Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109080
Revised: August 4, 2025
Accepted: September 16, 2025
Published online: October 15, 2025
Processing time: 113 Days and 2 Hours
Dry eye, also known as keratoconjunctival dryness, refers to a group of conditions that lead to eye discomfort and visual dysfunction. Being one of the most common complications of diabetes, it can lead to vision loss and, in severe cases, blindness in patients with diabetes.
To investigate ocular dryness manifestations, assess corneal neuropathy, and identify associated influencing factors in patients with type 2 diabetes (T2D) complicated with comorbid dry eye syndrome (DES).
Data from 81 patients with T2D admitted to Xianyang First People’s Hospital between January 2022 and June 2023 (18 months) were retrospectively reviewed. Patients were divided into the DES and non-DES groups. Additionally, 50 indi
The T2D + DES group showed an increase in the SPEED score, along with a decrease in the NIBUT and SIt wetting length, compared with the non-DES and control groups (P < 0.05); however, no marked inter-group differences were noted for fluorescein staining test scores between T2D + DES group and DES group. Compared with the non-DES groups, the DES group exhibited reductions in density, length, and number of the main nerves, as well as an increase in nerve tortuosity (all P < 0.05), and all these changes were more pronounced in the non-DES group than in the DES group (all P < 0.05). In the DES group, the SPEED score demonstrated a significant negative correlation with nerve density and the length and number of the main nerves but a positive correlation with nerve tortuosity. Conversely, both the NIBUT and SIt wetting length showed a positive association with the density and number of the main nerves; however, the SIt wetting length demonstrated an inverse correlation with nerve tortuosity. Multivariate modeling identified several independent risk factors for DES in T2D, such as age, diabetes duration, lacrimal gland dysfunction, and insufficient insulin secretion, as well as fasting blood glucose and glycated hemoglobin.
Patients with T2D are more susceptible to DES. The T2D + DES group exhibited significant reductions in the density, length, and count, along with increased tortuosity, of the main nerve. These corneal nerve changes are also intimately linked to the severity of DES.
Core Tip: Patients with diabetes are more likely to have dry eyes, experience a burning sensation and foreign body sensation, exhibit corneal epithelial changes, and have a significantly higher incidence of dry eye than those without diabetes. In this study, dry eye and corneal neuropathy symptoms were observed in patients with both dry eye and type 2 diabetes, and the correlation between the two was analyzed. This study also actively explored and summarized relevant factors influencing the occurrence of dry eye in patients with diabetes to provide effective preventive treatment and improve patients’ quality of life.
- Citation: Han WT, Zhao J, Feng WL, Ma WT. Type 2 diabetes complicated by dry eye syndrome: Analysis of dry eye symptoms, corneal neuropathy, and influencing factors. World J Diabetes 2025; 16(10): 109080
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/109080.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.109080
Dry eye syndrome (DES), or keratoconjunctivitis sicca, is a multifactorial ocular condition characterized by tear film instability and/or ocular surface damage caused by abnormalities in the quantity, quality, or fluid dynamics of tears, resulting in ocular discomfort and visual impairment[1]. This syndrome is clinically characterized by symptoms such as ocular itching, dryness, a persistent foreign body sensation, and a burning feeling, accompanied by increased sensitivity to external stimuli[2]. Insufficient basal tear production and severe ocular dryness can trigger reflex tearing, leading to intermittent episodes of excessive tearing[3]. Multiple factors affect DES development, such as environmental influences, individual lifestyle factors, and presence of localized or systemic comorbidities[4,5].
Diabetes is a chronic metabolic condition marked by hyperglycemia and has a multifactorial etiology. It can result in neuropathic, nephropathic, and microvascular complications, significantly impairing patients’ life quality and survival[6]. With the increasing prevalence of diabetes, associated ocular complications have attracted growing clinical attention, making diabetes-related ocular surface disorders a priority in ophthalmology research[7,8]. In particular, DES is one of the most prevalent complications among patients with diabetes. It leads not only to visual deterioration but may, in severe cases, result in blindness, leading to considerable deterioration of the quality of life among patients[9].
In 2007, the International Dry Eye Workshop redefined DES as a multifactorial ocular surface disorder that featured compromised tear film stability, high tear osmolarity, ocular surface injury with inflammation, ocular discomfort, and deterioration of visual function. With an intricate etiology, DES relates closely to the patient’s sex, age, endocrine conditions, autoimmune diseases, etc.[10,11]. With in-depth research and extensive discussion on the etiology and pathological mechanisms of DES, studies have revealed that patients with diabetes are particularly susceptible to symptoms such as dry eyes, burning/foreign body sensations, and corneal epithelium alterations. Notably, the prevalence of DES among patients with diabetes is 1.15-fold among those without diabetes[12]. DES disrupts film stability and causes ocular surface inflammation, leading to corneal neuropathy characterized by corneal nerve length/density reductions and morphological abnormalities such as heightened tortuosity. Corneal nerve damage, particularly decreased nerve density, severely affects the subjective visual quality of patients with DES[13]. A study indicated that 15%-33% of patients with diabetes aged ≥ 65 years have DES, affecting 50% more women than men[14]. In patients with diabetes, reduced corneal sensitivity and poor reflex tear production may also contribute to DES development[15]. Both inflammation and immunity are implicated in the pathogenesis of DES. Hyperglycemia triggers an inflammatory cascade in the functional units of the tear duct, subsequently triggering innate and adaptive immune responses[16]. The inflammatory component of diabetic complications is associated with alterations in corneal immune cells. Furthermore, it leads to quantitative and qualitative abnormalities in tear production, reduced corneal sensitivity, and poor adhesion of regenerating epithelial cells[17]. Additionally, diabetes-induced oxidative stress contributes to the development of DES, and this relationship was examined previously[18].
Therefore, actively exploring and summarizing DES-associated factors in patients with diabetes for prompt delivery of effective preventive and therapeutic measures is essential to safeguarding patients’ quality of life. In this study, DES and corneal neuropathy symptoms were primarily observed in patients with type 2 diabetes (T2D), and their correlations were analyzed. Moreover, a comprehensive analysis of patients’ clinical data was conducted to identify factors con
Data from 81 patients with T2D admitted to Xianyang First People’s Hospital between January 2022 and June 2023 (18 months) were retrospectively reviewed. Twenty-seven of them were definitively diagnosed with DES. Based on the occurrence of DES, patients were divided into the DES and non-DES groups.
Inclusion criteria: (1) All patients satisfied the T2D diagnostic criteria; (2) The DES group met the corresponding diagnostic standards; (3) Age was between 18 and 65 years; (4) No evidence of active ocular inflammation, local application of eye drops, and history of contact lens wear were observed in the preceding 3 months; (5) Patients had no prior history of eye surgery or trauma; and (6) Clinical records were complete.
Exclusion criteria: (1) Other forms of diabetes, such as type 1 diabetes and gestational diabetes; (2) Use of medications that could potentially affect DES development; (3) Presence of ocular surface infections, such as conjunctivitis and keratitis, or other intraocular inflammatory diseases, like glaucoma, cataracts, and retinopathy; (4) A history of eye trauma or having undergone eye surgery within the past 3 months; (5) Recent (within 3 months) contact lens wear; (6) Best-corrected visual acuity < 20/20; (7) History of autoimmune disorders, severe hepatic and renal dysfunction, or malignant neoplasms; and (8) Incomplete clinical data.
Additionally, a control group was employed, which included 50 volunteers who concurrently underwent physical examinations at our hospital. The general characteristics of the groups were not significantly different, ensuring comparability.
T2D diagnostic criteria: Random blood glucose level ≥ 11.1 mmol/L, or fasting blood glucose (FBG) level ≥ 7.0 mmol/L, or 2-hour postprandial blood glucose level ≥ 11.1 mmol/L, or glycated hemoglobin (HbA1c) level ≥ 6.5%.
DES diagnostic criteria: Patients must report subjective symptoms, such as dryness, foreign body sensation, burning sensation, eye fatigue, discomfort, eye redness, and fluctuating vision, and meet one of the following objective criteria: Noninvasive tear film breakup time (NIBUT) ≤ 5 seconds, or Schirmer I test (SIt) (without topical anesthesia) ≤ 5 mm/5 minutes, or if subjective symptoms are present: 5 seconds < NIBUT ≤ 10 seconds, and 5 mm/5 minutes < SIt ≤ 10 mm/5 minutes, and positive corneal and conjunctival fluorescein staining.
General information: Based on electronic medical records, patients’ general information, such as age, sex, body mass index, and diabetes duration, was gathered.
Biochemical data: FBG and HbA1c levels were measured. From each participant, venous blood samples were collected early in the morning on an empty stomach. HbA1c was determined by high-performance liquid chromatography, and FBG levels were measured using the glucose hexokinase method.
DES-related examination indicators: These included dry eye symptom evaluation results, NIBUT, SIt (tear secretion test), corneal fluorescence staining results, and corneal neuropathy assessment results.
Standard patient evaluation of eye dryness questionnaire: This questionnaire mainly assesses the following four groups of symptoms: Dry eyes, foreign body sensation, pain or irritation, and burning sensation or tearing. Participants were asked about the presence of these symptoms during activities, such as reading, using electronic screens, and nighttime driving, and exposure to arid or sandy environments. Each symptom is graded on a scale of 0-3 based on the frequency of occurrence: 0, never; 1, occasionally; 2, frequently; and 3, constantly. Severity is also rated on a 4-point scale: 0, none or mild discomfort; 1, discomfort without affecting daily life; 2, discomfort affecting quality of life; and 3, intolerable discomfort preventing normal activities. The maximum cumulative score of the questionnaire is 24 points.
NIBUT: The rounded tip of a premoistened fluorescein sodium ophthalmic test strip was gently inserted into the lower palpebral conjunctiva. Then, the patient was instructed to blink slowly 3-4 times to ensure the uniform distribution of fluorescein across the ocular surface. Subsequently, the patient was asked to maintain a forward-gazing position. Using the cobalt-blue illumination of a slit lamp, the duration between the final blink and initial appearance of tear film dark spots was precisely quantified.
SIt: The folded leading edge of a Schirmer test strip was carefully applied to the outer one-third of the lower palpebral conjunctiva. The patient was instructed to close both eyes gently. As the tears were secreted, the fluorescein on the strip gradually diffused downward. After 5 minutes, the distance of diffusion was measured, and the reading represented the tear secretion value.
Corneal fluorescein staining test: After NIBUT measurement, corneal epithelial staining was observed. A score of 2 points was assigned if ≥ 8 stained spots were observed, indicating a positive result. A score > 5 points was considered strongly positive.
Corneal neuropathy evaluation: The assessment involved corneal confocal microscopy. The patient was seated in an upright position. First, to anesthetize the eye under examination, 0.5% tetracaine hydrochloride was instilled onto the ocular surface. Then, carbomer gel (Bausch & Lomb, Germany) was evenly applied to the lens surface, and a disposable corneal contact cap was properly installed. The patient was then instructed to firmly fix the forehead and chin on the forehead rest and chin support, respectively. Subsequently, the lens position and focus were precisely adjusted to capture clear images. During imaging, the patient was required to remain completely still. For each eye, 30-40 images were captured, starting from the superficial epithelium at the corneal center and extending to the endothelium. Images were selectively obtained from the basal layer, which lies between the corneal basal epithelium and Bowman’s membrane and is parallel to the corneal surface. Images with noticeable artifacts were excluded. The Image J semiautomatic image-processing software, in combination with the Neuron J plug-in, was utilized to trace the density of the subbasement membrane nerve. Specifically, the density (number/mm2) and length of the main nerve and the straight-line distance between the start and end points of the main nerves were measured. The tortuosity of the main nerves was calculated as the ratio of their length to the straight-line distance between the start and end points. Two attending physicians from the ophthalmology department of our hospital performed the abovementioned measurement tasks independently. If the error between their two results exceeded 10%, an associate chief physician would be involved in the analysis, and the average value of the two most closely matched results would be adopted.
This study employed IBM SPSS Statistics for Windows version 25.0 for statistical analyses. Normally distributed continuous variables (mean ± SD) were analyzed by one-way analysis of variance for multigroup comparisons and independent-sample t-tests for between-group comparisons. Categorical variables, expressed as the case number and corresponding percentage [n (%)], were comparatively assessed by χ2 tests. Pearson correlation analysis explored relationships among variables. Logistic regression analysis was conducted to identify factors contributing to DES in patients with diabetes. All statistical tests rely on a P < 0.05 significance level.
Among the 81 patients with diabetes, 27 had DES, resulting in an incidence rate of 33.3%. A comprehensive comparison was conducted across three groups regarding the standard patient evaluation of eye dryness (SPEED) scores, NIBUT, SIt wetting length, and fluorescein staining test (FLS) scores. Compared with the control group, the DES and non-DES diabetic groups exhibited a significant increase in SPEED and FLS scores and a significant reduction in NIBUT and SIt values (P < 0.05). The DES group demonstrated higher SPEED scores but lower NIBUT and SIt values than the non-DES group (P < 0.05); however, no marked intergroup differences were noted for FLS scores (P > 0.05), as shown in Table 1.
| SPEED score | NIBUT (second) | SIt (mm) | FLS score | |
| DES group (n = 27) | 14.19 ± 2.75a,b | 6.70 ± 1.46a,b | 7.63 ± 1.45a,b | 1.93 ± 0.78a |
| Non-DES group (n = 54) | 5.22 ± 1.13a | 10.20 ± 1.84a | 10.67 ± 1.53a | 1.89 ± 1.11a |
| Control group (n = 50) | 4.32 ± 1.19 | 12.04 ± 1.48 | 12.16 ± 1.30 | 0.42 ± 0.50 |
| F | 368.6 | 91.56 | 103.5 | 46.51 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
FBG and HbA1c levels in the DES and non-DES groups were markedly increased compared with those in the control group (P < 0.05), with their levels being even higher in the DES group than in the non-DES group (P < 0.05), as presented in Table 2.
Compared with the non-DES group, the DES group demonstrated a significant decrease in density, length, and number of the main nerves, as well as an evident augmentation in main nerve tortuosity (P < 0.05), as detailed in Table 3.
| Nerve density (μm/mm2) | Main nerve length (μm) | Number of main nerves (pieces) | Main nerve tortuosity (%) | |
| DES group (n = 27) | 884.23 ± 25.09 | 565.41 ± 36.08 | 2.11 ± 0.42 | 1.17 ± 0.04 |
| Non-DES group (n = 54) | 7944.76 ± 231.55 | 1388.74 ± 55.89 | 5.94 ± 1.52 | 1.11 ± 0.05 |
| t value | 157.5 | 69.53 | 12.80 | 5.544 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
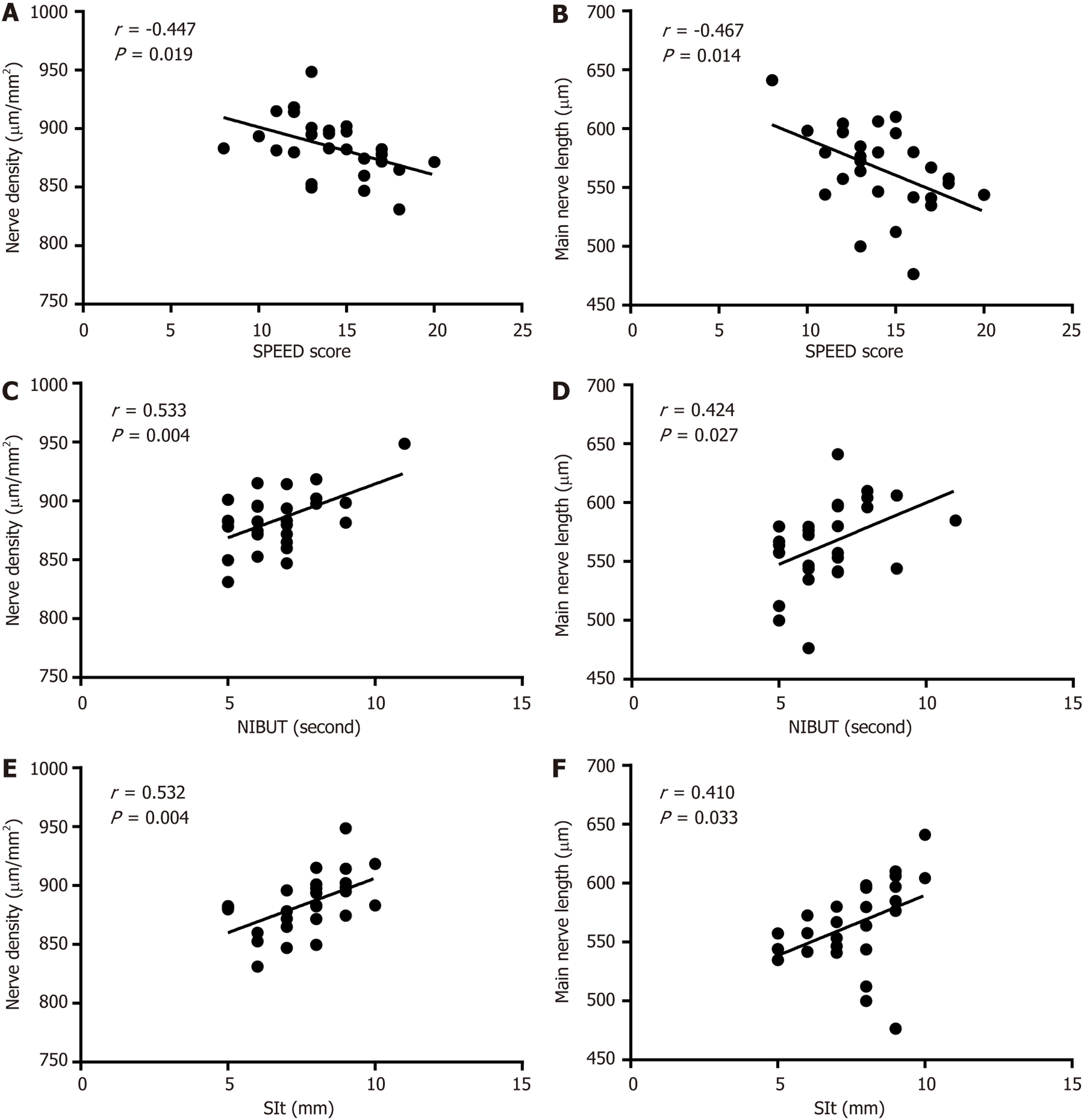
In the DES group, the SPEED score showed a negative correlation with the density, length, and number of the main nerves (r = -0.447, -0.467, and -0.382, respectively, all P < 0.05), and a positive correlation with main nerve tortuosity (r = 0.511, P < 0.05). The NIBUT score showed a positive correlation with the density, length, and number of the main nerves (r = 0.533, 0.424, and 0.428, respectively, all P < 0.05) and a negative correlation with main nerve tortuosity (r = -0.417, P < 0.05). The SIt was positively correlated with the density, length, and number of the main nerves (r = 0.532, 0.410, and 0.447, respectively, all P < 0.05) and inversely linked to main nerve tortuosity (r = -0.684, P < 0.05). See Figure 1 for details.
The analysis revealed significant differences between the DES and non-DES groups regarding age, diabetes duration, lacrimal gland dysfunction, insufficient insulin secretion, disease awareness level, and medication adherence (all P < 0.05). See Table 4 for details.
| DES group (n = 27) | Non-DES group (n = 54) | χ2/t | P value | |
| Sex | 0.225 | 0.635 | ||
| Male | 13 (48.1) | 23 (42.6) | ||
| Female | 14 (51.9) | 31 (57.4) | ||
| Age (years) | 59.59 ± 3.49 | 5.80 ± 3.01 | 3.739 | 0.0003 |
| Duration of diabetes (year) | 7.74 ± 2.18 | 5.29 ± 1.64 | 5.641 | < 0.0001 |
| Hypertension | 0.105 | 0.747 | ||
| Yes | 11 (40.7) | 20 (37.0) | ||
| No | 16 (59.3) | 34 (63.0) | ||
| Lacrimal gland dysfunction | 12.501 | 0.0004 | ||
| Yes | 18 (66.7) | 14 (25.9) | ||
| No | 9 (33.3) | 40 (74.1) | ||
| Insufficient insulin secretion | 6.488 | 0.011 | ||
| Yes | 21 (77.8) | 26 (48.1) | ||
| No | 6 (22.2) | 28 (51.9) | ||
| Disease awareness level | 4.365 | 0.037 | ||
| Low | 15 (55.6) | 17 (31.5) | ||
| High | 12 (44.4) | 37 (68.5) | ||
| Medication compliance | 5.625 | 0.018 | ||
| Good | 10 (37.0) | 35 (64.8) | ||
| Poor | 17 (63.0) | 19 (35.2) |
Multivariate logistic regression was performed, with DES occurrence in patients with T2D as the dependent variable (0, without DES; 1, with DES) and the significant indicators from Tables 2 and 4 as independent variables. Assignments are detailed in Table 5. Age, diabetes duration, lacrimal gland dysfunction, insufficient insulin secretion, FBG, and HbA1c independently influenced the development of DES in T2D. See Table 5 for details.
| Variable | Assignment | β | SE | Wald | P value | HR | 95%CI |
| Age | Continuous variable | 0.347 | 0.152 | 5.192 | 0.023 | 1.415 | 1.050-1.907 |
| Duration of diabetes | Continuous variable | 0.721 | 0.298 | 5.859 | 0.015 | 2.057 | 1.147-3.689 |
| Lacrimal gland dysfunction | 0 = no, 1 = yes | 2.134 | 1.027 | 4.317 | 0.038 | 8.450 | 1.129-63.262 |
| Insufficient insulin secretion | 0 = no, 1 = yes | 2.269 | 1.123 | 4.086 | 0.043 | 9.673 | 1.072-87.319 |
| Disease awareness level | 0 = high, 1 = low | 0.720 | 1.010 | 0.509 | 0.476 | 2.005 | 0.284-14.864 |
| Medication compliance | 0 = good, 1 = poor. | 0.397 | 0.911 | 0.190 | 0.663 | 1.487 | 0.249-8.874 |
| FBG | Continuous variable | 0.815 | 0.298 | 7.482 | 0.006 | 2.259 | 1.260-4.049 |
| HbA1c | Continuous variable | 1.396 | 0.698 | 4.006 | 0.045 | 4.041 | 1.029-15.862 |
| Constant | - | -45.302 | 13.712 | 10.916 | 0.001 | 0.000 | - |
In recent years, with the continuous improvement of the living environment and transformation of lifestyle patterns, the prevalence of diabetes in clinical settings has shown a conspicuous upward trajectory. Diabetes, a chronic endo
In this study, among the 81 patients with diabetes, 27 had DES, resulting in an incidence rate of 33.3%. In a retr
Finally, a comprehensive analysis was conducted on DES drivers in patients with T2D. The outcomes revealed that age, diabetes duration, lacrimal gland dysfunction, insufficient insulin secretion, FBG levels, and HbA1c were all independent determinants of DES development in patients with T2D. Evidently, DES susceptibility progressively increased with longer diabetic duration and worsening glycemic control. Lacrimal gland dysfunction occurs owing to the influence of neuropathy, metabolic disorders, or inflammation. Under these circumstances, the lacrimal gland may fail to maintain normal tear volume, or the tear composition may be altered. Consequently, the tears can no longer effectively lubricate and protect the eyeball surface, thus leading to sensations of dryness and irritation[27]. Insulin serves as a pivotal hormone in the regulation of blood glucose homeostasis. Accordingly, inadequate insulin secretion inevitably causes an increase in blood glucose levels. The ensuing hyperglycemic state may inflict damage upon blood vessels and nerves, particularly those responsible for supplying the ocular region and lacrimal glands[28]. Notably, Rocha et al[29] detected the presence of insulin in human tears and the existence of insulin-like growth factor receptors in both the human cornea and conjunctiva. Insulin is also vital in maintaining tear film stability and the ocular surface microenvironment. Compared with healthy individuals, patients with diabetes exhibit notably lower insulin content in their tears. This deficiency directly contributes to compromised tear film stability and a suboptimal ocular surface environment in patients with diabetes. The importance of glucose for the proper functioning of corneal cells is well known. In the eye, glucose uptake is independent of insulin; it is mediated by active glucose transporter 1[30]. Since the first description of the use of topical insulin in the treatment of corneal ulcers, topical insulin has been found to improve tear film stability[31,32]. Furthermore, high FBG and HbA1c levels are reliable markers of ineffective blood glucose management in patients with T2D. Extended periods of hyperglycemia can damage the microvasculature and disrupt the normal blood circulation of the eyes, potentially causing substantial harm to various ocular tissues such as the lacrimal glands[33]. Moreover, hyperglycemia may trigger an inflammatory response in the eyes, further exacerbating DES symptoms.
This study has some limitations. First, small sample sizes may affect the results, and a significant correlation between dry eye and diabetes duration must be observed in further studies with larger sample sizes. Second, this was a retrospective study; thus, information on diabetes-related clinical parameters was deficient. For example, the presence or absence of peripheral neuropathy in patients could be a confounder that affects the results, which must be confirmed in future studies.
In this study, a tangible correlation has been established between patients with T2D and DES, with these patients concurrently presenting corneal neuropathy of diverse severity levels. For patients with T2D characterized by a protracted disease duration and suboptimal glycemic control, regular funduscopic examinations and taking into full consideration the potential ocular surface disorders that might be triggered are necessary. This comprehensive approach is pivotal for ensuring the timely detection and prompt intervention of DES in patients with diabetes, thereby optimizing their ocular health management and overall prognosis.
| 1. | Mohamed HB, Abd El-Hamid BN, Fathalla D, Fouad EA. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur J Pharm Sci. 2022;175:106206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Huang R, Su C, Fang L, Lu J, Chen J, Ding Y. Dry eye syndrome: comprehensive etiologies and recent clinical trials. Int Ophthalmol. 2022;42:3253-3272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 3. | Yu K, Bunya V, Maguire M, Asbell P, Ying GS; Dry Eye Assessment and Management Study Research Group. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology. 2021;128:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Qian L, Wei W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS One. 2022;17:e0271267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 5. | I Y Hasan ZA. Dry eye syndrome risk factors: A systemic review. Saudi J Ophthalmol. 2021;35:131-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18:525-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 589] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 7. | Kovacova A, Shotliff K. Eye problems in people with diabetes: more than just diabetic retinopathy. Pract Diabetes. 2022;39:34-39. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Feldman-Billard S, Dupas B. Eye disorders other than diabetic retinopathy in patients with diabetes. Diabetes Metab. 2021;47:101279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Zhmud T, Malachkova N, Rejdak R, Costagliola C, Concilio M, Drozhzhyna G, Toro Mario D, Veretelnyk S. Corrigendum: Dry eye disease severity and impact on quality of life in type II diabetes mellitus. Front Med (Lausanne). 2023;10:1218260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Aragona P, Giannaccare G, Rolando M. Special Issue "Managing Dry Eye Disease over Time: An Italian Consensus Conference". J Clin Med. 2022;11:2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Deo N, Nagrale P. Dry Eye Disease: An Overview of Its Risk Factors, Diagnosis, and Prevalence by Age, Sex, and Race. Cureus. 2024;16:e54028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Zou X, Lu L, Xu Y, Zhu J, He J, Zhang B, Zou H. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol. 2018;18:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Ma J, Wei S, Jiang X, Chou Y, Wang Y, Hao R, Yang J, Li X. Evaluation of objective visual quality in dry eye disease and corneal nerve changes. Int Ophthalmol. 2020;40:2995-3004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol. 2016;2016:8201053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 15. | Alhalwani AY, Jambi S, Borai A, Khan MA, Almarzouki H, Elsayid M, Aseri AF, Taher NO, Alghamdi A, Alshehri A. Assessment of the systemic immune-inflammation index in type 2 diabetic patients with and without dry eye disease: A case-control study. Health Sci Rep. 2024;7:e1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 17. | Brar GK, Bawa M, Chadha C, Gupta T, Kaur H. Proportion of dry eye in type II diabetics. J Family Med Prim Care. 2024;13:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetol. 2021;2:36-50. [DOI] [Full Text] |
| 20. | Mussi N, Haque W, Robertson DM. The Association Between Risk Factors for Metabolic Syndrome and Meibomian Gland Disease in a Dry Eye Cohort. Clin Ophthalmol. 2021;15:3821-3832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Pan LY, Kuo YK, Chen TH, Sun CC. Dry eye disease in patients with type II diabetes mellitus: A retrospective, population-based cohort study in Taiwan. Front Med (Lausanne). 2022;9:980714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 22. | Abu EK, Ofori AO, Boadi-Kusi SB, Ocansey S, Yankah RK, Kyei S, Awuku AY. Dry eye disease and meibomian gland dysfunction among a clinical sample of type 2 diabetes patients in Ghana. Afr Health Sci. 2022;22:293-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Singh S, Shanbhag SS, Basu S. Tear secretion from the lacrimal gland: variations in normal versus dry eyes. Br J Ophthalmol. 2022;106:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Xue J, Zhang B, Dou S, Zhou Q, Ding M, Zhou M, Wang H, Dong Y, Li D, Xie L. Revealing the Angiopathy of Lacrimal Gland Lesion in Type 2 Diabetes. Front Physiol. 2021;12:731234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Yazdani-Ibn-Taz MK, Han MM, Jonuscheit S, Collier A, Nally JE, Hagan S. Patient-reported severity of dry eye and quality of life in diabetes. Clin Ophthalmol. 2019;13:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Wan L, Bai X, Zhou Q, Chen C, Wang H, Liu T, Xue J, Wei C, Xie L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int J Biol Sci. 2022;18:809-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 27. | Qi D, Zou S, Lu D, Pei X, Huang S, Huang DL, Liu J, Si H, Li Z. Long-term high fructose intake promotes lacrimal gland dysfunction by inducing gut dysbiosis in mice. Exp Eye Res. 2023;234:109573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 28. | Burgos-Blasco B, Diaz-Valle D, Rego-Lorca D, Perez-Garcia P, Puebla-Garcia V, Fernandez-Vigo JI, Benitez-Del-Castillo JM, Gegundez-Fernandez JA. Topical insulin, a novel corneal epithelial regeneration agent in dry eye disease. Eur J Ophthalmol. 2024;34:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJ, Velloso LA. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest Ophthalmol Vis Sci. 2002;43:963-967. [PubMed] |
| 30. | Stuard WL, Titone R, Robertson DM. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front Endocrinol (Lausanne). 2020;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Britten-Jones AC, Wang MTM, Samuels I, Jennings C, Stapleton F, Craig JP. Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management. Medicina (Kaunas). 2024;60:1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 32. | Vicario-de-la-Torre M, Puebla-García V, Ybañez-García L, López-Cano JJ, González-Cela-Casamayor MA, Brugnera M, Burgos-Blasco B, Díaz-Valle D, Gegúndez-Fernández JA, Benítez-Del-Castillo JM, Herrero-Vanrell R. Topical Insulin Eye Drops: Stability and Safety of Two Compounded Formulations for Treating Persistent Corneal Epithelial Defects. Pharmaceutics. 2024;16:580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Alfuraih S, Tran A, Kim L, Ansari R, Sharma A. Hyperglycemia causes differential change in macrophage population in the lacrimal gland, conjunctiva and cornea. Front Immunol. 2024;15:1505508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/