Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108714
Revised: July 4, 2025
Accepted: September 1, 2025
Published online: October 15, 2025
Processing time: 177 Days and 3.7 Hours
Diabetes mellitus (DM) and its complications continue to impose a substantial burden on healthcare systems worldwide. Diabetic neuropathy (DN) is one of the most common chronic microvascular and neurodegenerative complications of DM. It is clinically characterized by allodynia, hyperalgesia, and abnormal or absent nerve fiber sensation, which collectively contribute to poor quality of life, sleep disturbances, depression, and increased mortality. Although several phar
Core Tip: This paper critically reviews the role of astrocytes in the mechanism of diabetic neuropathy (DN). We discuss the signaling pathways, protein kinases, receptors, and mediators that are involved in the pathophysiology of DN. We also explore research that focuses on astrocyte activation as a potential treatment for DN.
- Citation: Haris K, Long I. Role of astrocytes in diabetic neuropathy: Review of their involvement in disease mechanisms. World J Diabetes 2025; 16(10): 108714
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/108714.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.108714
Diabetes mellitus (DM) is a common metabolic disorder characterized by persistent hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Globally, more than 589 million adults were affected in 2025, with projections rising to 853 million by 2050[1]. Chronic hyperglycemia contributes to both macrovascular and microvascular complications, of which diabetic neuropathy (DN) is one of the most common and disabling, affecting up to 50% of diabetic individuals[2,3].
DN presents with symptoms such as numbness, allodynia, and hyperalgesia, leading to substantial impairment in daily functioning and quality of life[4]. While neuronal injury has been the primary focus in DN research, growing evidence suggests that astrocytes, key glial cells in the central nervous system (CNS), play a pivotal role in DN path
To compile relevant literature for this review, a comprehensive search was conducted using multiple scientific databases, including PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar. The search covered publications from 2000 to 2024, with a focus on recent developments. The search used different combinations of keywords and Boolean operators, such as “astrocytes”, “diabetic neuropathy”, “glial cells”, “neuroinflammation”, “reactive astrogliosis”, “oxidative stress”, “blood-brain barrier”, “JAK/STAT3”, “CX36”, and “neuropathic pain”. Titles and abstracts were screened for relevance, and full-text articles were retrieved for detailed evaluation. We focused on original studies that were reviewed by experts, systematic reviews, and important experimental research using animal models or human subjects related to how astrocytes are involved in DN. Reference lists of included articles were also manually screened to identify additional relevant sources.
Neuropathic pain is caused by abnormal neuronal responses along the pain pathway and serves under pathological pain. It is often cardinally characterized by lancinating sensation or burning pain, as well as abnormal sensory symptoms such as allodynia and hyperalgesia[8]. Allodynia, which refers to pain caused by a normally non-noxious stimulation, and hyperalgesia, which is an enhanced sensitivity to pain from a typically painful stimulus, are notable symptoms observed in individuals suffering from neuropathic pain[5].
DN is a neuropathic pain syndrome that affects individuals with diabetes, leading to various complications, ultimately lowering their quality of life. The primary factor contributing to the pathophysiology of DN is hyperglycemia, which contributes to inflammation and neurotoxicity[9]. In addition, hyperglycemia in patients with diabetes reportedly activates astrocytes and microglia, leading to the release of reactive oxygen species (ROS) that serve as hallmarks of inflammation[10]. DN refers to the painful sensation resulting from injury to the peripheral somatosensory system due to diabetes[4]. DN is not a rare condition but a significant health issue that affects a substantial portion of diabetic individuals, hence it is important to note the variability in DN incidence among individuals with diabetes. Data show that one-third of them experience DN[2,3], whereas the incidence of DN among all individuals with DM ranges from 10% to 50%. It is estimated that 50% of the patients develop evidence of nerve damage after over 25 years of having DM[9]. A recent survey conducted by Viatris, an American multinational pharmaceutical and healthcare firm, revealed that 68% of patients in Malaysia who have DN were aware of the link between pain sensations and diabetes before being diagnosed. Unfortunately, no recommended medication is approved worldwide that specifically blocks the pathological processes leading to excruciating DN[7]. This high prevalence underscores the urgent need for effective treatments. Much of the research on DN pathogenesis has focused on the role of neurons and Schwann cells. However, there is growing recognition on the function of glial cells, especially astrocytes, as active players in neuropathic pain and neurodegeneration. Thus, this review critically analyzes the role of astrocytes in DN.
Glial cells, which include astrocytes, microglia, and oligodendrocytes, are non-neuronal cells that play essential roles in maintaining homeostasis, supporting neuronal function, and regulating immune responses in the CNS[11]. Morphologically, these cells significantly differ: Astrocytes exhibit a star-shaped architecture with numerous processes, microglia possess small, and motile cell bodies with ramified branches; whereas oligodendrocytes display a compact morphology tailored for myelin production[12]. Functionally, glial cells contribute to synaptic modulation, metabolic support, blood-brain barrier (BBB) integrity, and clearance of cellular debris. In recent years, accumulating evidence has emphasized the active involvement of glial cells in the pathogenesis of neurodegenerative disorders, including DN, through mechanisms such as oxidative stress, neuroinflammation, and glial reactivity[13]. This broader understanding underscores the importance of examining glial cell-specific responses, particularly those of astrocytes, in the context of DN.
Astrocytes are found exclusively in the CNS. They are derived from neural tube progenitor cells like other macroglia such as oligodendrocytes and ependymal cells. Similar to oligodendrocytes and ependymal cells, astrocytes originate during early development through the influence of specific signaling pathways, including neurogenic locus notch homolog (Notch), bone morphogenetic protein, and sonic hedgehog. These pathways guide precursor cell migration and differentiation across specific CNS regions[14]. The macroglia group of glial cells, which includes astrocytes, is distinct from the smaller microglia. Astrocytes are the largest glial cells in CNS. They comprise more than half of the glial population and are the most varied in terms of both shape and function. They exhibit extensive functional diversity and region-specific specialization, now recognized as a form of glial plasticity. Emerging data underscore that astrocyte heterogeneity may contribute to region-specific vulnerability in neurodegenerative conditions, including DN[15,16]. The term "astrocyte" is derived from the Greek words "astro" (meaning star) and "cyte" (meaning cell), related to its structure like star shape[17].
The main characteristic of astrocytes is their branching processes that emanate from the cell body. These processes terminate in specialized end-feet, known as the "astrocyte end-foot", which interact with capillaries, neurons, and the extracellular matrix create cell-to-cell signaling[18]. The nucleus of the astrocytes is big, ovoid or spherical, and faintly stained. Conventional stains make it impossible to view the processes under a microscope, while gold or silver stains make them easily visible. Glial fibrillary acidic protein (GFAP) remains a classic marker for astrocyte identification, although recent studies advocate combining GFAP with markers like aldehyde dehydrogenase 1 family member L1 or S100 calcium (Ca2+)-binding protein beta to distinguish astrocyte subtypes more accurately[6].
There are two astrocyte subtypes, known as protoplasmic, found in gray matter surrounding neurons and the fibrous, located between axons in the white matter. Under a standard microscope, protoplasmic astrocytes have a distinctive star-like appearance due to their numerous short processes with numerous branches reaching in all directions. Numerous organelles visible under an electron microscope suggest that protoplasmic astrocytes have high metabolic rates and are always activated. Compared to protoplasmic astrocytes, fibrous astrocytes have fewer, longer, and less branching processes. Additionally, under an electron microscope, they have less organelles[19].
Astrocytes not only provide structural and metabolic support but also serve as active participants in CNS signaling. Notably, astrocytes exhibit plasticity, altering morphology, protein expression, and function in response to injury, inflammation, or metabolic stress. In the diabetic CNS, astrocytic plasticity can be maladaptive, contributing to neuroinflammation and neuropathic pain[10].
BBB disruption in diabetes: Traditionally, astrocytes have been recognized for their role in forming and maintaining the BBB through their perivascular end-feet, which encase CNS capillaries and support the endothelial tight junctions essential for selective permeability[10]. However, in the context of diabetes, chronic hyperglycemia and metabolic dysregulation severely compromise BBB integrity, transforming astrocytes from protectors into facilitators of neuropathological processes. Diabetes-induced BBB disruption is now attributed to several interlinked mechanisms. Elevated glucose levels promote the accumulation of advanced glycation end products (AGEs), which bind to their receptors on endothelial and glial cells, triggering oxidative stress and inflammatory signaling cascades. These changes degrade tight junction proteins such as occludin and claudin-5, increasing BBB permeability[10].
Additionally, insulin resistance impairs the regulatory functions of astrocytes, disrupting their ability to buffer ions and support endothelial health. Studies show that insulin signaling in astrocytes modulates aquaporin-4 and glucose transporter 1 expression, essential for fluid and glucose homeostasis at the BBB. In diabetic models, astrocytic dysfunction results in perivascular edema, heightened leukocyte infiltration, and increased expression of proinflammatory cytokines like interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α)[6]. Astrocyte end-foot detachment from capillary walls, a hallmark of BBB breakdown, has been observed in streptozotocin-induced diabetic rats, correlating with increased GFAP expression and neuroinflammation[20]. These pathological changes not only compromise barrier selectivity but also facilitate the entry of peripheral immune mediators, exacerbating neuronal damage in DN. This shift from a purely anatomical function to a pathological role underscores the need to consider BBB disruption as a dynamic and central feature in the progression of diabetic CNS complications.
Nutrient supplementation: It is well known that because neurons and glial cells have a high metabolic rate, therefore the nervous tissue needs a constant supply of nutrients. Astrocytes are the first to interact with blood macromolecules through their participation in the BBB. An astrocyte acts as a mediator, delivering metabolites and other substances to all other cells based on the requirements of neighboring cells. For instance, an astrocyte initially absorbs blood-derived cholesterol, which is subsequently transferred to neighboring oligodendrocytes for the purpose of myelination. At the same time, the membrane of astrocytes has gates and ion pumps[21]. This is because of their function in extracellularly preserving the required ion concentrations. The development of an ion gradient that is utilized for neuron action potentials depends on this. Astrocytes oversee and maintaining the appropriate level of extracellular potassium (K+). Additionally, astrocytes prevent neuronal injury by directing harmful byproducts away from the nervous tissue and via the BBB[22].
Promote synapse formation: Astrocytes also involve helping neurons communicate with one another at every stage of the development of the CNS. Through signaling, astrocyte processes direct growing axons to the synapse site. The same method of action is responsible for astrocyte function in axon repair and reconnection following damage, as well as their part in neuronal plasticity in the adult CNS. Astrocyte not only contributes to the synapse's formation but also helps it function by recycling and absorbing neurotransmitters, controlling the metabolism of pre- and post-synapse cells, and utilizing signaling factors to either upregulate or downregulate the synapse[23].
Regulating cerebral blood flow: Implications for pancreatic metabolic homeostasis: An astrocyte can sense the metabolic rate of neighboring neuronal cells and employ endothelial factors, changes in Ca2+ concentrations, and other substances called gliotransmitters to instruct blood vessels to dilate or constrict. To achieve optimal efficiency, the blood flow in various brain regions is controlled based on which regions are more active at any given time. A single cell initiates the process, but it is intensified by specialized gap junctions between astrocytes that enable them to all activate simultaneously and send synchronized signals to neighboring cells[24]. This timed activation is a type of functional syncytium that speeds up all the astrocytes' functions to being crucial for controlling blood flow in the CNS. According to recent studies, astrocytes actively communicate with neurons and emit gliotransmitters, which are signaling molecules that modify relationships between neurons and between neurones and glial cells. Research is ongoing to determine the full degree of astrocyte influence on neuronal function and nervous tissue organization. For example, it is believed that the processes of a single astrocyte interact with millions of synapses, demonstrating the astounding complexity of astrocytes[6].
Protect the neuron during injury and toxicity: Astrocytes are crucial to the reaction that the nervous system produces in the event of injury or toxicity. Reactive astrocytes, which are more active, are created when astrocytes are triggered. This causes inflammation, increases BBB permeability, and releases neurotoxic chemicals that destroy damaged neurons. Astrocytic scars are produced by astrocytes in response to a lesion in the brain or spinal cord. Particularly in cases of acute ischemic injury, a flat, peripherally positioned nucleus gives the impression of inflated astrocytes. These cells are known as gemistocytes. Astrocytes interact with microglia and display similar activation patterns in the case of a pathogen invasion in the CNS. They mediate reactions and coordinate nerve tissue regeneration once the threat has been neutralized[25].
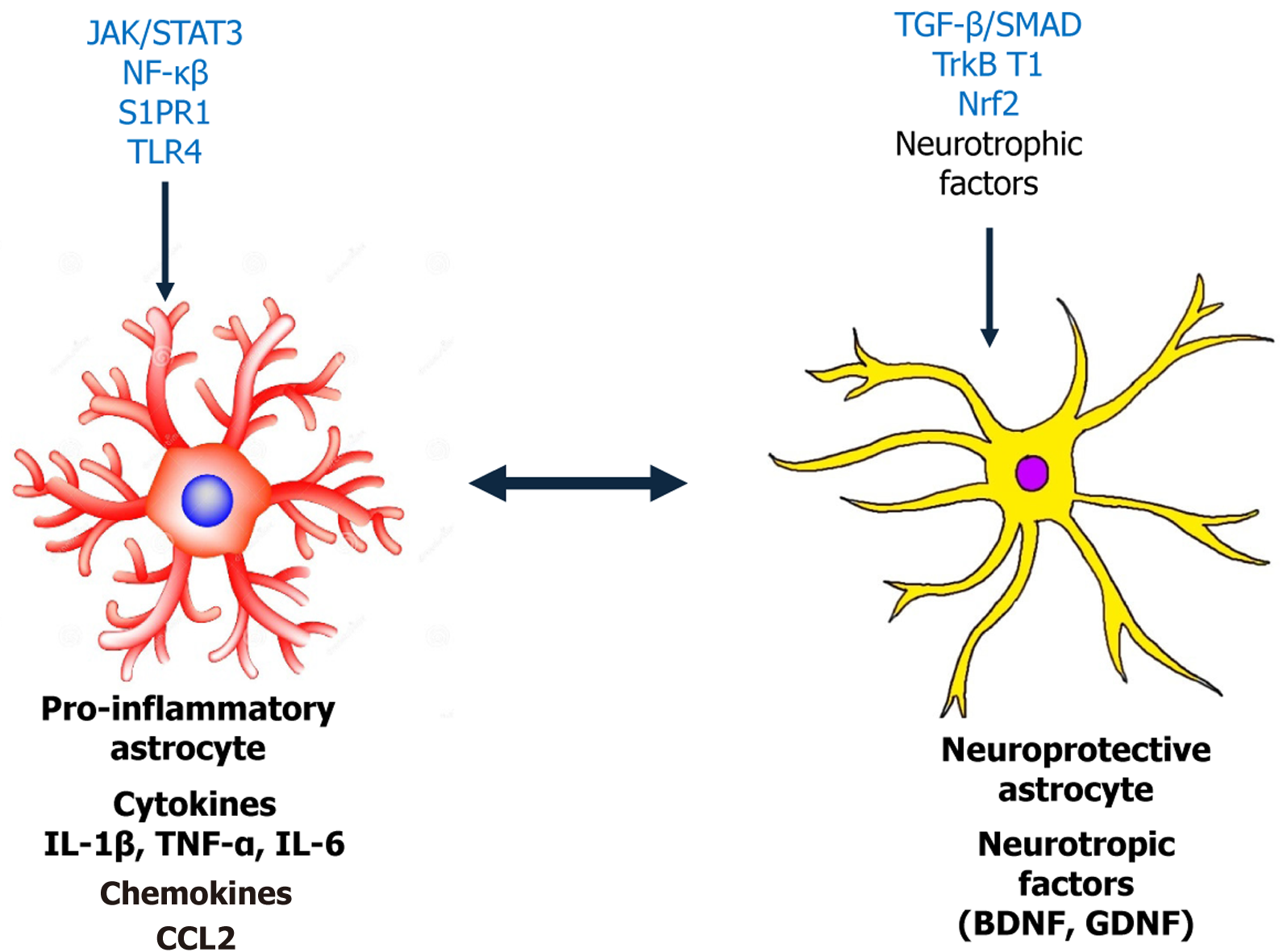
In summary, the physiology function of astrocytes is to facilitate neuronal communication, maintain homeostasis in neural tissue, and fortify the boundaries governing the interaction between the body and the CNS. Astrocytes are essential for communicating between neurons, preserving homeostasis within neural tissue, and strengthening the barriers that control how the CNS interacts with the body[16] (Figure 1).
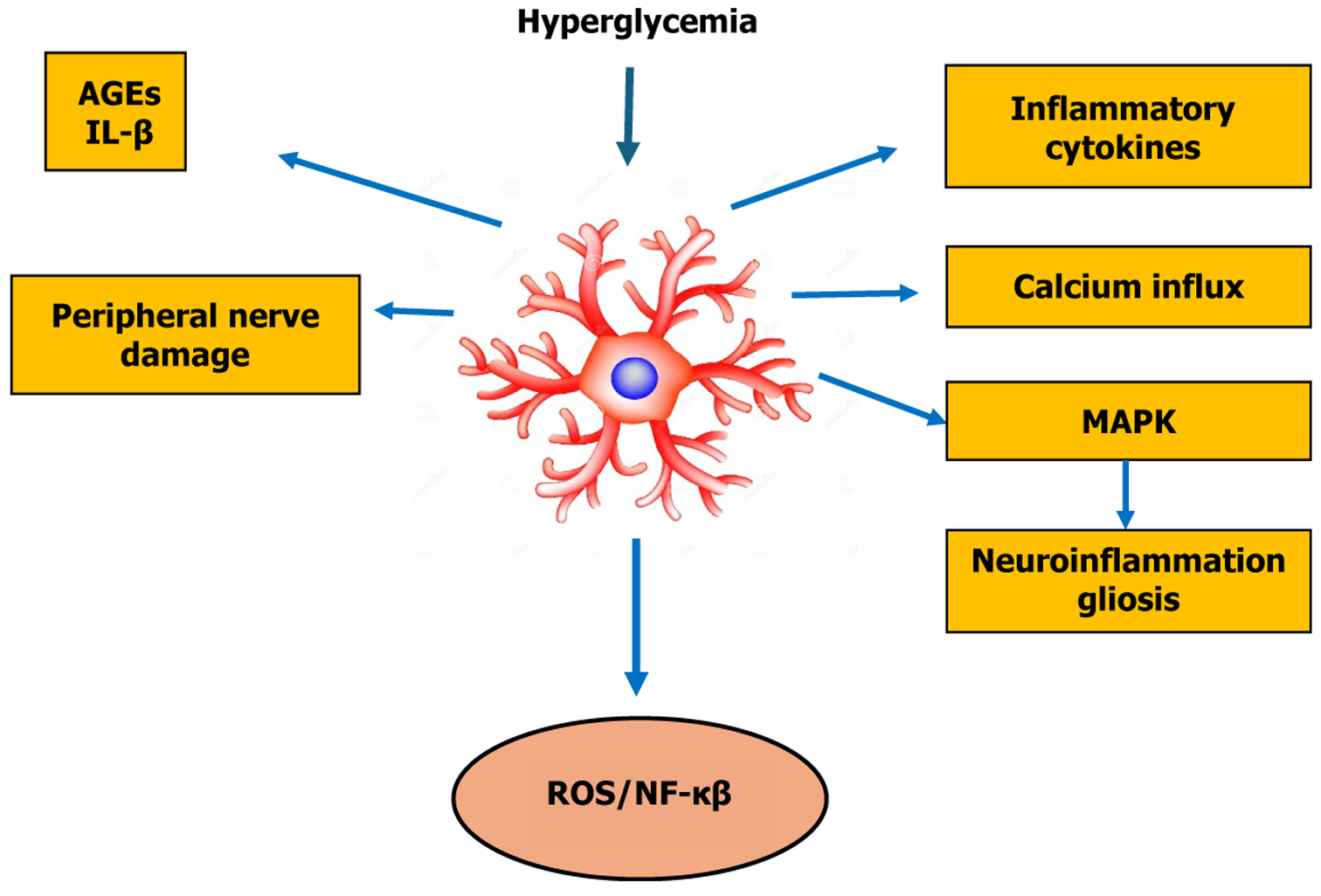
Hyperglycemia is a primary pathophysiological factor in the development of DN. Hyperglycemia can activate some major pathways like polyol pathway[26], AGE[27], mitogen-activated protein kinase (MAPK)[28] and poly-ADP ribose polymerase[29]. The combined impact of these cascades produces intense oxidative stress, cytokine release and neuroinflammation[30]. Studies have demonstrated that hyperglycemia in diabetes has activated glial cells (microglia and astrocytes) and released ROS that cause neurotoxicity, stimulated the release of proinflammatory cytokines and chemokines such as IL-1β, IL-6, TNF-α, prostaglandin E2, and nitric oxide (NO)[31,32], which exacerbates neuronal damage and neuropathic pain. Nerve is made up of a cluster of nerve fibers, each of which contains many neurons. Axons are the elongated portions of the neuron that transmit signals. Schwann cells, the supporting glial cells of the peripheral nervous system, generate a myelin sheath by wrapping around axons to form a thick insulating layer that speeds up nerve signal transmission. The degeneration and apoptosis of Schwann cell are prominent features of the onset and progression of DN[33]. As previously demonstrated in animal models and human patients, significant neuron damage, as defined by a decrease in nerve fiber density, was detected in DN sciatic nerve tissues[34,35].
Prolong hyperglycemia in patients with diabetes reportedly activates astrocytes and microglia, leading to the release of ROS that serves as hallmarks of inflammation[10]. A study has showed that hyperglycemia can induce astrocytic activation via NF-κB signaling, promoting neuroinflammation and contributing to DN progression[36]. It has been demonstrated that after this inflammation, astrocytes experience changes in their morphology and behavior, including translation and transcription, to become reactive astrocytes. The process involves phosphorylation of signaling molecules and an increase in intracellular Ca2+ flux that can completed in a minute. Certain activation stages require extended periods to complete, taking tens of minutes for translational regulation and several hours for transcriptional regulation. It may take tens of hours or even days for prolonged activation states, recognized as a reactive astrogliosis, to be manifest, that crucial for maintaining neuropathic pain. Cellular hypertrophy, hyperplasia, increased GFAP expression and proliferation, and in extreme cases, scar formation are the hallmarks of reactive astrogliosis, which is a finely graded continuum of progressive cellular and molecular changes in relation to the severity of injury[37].
Besides inflammatory signaling cascade, studies have demonstrated that oxidative stress in diabetic conditions induces astrocyte apoptosis primarily through the intrinsic mitochondrial pathway, whereby mitochondrial dysfunction and sirtuin 1 downregulation led to increased p53 acetylation, impairing astrocytic support and contributing to sensory neuron degeneration[38,39]. These findings confirm that astrocytes not only initiate inflammatory signaling cascades but also undergo programmed cell death in the diabetic milieu, both of which synergistically aggravate DN.
Astrogliosis and the mechanism of DN: After astrocytes became reactive astrogliosis, its lose their ability to maintain the homeostatic concentrations of extracellular K+ and glutamate, leading to neuronal hyperexcitability[40]. This occurred through releasing many chemokines from astrocytes, which act as neuromodulators and potentiate excitatory synaptic transmission in the spinal cord pain circuitry[41]. Astrocytes can also signal directly to neurons through physically coupled networks mediated by gap junctions to facilitate intercellular transmission. Gap junction communication is mediated by connexin 43 (Cx43), the predominant Cx expressed in astrocytes. DN induces the persistent upregulation of Cx43 in astrocytes and switches the function of Cx43 from gap junction communication to paracrine modulation[41]. This paracrine regulation leads to the increased release of glutamate, ATP, and chemokines through a paracrine mechanism[42]. Furthermore, DN upregulates C-X-C motif chemokine ligand 13 (CXCL13) in spinal cord neurons, which can activate astrocytes via C-C chemokine receptor type 5 (CCR5) to maintain neuropathic pain[43]. Thus, chemokines facilitate neuropathic pain via bi-directional neuron-astrocyte interactions.
DN also induces spinal cord and cortical astrocytes to upregulate thrombospondin-4, which promotes neuropathic pain through the formation of new synapses and rewiring of somatosensory cortical circuits[44]. Notably, a single human astrocyte may contact more than 1 million synapses, and such complexity points to a more important role of astrocytes in humans that bears further investigation. Compared to microglial activation, astrocyte activation in chronic pain con
Astrocytes and signaling pathways involved in DN: Proinflammatory cytokines, including IL-1β, TNF-α, and IL-6 as well as gene transcription factors like signal transducer and activator of transcription 3 (STAT3) are examples of chemical signals that regulate astrocyte activity[46]. Astrocyte activation in DN is orchestrated by a complex interplay of cytokines, transcription factors, and signaling cascades. The key regulatory pathways include Janus kinase (JAK)/STAT3, Notch-Oligodendrocyte transcription factor 2 (Olig2), sphingosine-1-phosphate receptor 1 (S1PR1), transforming growth factor beta (TGF-β)/SMAD, Ras-related C3 botulinum toxin substrate 1-G1 to S phase transition 1 (GSPT1), and c-Jun N-terminal kinase (JNK) signaling pathway. These networks modulate astrocytic phenotypic transformation, promoting neuroinflammation and synaptic remodeling that underlie neuropathic pain.
In 2011, Tsuda et al[47], showed that the JAK/STAT3 signaling pathway regulates astrocyte proliferation in neu
Olig2 is a crucial transcription factor for astrocyte proliferation and development. In 2008, Chen et al[49] discovered that reactive astrocyte proliferation decreased when Olig2 was ablated, indicating that Olig2 is essential for reactive astrocyte proliferation. Whereas Notch molecules may help reactive astrocytes proliferate and become functionally specialized by controlling the nuclear cytoplasmic translocation of Olig2, as evidenced by a BrdU labeling experiment[50]. Proliferative reactive astrocytes were significantly reduced in mice with selective Notch 1 knockout in GFAP-positive reactive astrocytes[51]. By suppressing the mRNA expression of Olig2, a marker of astrocyte activation, the mechanical and thermal hyperalgesia brought on by chronic constriction injury (CCI) in the rat model was successfully reduced[52]. The results indicate that astrocyte activation and the development of neuropathic pain are significantly influenced by the Notch/Olig2 signaling pathway.
In 2019, Chen et al[53] discovered that sphingosine-1-phosphate, which is generated in the spinal dorsal horn of CCI and SNI rat model, causes neuropathic pain by specifically activating S1PR1 present in astrocytes. The neuropathic pain symptoms in CCI and SNI rat models were attenuated after treatment with astrocyte-specific S1PR1 knockout and IL-10, is a cellular factor responsible for neuroprotection and combating inflammatory infections[53]. It is believed that the primary cellular substrates for S1PR1 activity are astrocytes. Therefore, astrocytes have been important for the mechanism of neuropathic pain through S1PR1, and this signaling pathway may be a novel therapeutic target for neuropathic pain.
TGF-β is one of the growth factors involved in body processes such as regulating growth, illness, and wound healing. Following CNS injury, TGF-β is rapidly activated, triggering activation of the SMAD family of transcription factors in astrocytes[46]. In 2010, Ranaivo et al[54] successfully activated the SMAD pathway downstream of the TGF-β receptor. The knockdown of repulsive guidance molecule a (RGMa) has been shown to eliminate TGF-β1-induced astrocyte proliferation and activation[54]. It has also been demonstrated that RGMa can enhance TGF-β1/SMAD2/3 signal transduction by simultaneously binding to SMAD2/3 and activin receptor-like kinase 5, thereby forming a composite complex[55]. These findings suggest that the TGF/SMAD signaling pathway could serve as a key target for modulating astrocyte acti
As a member of the Ras homolog gene family, the small GTP-binding protein RhoA is abundantly expressed in astrocytes, and through its downstream effector Rho-associated kinase (ROCK), regulates myosin and actin dynamics to control cytoskeletal organization[56]. Following injury, the expression of RhoA and Rac family small GTPase 1 (Rac1) in the glial scar region is markedly upregulated[57]. Experimental evidence indicates that activation of the RhoA/ROCK pathway, along with its upstream and downstream effectors, contributes to both the initiation and maintenance of neuropathic pain[58]. Inhibition of ROCK induces rapid and reversible astral realignment in cultured astrocytes and enhances their migratory capacity. In one study, Rac1 knockdown or knockout astrocytes exhibited significantly delayed cell cycle progression, reduced migration, and decreased expression of GSPT1. Moreover, these cells showed diminished GSPT1 expression and responsiveness to lipopolysaccharide stimulation, suggesting that GSPT1 functions as a downstream target of Rac1[59]. Therefore, the Rac1-GSPT1 axis, represents a novel signaling pathway involved in astrocyte activation after CNS injury. However, the direct role of GSPT1 in mediating neuropathic pain remains to be elucidated.
Recent studies have expanded this paradigm. A study demonstrated that mitochondrial dysfunction and altered fission/fusion dynamics in reactive astrocytes can contribute to redox imbalance and bioenergetic failure in diabetic models, ultimately exacerbating neuronal injury and pain signaling. Mitochondrial fission factor and dynamin-related protein 1 were identified as upstream regulators of astrocytic stress responses, highlighting the metabolic vulnerability of glia in DN[60]. Furthermore, a recent study revealed that glial-derived exosomes are active mediators of neuron-glia communication in diabetes. These exosomes, enriched with proinflammatory miRNAs and metabolic enzymes, promote neuronal hyperexcitability and sustain neuropathic pain circuits. Inhibition of exosome release from astrocytes reduced mechanical allodynia and decreased spinal cord levels of IL-6 and C-C motif chemokine ligand 2 (CCL2), underscoring the importance of extracellular vesicle-mediated signaling in DN pathophysiology[61]. Together, these pathways and mechanisms underscore the multifaceted contribution of astrocytes to DN, bridging intracellular signaling with intercellular communication and metabolic homeostasis. Targeting these signaling axes offers promising therapeutic avenues to mitigate glial-driven neuroinflammation and neuronal sensitization in DN. Figure 2 illustrates the specific molecular and signaling mechanisms of astrocytes involved in the pathogenesis and progression of DN.
Astrocytes and intracellular kinases, receptors, and proinflammatory cytokines in DN: Apart from the signaling pathway, astrocytes also exhibit pathological changes and contribute to DN through intracellular kinases, channels, receptors, and transcription factors. The regulation of diabetic neuropathic pain is strongly influenced by the MAPK family, which comprises p38, extracellular signal-regulated kinase (ERK), and JNK[62]. Notably, phosphorylated ERK levels have been found to increase in spinal astrocytes during the late phase of pain induced by complete Freund’s adjuvant[63]. A study reported that the mitogen-activated ERK kinase inhibitor U0126 suppresses astrocyte activation and reduces the production of proinflammatory cytokines, including IL-1β and IL-6[64].
Toll-like receptor 4 (TLR4) is abundantly expressed in astrocytes. The presence of TLRs enables astrocytes to release inflammatory mediators closely associated with neuropathic pain, including IL-6, monocyte chemoattractant protein-1 (MCP-1), and NO[65]. Experimental studies in TLR2 knockout mice have demonstrated that TLR2 expression is critical for the development of neuropathic pain. Collectively, these findings indicate that both TLR2 and TLR4 contribute to the initiation, progression, and maintenance of pain following nerve injury[66].
In addition to TLRs, tyrosine receptor kinase B (TrkB) in its truncated isomeric form has also been linked to the pat
IL-1β is a well-known cytokine that causes inflammation. After peripheral nerve injuries, astrocytes in the spinal cord have been shown to have increased IL-1β. There is growing evidence that IL-1β plays a role in sensitivity to pain[71]. The administration of IL-1β receptor antagonists intrathecally reduces neuropathic and inflammatory pain. Moreover, mice lacking the IL-1β type I receptor, or those administered an IL-1β antagonist, exhibited a marked reduction in neuropathic pain. Conversely, intrathecal injection of IL-1β induced pronounced pain hypersensitivity[72].
MCP-1, also recognized as CCL2, is mostly produced by astrocytes. It has also been discovered that peripheral nerve injury causes a notable rise in JNK-dependent CCL2 in spinal astrocytes, which directly modulates spinal neurons to cause neuropathic pain. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl D-aspartate (NMDA)-induced spinal dorsal horn neuron inward currents can be quickly increased by CCL2. This suggests increased synaptic glutamate transmission, which is significantly linked to a considerable decrease in nociceptive feeling and an increase in central sensitization[62]. To further assist the development of pain, CCL2 may also promote the differentiation and division of the spinal microglia[73].
Proteases that are expressed in astrocytes, including tissue-type plasminogen activators and matrix metalloproteinases (MMPs), have been shown to be important in the development of astrocyte-driven neuropathic pain[62]. The deve
A rat model of the spinal nerve ligation has recently shown a strong correlation between astrocyte activation and brain-derived neurotrophic factor (BDNF), which binds to TrkB. Several experimental studies have shown that the BDNF inhibitor ANA-12 can suppress astrocyte activation in the dorsal horn of the spinal cord, thereby reducing or even preventing the development of mechanical nociceptive hypersensitivity. Astrocyte activation has been associated with increased levels of proinflammatory cytokines and BDNF[75]. Fluorocitrate also reportedly reverses mechanical pain hypersensitivity by inhibiting astrocyte activation and subsequently reducing BDNF levels. These effects are mediated by the binding of BDNF to TrkB.T1, the sole TrkB receptor subtype expressed on astrocytes[76]. Furthermore, astrocyte-derived BDNF has been found to enhance NMDA receptor expression in excitatory neurons and diminish inhibitory neuronal activity by inducing depolarizing shifts in the gamma aminobutyric acid (GABA) reversal potential and reducing presynaptic GABA conductance. Collectively, these actions increase synaptic activity in excitatory neurons, thereby facilitating the onset and progression of neuropathic pain[77].
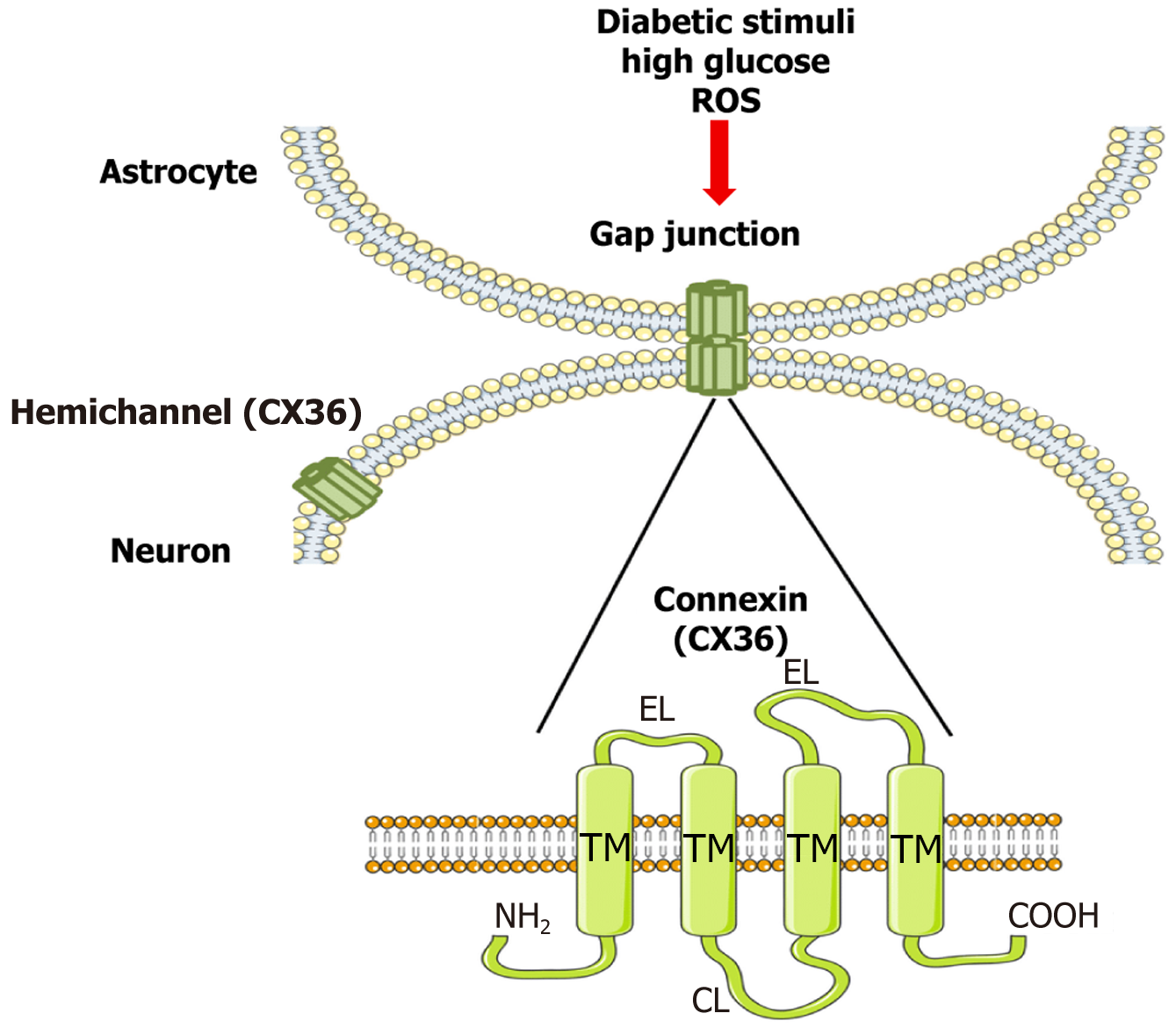
(1) CX36 expression in neurovascular units: Additionally, astrocytes create a network of gap junctions. Among other connexins, the primary structural elements of gap junctions include Cx26, Cx29, Cx30, Cx32, and Cx36. Astrocytes are the only cells that express Cx30 and Cx43[78]. In addition to controlling gap junction function, astrocyte connexins function as semi-channels that allow chemicals like ATP to be transferred and released from inside to outside of cells[79]. This regulates synaptic transmission and, by directly interacting with damaged neurons, causes pain[80]. Although CX43 is the dominant astrocytic connexin, recent studies have uncovered functionally relevant expression of CX36 in astrocytes, particularly within the neurovascular unit during metabolic stress. In diabetic conditions, hyperglycemia and oxidative stress upregulate proinflammatory mediators such as IL-1β and TNF-α, which modulate connexin expression. CX36 expression is altered in diabetic spinal cord tissues, with disrupted localization and reduced coupling efficiency. Moreover, inflammatory signals may impair connexin turnover or phosphorylation, altering their trafficking and assembly. Studies suggest that impaired CX36-mediated gap junctions under hyperglycemic stress diminish homeostatic glial support and contribute to maladaptive network activity in DN. (2) Functional consequences for glial-neuronal coupling: CX36 forms hexameric connexons that dock with counterparts on adjacent cells to form intercellular channels, enabling astrocytes to propagate calcium waves, buffer extracellular potassium, and release ATP and glutamate. In healthy tissue, this synchronization ensures neural-glial metabolic coordination. However, in DN, impaired CX36 function leads to desynchronized glial activity, glutamate excitotoxicity, and increased neuronal hyperexcitability, which are hallmarks of central sensitization.
Experimental models have shown that CX36 dysfunction results in elevated ATP and glutamate release, which activate purinergic and NMDA receptors on dorsal horn neurons, amplifying nociceptive signaling. Additionally, CX36-mediated coupling regulates the astrocytic response to mechanical and inflammatory stimuli. Disruption of this network due to altered phosphorylation (e.g., by calcium/calmodulin-dependent protein kinase II or protein kinase A) or oxidative modification reduces junctional conductance and impairs glial buffering capacity[81].
In diabetic states, the resulting glial uncoupling and ion dysregulation not only potentiate pain signaling but also compromise BBB stability and increase cytokine leakage, further exacerbating neuroinflammation. Together, these findings underscore the pathological role of CX36 dysregulation in DN and highlight it as a potential therapeutic target (Figure 3).
In summary, the following three activation states are generally thought to be how astrocytes involves to regulate DN: (1) Alterations in glial signaling pathways, including alterations in the expression of transcription factors and the phosphorylation level of MAPK; (2) Alterations in receptor and channel protein expression, including the downregulation of glutamate transporters and the upregulation of gap junction proteins and inflammatory factor receptors; and (3) Ongoing production and release of various glia-derived factors, including cytokines, chemokines, and proteinases.
Astrocytes are increasingly recognized as critical players in the pathogenesis of DN, prompting interest in targeting these glial cells for therapeutic intervention. Pharmacological agents such as minocycline and fluocinolone have been shown to modulate astrocytic activity by attenuating neuroinflammation and glial reactivity, thereby offering potential neuroprotective benefits in DN models[82,83]. In parallel, natural compounds like curcumin and resveratrol exhibit astroprotective properties, largely attributed to their antioxidant and anti-inflammatory effects, possibly through the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and inhibition of NF-κB signaling[84,85]. These pathways, including JAK/STAT, NF-κB, and Nrf2, have emerged as central regulators of astrocyte function and are key therapeutic targets in the context of DN[47]. Beyond small molecules, experimental gene therapy and RNA-based approaches, such as small interfering RNA and antisense oligonucleotides are being investigated to modulate astrocyte-specific gene expression and functional responses[86]. However, current therapies face limitations, including poor astrocyte specificity, insufficient BBB permeability, and inadequate long-term efficacy, which hamper their translational potential[87]. One critical gap in knowledge is the absence of astrocyte-specific biomarkers in DN, which hinders accurate assessment and targeted intervention[88]. Moreover, there is a need for refined animal models that better mimic astrocyte-targeted pathology in DN, as current models often fail to capture the complexity of human disease. The heterogeneity of ast
| Name of treatment | Target treatment | Target cells | Mediator/cells, receptor regulated via activation | Molecular mechanisms | Ref. |
| MK-801 | Protein kinase-MAPKs | Microglia, neuron | NMDA receptor | Protein kinases | Daulhac et al[91], 2006 |
| PARP inhibitor, 1,5-isoquinolinediol | Enzyme- poly (ADP-ribose) polymerase (PPAR) | Microglia, neuron, astrocytes | Isoprostane, 8-hydroxy-2′-deoxyguanosine excretion, 4-hydroxynonenal adduct accumulation | Enzymes | Lupachyk et al[92], 2011 |
| MK-801 | Receptor-NMDA receptor | Neuron, astrocytes | ERK1/2, NMDA receptor, nNOS, iNOS | Receptor-related mechanisms | Dauch et al[93], 2012 |
| U0126 | Extracellular signal-regulated protein kinase | Neuron, astrocytes | p-ERK, Iba1, GFAP, NeuN | Protein kinases | Xu et al[94], 2014 |
| levetiracetam | Channels-calcium channel | Microglia, astrocytes | CD11b and GFAP expression | Ion channels | Reda et al[95], 2016 |
| Lycopene | Gap junction- Connexin43 | Astrocytes | GFAP and TNF-α expression | Gap junctions | Zhang et al[96], 2016 |
| Dexmedetomidine | Receptor-α2 adrenergic agonist | Astrocytes, microglial | Iba1, GFAP, IL-1β, TNF-α, glutamate | Receptor-related mechanisms | Lu et al[97], 2017 |
| Mesenchymal stem/stromal cells | Cytokines-IL-1β, and TNF-α | Astrocytes, microglia | Iba1, GFAP, catalase, glutathione peroxidase, superoxide dismutase, Nrf2, MDA, IL-1β, TNF-α | Cytokines signaling | Evangelista et al[98], 2018 |
| Duloxetine | Receptor-Serotonin noradrenaline reuptake inhibitor | Astrocytes, microglia | CD11, GFAP, sciatic mRNA expression of NGF | Receptor-related mechanisms | Tawfik et al[99], 2018 |
| Dexmedetomidine | Signaling pathway-Wnt 10a/β-Catenin | Astrocytes | TNF-α, IL-1β, GFAP, Wnt 10a and β-catenin | Signaling pathways | Zhong et al[100], 2018 |
| Ammoxetine | Receptor-Serotonin and norepinephrine reuptake inhibitors | Astrocytes, microglia | Iba1, GFAP, MAPKs, NF-κβ, IL-1β, TNF-α, IL-6 | Receptor-related mechanisms | Zhang et al[101], 2018 |
| Hydrogen sulfide donor GYY4137 | Cytokines-proinflammatory mediators | Astrocytes, microglia | Iba1, GFAP, neuron, IL-1β, IL-6, TNF-α | Cytokines signaling | Shayea et al[102], 2020 |
| Curcumin | Protein kinases-c-Jun N-terminal kinase | Astrocytes, neurons | p-JNK expression | Protein kinases | Park et al[103], 2021 |
| Fluorocitrate | Selective inhibitor of astrocyte activation | Astrocytes | GFAP expression | Selective inhibitors | Liu et al[104], 2022 |
| Semaglutide | GLP-1 receptor agonists | Astrocytes, microglia | HbA1c, AGEs, Iba1, GFAP, IL-1β, IL-6, TNF-α | GLP-1 receptor agonists | Lee et al[105], 2024 |
Astrocytes have emerged as central yet historically underappreciated contributors to the pathogenesis of DN. Traditionally overshadowed by neurons and microglia in neurodegenerative research, astrocytes are now recognized for their active involvement in neuroinflammation, oxidative stress, and neuronal support failure in DN. Despite growing evidence, current therapies largely overlook astrocytic mechanisms, underscoring a critical gap in both basic research and clinical translation. However, significant challenges remain, including a lack of astrocyte-specific biomarkers, insufficient animal models that accurately replicate astrocytic involvement in DN, and limited understanding of astrocyte heterogeneity and glia-neuron-microglia crosstalk. Addressing these limitations through focused research will be essential for the development of effective, astrocyte-targeted therapies. Advocacy for this shift in perspective is crucial to uncovering new mechanisms and therapeutic opportunities in DN.
| 1. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. 2024;404:2077-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 382] [Article Influence: 191.0] [Reference Citation Analysis (1)] |
| 2. | Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One. 2013;8:e74195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Robertson DB. Dental Caries. Dent Regist. 1898;52:368-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 2019;20:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 365] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 6. | Yuan Y, Liu H, Dai Z, He C, Qin S, Su Z. From Physiology to Pathology of Astrocytes: Highlighting Their Potential as Therapeutic Targets for CNS Injury. Neurosci Bull. 2025;41:131-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Szewczyk AK, Jamroz-Wiśniewska A, Haratym N, Rejdak K. Neuropathic pain and chronic pain as an underestimated interdisciplinary problem. Int J Occup Med Environ Health. 2022;35:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3304] [Cited by in RCA: 3211] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 10. | Fang XX, Wang H, Song HL, Wang J, Zhang ZJ. Neuroinflammation Involved in Diabetes-Related Pain and Itch. Front Pharmacol. 2022;13:921612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Cibelli A, Spray DC, Mola MG. Editorial: Glial cells in homeostasis, neurodevelopment, and repair. Front Cell Neurosci. 2025;19:1575105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front Cell Dev Biol. 2016;4:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Planas AM. Role of microglia in stroke. Glia. 2024;72:1016-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | Verkhratsky A, Nedergaard M. Physiology of Astroglia. Physiol Rev. 2018;98:239-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1219] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 15. | Bugiani M, Plug BC, Man JHK, Breur M, van der Knaap MS. Heterogeneity of white matter astrocytes in the human brain. Acta Neuropathol. 2022;143: 159-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Valles SL, Singh SK, Campos-Campos J, Colmena C, Campo-Palacio I, Alvarez-Gamez K, Caballero O, Jorda A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int J Mol Sci. 2023;24:8434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 17. | Vasile F, Dossi E, Rouach N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct. 2017;222:2017-2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 18. | Wei J, Wang M, Li S, Han R, Xu W, Zhao A, Yu Q, Li H, Li M, Chi G. Reprogramming of astrocytes and glioma cells into neurons for central nervous system repair and glioblastoma therapy. Biomed Pharmacother. 2024;176:116806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Nguyen H, Zerimech S, Baltan S. Astrocyte Mitochondria in White-Matter Injury. Neurochem Res. 2021;46:2696-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Sato Y, Falcone-Juengert J, Tominaga T, Su H, Liu J. Remodeling of the Neurovascular Unit Following Cerebral Ischemia and Hemorrhage. Cells. 2022;11:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Werkman IL, Kövilein J, de Jonge JC, Baron W. Impairing committed cholesterol biosynthesis in white matter astrocytes, but not grey matter astrocytes, enhances in vitro myelination. J Neurochem. 2021;156:624-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Murakami S, Kurachi Y. Mechanisms of astrocytic K(+) clearance and swelling under high extracellular K(+) concentrations. J Physiol Sci. 2016;66:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Barker AJ, Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist. 2010;16:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Szabó Z, Héja L, Szalay G, Kékesi O, Füredi A, Szebényi K, Dobolyi Á, Orbán TI, Kolacsek O, Tompa T, Miskolczy Z, Biczók L, Rózsa B, Sarkadi B, Kardos J. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci Rep. 2017;7:6018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3031] [Cited by in RCA: 3843] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 26. | Ramana KV. Aldose Reductase: New Insights for an Old Enzyme. Biomol Concepts. 2011;2:103-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Puddu A, Sanguineti R, Durante A, Nicolò M, Viviani GL. Vascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularization. Mol Vis. 2012;18:2509-2517. [PubMed] |
| 28. | Lim AK, Ma FY, Nikolic-Paterson DJ, Ozols E, Young MJ, Bennett BL, Friedman GC, Tesch GH. Evaluation of JNK blockade as an early intervention treatment for type 1 diabetic nephropathy in hypertensive rats. Am J Nephrol. 2011;34:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Abdelkader NF, Ibrahim SM, Moustafa PE, Elbaset MA. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-κB/Nrf2 and TGF-β/PKC/TRPV1 signaling pathways. Biomed Pharmacother. 2022;145:112395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Ab Hamid N, Omar N, Ismail CAN, Long I. Insight of Mechanism and Signalling Pathway in Pathogenesis of Diabetic Neuropathy: A Review. IIUM Med J Malays. 2021;20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Zhao H, Alam A, Chen Q, A Eusman M, Pal A, Eguchi S, Wu L, Ma D. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 2017;118:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Wang J, Li G, Wang Z, Zhang X, Yao L, Wang F, Liu S, Yin J, Ling EA, Wang L, Hao A. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Zhang X, Liang Z, Zhou Y, Wang F, Wei S, Tan B, Guo Y. Artesunate Inhibits Apoptosis and Promotes Survival in Schwann Cells via the PI3K/AKT/mTOR Axis in Diabetic Peripheral Neuropathy. Biol Pharm Bull. 2023;46:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 34. | Jende JME, Kender Z, Mooshage C, Groener JB, Alvarez-Ramos L, Kollmer J, Juerchott A, Hahn A, Heiland S, Nawroth P, Bendszus M, Kopf S, Kurz FT. Diffusion Tensor Imaging of the Sciatic Nerve as a Surrogate Marker for Nerve Functionality of the Upper and Lower Limb in Patients With Diabetes and Prediabetes. Front Neurosci. 2021;15:642589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Colcimen N, Altindag F. Evaluation of the effects of sinapic acid and ellagic acid on sciatic nerve in experimental diabetic rats by immunohistochemical and stereological methods. J Chem Neuroanat. 2023;131:102274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Abdyeva A, Kurtova E, Savinkova I, Galkov M, Gorbacheva L. Long-Term Exposure of Cultured Astrocytes to High Glucose Impact on Their LPS-Induced Activation. Int J Mol Sci. 2024;25:1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Bhatt M, Sharma M, Das B. The Role of Inflammatory Cascade and Reactive Astrogliosis in Glial Scar Formation Post-spinal Cord Injury. Cell Mol Neurobiol. 2024;44:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 38. | Bheereddy P, Yerra VG, Kalvala AK, Sherkhane B, Kumar A. SIRT1 Activation by Polydatin Alleviates Oxidative Damage and Elevates Mitochondrial Biogenesis in Experimental Diabetic Neuropathy. Cell Mol Neurobiol. 2021;41:1563-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Chistyakov DV, Azbukina NV, Astakhova AA, Polozhintsev AI, Sergeeva MG, Reiser G. Toll-like receptors control p38 and JNK MAPK signaling pathways in rat astrocytes differently, when cultured in normal or high glucose concentrations. Neurochem Int. 2019;131:104513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Price BR, Johnson LA, Norris CM. Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease. Ageing Res Rev. 2021;68:101335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 41. | Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 42. | Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781-13792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 43. | Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29: 4096-4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 488] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 44. | Düzenli N, Can C, Önal A. Blockage of thrombospondin 4 secreted by spinal astrocytes may be a promising therapeutic target in the treatment of neuropathic pain. Explor Neuroprot Ther. 2022;22:226-241. [DOI] [Full Text] |
| 45. | Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, Inada H, Roh SE, Kim SJ, Lee G, Bae H, Moorhouse AJ, Mikoshiba K, Fukazawa Y, Koizumi S, Nabekura J. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126:1983-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 46. | Koyama Y. Signaling molecules regulating phenotypic conversions of astrocytes and glial scar formation in damaged nerve tissues. Neurochem Int. 2014;78:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 48. | Liu M, Cheng X, Yan H, Chen J, Liu C, Chen Z. MiR-135-5p Alleviates Bone Cancer Pain by Regulating Astrocyte-Mediated Neuroinflammation in Spinal Cord through JAK2/STAT3 Signaling Pathway. Mol Neurobiol. 2021;58:4802-4815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Chen Y, Miles DK, Hoang T, Shi J, Hurlock E, Kernie SG, Lu QR. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci. 2008;28:10983-10989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Marumo T, Takagi Y, Muraki K, Hashimoto N, Miyamoto S, Tanigaki K. Notch signaling regulates nucleocytoplasmic Olig2 translocation in reactive astrocytes differentiation after ischemic stroke. Neurosci Res. 2013;75:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Shimada IS, Borders A, Aronshtam A, Spees JL. Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke. 2011;42:3231-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Bertozzi MM, Rossaneis AC, Fattori V, Longhi-Balbinot DT, Freitas A, Cunha FQ, Alves-Filho JC, Cunha TM, Casagrande R, Verri WA Jr. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem Biol Interact. 2017;273:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Chen Z, Doyle TM, Luongo L, Largent-Milnes TM, Giancotti LA, Kolar G, Squillace S, Boccella S, Walker JK, Pendleton A, Spiegel S, Neumann WL, Vanderah TW, Salvemini D. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A. 2019;116:10557-10562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 54. | Ralay Ranaivo H, Patel F, Wainwright MS. Albumin activates the canonical TGF receptor-smad signaling pathway but this is not required for activation of astrocytes. Exp Neurol. 2010;226:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Zhang R, Wu Y, Xie F, Zhong Y, Wang Y, Xu M, Feng J, Charish J, Monnier PP, Qin X. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018;25:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Lu W, Chen Z, Wen J. RhoA/ROCK signaling pathway and astrocytes in ischemic stroke. Metab Brain Dis. 2021;36:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Erschbamer MK, Hofstetter CP, Olson L. RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. J Comp Neurol. 2005;484:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Hang LH, Shao DH, Chen Z, Sun WJ. Spinal RhoA/Rho kinase signalling pathway may participate in the development of bone cancer pain. Basic Clin Pharmacol Toxicol. 2013;113:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Ishii T, Ueyama T, Shigyo M, Kohta M, Kondoh T, Kuboyama T, Uebi T, Hamada T, Gutmann DH, Aiba A, Kohmura E, Tohda C, Saito N. A Novel Rac1-GSPT1 Signaling Pathway Controls Astrogliosis Following Central Nervous System Injury. J Biol Chem. 2017;292:1240-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N. Mitochondria and sensory processing in inflammatory and neuropathic pain. Front Pain Res (Lausanne). 2022;3:1013577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Akbari-Gharalari N, Khodakarimi S, Nezhadshahmohammad F, Karimipour M, Ebrahimi-Kalan A, Wu J. Exosomes in neuron-glia communication: A review on neurodegeneration. Bioimpacts. 2024;14:30153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 62. | Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 493] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 63. | Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Zhong Y, Chen J, Chen J, Chen Y, Li L, Xie Y. Crosstalk between Cdk5/p35 and ERK1/2 signalling mediates spinal astrocyte activity via the PPARγ pathway in a rat model of chronic constriction injury. J Neurochem. 2019;151:166-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Thakur KK, Saini J, Mahajan K, Singh D, Jayswal DP, Mishra S, Bishayee A, Sethi G, Kunnumakkara AB. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol Res. 2017;115:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975-14983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 67. | Renn CL, Leitch CC, Dorsey SG. In vivo evidence that truncated trkB.T1 participates in nociception. Mol Pain. 2009;5:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Holt LM, Hernandez RD, Pacheco NL, Torres Ceja B, Hossain M, Olsen ML. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. Elife. 2019;8:e44667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 69. | Matyas JJ, O'Driscoll CM, Yu L, Coll-Miro M, Daugherty S, Renn CL, Faden AI, Dorsey SG, Wu J. Truncated TrkB.T1-Mediated Astrocyte Dysfunction Contributes to Impaired Motor Function and Neuropathic Pain after Spinal Cord Injury. J Neurosci. 2017;37:3956-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Dorsey SG, Renn CL, Carim-Todd L, Barrick CA, Bambrick L, Krueger BK, Ward CW, Tessarollo L. In vivo restoration of physiological levels of truncated TrkB.T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron. 2006;51:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189-5194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 974] [Cited by in RCA: 969] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 73. | Nakagawa T, Kaneko S. Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci. 2010;114:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 612] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 75. | Chiang CY, Sessle BJ, Dostrovsky JO. Role of astrocytes in pain. Neurochem Res. 2012;37:2419-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Wu J, Renn CL, Faden AI, Dorsey SG. TrkB.T1 contributes to neuropathic pain after spinal cord injury through regulation of cell cycle pathways. J Neurosci. 2013;33:12447-12463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Zhou W, Xie Z, Li C, Xing Z, Xie S, Li M, Yao J. Driving effect of BDNF in the spinal dorsal horn on neuropathic pain. Neurosci Lett. 2021;756:135965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev. 2004;47:191-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 79. | Xing L, Yang T, Cui S, Chen G. Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front Mol Neurosci. 2019;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 80. | D'hondt C, Iyyathurai J, Himpens B, Leybaert L, Bultynck G. Cx43-hemichannel function and regulation in physiology and pathophysiology: insights from the bovine corneal endothelial cell system and beyond. Front Physiol. 2014;5:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Muñoz MF, Griffith TN, Contreras JE. Mechanisms of ATP release in pain: role of pannexin and connexin channels. Purinergic Signal. 2021;17:549-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 82. | Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 83. | Tiwari AP, Tristan LJC, Albin B, Yang IH. Fluocinolone Acetonide Enhances Anterograde Mitochondria Trafficking and Promotes Neuroprotection against Paclitaxel-Induced Peripheral Neuropathy. ACS Chem Neurosci. 2023;14:2208-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 84. | Elsayed HRH, Rabei MR, Elshaer MMA, El Nashar EM, Alghamdi MA, Al-Qahtani Z, Nabawy A. Suppression of neuronal apoptosis and glial activation with modulation of Nrf2/HO-1 and NF-kB signaling by curcumin in streptozotocin-induced diabetic spinal cord central neuropathy. Front Neuroanat. 2023;17:1094301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 85. | Zhang W, Yu H, Lin Q, Liu X, Cheng Y, Deng B. Anti-inflammatory effect of resveratrol attenuates the severity of diabetic neuropathy by activating the Nrf2 pathway. Aging (Albany NY). 2021;13:10659-10671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 86. | Mata M, Chattopadhyay M, Fink DJ. Gene therapy for the treatment of diabetic neuropathy. Curr Diab Rep. 2008;8:431-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Valori CF, Possenti A, Brambilla L, Rossi D. Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders. Cells. 2021;10:2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Fujita Y, Murakami T, Nakamura A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int J Mol Sci. 2021;22:2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Zhou B, Zuo YX, Jiang RT. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther. 2019;25:665-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 90. | Henn RE, Noureldein MH, Elzinga SE, Kim B, Savelieff MG, Feldman EL. Glial-neuron crosstalk in health and disease: A focus on metabolism, obesity, and cognitive impairment. Neurobiol Dis. 2022;170:105766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 91. | Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 92. | Lupachyk S, Shevalye H, Maksimchyk Y, Drel VR, Obrosova IG. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: correlation with peripheral nerve function. Free Radic Biol Med. 2011;50:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 93. | Dauch JR, Yanik BM, Hsieh W, Oh SS, Cheng HT. Neuron-astrocyte signaling network in spinal cord dorsal horn mediates painful neuropathy of type 2 diabetes. Glia. 2012;60:1301-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Xu X, Chen H, Ling BY, Xu L, Cao H, Zhang YQ. Extracellular signal-regulated protein kinase activation in spinal cord contributes to pain hypersensitivity in a mouse model of type 2 diabetes. Neurosci Bull. 2014;30:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Reda HM, Zaitone SA, Moustafa YM. Effect of levetiracetam versus gabapentin on peripheral neuropathy and sciatic degeneration in streptozotocin-diabetic mice: Influence on spinal microglia and astrocytes. Eur J Pharmacol. 2016;771:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Zhang FF, Morioka N, Kitamura T, Fujii S, Miyauchi K, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. Lycopene ameliorates neuropathic pain by upregulating spinal astrocytic connexin 43 expression. Life Sci. 2016;155:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Lu Y, Lin B, Zhong J. The Therapeutic Effect of Dexmedetomidine on Rat Diabetic Neuropathy Pain and the Mechanism. Biol Pharm Bull. 2017;40:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Evangelista AF, Vannier-Santos MA, de Assis Silva GS, Silva DN, Juiz PJL, Nonaka CKV, Dos Santos RR, Soares MBP, Villarreal CF. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation. 2018;15:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 99. | Tawfik MK, Helmy SA, Badran DI, Zaitone SA. Neuroprotective effect of duloxetine in a mouse model of diabetic neuropathy: Role of glia suppressing mechanisms. Life Sci. 2018;205:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Zhong JM, Lu YC, Zhang J. Dexmedetomidine Reduces Diabetic Neuropathy Pain in Rats through the Wnt 10a/β-Catenin Signaling Pathway. Biomed Res Int. 2018;2018:9043628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Zhang TT, Xue R, Fan SY, Fan QY, An L, Li J, Zhu L, Ran YH, Zhang LM, Zhong BH, Li YF, Ye CY, Zhang YZ. Ammoxetine attenuates diabetic neuropathic pain through inhibiting microglial activation and neuroinflammation in the spinal cord. J Neuroinflammation. 2018;15:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Shayea AMF, Mousa AMA, Renno WM, Nadar MS, Qabazard B, Yousif MHM. Chronic Treatment With Hydrogen Sulfide Donor GYY4137 Mitigates Microglial and Astrocyte Activation in the Spinal Cord of Streptozotocin-Induced Diabetic Rats. J Neuropathol Exp Neurol. 2020;79:1320-1343. [PubMed] [DOI] [Full Text] |
| 103. | Park H, Lee JH, Sim JH, Park J, Choi SS, Leem JG. Effects of Curcumin Treatment in a Diabetic Neuropathic Pain Model of Rats: Involvement of c-Jun N-Terminal Kinase Located in the Astrocytes and Neurons of the Dorsal Root Ganglion. Pain Res Manag. 2021;2021:8787231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 104. | Liu X, He J, Gao J, Xiao Z. Fluorocitrate and neurotropin confer analgesic effects on neuropathic pain in diabetic rats via inhibition of astrocyte activation in the periaqueductal gray. Neurosci Lett. 2022;768:136378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Lee SO, Kuthati Y, Huang WH, Wong CS. Semaglutide Ameliorates Diabetic Neuropathic Pain by Inhibiting Neuroinflammation in the Spinal Cord. Cells. 2024;13:1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/