Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108346
Revised: May 28, 2025
Accepted: August 26, 2025
Published online: October 15, 2025
Processing time: 187 Days and 1.1 Hours
Diabetic foot ulcer is the most prevalent and serious lower-limb complication among individuals with diabetes, and it significantly contributes to the incidence of non-traumatic amputations. The repeated failure of diabetic wounds to heal can result in diabetic foot ulcers, inflicting considerable physical suffering and imposing substantial economic burdens on both patients and global healthcare systems because of the complexity and high costs of treatment. The mechanisms underlying the impaired healing of diabetic wounds are intricate and incom
Core Tip: Repeated failure of diabetic wounds to heal is a critical global health issue that significantly contributes to the incidence of non-traumatic amputations. However, the mechanisms underlying the impaired healing of diabetic wounds are intricate and incompletely elucidated. In this review, we analyze the pivotal roles of histone deacetylases in the critical events in diabetic wound healing.
- Citation: Zhang F, Ma HG, Zhang B, Jiang LL, Nie KY, Deng CL, Liu Y. Potential roles of histone deacetylases in diabetic wound healing. World J Diabetes 2025; 16(10): 108346
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/108346.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.108346
Diabetic foot ulcer (DFU) is the most common and serious lower-limb complication in individuals with diabetes and a major cause of non-traumatic amputations[1]. The global prevalence of diabetes remains grim, with 9.3% (463 million people) of the population affected in 2019, and projections suggesting an increase to 10.2% (578 million people) in 2030 and 10.9% (700 million people) by 2045[2,3]. DFUs occur in 19%-34% of patients with diabetes, and they generally lead to varying degrees of amputation. Each year, approximately 1.6 million individuals globally undergo amputation, 33% of which are major amputations[4]. Furthermore, the mortality rate within 5 years following diabetes-related amputations exceeds 70%[1]. Multiple factors lead to persistent non-healing of diabetic wounds, which ultimately results in the formation of DFUs[5], including impaired leukocyte function[6], reduced growth factor secretion[7,8], impaired ma
Persistent hyperglycemia and prolonged elevation of free fatty acid levels in patients with diabetes can trigger the excessive release of inflammatory molecules, impair leukocyte and macrophage function, and disrupt immune responses, resulting in a chronic inflammatory state that prevents the transition of wounds to the proliferative phase of healing[6,13,14]. Additionally, patients with diabetes exhibit a higher incidence of macrovascular and peripheral artery diseases, like stenosis or occlusion of aortoiliac artery, femoral artery, popliteal artery, infrapopliteal artery and foot artery, than individuals without diabetes, and microcirculatory insufficiency potentially occurs early in the disease course. Pathological changes, including capillary shrinkage, basement membrane thickening, and arterial hyalinization (a pathological change in which homogeneous and transparent hyaline material deposition result in arterial wall thickening and decreased elasticity), are common in diabetes[8,15]. Long-term hyperglycemia can also lead to peripheral neuro
In recent decades, substantial efforts have been dedicated to exploring potential strategies to promote diabetic wound healing, with increasing attention devoted to the effects of epigenetic modifications[23-25]. Diabetes-induced epigenetic dysregulation, such as methylation, phosphorylation, ubiquitination, and acetylation, significantly contributes to delayed wound healing in patients with diabetes. Targeted regulation of specific epigenetic modifications can alter the healing process and improve outcomes[26]. For example, decreased expression of the N6-methyladenosine (m6A) reader YTHDC1 was detected both in vitro in hyperglycemia-treated epidermal cells and in vivo in diabetic wounds, and the decrease in m6A level caused by YTHDC1 downregulation significantly delayed skin wound healing[23]. Phosph
As important epigenetic regulators, histone deacetylases (HDACs) remove acetyl or acyl groups from lysine residues of proteins to regulate various biological processes, including transcription, cell death, and metabolism[26,30-32]. Crosstalk exists between different epigenetic regulators. For example, HDAC inhibition triggered the elevation of m6A RNA modification in ocular melanoma as well as altered DNA and histone methylation in mouse models of Huntington’s disease[33,34]. In neurons, HDAC2 phosphorylation at Y222 contributes to maintaining HDAC2 protein expression. In parathyroid hormone signaling, protein kinase A promotes HDAC4 nuclear export by phosphorylating HDAC4 at S740[35]. HDAC/H3K27ac-mediated transcription of nicotinamide adenine dinucleotide: Ubiquinone oxidoreductase subunit A3 suppresses apoptosis, eliminates reactive oxygen species (ROS), improves mitochondrial function and oxidative phosphorylation, protecting human nucleus pulposus cells against high glucose-induced injuries[36]. In addition, the E3 ubiquitin ligase parkin interacts with acetylase acetyl-CoA acetyltransferase 1 and deacetylase HDAC2. Therefore, inhibiting HDAC activity mediates parkin acetylation and activates mitophagy, inhibiting cervical cancer cell proliferation[37]. As the regulation of methylation, phosphorylation and ubiquitination play important roles in diabetic wound healing, the above results suggest potential roles of HDACs in diabetic wound healing. HDACs can target angiogenic markers such as vascular endothelial growth factor (VEGF) and HIF-1α, and their targeted regulation might play a critical role in angiogenesis. Additionally, HDACs are involved in the polarization of macrophages from M1 to M2, a key event in wound healing. Furthermore, HDACs are associated with insulin release, thus contributing to glucose homeostasis and playing roles in the development of diabetes mellitus and its complications[38-40]. However, the roles of HDACs in diabetic wound healing are incompletely understood. This review summarizes the current research on HDAC functions in diabetic wound healing, discusses potential HDAC targets, and explores their therapeutic potential, thereby offering new insights for addressing impaired diabetic wound healing.
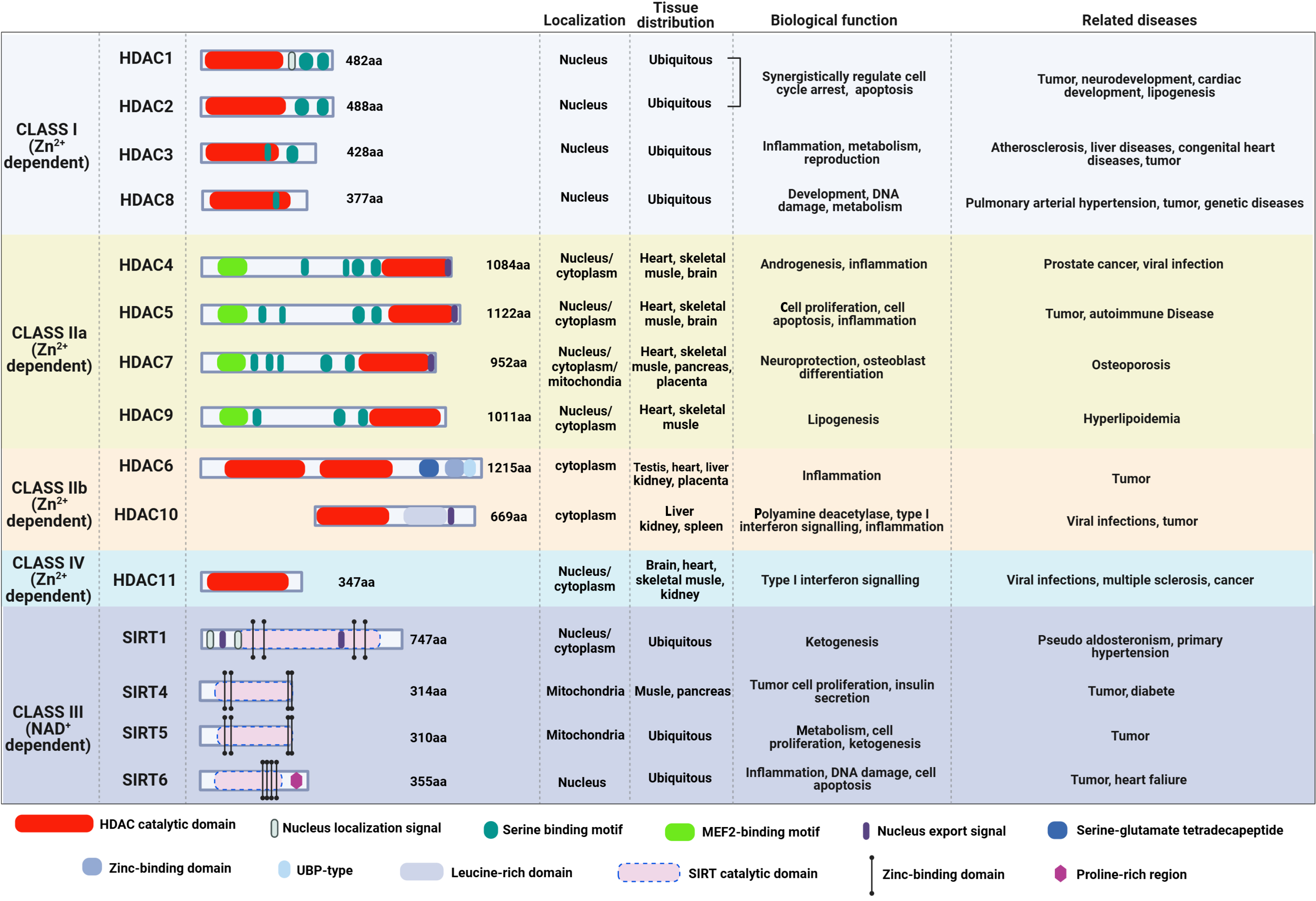
HDACs are highly evolutionarily conserved[36]. Eighteen HDACs have been identified, and they are divided into four classes based on sequence and functional homology. Class I HDACs, namely HDAC1, HDAC2, HDAC3, and HDAC8, share homology in the catalytic pocket with the yeast enzyme reduced potassium dependency 3. Primarily localized in the nucleus, class I HDACs regulate cell cycle, apoptosis, development, metabolism, and disease processes through chromatin modification and transcriptional regulation[41-43]. Class II HDACs have six members: HDAC4, HDAC5, HDAC6, HDAC7, HDAC9 and HDAC10. Among them, HDAC4, HDAC5, HDAC7, and HDAC9 are further grouped into class IIa, whereas HDAC6 and HDAC10 are categorized into class IIb. All of class II HDACs are similar to the yeast protein HDA1. By deacetylating histone and non-histone substrates, class II HDACs play important roles in tissue development, transcriptional repression, and cytoplasmic functions[42,43]. Class III HDACs, which are also known as sirtuins (SIRTs), consist of seven members: SIRT1-7. They are nicotinamide adenine dinucleotide-dependent and have various tissue specificities and subcellular localization, where they play critical roles in aging, metabolism, genome stability, and inflammation[41,44]. Meanwhile, the only class IV HDAC is HDAC11, which can localize to both the nucleus and cytoplasm[38,41,42]. In addition to deacetylase activity, HDAC11 has defatty-acylase activity, indicating its broad physiological functions and pleiotropic regulatory roles in immunity, metabolism, cancer, and nervous system function[45]. The structure, cellular localization, tissue distribution, and biological functions of the four HDAC classes and their involvement in various disease are presented in Figure 1.
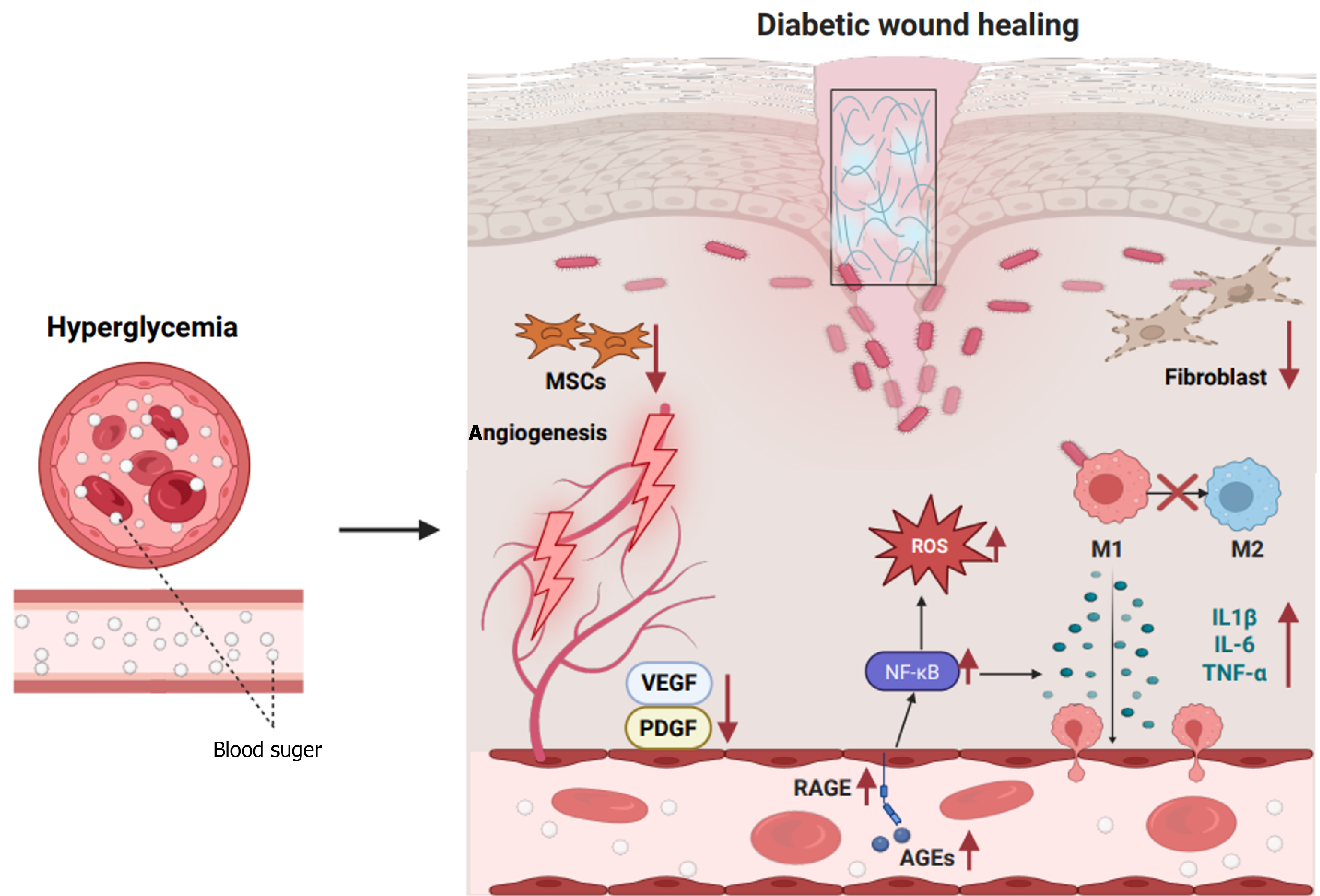
Impaired wound healing is a common and severe complication of diabetes, characterized by a complex pathophysiology involving dysregulated inflammatory responses, impaired angiogenesis, and abnormal epithelial regeneration.
Chronic inflammation is a central feature of delayed healing in diabetic wounds, with mechanisms involving disruption in both the innate and adaptive immune systems. Hyperglycemia promotes the accumulation of advanced glycation end-products (AGEs), which activate the receptor of AGE (RAGE) signaling pathway, leading to the persistent release of nuclear factor kappa-B (NF-κB)-dependent pro-inflammatory factors, such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and IL-1β[8]. Clinical studies demonstrated that IL-1β levels in wound exudates from patients with DFUs are 2-3-fold higher than those in individuals without diabetes, and this increase is negatively correlated with healing time[46]. Furthermore, neutrophil chemotaxis and phagocytic function are impaired in the diabetic state, and apoptosis is delayed, resulting in excessive production of ROS and proteases [e.g., matrix metalloproteinase (MMP)-8], further damaging the ECM and inhibiting re-epithelialization[47]. Abnormal macrophage polarization is another key factor in diabetic wound healing. In normal healing, the dynamic transition of macrophages from pro-inflammatory (M1) to anti-inflammatory/repair (M2) phenotype is crucial for resolving inflammation. However, in diabetic wounds, hyperglycemia and oxidative stress inhibit M2 polarization by suppressing the peroxisome proliferators-activated receptor-γ and signal transducer and activator of transcription (STAT) 6 signaling pathways[48]. Animal studies revealed that M1 marker expression [inducible nitric oxide synthase, cluster of differentiation (CD) 86] increased in mice with diabetes, whereas M2 marker expression [arginase-1 (Arg-1), CD206] decreased. This imbalance in the M1/M2 ratio is directly associated with increased wound area[49]. Additionally, macrophages in patients with diabetes exhibit impaired efferocytosis, leading to the accumulation of necrotic tissue and chronic inflammation[50]. Recent research demonstrated that neu
Angiogenesis is also impaired in diabetic wounds, and this impairment involves endothelial dysfunction, growth factor signaling inhibition, and abnormal perivascular matrix. Hyperglycemia reduces HIF-1α stability, resulting in decreased VEGF and platelet-derived growth factor (PDGF) expression[16]. In mice with diabetes, the half-life of HIF-1α in wounds decreased by 50%, and VEGF messenger RNA levels decreased by 60%[53]. Additionally, AGEs activate nicotinamide adenine dinucleotide phosphate oxidase by binding to RAGE, thereby increasing ROS production and leading to the uncoupling of endothelial nitric oxide synthase and a decrease in nitric oxide (NO) bioavailability[53]. Diabetes-specific microvascular lesions exacerbate ischemia. Histopathological analysis revealed that capillary basement membranes are thickened in patients with DFUs, and the expression of endothelial junction proteins (such as vascular endothelial-cadherin) is reduced, increasing vascular permeability and plasma leakage[54]. Furthermore, pericyte dropout is common in both diabetic retinopathy and wound vasculature degradation. Studies suggest that hyperglycemia induces pericyte apoptosis via activation of the protein kinase C-β signaling pathway, compromising vascular stability[55]. Recent studies also highlighted the role of non-coding RNAs in angiogenesis regulation. For instance, miR-200b is upregulated in diabetic wounds, and it inhibits endothelial cell migration by targeting GATA2 and VEGFR2[56]. Conversely, miR-126-3p is downregulated in diabetes, resulting in impaired angiopoietin-1 signaling[57].
Abnormalities in epidermal regeneration, primarily impaired keratinocyte function and diminished stem cell repair capacity, also occur in diabetic wounds. Integrin α5β1, a core receptor for keratinocyte adhesion to the ECM, is activated by the phosphorylation of focal adhesion kinase (FAK) at phospho-FAK (Tyr397). Research has demonstrated that integrin α5β1 expression is downregulated in keratinocytes from patients with diabetes, leading to decreased FAK phosphorylation, causing weakened cell-matrix adhesion and significantly slower cell migration[58]. In vitro scratch assays confirmed that under high glucose conditions (25 mmol/L glucose), keratinocyte migration is significantly slowed compared with that in the normal glucose group (5.5 mmol/L), and directional migration was impaired[59]. This phenomenon is closely linked to hyperglycemia-induced oxidative stress. Specifically, ROS activate the p38 mitogen-activated protein kinase pathway, thereby inhibiting the activity of small G proteins (Rac1/Cdc42) and disrupting cytoskeletal rearrangement. The basement membrane serves as a scaffold for keratinocyte migration, and its integrity depends on stable expression of laminin-332 and type IV collagen. In diabetes, abnormal basement membrane components represent another key factor limiting epidermal regeneration. Prior studies illustrated that laminin-332 and type IV collagen expression is reduced in diabetic wounds, whereas MMP-9 activity is increased, leading to a loose basement membrane structure that hinders directional keratinocyte migration[58,60]. Additionally, the repair capacity of epidermal stem cells (EpSCs) and mesenchymal stem cells (MSCs) is significantly diminished in diabetes. EpSCs, located in the basal layer of the epidermis, rely on Wnt/β-catenin signaling for self-renewal. Hyperglycemia activates glycogen synthase kinase-3β, thereby promoting β-catenin phosphorylation and degradation, and repressing EpSC proliferation[61]. Flow cytometry revealed reduced Ki-67 positivity in EpSCs from mice with diabetes compared with control mice. MSC paracrine function is also suppressed in diabetes. In prior research, diabetic bone marrow MSCs exhibited a 60% reduction in the secretion of stromal cell-derived factor-1α and VEGF, impairing their homing ability[62]. Mechanistically, hyperglycemia excessively activates the mechanistic target of rapamycin complex 1 signaling pathway, inducing autophagic flux blockage in MSCs and promoting a senescence-associated secretory phenotype[63]. The characteristics of wound healing in diabetes are presented in Figure 2.
The transformation of macrophages from the M1 phenotype to the M2 phenotype is critical for wound healing, as this polarization promotes the non-inflammatory clearance of neutrophils, expression of anti-inflammatory mediators, and production of growth factors, such as IL-10, transforming growth factor (TGF) β1, VEGF, and insulin-like growth factor 1, which promote wound healing[64,65]. Mullican et al[66] reported that HDAC3 deletion in macrophages leads to increased inflammatory gene expression, demonstrating the role of HDAC3 in restraining the M1-to-M2 polarization of ma
In RAW 264.7 and human THP-1-derived macrophages, HDAC9 knockout increased M2 marker expression, de
SIRTs also play pivotal roles in inflammation regulation. SIRT1 promotes M2-type anti-inflammatory macrophage differentiation by enhancing adenosine 5’-monophosphate-activated protein kinase (AMPK) signaling and inhibiting the mammalian target of rapamycin (mTOR) pathway. SIRT1 also activates peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α, improves mitochondrial biosynthesis, reduces ROS accumulation, and thus inhibits NLRP3 inflammasome activation[71-73]. SIRT2 reduced inflammation in gout models by deacetylating NLRP3, inhibiting NLRP3 oligomerization and suppressing IL-1β maturation[74,75]. By deacetylating SOD2 and IDH2, SIRT3 enhanced antioxidant defenses and reduced ROS-induced activation of NLRP3 inflammasome, thereby protecting against sepsis and atherosclerosis[76,77]. In addition, SIRT3 can inhibit macrophage polarization to the pro-inflammatory M1 type, and miR-421 delivery to macrophages inhibits SIRT3, thus increasing M1-type macrophage polarization[78]. SIRT6 has been shown to inhibit TNF-α and IL-8 expression and delay aging-related inflammation by directly binding to the promoters of NF-κB target genes and deacetylating histone H3 Lysine 9[79].
As the only class IV HDAC[80], HDAC11 primarily regulates metabolic inflammation by controlling IL-10 released by antigen-presenting cells. It plays an important role in regulating macrophage phenotype in several diseases. HDAC11 inhibition can increase IL-10 expression in macrophages and polarize them to the M2 type, whereas HDAC11 overexpression can reduce IL-10 expression[39]. Inhibition of HDAC11 promoted the anti-inflammatory properties of ma
The exact mechanism of HDACs in regulating diabetic wound inflammation is still unclear. However, the studies reported above have suggested its great potential in diabetic wound healing. To clarify these effects, alterations of HDAC expression in critical cells (such as macrophages, vascular endothelial cells and epithelial cells) treated with high glucose and in animal models of diabetes warrant further exploration. The impact of HDAC regulation on inflammatory factor expression, signaling pathways, and wound healing outcomes, such as the rate and quality of healing, remains to be explored. Notably, studies incorporating clinical samples and data, such as analyses of skin tissue or blood from patients with DFU, would make the results more convincing.
The stages of wound healing are distinct but overlapping. Prolonged inflammation in the wound contributes to impaired angiogenesis and re-epithelialization. Macrophages regulate the behavior of endothelial cells, fibroblasts and epithelial cells. Consequently, macrophage depletion by pharmacological agents may reduce angiogenesis and cause abnormal ECM deposition in the wound bed, leading to impaired keratinocyte migration, proliferation, and gap junctions[89]. Chronic inflammation also impairs M2 macrophage polarization, resulting in reduced release of growth factors such as VEGF, PDGF, members of the epidermal growth factor family (including EGF, heparin binding-EGF, and TGF-α), and fibroblast growth factors (particularly FGF2 and keratinocyte growth factor). This deficiency hinders neovascularization and disrupts epithelial tongue migration. Injury causes neutrophil recruitment to the wound site. In chronic wounds, the production of neutrophil-derived proteolytic enzymes is abnormally increased, resulting in decreased growth factors and receptor expression, impairing the angiogenesis processes and blood flow[90]. Thus, the regulation of HDACs in inflammation may directly or indirectly influence angiogenesis and epithelial renewal.
Neovascularization is a key event in wound healing. Several studies suggested that HDACs play important roles in angiogenesis by regulating acetylation in both histone and non-histone proteins. The function of HDACs in angiogenesis is subtype-specific and disease context-dependent, meaning that they can either promote or inhibit vessel regeneration[91,92]. By deacetylating histones, HDAC1 and HDAC3 can inhibit the expression of anti-angiogenic genes, such as thrombospondin-1, and promote endothelial cell proliferation and migration[93]. Under hypoxia, HDAC7 is recruited to the VEGF promoter region by HIF-1α to enhance VEGF expression and promote angiogenesis, whereas HDAC4 enhances VEGF expression by deacetylating HIF-1α and enhancing its stability[94,95]. HDAC6 was reported to deacetylate α-tubulin, causing changes in cytoskeletal dynamics and enhancing endothelial cell migration[96]. Besides, HDAC9 upregulation was involved in AGE-induced angiogenesis[92]. Silencing of HDAC9 inhibited VEGF-A expression in diabetic retinopathy, which is important for angiogenesis[97]. HDAC5 plays an antiangiogenic role in vascular homeostasis. Deletion of HDAC5 increased the expression of FGF2 and angiogenic guidance factor Slit2, thereby promoting endothelial cell migration, sprouting, and tube formation[98].
Deletion of SIRT1 downregulated angiogenic genes and reduced angiogenic sprouting, and SIRT1 abolished anti-angiogenic effects by deacetylating the anti-angiogenic factor forkhead transcription factor O1[99]. SIRT1 also promoted cardiac angiogenesis in rats with type 2 diabetes by deacetylating HIF-1α and inhibiting its degradation[100]. It has been reported that SIRT2 activity is reduced in aged aortas. SIRT2 deficiency has been shown to increase mitochondrial oxidative stress, aggravate aging-induced arterial stiffness, and accelerate vascular aging, suggesting its therapeutic value in vascular rejuvenation[101]. Concerning angiogenesis inhibition, HDAC9 contributes to oxygen-glucose deprivation-induced endothelial cell dysfunction and exacerbates endothelial injury in cerebral ischemia/reperfusion injury[102]. In pericytes, HDAC7 impaired vascular maturation by deacetylating PDGF receptor-β, thereby inhibiting its phosph
Studies reported that HDAC2 activity was increased in aortic endothelial cells of diabetic patients, and HDAC2 overexpression increased hyperglycemia-induced vascular injury and abolished the vasculo-protective effect of adiponectin[105]. The HDAC1 inhibitor 1,3-Diphenylurea exhibited wound healing effects in human immortalized keratinocyte (HaCaT) cells, promoted HaCaT cell migration and increased LL-37 and VEGF expression in both HaCaT cells and primary keratinocytes from patients with DFU, and promoted angiogenesis in a mouse aortic ring model[106]. However, the exact characteristics of HDACs in diabetic wound angiogenesis are not yet clear. Further investigations are needed to elucidate the role of HDACs in regulating vascular endothelial cell function, as well as angiogenic and anti-angiogenic factors, in both in vitro and in vivo diabetic wound models.
Dysregulation of HDAC activity results in skin cell dysfunction. HDAC1 deletion leads to abnormal epidermal thickness with hyperkeratosis, hair follicle dystrophy, alopecia, claw dystrophy, and abnormal pigmentation in mice during skin morphogenesis and homeostasis. Double deletion of HDAC1 and HDAC2 was associated with more severe symptoms[107]. Regarding skin regeneration, HDAC1 and HDAC2 are abundantly expressed in the migrating epithelial tongue. According to prior research, hair follicle stem cell (HFSC) activation is also related to histone H4 acetylation. Global H4 deacetylation occurs after the initial injury and contributes to increased HFSC activity, thus promoting proliferation and migration to the wound edge to promote healing[108,109].
Qin et al[110] reported that HDAC6 reversed impaired fibroblast function in aged mice, thereby promoting fibroblast migration and differentiation and accelerating wound healing in aged mice. Interestingly, in diabetic wounds, HDAC6 inhibition induced collagen deposition, angiogenesis, and fibrotic factor expression in the late phase of healing, consequently promoting wound healing in diabetic mice[111].
The importance of SIRT1 in keratinocyte migration and differentiation during wound healing has been reported. Following SIRT1 knockdown in epithelial cells, p53 deacetylation and autophagy-related protein expression were reduced, and FGF21-induced autophagy was inhibited during wound healing. As a result, the pro-healing effect of FGF21 was abolished[112]. Meanwhile, SIRT1 activation promoted the self-renewal and differentiation of type 2 alveolar epithelial cells in patients with idiopathic pulmonary fibrosis and aged mice[113]. Yang et al[114] reported that SIRT3 protected retinal pigment epithelial cells from high glucose-induced injury by activating the AMPK/mTOR/ULK1 signaling pathway. SIRT3 overexpression activated the forkhead box protein O3/PTEN-induced putative kinase protein 1/parkin pathway, promoting diabetic corneal epithelial wound healing by stimulating mitophagy[115]. It has been reported that SIRT6 is responsible for maintaining epithelial integrity and corneal transparency, and SIRT6 deficiency led to Notch signaling pathway dysfunction and delayed healing after corneal epithelial injury[116].
In age-related macular degeneration, HDAC11 overexpression partially contributed to reduced chromatin accessibility, leading to retinal pigment epithelial cell dysfunction and disease onset[117]. HDAC11 also participates in intestinal epithelial barrier dysfunction. An interaction with the vitamin D receptor prevented HDAC11 from binding the promoters of zona occluden-1, claudin-5 and occludin, thereby maintaining epithelial barrier integrity[118]. Furthermore, HDAC11 upregulation was observed in renal tubular epithelial cells in different animal models of renal fibrosis, and HDAC11 inhibition inhibited the pro-fibrogenic response and ameliorated renal fibrosis development[119].
It is still not clear how HDACs affect epithelial cell function and biological behavior under high glucose and in diabetic wound skin. Local injection of small interfering RNA or clustered regularly interspaced short palindromic repeats-associated protein 9 technology for local selective knockout in the skin or lentiviral transfection for local overexpression could be alternative research methods for further study[120].
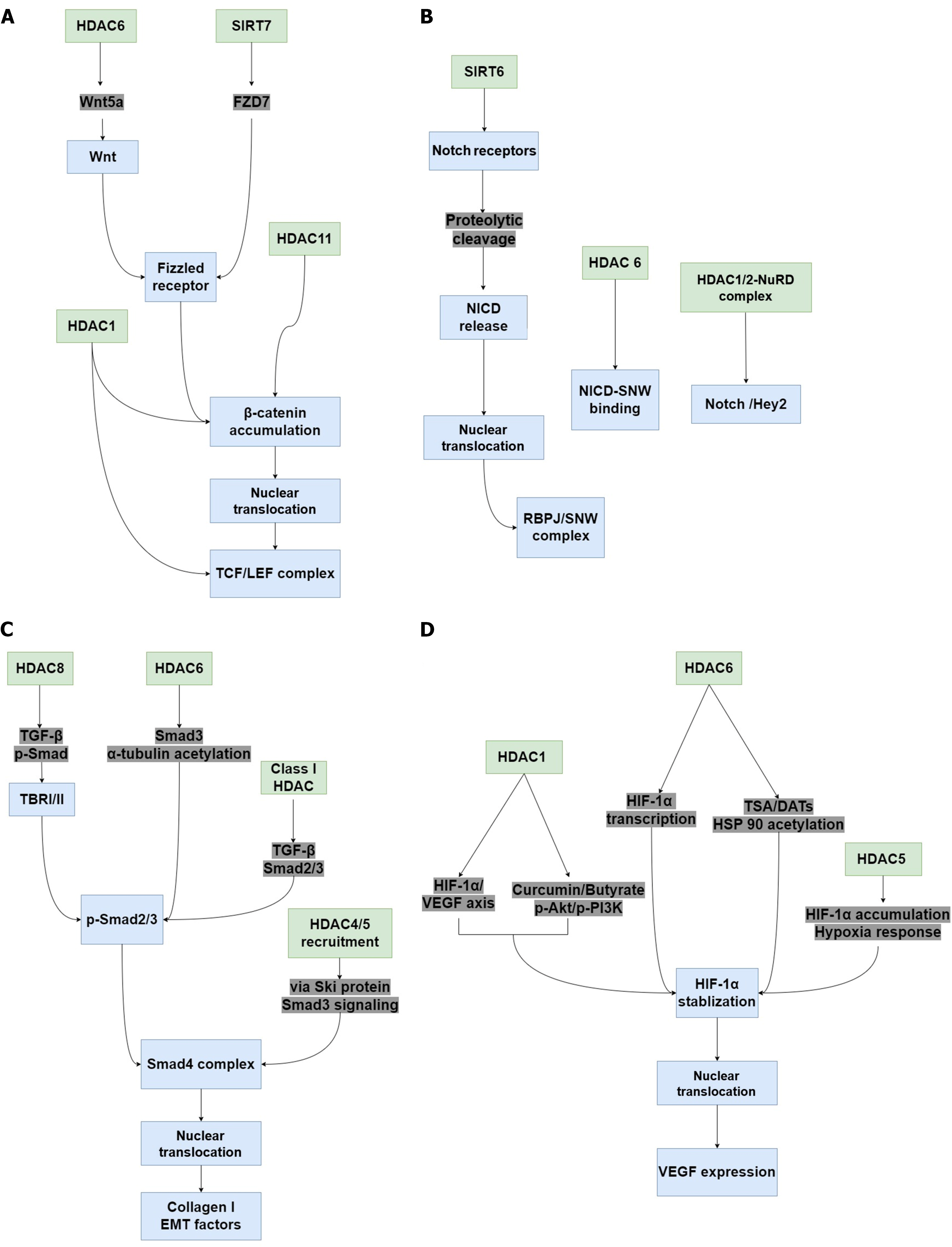
The wound healing process involves numerous signaling pathways, and the dysregulation of any of these pathways affects wound healing. Evidence suggests that Wnt/β-catenin pathway activation promotes skin tissue repair by regulating cell proliferation, hair regeneration, EpSC function, and angiogenesis, and is one of the main ways to regulate wound healing, improve wound angiogenesis and epithelial remodeling[121-123]. Dysregulation of the Wnt/β-catenin signaling pathway led to impaired diabetic wound healing, whereas Wnt/β-catenin signaling activation significantly promoted diabetic skin wound healing[124]. During diabetic wound healing, the size of the ulcer is closely related to β-catenin protein expression[125]. Multiple studies have reported the regulation of HDACs in Wnt/β-catenin signaling. HDAC1 inhibition caused Wnt/β-catenin pathway activation by upregulating TCF4, LEF1, and β-catenin, thereby enhancing chimeric antigen receptor T cell function[126]. Inhibiting HDAC activity with trichostatin A (TSA) increased the expression of the transcription factor TCF and activated Wnt/β-catenin signaling in HepG2 cells[127]. HDAC6 contributed to Wnt signaling activation by increasing Wnt5a expression and promoted cervical cancer progression[128]. HDAC6 activation stimulated c-Myc expression, the downstream proto-oncogene of the Wnt/β-catenin signaling pathway, and promoted tumor growth[129]. HDAC6 deacetylase activity enhanced β-catenin stability, thereby activating the targets of β-catenin signaling. HDAC6 downregulation resulted in increased β-catenin acetylation and degradation, thereby inhibiting Wnt/β-catenin signaling pathway activation and significantly suppressing TGF-β1-induced epithelial-mesenchymal transition (EMT) and metastasis[130]. In HCC cells, SIRT7 promoted FZD7 expression, contributing to β-catenin stability and activation to promote HCC cell migration. Knockdown of SIRT7 decreased β-catenin stability, nuclear localization, and activation[131]. In an inflammatory bowel disease model, SIRT2 inhibited Wnt/β-catenin signaling activation[132]. Meanwhile, butyrate treatment suppressed HDAC11 expression and activated Wnt/β-catenin signaling in vascular smooth muscle cells[133].
Notch signaling is activated by the interaction of membrane-bound Notch receptors (Notch-1-4) on adjacent cells with their ligands (Jagged-1-2 and Delta-like-1/3/4)[134]. Notch signaling participates in wound healing by actively regulating angiogenesis, cell migration, and inflammation[135]. However, Notch signaling is over-activated in diabetic skin, leading to impaired diabetic wound healing[120]. HDACs regulate Notch signaling in various manners. In peripheral neu
Activation of the TGF-β/suppressor of mother against decapentaplegic (Smad) signaling pathway plays important role in wound healing, as it promotes keratinocyte proliferation and migration and participates in the maturation of granulation tissue. The pathway is activated by TGF-β ligand binding to the TGF-β receptor I/II (TBR I/II), which leads to Smad protein phosphorylation, initiating their nuclear translocation and target gene activation. However, TGF-β/Smad signaling is inhibited in diabetic wounds, resulting in delayed wound healing[141,142]. The regulation of HDACs in the TGF-β/Smad signaling pathway suggests its potential role in diabetic wound healing. Inhibition of HDAC6 impaired TGF-β-induced EMT through Smad3 inactivation in A549 cells, resulting in α-tubulin acetylation and impairing mesenchymal stress fiber formation. Inhibiting HDAC10 activity also decreased TGF-β1-induced type 1 collagen expression[143]. In human mammary fibroblasts, high-dose glutamine supplementation significantly inhibited class I HDAC activity, thereby inactivating TGF-β-Smad2/3 signaling. This is important for maintaining the myofibroblast state[144]. In fibrotic buccal mucosal fibroblasts (fBMFs), HDAC8 knockdown significantly reduced TGF-β secretion and the expression of SNAIL and phospho-Smad, thereby inhibiting collagen gel contraction and decreasing wound healing in fBMFs[145]. By recruiting HDAC4 and HDAC5, the oncoprotein Ski, inhibited TGF-β/Smad3 signaling, thereby accelerating chondrocyte differentiation[146]. In 2.5-micrometer particulate matter (PM2.5)-induced lung injury mouse models, HDAC3 knockout reduced TGF-β and Smad2/3 protein expression. Consistently, HDAC3 deficiency inhibited TGF-β/Smad2/3 signaling pathway activation and ameliorated PM2.5-induced inflammation response in lung epithelial cells[147].
Dysregulation of HIF-1α/VEGF signaling delayed diabetic wound healing due to impaired angiogenesis in response to hypoxia and a failure to upregulate VEGF[148]. By inhibiting HDAC1 activity, curcumin and sodium butyrate sup
Many studies have reported the application of HDAC inhibitors in the treatment of different diseases, and some inhibitors have been approved by the Food and Drug Administration for clinical use (Table 1). Although there are no clinical studies directly testing wound healing, a series of preclinical animal studies demonstrated the great potential of HDAC inhibitors in regulating wound healing.
| Inhibitor | Target | Application | Ref. |
| Vorinostat (SAHA) | Broad-spectrum, mainly targets HDAC1, HDAC2, HDAC3, and HDAC6 | Relapsed or refractory cutaneous T-cell lymphoma, multiple myeloma | Kim et al[163]; Olsen et al[164]; Sborov et al[165] |
| Romidepsin (FK228) | Selectively targets HDAC1 and HDAC2 | Relapsed or refractory cutaneous T cell lymphoma and peripheral T cell lymphoma, multiple myeloma | Piekarz et al[166]; Harrison et al[167] |
| Belinostat (PXD101) | Broad-spectrum, targets HDAC1-9 | Relapsed or refractory peripheral T cell lymphoma | O'Connor et al[168] |
| Panobinostat (LBH589) | Broad-spectrum, targets HDAC1-11 | Relapsed or relapsed and refractory multiple myeloma | San-Miguel et al[169] |
| Givinostat (DUVYZAT™) | Pan-HDAC inhibitor | Duchenne muscular dystrophy | Lamb[170] |
| Chidamide | Class I HDAC inhibitor | Relapsed or refractory extranodal natural killer T-cell lymphoma, relapsed or refractory peripheral T cell lymphoma | Shi et al[171]; Gao et al[172] |
The regulation of specific HDACs can have completely opposite effects on skin wound healing, either ameliorating or delaying healing, and even the same HDAC inhibitor can have differential effects. For example, sodium valproate prevented tail regeneration in amphibians but reduced apoptosis in mice[108].
By increasing histone H3 and H4 acetylation, vorinostat decreased collagen deposits at the site of sclerotomy and reduced F-actin and α-smooth muscle actin expression in eye tissue, thereby preventing excessive wound healing and scar formation in a rabbit model of glaucoma filtration surgery[153]. Furthermore, in the treatment of triple-negative breast cancer, vorinostat decreased the deleterious effect of paclitaxel on wound healing[154]. Givinostat was reported to ameliorate glycemic control and restore insulin secretion in diabetic mice that underwent bone marrow transplantation, thereby achieving complete remission of diabetes, suggesting its pivotal role in diabetes and diabetic complications[155]. Treatment with givinostat and vorinostat increased TGF-β1 and IL-8 secretion, thereby improving epithelial wound healing in a mouse model of dextran sodium sulfate-stressed inflammatory bowel disease[156].
It has been reported that the class I HDAC selective inhibitor entinostat, promoted the healing process in mouse skin excisional wounds. However, this effect was abolished by sirtinol, a class III HDAC inhibitor that delayed skin repair by inhibiting SIRT activity, which enhanced cell motility, stimulated keratinocyte proliferation and promoted skin regeneration via endothelial NO synthase phosphorylation and NO production[157].
Topical treatment of TSA, a representative HDAC inhibitor, increased macrophage plasticity in wound beds and achieved better reconstitution in both epithelial and dermal layers in acute wounds compared with control[31]. TSA-pretreated bone marrow MSC-derived exosomes contributed to the polarization of M1 macrophages to M2 macrophages and promoted the migration and angiogenesis of human umbilical vein endothelial cells and formation of collagen in vivo, thereby further promoting wound healing[158]. Additionally, inhibition of HDAC activity by TSA may change bone marrow myeloid progenitor cell behavior and promote the expansion of the Ly6Clow subset, which produces a variety of pro-healing mediators, consequently promoting tissue regeneration and wound healing[31]. TSA can also enhance skin repair by promoting keratinocyte proliferation[159]. TSA treatment can also promote cell proliferation and collagen deposition and expand stem cell populations in digital amputation wounds in mice and frogs[108].
In chronic wounds in human, endoplasmic reticulum stress impairs keratinocyte and fibroblast migration, resulting in prolonged wound healing, the HDAC inhibitor 4-phenylbutyrate significantly rescued keratinocyte migration from elderly leg amputation donors and improved re-epithelialization in an ex vivo human skin wound healing model, suggesting its promising use for wound healing[160].
Although the critical roles of HDAC inhibitors in wound healing are emerging, there are still very few direct studies of HDAC inhibitors in diabetic wound healing models. To reveal the roles that they play in healing in a diabetic setting, explorations of the effects of HDAC inhibitors on diabetic skin coloboma wound healing are necessary, as it would allow for the mechanistic exploration of various key cells involved in healing, including epithelial cells, vascular endothelial cells, macrophages and fibroblasts. After verifying its efficacy and safety in animal models, a small-sample clinical study can be conducted to evaluate the safety and efficacy of HDAC inhibitors in patients with DFU. For example, a single-center, randomized, double-blind, placebo-controlled phase I/II exploratory clinical trial in which topical application of the HDAC inhibitor TSA or HDAC11 selective inhibitor FT895 in DFU patients are considered the treatment group. However, many studies are needed to achieve this goal.
In addition to the known HDAC inhibitors, efforts to identify new HDAC inhibitors are ongoing. In recent years, artificial intelligence (AI) technology has significantly accelerated the drug discovery process by integrating multi-source data and algorithm optimization. Generative adversarial networks and variational autoencoders can generate entirely new molecular structures with HDAC inhibition potential. AI models can rapidly predict the inhibitory activity and selectivity of new compounds by analyzing the structure-activity relationship of known HDAC inhibitors. For example, deep learning-based frameworks can identify key pharmacophore features and optimize the binding affinity of candidate molecules. AI can simultaneously optimize the properties of compounds, including absorption, metabolism, and toxicity, through multi-task models to reduce the risk of later research and development failure. AI-generated molecules exhibited comparable activity to known inhibitors in vitro, along with superior drug-like properties[161,162]. Thus, the application of AI in HDAC inhibitor development will also be a hot topic for future research.
HDACs play pivotal regulatory roles in the healing processes, including inflammation resolution, vascular repair and neovascularization, and tissue regeneration. Yet now, studies about the exact mechanism of HDACs and its inhibitors in regulating diabetic wound healing are still rare. Both animal and clinical studies are lacking. This review synthesized current knowledge on HDACs in diabetic wound healing and provided a roadmap for the translation of epigenetic insights in diabetic wound into clinical breakthroughs. To elucidate the roles of HDACs in diabetic wound healing, both molecular mechanism studies in cells and animal models and human tissue specimens and clinical efficacy studies are essential. Although HDAC inhibitors have tremendous potential as candidate drugs for effective treatment of diabetic wounds, there is a lack of adequate animal studies to evaluate the optimal drug delivery method, and clinical efficacy and safety. Further research is needed to clarify the specific roles of different HDAC isoforms, develop tissue-specific delivery systems, and promote individualized therapeutic strategies based on HDAC regulation.
| 1. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2574] [Article Influence: 286.0] [Reference Citation Analysis (2)] |
| 2. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6389] [Article Influence: 912.7] [Reference Citation Analysis (12)] |
| 3. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1401] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 4. | Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 798] [Article Influence: 266.0] [Reference Citation Analysis (2)] |
| 5. | Li Y, Xu Y, Liu X, Yan X, Lin Y, Tan Q, Hou Y. mTOR inhibitor INK128 promotes wound healing by regulating MDSCs. Stem Cell Res Ther. 2021;12:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Gary Sibbald R, Woo KY. The biology of chronic foot ulcers in persons with diabetes. Diabetes Metab Res Rev. 2008;24 Suppl 1:S25-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1737] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 9. | Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 422] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 599] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 13. | Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 716] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 14. | Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (2)] |
| 15. | Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11:2301-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18:1419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 687] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Nie X, Shi X, Zhao J, Chen Y, Yao Q, Sun C, Yang J. Regulatory Mechanisms of the Wnt/β-Catenin Pathway in Diabetic Cutaneous Ulcers. Front Pharmacol. 2018;9:1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3609] [Cited by in RCA: 4532] [Article Influence: 251.8] [Reference Citation Analysis (0)] |
| 20. | Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: Focusing on diabetic wound healing. J Dermatol Sci. 2016;84:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, Brandner JM. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med. 2011;15:861-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Liang D, Lin WJ, Ren M, Qiu J, Yang C, Wang X, Li N, Zeng T, Sun K, You L, Yan L, Wang W. m(6)A reader YTHDC1 modulates autophagy by targeting SQSTM1 in diabetic skin. Autophagy. 2022;18:1318-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 24. | Chen C, Liu T, Tang Y, Luo G, Liang G, He W. Epigenetic regulation of macrophage polarization in wound healing. Burns Trauma. 2023;11:tkac057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 25. | Nascimento-Filho CHV, Silveira EJD, Goloni-Bertollo EM, de Souza LB, Squarize CH, Castilho RM. Skin wound healing triggers epigenetic modifications of histone H4. J Transl Med. 2020;18:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Yu H, Wang Y, Wang D, Yi Y, Liu Z, Wu M, Wu Y, Zhang Q. Landscape of the epigenetic regulation in wound healing. Front Physiol. 2022;13:949498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Xia G, Chen Y, Xia H, Xu J, Guo L, Lin S, Liu Y. Purpurolide C-based microneedle promotes macrophage-mediated diabetic wound healing via inhibiting TLR4-MD2 dimerization and MYD88 phosphorylation. Acta Pharm Sin B. 2023;13:5060-5073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 28. | Gao JJ, Wu FY, Liu YJ, Li L, Lin YJ, Kang YT, Peng YM, Liu YF, Wang C, Ma ZS, Cao Y, Cao HY, Mo ZW, Li Y, Ou JS, Ou ZJ. Increase of PCSK9 expression in diabetes promotes VEGFR2 ubiquitination to inhibit endothelial function and skin wound healing. Sci China Life Sci. 2024;67:2635-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Yang Y, Huang K, Wang M, Wang Q, Chang H, Liang Y, Wang Q, Zhao J, Tang T, Yang S. Ubiquitination Flow Repressors: Enhancing Wound Healing of Infectious Diabetic Ulcers through Stabilization of Polyubiquitinated Hypoxia-Inducible Factor-1α by Theranostic Nitric Oxide Nanogenerators. Adv Mater. 2021;33:e2103593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 30. | Omidkhah N, Ghodsi R. NO-HDAC dual inhibitors. Eur J Med Chem. 2022;227:113934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Cabanel M, da Costa TP, El-Cheikh MC, Carneiro K. The epigenome as a putative target for skin repair: the HDAC inhibitor Trichostatin A modulates myeloid progenitor plasticity and behavior and improves wound healing. J Transl Med. 2019;17:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Biersack B, Polat S, Höpfner M. Anticancer properties of chimeric HDAC and kinase inhibitors. Semin Cancer Biol. 2022;83:472-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Jia H, Morris CD, Williams RM, Loring JF, Thomas EA. HDAC inhibition imparts beneficial transgenerational effects in Huntington's disease mice via altered DNA and histone methylation. Proc Natl Acad Sci U S A. 2015;112:E56-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Zhuang A, Gu X, Ge T, Wang S, Ge S, Chai P, Jia R, Fan X. Targeting histone deacetylase suppresses tumor growth through eliciting METTL14-modified m(6) A RNA methylation in ocular melanoma. Cancer Commun (Lond). 2023;43:1185-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 35. | Bahl S, Seto E. Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance. Cell Mol Life Sci. 2021;78:427-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Zheng C, Guo D, Zhang T, Hu W, Zhang B, Feng H, Gao Y, Yang G. HDAC/H3K27ac-mediated transcription of NDUFA3 exerts protective effects on high glucose-treated human nucleus pulposus cells through improving mitochondrial function. Sci Rep. 2024;14:21165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Sun X, Shu Y, Ye G, Wu C, Xu M, Gao R, Huang D, Zhang J. Histone deacetylase inhibitors inhibit cervical cancer growth through Parkin acetylation-mediated mitophagy. Acta Pharm Sin B. 2022;12:838-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 38. | Makkar R, Behl T, Arora S. Role of HDAC inhibitors in diabetes mellitus. Curr Res Transl Med. 2020;68:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J Immunol. 2017;199:1505-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 40. | Xiong J, Ma F, Ding N, Xu L, Ma S, Yang A, Hao Y, Zhang H, Jiang Y. miR-195-3p alleviates homocysteine-mediated atherosclerosis by targeting IL-31 through its epigenetics modifications. Aging Cell. 2021;20:e13485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Schiöth HB. Recent developments of HDAC inhibitors: Emerging indications and novel molecules. Br J Clin Pharmacol. 2021;87:4577-4597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 42. | Ramaiah MJ, Tangutur AD, Manyam RR. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021;277:119504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 231] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 43. | Cheng B, Pan W, Xiao Y, Ding Z, Zhou Y, Fei X, Liu J, Su Z, Peng X, Chen J. HDAC-targeting epigenetic modulators for cancer immunotherapy. Eur J Med Chem. 2024;265:116129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 44. | Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 1066] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 45. | Núñez-Álvarez Y, Suelves M. HDAC11: a multifaceted histone deacetylase with proficient fatty deacylase activity and its roles in physiological processes. FEBS J. 2022;289:2771-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 47. | Ma XX, Liu QK, Kuai L, Ma X, Luo Y, Luo Y, Song JK, Fei XY, Jiang JS, Wang MX, Shen F, Ru Y, Li B. The role of neutrophils in diabetic ulcers and targeting therapeutic strategies. Int Immunopharmacol. 2023;124:110861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 48. | Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 880] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 49. | Wolf SJ, Melvin WJ, Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin Cell Dev Biol. 2021;119:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 50. | Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 1029] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 51. | Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 52. | Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, Albiero M, Vigili de Kreutzenberg S, Avogaro A, Fadini GP. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 53. | Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, Pereira T, Ylä-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105:19426-19431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 430] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 54. | Pyšná A, Bém R, Němcová A, Fejfarová V, Jirkovská A, Hazdrová J, Jude EB, Dubský M. Endothelial Progenitor Cells Biology in Diabetes Mellitus and Peripheral Arterial Disease and their Therapeutic Potential. Stem Cell Rev Rep. 2019;15:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Li P, Fan H. Pericyte Loss in Diseases. Cells. 2023;12:1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 56. | Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286:2047-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 57. | Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 58. | Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 371] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 59. | Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190:589-594. [PubMed] [DOI] [Full Text] |
| 60. | Moore BA, Manthey CL, Johnson DL, Bauer AJ. Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology. 2011;141:1283-1292, 1292.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, Ma TL. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front Cell Dev Biol. 2022;10:812262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 62. | Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 528] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 63. | Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013;21:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 64. | Al Sadoun H. Macrophage Phenotypes in Normal and Diabetic Wound Healing and Therapeutic Interventions. Cells. 2022;11:2430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 65. | Wu X, He W, Mu X, Liu Y, Deng J, Liu Y, Nie X. Macrophage polarization in diabetic wound healing. Burns Trauma. 2022;10:tkac051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 66. | Mullican SE, Gaddis CA, Alenghat T, Nair MG, Giacomin PR, Everett LJ, Feng D, Steger DJ, Schug J, Artis D, Lazar MA. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 67. | He R, He Z, Zhang T, Liu B, Gao M, Li N, Geng Q. HDAC3 in action: Expanding roles in inflammation and inflammatory diseases. Cell Prolif. 2025;58:e13731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Liu Y, Du M, Lin HY. Histone deacetylase 9 deficiency exaggerates uterine M2 macrophage polarization. J Cell Mol Med. 2021;25:7690-7708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Chang P, Li H, Hu H, Li Y, Wang T. The Role of HDAC6 in Autophagy and NLRP3 Inflammasome. Front Immunol. 2021;12:763831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 70. | Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 763] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 71. | Xia DY, Yuan JL, Jiang XC, Qi M, Lai NS, Wu LY, Zhang XS. SIRT1 Promotes M2 Microglia Polarization via Reducing ROS-Mediated NLRP3 Inflammasome Signaling After Subarachnoid Hemorrhage. Front Immunol. 2021;12:770744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 72. | Jafarzadeh S, Nemati M, Zandvakili R, Jafarzadeh A. Modulation of M1 and M2 macrophage polarization by metformin: Implications for inflammatory diseases and malignant tumors. Int Immunopharmacol. 2025;151:114345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 73. | Kaukonen R, Mai A, Georgiadou M, Saari M, De Franceschi N, Betz T, Sihto H, Ventelä S, Elo L, Jokitalo E, Westermarck J, Kellokumpu-Lehtinen PL, Joensuu H, Grenman R, Ivaska J. Normal stroma suppresses cancer cell proliferation via mechanosensitive regulation of JMJD1a-mediated transcription. Nat Commun. 2016;7:12237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 74. | Akira S, Misawa T, Satoh T, Saitoh T. Macrophages control innate inflammation. Diabetes Obes Metab. 2013;15 Suppl 3:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Zhao Q, Xia N, Xu J, Wang Y, Feng L, Su D, Cheng Z. Pro-Inflammatory of PRDM1/SIRT2/NLRP3 Axis in Monosodium Urate-Induced Acute Gouty Arthritis. J Innate Immun. 2023;15:614-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 76. | Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2754] [Cited by in RCA: 3194] [Article Influence: 245.7] [Reference Citation Analysis (2)] |
| 77. | Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, Billings FT 4th, Verdin E, Auwerx J, Harrison DG, Dikalov SI. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ Res. 2020;126:439-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 78. | Yang L, Liu S, He Y, Gan L, Ni Q, Dai A, Mu C, Liu Q, Chen H, Lu H, Sun R. Exosomes regulate SIRT3-related autophagy by delivering miR-421 to regulate macrophage polarization and participate in OSA-related NAFLD. J Transl Med. 2024;22:475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 79. | Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 904] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 80. | Liu SS, Wu F, Jin YM, Chang WQ, Xu TM. HDAC11: a rising star in epigenetics. Biomed Pharmacother. 2020;131:110607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 81. | Heim CE, Bosch ME, Yamada KJ, Aldrich AL, Chaudhari SS, Klinkebiel D, Gries CM, Alqarzaee AA, Li Y, Thomas VC, Seto E, Karpf AR, Kielian T. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat Microbiol. 2020;5:1271-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 82. | Núñez-Álvarez Y, Hurtado E, Muñoz M, García-Tuñon I, Rech GE, Pluvinet R, Sumoy L, Pendás AM, Peinado MA, Suelves M. Loss of HDAC11 accelerates skeletal muscle regeneration in mice. FEBS J. 2021;288:1201-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Teng L, Li Z, Shi Y, Gao Z, Yang Y, Wang Y, Bi L. Development and validation of a microenvironment-related prognostic model for hepatocellular carcinoma patients based on histone deacetylase family. Transl Oncol. 2022;26:101547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Wu H, Yin X, Zhao X, Wu Z, Xiao Y, Di Q, Sun P, Tang H, Quan J, Chen W. HDAC11 negatively regulates antifungal immunity by inhibiting Nos2 expression via binding with transcriptional repressor STAT3. Redox Biol. 2022;56:102461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 85. | Bagchi RA, Ferguson BS, Stratton MS, Hu T, Cavasin MA, Sun L, Lin YH, Liu D, Londono P, Song K, Pino MF, Sparks LM, Smith SR, Scherer PE, Collins S, Seto E, McKinsey TA. HDAC11 suppresses the thermogenic program of adipose tissue via BRD2. JCI Insight. 2018;3:e120159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Sun L, Marin de Evsikova C, Bian K, Achille A, Telles E, Pei H, Seto E. Programming and Regulation of Metabolic Homeostasis by HDAC11. EBioMedicine. 2018;33:157-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 87. | Chen H, Xie C, Chen Q, Zhuang S. HDAC11, an emerging therapeutic target for metabolic disorders. Front Endocrinol (Lausanne). 2022;13:989305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Fan XD, Wan LL, Duan M, Lu S. HDAC11 deletion reduces fructose-induced cardiac dyslipidemia, apoptosis and inflammation by attenuating oxidative stress injury. Biochem Biophys Res Commun. 2018;503:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 658] [Article Influence: 329.0] [Reference Citation Analysis (0)] |
| 90. | Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1857] [Article Influence: 265.3] [Reference Citation Analysis (0)] |
| 91. | Zhang X, Lu J, Zhang Q, Luo Q, Liu B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol Res. 2021;54:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 92. | Chen Z, Lin B, Yao X, Weng J, Liu J, He Q, Song K, Zhou C, Zuo Z, Huang X, Liu Z, Huang Q, Xu Q, Guo X. Endothelial β-catenin upregulation and Y142 phosphorylation drive diabetic angiogenesis via upregulating KDR/HDAC9. Cell Commun Signal. 2024;22:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 93. | Aurora AB, Biyashev D, Mirochnik Y, Zaichuk TA, Sánchez-Martinez C, Renault MA, Losordo D, Volpert OV. NF-kappaB balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2010;116:475-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1alpha and increases transcriptional activity. J Biol Chem. 2004;279:41966-41974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Bodily JM, Mehta KP, Laimins LA. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 2011;71:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 96. | Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 97. | Cilenšek I, Lapuh V, Globočnik Petrovič M, Petrovič D. HDAC9 rs11984041 polymorphism is associated with diabetic retinopathy in Slovenian patients with type 2 diabetes mellitus. Gene. 2021;796-797:145802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Urbich C, Rössig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669-5679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 99. | Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 100. | Sarlak Z, Eidi A, Ghorbanzadeh V, Moghaddasi M, Mortazavi P. miR-34a/SIRT1/HIF-1α axis is involved in cardiac angiogenesis of type 2 diabetic rats: The protective effect of sodium butyrate combined with treadmill exercise. Biofactors. 2023;49:1085-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Wang X, Li XK, Lv SJ, Wang HP, Liu Y, Zhou J, Gong H, Chen XF, Ren SC, Zhang H, Dai Y, Cai H, Yan B, Chen HZ, Tang X. Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice. Eur Heart J. 2023;44:2746-2759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 102. | Shi W, Wei X, Wang Z, Han H, Fu Y, Liu J, Zhang Y, Guo J, Dong C, Zhou D, Zhou Q, Chen Y, Yi F. HDAC9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2016;20:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 103. | Mottet D, Bellahcène A, Pirotte S, Waltregny D, Deroanne C, Lamour V, Lidereau R, Castronovo V. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res. 2007;101:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 104. | Yao F, Jin Z, Zheng Z, Lv X, Ren L, Yang J, Chen D, Wang B, Yang W, Chen L, Wang W, Gu J, Lin R. HDAC11 promotes both NLRP3/caspase-1/GSDMD and caspase-3/GSDME pathways causing pyroptosis via ERG in vascular endothelial cells. Cell Death Discov. 2022;8:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 105. | Du Y, Duan Y, Zhao J, Liu C, Zhang Z, Zhang J, Meng Z, Wang X, Lau WB, Xie D, Lopez BL, Christopher TA, Gao E, Koch WW, Liu H, Liu D, Ma XL, Gu G, Wang Y. Dysfunctional APPL1-Mediated Epigenetic Regulation in Diabetic Vascular Injury. Arterioscler Thromb Vasc Biol. 2023;43:e491-e508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 106. | Santos-Mena A, Gonzalez-Muñiz O, Rodríguez-Carlos A, Guerrero AR, Mendieta CR, Jacobo Delgado YM, Muñoz GS, Rosenstein Y, Trujillo-Paez V, Portales-Perez D, de Jesus Gonzalez LA, Calvillo R, Gonzalez-Curiel I, Vitales-Noyola M, Rivas-Santiago B. Wound Healing Effect of HDACi Repositioned Molecules in the Therapy for Chronic Wounds Models. Exp Dermatol. 2025;34:e70060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 107. | Hughes MW, Jiang TX, Lin SJ, Leung Y, Kobielak K, Widelitz RB, Chuong CM. Disrupted ectodermal organ morphogenesis in mice with a conditional histone deacetylase 1, 2 deletion in the epidermis. J Invest Dermatol. 2014;134:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Lewis CJ, Stevenson A, Fear MW, Wood FM. A review of epigenetic regulation in wound healing: Implications for the future of wound care. Wound Repair Regen. 2020;28:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Kang S, Chovatiya G, Tumbar T. Epigenetic control in skin development, homeostasis and injury repair. Exp Dermatol. 2019;28:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Qin YM, Li P, Mu XP, Li ZM, Sun C, Xue WL, Sun J, Bai JJ, Zhu YC, Wang MJ. Histone deacetylase 6 promotes skin wound healing by regulating fibroblast migration and differentiation in aged mice. Sheng Li Xue Bao. 2022;74:979-992. [PubMed] |
| 111. | Karnam K, Sedmaki K, Sharma P, Routholla G, Goli S, Ghosh B, Venuganti VVK, Kulkarni OP. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1β secretion in diabetic mice. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 112. | Chen X, Tong G, Fan J, Shen Y, Wang N, Gong W, Hu Z, Zhu K, Li X, Jin L, Cong W, Xiao J, Zhu Z. FGF21 promotes migration and differentiation of epidermal cells during wound healing via SIRT1-dependent autophagy. Br J Pharmacol. 2022;179:1102-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 113. | Liang J, Huang G, Liu X, Taghavifar F, Liu N, Wang Y, Deng N, Yao C, Xie T, Kulur V, Dai K, Burman A, Rowan SC, Weigt SS, Belperio J, Stripp B, Parks WC, Jiang D, Noble PW. The ZIP8/SIRT1 axis regulates alveolar progenitor cell renewal in aging and idiopathic pulmonary fibrosis. J Clin Invest. 2022;132:e157338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 114. | Yang W, Qiu C, Lv H, Zhang Z, Yao T, Huang L, Wu G, Zhang X, Chen J, He Y. Sirt3 Protects Retinal Pigment Epithelial Cells From High Glucose-Induced Injury by Promoting Mitophagy Through the AMPK/mTOR/ULK1 Pathway. Transl Vis Sci Technol. 2024;13:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 115. | Hu J, Kan T, Hu X. Sirt3 regulates mitophagy level to promote diabetic corneal epithelial wound healing. Exp Eye Res. 2019;181:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Hu X, Zhu S, Liu R, Miller JD, Merkley K, Tilton RG, Liu H. Sirt6 deficiency impairs corneal epithelial wound healing. Aging (Albany NY). 2018;10:1932-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Wang J, Zibetti C, Shang P, Sripathi SR, Zhang P, Cano M, Hoang T, Xia S, Ji H, Merbs SL, Zack DJ, Handa JT, Sinha D, Blackshaw S, Qian J. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat Commun. 2018;9:1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 118. | Liu FH, Li SS, Li XX, Wang S, Li MG, Guan L, Luan TG, Liu ZG, Liu ZJ, Yang PC. Vitamin D3 induces vitamin D receptor and HDAC11 binding to relieve the promoter of the tight junction proteins. Oncotarget. 2017;8:58781-58789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Mao L, Liu L, Zhang T, Qin H, Wu X, Xu Y. Histone Deacetylase 11 Contributes to Renal Fibrosis by Repressing KLF15 Transcription. Front Cell Dev Biol. 2020;8:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 120. | Zheng X, Narayanan S, Sunkari VG, Eliasson S, Botusan IR, Grünler J, Catrina AI, Radtke F, Xu C, Zhao A, Ekberg NR, Lendahl U, Catrina SB. Triggering of a Dll4-Notch1 loop impairs wound healing in diabetes. Proc Natl Acad Sci U S A. 2019;116:6985-6994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 121. | Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, Chen L, Qi S, Liu X, Wang P, Tang J, Xie J. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015;6:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 122. | Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847-10854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 123. | Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, Nace A, Zhang X, Baratono S, Wang F, Ornitz DM, Millar SE, Cotsarelis G. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 124. | Nie X, Zhang H, Shi X, Zhao J, Chen Y, Wu F, Yang J, Li X. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/β-catenin signaling pathway. Int Immunopharmacol. 2020;79:106109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 125. | McBride JD, Jenkins AJ, Liu X, Zhang B, Lee K, Berry WL, Janknecht R, Griffin CT, Aston CE, Lyons TJ, Tomasek JJ, Ma JX. Elevated circulation levels of an antiangiogenic SERPIN in patients with diabetic microvascular complications impair wound healing through suppression of Wnt signaling. J Invest Dermatol. 2014;134:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Zhu M, Han Y, Gu T, Wang R, Si X, Kong D, Zhao P, Wang X, Li J, Zhai X, Yu Z, Lu H, Li J, Huang H, Qian P. Class I HDAC inhibitors enhance antitumor efficacy and persistence of CAR-T cells by activation of the Wnt pathway. Cell Rep. 2024;43:114065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 127. | Shi QQ, Zuo GW, Feng ZQ, Zhao LC, Luo L, You ZM, Li DY, Xia J, Li J, Chen DL. Effect of trichostatin A on anti HepG2 liver carcinoma cells: inhibition of HDAC activity and activation of Wnt/β-Catenin signaling. Asian Pac J Cancer Prev. 2014;15:7849-7855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Shao Y, Zhu F, Zhu S, Bai L. HDAC6 suppresses microRNA-199a transcription and augments HPV-positive cervical cancer progression through Wnt5a upregulation. Int J Biochem Cell Biol. 2021;136:106000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Zhang X, Guo N, Jin H, Liu R, Zhang Z, Cheng C, Fan Z, Zhang G, Xiao M, Wu S, Zhao Y, Lu X. Bisphenol A drives di(2-ethylhexyl) phthalate promoting thyroid tumorigenesis via regulating HDAC6/PTEN and c-MYC signaling. J Hazard Mater. 2022;425:127911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 130. | Wang Y, Ha M, Li M, Zhang L, Chen Y. Histone deacetylase 6-mediated downregulation of TMEM100 expedites the development and progression of non-small cell lung cancer. Hum Cell. 2022;35:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Gu Y, Wang Z, Liang G, Peng J, Zhang X, Yu T, Ding C, Li Z. SIRT7 stabilizes β-catenin and promotes canonical Wnt activation via upregulating FZD7. Life Sci. 2024;359:123240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 132. | Li C, Zhou Y, Rychahou P, Weiss HL, Lee EY, Perry CL, Barrett TA, Wang Q, Evers BM. SIRT2 Contributes to the Regulation of Intestinal Cell Proliferation and Differentiation. Cell Mol Gastroenterol Hepatol. 2020;10:43-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 133. | Zhong H, Yu H, Chen J, Mok SWF, Tan X, Zhao B, He S, Lan L, Fu X, Chen G, Zhu D. The short-chain fatty acid butyrate accelerates vascular calcification via regulation of histone deacetylases and NF-κB signaling. Vascul Pharmacol. 2022;146:107096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 134. | Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017;97:1235-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 745] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 135. | Kimball AS, Joshi AD, Boniakowski AE, Schaller M, Chung J, Allen R, Bermick J, Carson WF 4th, Henke PK, Maillard I, Kunkel SL, Gallagher KA. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Front Immunol. 2017;8:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 136. | Wu LM, Wang J, Conidi A, Zhao C, Wang H, Ford Z, Zhang L, Zweier C, Ayee BG, Maurel P, Zwijsen A, Chan JR, Jankowski MP, Huylebroeck D, Lu QR. Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat Neurosci. 2016;19:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 137. | Xia S, Menden HL, Mabry SM, Sampath V. HDAC6 and ERK/ADAM17 Regulate VEGF-Induced NOTCH Signaling in Lung Endothelial Cells. Cells. 2023;12:2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 138. | Farhadipour M, Arnauts K, Clarysse M, Thijs T, Liszt K, Van der Schueren B, Ceulemans LJ, Deleus E, Lannoo M, Ferrante M, Depoortere I. SCFAs switch stem cell fate through HDAC inhibition to improve barrier integrity in 3D intestinal organoids from patients with obesity. iScience. 2023;26:108517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 139. | Subramani P, Nagarajan N, Mariaraj S, Vilwanathan R. Knockdown of sirtuin6 positively regulates acetylation of DNMT1 to inhibit NOTCH signaling pathway in non-small cell lung cancer cell lines. Cell Signal. 2023;105:110629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 140. | Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi-Kocal G, Rickelt S, Butty VL, Moreno-Serrano M, Iqbal AM, Bauer-Rowe KE, Imada S, Ulutas MS, Mylonas C, Whary MT, Levine SS, Basbinar Y, Hynes RO, Mino-Kenudson M, Deshpande V, Boyer LA, Fox JG, Terranova C, Rai K, Piwnica-Worms H, Mihaylova MM, Regev A, Yilmaz ÖH. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell. 2019;178:1115-1131.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 141. | Zhang F, Ren Y, Liu P, Ren Y, Wang D. Expression of TGF-β1 and miRNA-145 in patients with diabetic foot ulcers. Exp Ther Med. 2016;11:2011-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 142. | Nie X, Zhao J, Ling H, Deng Y, Li X, He Y. Exploring microRNAs in diabetic chronic cutaneous ulcers: Regulatory mechanisms and therapeutic potential. Br J Pharmacol. 2020;177:4077-4095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 143. | Yu H, Liu S, Wang S, Gu X. A narrative review of the role of HDAC6 in idiopathic pulmonary fibrosis. J Thorac Dis. 2024;16:688-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 144. | Mezawa Y, Wang T, Daigo Y, Takano A, Miyagi Y, Yokose T, Yamashita T, Yang L, Maruyama R, Seimiya H, Orimo A. Glutamine deficiency drives transforming growth factor-β signaling activation that gives rise to myofibroblastic carcinoma-associated fibroblasts. Cancer Sci. 2023;114:4376-4387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 145. | Yang HW, Sun YH, Fang CY, Ohiro Y, Liao HY, Liao YW, Kao YH, Yu CC. Downregulation of histone deacetylase 8 (HDAC8) alleviated the progression of oral submucous fibrosis. J Dent Sci. 2023;18:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 146. | Kim KO, Sampson ER, Maynard RD, O'Keefe RJ, Chen D, Drissi H, Rosier RN, Hilton MJ, Zuscik MJ. Ski inhibits TGF-β/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J Cell Biochem. 2012;113:2156-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Gu LZ, Sun H, Chen JH. Histone deacetylases 3 deletion restrains PM2.5-induced mice lung injury by regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed Pharmacother. 2017;85:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 148. | Chen H, Cheng Y, Tian J, Yang P, Zhang X, Chen Y, Hu Y, Wu J. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 149. | Islam R, Dash D, Singh R. An antioxidant ameliorates allergic airway inflammation by inhibiting HDAC 1 via HIF-1α/VEGF axis suppression in mice. Sci Rep. 2023;13:9637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 150. | Chen S, Yin C, Lao T, Liang D, He D, Wang C, Sang N. AMPK-HDAC5 pathway facilitates nuclear accumulation of HIF-1α and functional activation of HIF-1 by deacetylating Hsp70 in the cytosol. Cell Cycle. 2015;14:2520-2536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 151. | Schoepflin ZR, Shapiro IM, Risbud MV. Class I and IIa HDACs Mediate HIF-1α Stability Through PHD2-Dependent Mechanism, While HDAC6, a Class IIb Member, Promotes HIF-1α Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc. J Bone Miner Res. 2016;31:1287-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 152. | Wei Z, Shan Y, Tao L, Liu Y, Zhu Z, Liu Z, Wu Y, Chen W, Wang A, Lu Y. Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol Carcinog. 2017;56:2317-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 153. | Sharma A, Anumanthan G, Reyes M, Chen H, Brubaker JW, Siddiqui S, Gupta S, Rieger FG, Mohan RR. Epigenetic Modification Prevents Excessive Wound Healing and Scar Formation After Glaucoma Filtration Surgery. Invest Ophthalmol Vis Sci. 2016;57:3381-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 154. | Marcotte JH, Rattigan DA, Irons RF, Cahill KW, Zhang P, Chang S, Koko KR, Gaughan JP, Carpenter JP, Brown SA, Budak-Alpdogan T. The Effect of the Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid and Paclitaxel Treatment on Full-Thickness Wound Healing in Mice. Ann Plast Surg. 2018;81:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 155. | Kojima H, Katagi M, Okano J, Nakae Y, Ohashi N, Fujino K, Miyazawa I, Nakagawa T. Complete remission of diabetes with a transient HDAC inhibitor and insulin in streptozotocin mice. Commun Biol. 2023;6:637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 156. | Friedrich M, Gerbeth L, Gerling M, Rosenthal R, Steiger K, Weidinger C, Keye J, Wu H, Schmidt F, Weichert W, Siegmund B, Glauben R. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFβ1 signalling in inflammation. Mucosal Immunol. 2019;12:656-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 157. | Spallotta F, Cencioni C, Straino S, Nanni S, Rosati J, Artuso S, Manni I, Colussi C, Piaggio G, Martelli F, Valente S, Mai A, Capogrossi MC, Farsetti A, Gaetano C. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J Biol Chem. 2013;288:11004-11012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 158. | Wang T, Xue Y, Zhang W, Zheng Z, Peng X, Zhou Y. Collagen sponge scaffolds loaded with Trichostatin A pretreated BMSCs-derived exosomes regulate macrophage polarization to promote skin wound healing. Int J Biol Macromol. 2024;269:131948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 159. | Spallotta F, Cencioni C, Straino S, Sbardella G, Castellano S, Capogrossi MC, Martelli F, Gaetano C. Enhancement of lysine acetylation accelerates wound repair. Commun Integr Biol. 2013;6:e25466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Bachar-Wikstrom E, Manchanda M, Bansal R, Karlsson M, Kelly-Pettersson P, Sköldenberg O, Wikstrom JD. Endoplasmic reticulum stress in human chronic wound healing: Rescue by 4-phenylbutyrate. Int Wound J. 2021;18:49-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 161. | Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers. 2021;25:1315-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 552] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 162. | Yu KH, Healey E, Leong TY, Kohane IS, Manrai AK. Medical Artificial Intelligence and Human Values. N Engl J Med. 2024;390:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 163. | Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, Whittaker S, Tokura Y, Vermeer M, Zinzani PL, Sokol L, Morris S, Kim EJ, Ortiz-Romero PL, Eradat H, Scarisbrick J, Tsianakas A, Elmets C, Dalle S, Fisher DC, Halwani A, Poligone B, Greer J, Fierro MT, Khot A, Moskowitz AJ, Musiek A, Shustov A, Pro B, Geskin LJ, Dwyer K, Moriya J, Leoni M, Humphrey JS, Hudgens S, Grebennik DO, Tobinai K, Duvic M; MAVORIC Investigators. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 476] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 164. | Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109-3115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 779] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 165. | Sborov DW, Benson DM, Williams N, Huang Y, Bowers MA, Humphries K, Efebera Y, Devine S, Hofmeister CC. Lenalidomide and vorinostat maintenance after autologous transplant in multiple myeloma. Br J Haematol. 2015;171:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 166. | Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, Jaffe ES, Ling A, Turner M, Peer CJ, Figg WD, Steinberg SM, Smith S, Joske D, Lewis I, Hutchins L, Craig M, Fojo AT, Wright JJ, Bates SE. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827-5834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 383] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 167. | Harrison SJ, Quach H, Link E, Seymour JF, Ritchie DS, Ruell S, Dean J, Januszewicz H, Johnstone R, Neeson P, Dickinson M, Nichols J, Prince HM. A high rate of durable responses with romidepsin, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma. Blood. 2011;118:6274-6283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 168. | O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, Bhat G, Choi MR, Walewski J, Savage K, Foss F, Allen LF, Shustov A. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33:2492-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 169. | San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn TN, Siritanaratkul N, Corradini P, Chuncharunee S, Lee JJ, Schlossman RL, Shelekhova T, Yong K, Tan D, Numbenjapon T, Cavenagh JD, Hou J, LeBlanc R, Nahi H, Qiu L, Salwender H, Pulini S, Moreau P, Warzocha K, White D, Bladé J, Chen W, de la Rubia J, Gimsing P, Lonial S, Kaufman JL, Ocio EM, Veskovski L, Sohn SK, Wang MC, Lee JH, Einsele H, Sopala M, Corrado C, Bengoudifa BR, Binlich F, Richardson PG. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 618] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 170. | Lamb YN. Givinostat: First Approval. Drugs. 2024;84:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 171. | Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Huang H, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (5)] |
| 172. | Gao Y, He H, Li X, Zhang L, Xu W, Feng R, Li W, Xiao Y, Liu X, Chen Y, Wang X, Bai B, Wu H, Cai Q, Li Z, Li J, Lin S, He Y, Ping L, Huang C, Mao J, Chen X, Zhao B, Huang H. Sintilimab (anti-PD-1 antibody) plus chidamide (histone deacetylase inhibitor) in relapsed or refractory extranodal natural killer T-cell lymphoma (SCENT): a phase Ib/II study. Signal Transduct Target Ther. 2024;9:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/