnormalities in DR[18]. Clinical studies have also investigated the link between CRP and DR. An observational case study involving 90 participants found that serum CRP levels were significantly higher in patients with proliferative DR (PDR) compared to diabetic patients without PDR and healthy controls. However, CRP levels did not significantly decrease after three months of laser treatment, indicating that while CRP may serve as a biomarker for the presence of PDR, it may not be useful for monitoring treatment response[19]. Interestingly, some studies have reported an inverse relationship between CRP levels and DR. A study analyzing data from the Multi-Ethnic Study of Atherosclerosis found that individuals with diabetes who had higher levels of CRP and BMI were less likely to have DR. This unexpected finding suggests a complex interplay between inflammation, body weight, and the development of DR, warranting further research to elucidate these relationships[20]. According to the accumulated evidence in literature, higher CRP has been associated with the presence and severity of DR, however, the exact role of CRP in its pathogenesis remains complex and not fully understood. Further research is needed to clarify the mechanisms by which CRP and inflammation contribute to DR and to determine the potential of CRP as a biomarker for diagnosis and monitoring of this condition.
CRP IN DPN
DPN is associated with high burden of inflammation as marked by CRP levels. Elevated CRP has been linked to the evolution and severity of DPN, which suggests significant role of inflammation in its progression. A study investigating the association between inflammation, microvascular reactivity, and peripheral diabetic neuropathy found that patients with neuropathy exhibited higher levels of inflammatory markers, including CRP, compared to non-neuropathic diabetic patients and healthy controls. Notably, patients with painful neuropathy had significantly higher CRP levels than those with painless neuropathy, indicating a potential link between CRP levels and neuropathic pain intensity[21]. Research has also explored the association between CRP and subclinical peripheral neuropathy in prediabetic individuals. Findings suggest that elevated CRP correlates with peripheral nerve dysfunction even before the clinical onset of diabetes, highlighting the role of inflammation in early nerve damage[22]. To sum up, elevated CRP levels are associated with the presence and severity of DPN, underscoring the role of systemic inflammation in its pathogenesis. Monitoring CRP levels may aid in identifying individuals at risk for developing DPN and in assessing the severity of existing neuropathic conditions.
HS-CRP IN T2DM
hs-CRP is a biomarker indicative of inflammation and being largely studied in connection to T2DM. High hs-CRP has been linked to an elevated risk of developing T2DM across diverse populations. In the Singapore Chinese Health Study, a prospective analysis demonstrated that higher plasma hs-CRP was linked to a greater risk of incident T2DM among Chinese individuals. This finding underscores the role of inflammation in the etiology-pathogenesis of T2DM within this demographic[5]. Similarly, the Jackson Heart Study, focusing on African American participants, found that elevated hs-CRP levels were significantly associated with the onset of T2DM. This study highlighted the importance of hs-CRP as a predictive marker for diabetes in African American communities[23]. Research involving urban North Indian populations also revealed a strong correlation between increased hs-CRP and T2DM. This suggests that inflammation is an important contributor to the development of T2DM across different ethnic groups[24]. Beyond its role in predicting the onset of T2DM, hs-CRP has been linked to complications arising from diabetes. A study examining patients with T2DM revealed that increased hs-CRP levels were related to an increased risk of DKD. This association emphasizes the potential of hs-CRP as a marker for identifying patients at higher risk for DKD, thereby facilitating early intervention strategies[25]. Further supporting the link between hs-CRP and T2DM, studies suggest that chronic low-grade inflammation contributes to the dual mechanisms of insulin resistance and β-cell dysfunction, both of which are central to the pathogenesis of T2DM. Elevated hs-CRP levels reflect this inflammatory state, providing insight into the underlying mechanisms of the disease[2]. It is obvious that hs-CRP serves as a valuable biomarker in understanding the inflammatory processes involved in T2DM. Moreover, a recent meta-analysis revealed that hs-CRP was an independent risk factor for diabetic complications in patients with T2DM[26]. Its elevation is consistently associated with an increased risk of developing T2DM and its complications across various populations. Monitoring hs-CRP levels could aid in identifying individuals at higher risk, allowing for targeted preventive measures and personalized therapeutic approaches.
HS-CRP IN DN
hs-CRP is extensively studied in DN. Increased hs-CRP has been consistently associated with the presence and advancement of DN. This suggests the important role of inflammation in DN pathogenesis. A meta-analysis demonstrated that patients with DN have markedly elevated hs-CRP concentrations compared to both healthy individuals and diabetic patients without nephropathy. This finding indicates that elevated hs-CRP levels are closely linked to the development of DN[16]. Further supporting this association, a study involving type 2 diabetic patients found that those with nephropathy exhibited higher serum hs-CRP levels than those without nephropathy. This suggests that increased hs-CRP concentrations may reflect the inflammatory processes contributing to renal damage in diabetic individuals[27]. Experimental research has provided insights into the mechanistic role of CRP in DN. Studies using diabetic mouse models expressing human CRP have shown that elevated CRP levels exacerbate renal inflammation and fibrosis. This progression is mediated through the activation of pathways such as CD32b, NF-κB, TGF-β/Smad3, and mTOR signaling, which are known to contribute to the pathophysiology of DN[14]. However, the predictive value of hs-CRP for the advancement of DN remains a topic of investigation. A prospective Japanese cohort study of patients with type 2 diabetes reported that high baseline hs-CRP was linked to an elevated risk of DN but did not predict its progression. This finding suggests that while hs-CRP may serve as a marker for the onset of DN, its role in monitoring disease progression requires further clarification[28]. These data suggest that high hs-CRP is related with the development and presence of DN, highlighting the significant role of inflammation in its pathogenesis. Monitoring hs-CRP concentrations in diabetic patients could aid in identifying those at higher risk for nephropathy, potentially guiding early intervention strategies.
HS-CRP IN DR
The hs-CRP is also studied in another microvascular complication of diabetes mellitus i.e., DR. Elevated hs-CRP concentrations have been linked to the presence and progression of DR, suggesting that inflammatory mechanisms play a crucial role in its pathophysiology. A systematic review and meta-analysis aimed to clarify the relationship between CRP levels and DR. The analysis revealed that patients with DR had significantly higher CRP levels compared to those without DR, supporting the notion that elevated CRP is associated with the development of retinopathy in diabetic individuals[29]. Animal studies have provided data of the mechanisms of the role of CRP in DR. Research utilizing human CRP transgenic rats demonstrated that elevated CRP levels exacerbated retinal inflammation and neovascularization. These effects were mediated through pathways such as oxidative stress and apoptosis, contributing to retinal damage[18]. However, the relationship between hs-CRP and DR is complex. A study examining the association between CRP, BMI, and DR found that while elevated CRP levels were initially associated with DR, this association was attenuated after adjusting for BMI. This suggests that BMI may confound the relationship between CRP and DR, indicating that the link between inflammation and retinopathy is influenced by multiple factors[20]. Current evidence indicate that elevated hs-CRP levels are associated with the presence and severity of DR, highlighting the role of inflammation in its pathogenesis. Monitoring hs-CRP concentrations in diabetic patients could aid in identifying those at higher risk for retinopathy, potentially guiding early intervention strategies. Yet, advanced research is needed to elucidate the precise mechanisms by which CRP contributes to DR and to determine its utility in predicting disease progression.
HS-CRP IN DPN
Another complication of T2DM, DPN, has been reported to be associated with hs-CRP levels. Increased serum hs-CRP levels have been linked to the presence and severity of DPN. This suggests that inflammation plays a significant role in its pathogenesis. A study examining microvascular reactivity and inflammatory cytokines in patients with DPN found that those with neuropathy exhibited higher serum hs-CRP levels compared to non-neuropathic diabetic patients and healthy control subjects. Notably, patients with painful neuropathy had markedly elevated hs-CRP concentrations than the patients with painless neuropathy, indicating a correlation between high hs-CRP and the presence of neuropathic pain[21]. Moreover, another research has suggested that increased hs-CRP was linked to the development and progression of DPN. In a study involving T2DM patients, those with polyneuropathy and neuropathic pain showed significantly higher hs-CRP levels compared to those without neuropathy. This finding suggests that elevated hs-CRP may reflect the inflammatory mechanisms contributing to nerve damage and pain in DPN[30]. The association between hs-CRP and DPN is further supported by evidence indicating that chronic subclinical inflammation contributes to the development of diabetic complications. High hs-CRP has been connected with endothelial dysfunction and vascular pathology, which are critical factors in the progression of neuropathy[2]. Hence, taking all together, in summary, the presence and severity of DPN is connected with elevated hs-CRP levels. Follow-up of hs-CRP concentrations in diabetic patients could aid in identifying those at higher risk for neuropathy, potentially guiding early intervention strategies.
CAR IN T2DM
The CRP to serum albumin ratio has emerged as an important biomarker for assessing systemic inflammation and nutritional status in multiple diseases, including T2DM. By integrating CRP levels with serum albumin, CAR offers a composite indicator that captures the interplay between inflammatory processes and nutritional health. The role of CAR, as a useful diagnostic or prognostic marker, has been established in various inflammatory conditions including cardiovascular disease[6], malignancy[31,32], infection[33], chronic obstructive pulmonary disease[34], autoimmune disorders[35], and cirrhosis[36]. Moreover, it has been considered as an independent predictive marker for mortality in intensive care population[37]. In the context of T2DM, low-grade chronic inflammation is a well-recognized contributor to the diabetic complications. An epidemiological study observed the incident diabetes mellitus and reported that compared to the lowest quartile of CAR group, highest quartile of CAR group had 1.6 times more risk for developing diabetes during 7.6 years of follow-up period[38]. Another study showed that patients with prediabetes and T2DM had significantly higher CAR levels compared to the controls[39]. This finding was confirmed by Caliskan et al’s study which reported 77.21% sensitivity and 52.44% specificity of CAR in discriminating T2DM[40]. There are also conflicting results in the literature. A study from Saudi Arabia reported that CAR levels of patients with T2DM and nondiabetic subjects were not significantly different[41]. Yet, the relationship between CAR and T2DM should be elaborated in future studies.
CAR IN DN
Since CAR has emerged as a significant biomarker in assessing inflammation and nutritional status[37], and since inflammation[42] and nutritional[43] markers are associated with DN, the relationship between the two is attracted by many researchers. Low-grade chronic inflammation is a well-recognized contributor to the development and progression of DN[44]. Accordingly, increased CAR has been linked to an increased risk of DN. A study demonstrated that T2DM patients with DN had markedly elevated CAR levels compared to those without DN. The research identified a CAR threshold of 0.82% as having optimal sensitivity and specificity for predicting the presence of DN, suggesting that CAR could serve as a valuable marker for early detection and management of this complication[45]. Moreover, a recent study reported that increased CAR was an independent risk factor for chronic kidney disease[46]. Another report from Turkey compared the CAR levels of diabetic patients with at least one microvascular complication to those with healthy subjects and reported that CAR was significantly higher in diabetic group with at least one chronic microvascular complication[47]. The relationship between elevated CRP levels and the risk of developing T2DM has been established. Studies have shown that higher CRP concentrations are linked to an increased risk of incident diabetes[4]. However, further research is needed to determine whether CAR offers additional predictive value over CRP alone in this context. We can conclude that CAR is a promising biomarker in the management of T2DM. Its elevation correlates with the presence of complications such as DN, highlighting its potential utility in early detection and risk stratification. Further studies are warranted to fully elucidate the role of CAR in predicting the onset of T2DM and its complications, which could enhance clinical decision-making and patient outcomes.
CAR IN DR
The CAR could be associated with DR, too, because DR has significant inflammatory burden. The role of CAR in DR has been explored to understand its potential as a predictive biomarker. Direct studies on CAR in DR are limited, but research on related retinal conditions provides some insights. For instance, a study investigating retinopathy of prematurity found that higher CAR values at the end of the first postnatal month were associated with the development of severe retinopathy of prematurity[48]. Although retinopathy of prematurity and DR are distinct conditions, both involve retinal neovascularization and inflammation, suggesting that CAR could potentially serve as a marker in retinal vascular diseases. Another study focusing on the relationship between CRP, BMI, and DR found that higher levels of CRP and BMI were associated with a lower prevalence of DR[20]. This counterintuitive finding suggests a complex interplay between inflammation, body weight, and the development of DR, indicating that elevated CRP alone may not directly correlate with increased DR risk. These evidence in literature suggest that elevated CRP levels have been associated with DR, however, the specific role of CAR in DR remains underexplored. The existing evidence highlights the need for further research to elucidate the relationship between CAR and DR, which could enhance early detection and management strategies for this complication.
CAR IN DPN
Recent studies have also explored the association between CAR and DPN, suggesting its potential role in early detection and management. A study by Aktas evaluated CAR levels in T2DM patients with and without diabetic neuropathy (DN). The findings revealed that patients with DN had significantly higher median CAR levels (2.19%) compared to those without DN (0.56%), indicating a strong association between elevated CAR and the presence of neuropathy. This suggests that CAR could serve as a reliable marker for identifying patients at risk for DPN[49]. Other studies supported this research. A study has demonstrated that increased CAR levels are related to the development and progression of DN, another microvascular complication of T2DM. A study reported that T2DM patients with nephropathy exhibited higher CAR levels than those without, highlighting the role of inflammation in diabetic complications[45]. The relationship between inflammation and DPN is further corroborated by evidence indicating that chronic subclinical inflammation contributes to the development of diabetic complications. Elevated CRP levels have been associated with endothelial dysfunction and vascular pathology, which are critical factors in the progression of neuropathy[2]. Taking together, elevated CAR levels are associated with the presence and severity of DPN, underscoring the role of inflammation in its pathogenesis. Physicians should monitor CAR levels in diabetic patients so this could aid in identifying those at higher risk for neuropathy, potentially guiding early intervention strategies.
CLR IN T2DM AND DIABETIC MICROVASCULAR COMPLICATIONS
The CLR has been investigated as a marker of systemic inflammation and immune response in various medical conditions. In the context of T2DM, chronic subclinical inflammation plays a pivotal role in the pathogenesis of insulin resistance and subsequent disease progression. CLR is associated with inflammatory diseases including chronic hepatitis C[50], chronic kidney disease[51], pancreatitis[52], and cardiac conditions[53]. While direct studies on CLR in T2DM are limited, related inflammatory markers provide insights into its potential relevance. CRP is an acute-phase reactant produced by the liver in response to inflammation. Elevated CRP levels have been associated with the development of T2DM, suggesting that inflammation contributes to insulin resistance and impaired glucose metabolism. A study highlighted that chronic subclinical inflammation, as indicated by increased CRP levels, is a triggering factor in the origin of T2DM[2]. Lymphocyte counts, on the other hand, reflect the status of the adaptive immune system. Alterations in lymphocyte counts have been observed in various inflammatory states, including diabetes. Research indicates that low lymphocyte count is associated with poor glycemic control and insulin resistance in T2DM patients, underscoring the link between inflammation and glucose metabolism[54]. Of note, another study revealed higher CLR levels in correlation with high blood glucose levels in diabetic tuberculosis patients[7]. Although specific research on CLR in T2DM is scarce, the individual components of this ratio, CRP and lymphocyte count, have demonstrated associations with the disease. Given that CLR combines these two parameters, it holds potential as a composite marker reflecting the balance between systemic inflammation and immune status in T2DM patients. Further studies are warranted to elucidate the clinical utility of CLR in predicting disease onset, monitoring progression, and tailoring therapeutic strategies in T2DM. Studies on CLR’s role in diabetic microvascular complications are still needed.
CONCLUSION
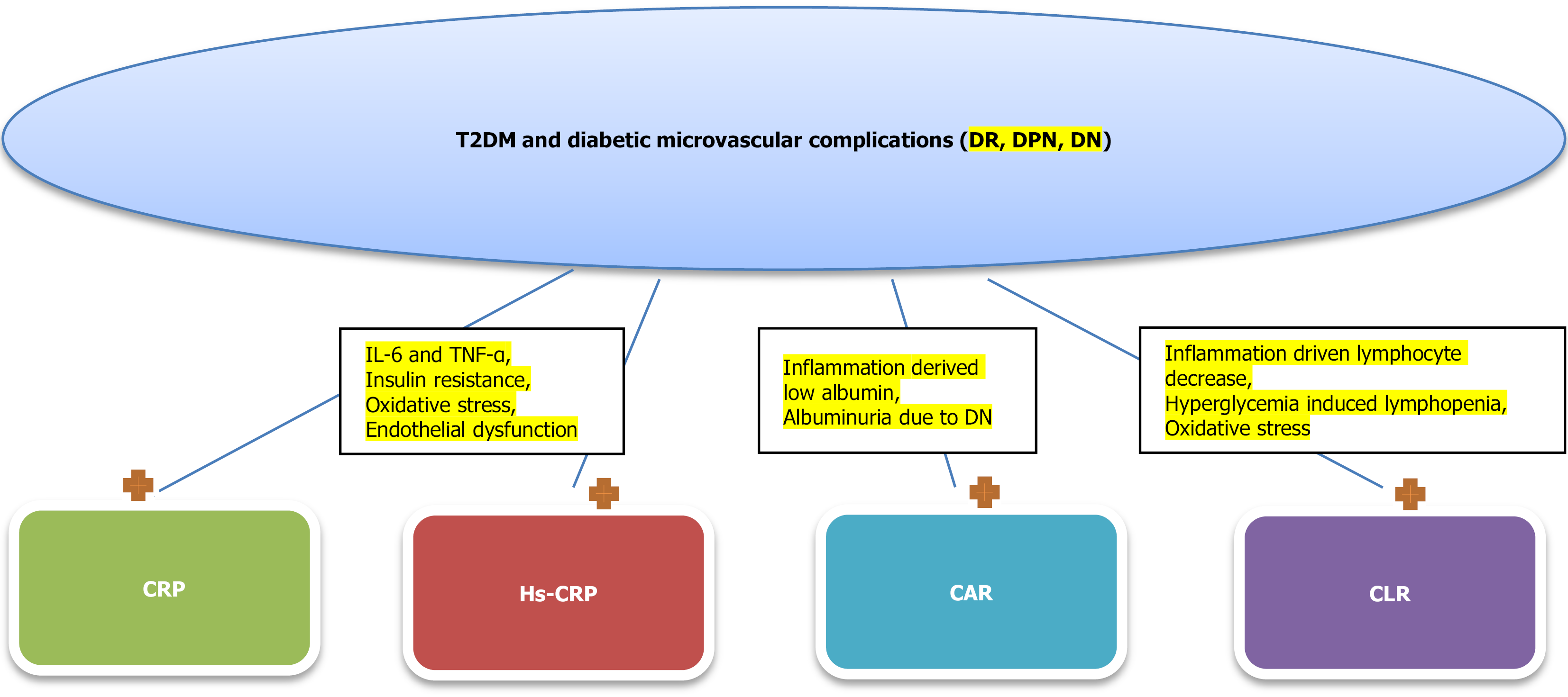
In conclusion, the biomarkers CRP, hs-CRP, CAR, and CLR have shown promising roles in the evaluation and management of T2DM and its associated chronic microvascular complications, including DN, retinopathy, and peripheral neuropathy. Elevated CRP and hs-CRP levels reflect the underlying systemic inflammation that contributes to the development and progression of these complications. The CAR and CLR offer additional insights into the inflammatory state and immune response in T2DM patients, providing a more comprehensive approach to risk stratification (Figure 1). While significant associations have been observed between these biomarkers and the presence and severity of diabetic complications, further research is required to fully elucidate their utility as predictive and diagnostic tools in clinical practice. Incorporating these biomarkers into routine clinical assessments could enhance early detection, enable better monitoring, and inform personalized treatment strategies for patients with T2DM, ultimately improving patient outcomes.
Figure 1 Association between C-reactive protein, high sensitivity C-reactive protein, C-reactive protein to lymphocyte count ratio and C-reactive protein to lymphocyte count ratio levels and type 2 diabetes mellitus and diabetic complications.
Both C-reactive protein, high sensitivity C-reactive protein, C-reactive protein to albumin ration and C-reactive protein to lymphocyte count ratio are increased in patients with type 2 diabetes mellitus and with diabetic microvascular complications. Moreover, elevated levels of these markers are associated with poor disease control in diabetic population. T2DM: Type 2 diabetes mellitus; CRP: C-reactive protein; Hs-CRP: High sensitivity C-reactive protein; CAR: C-reactive protein to albumin ratio; CLR: C-reactive protein to lymphocyte count ratio; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; DN: Diabetic nephropathy; DR: Diabetic retinopathy; DPN: Diabetic peripheral neuropathy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Federation of Internal Medicine.
Specialty type: Endocrinology and metabolism
Country of origin: Türkiye
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C, Grade C
Novelty: Grade C, Grade C, Grade C
Creativity or Innovation: Grade B, Grade C, Grade D
Scientific Significance: Grade B, Grade B, Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Jiang YC, PhD, Senior Researcher, China; Li Y, PhD, Researcher, China; Pappachan JM, MD, FRCP, MRCP, Professor, Senior Researcher, United Kingdom S-Editor: Qu XL L-Editor: A P-Editor: Yang YQ