©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 108714
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108714
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108714
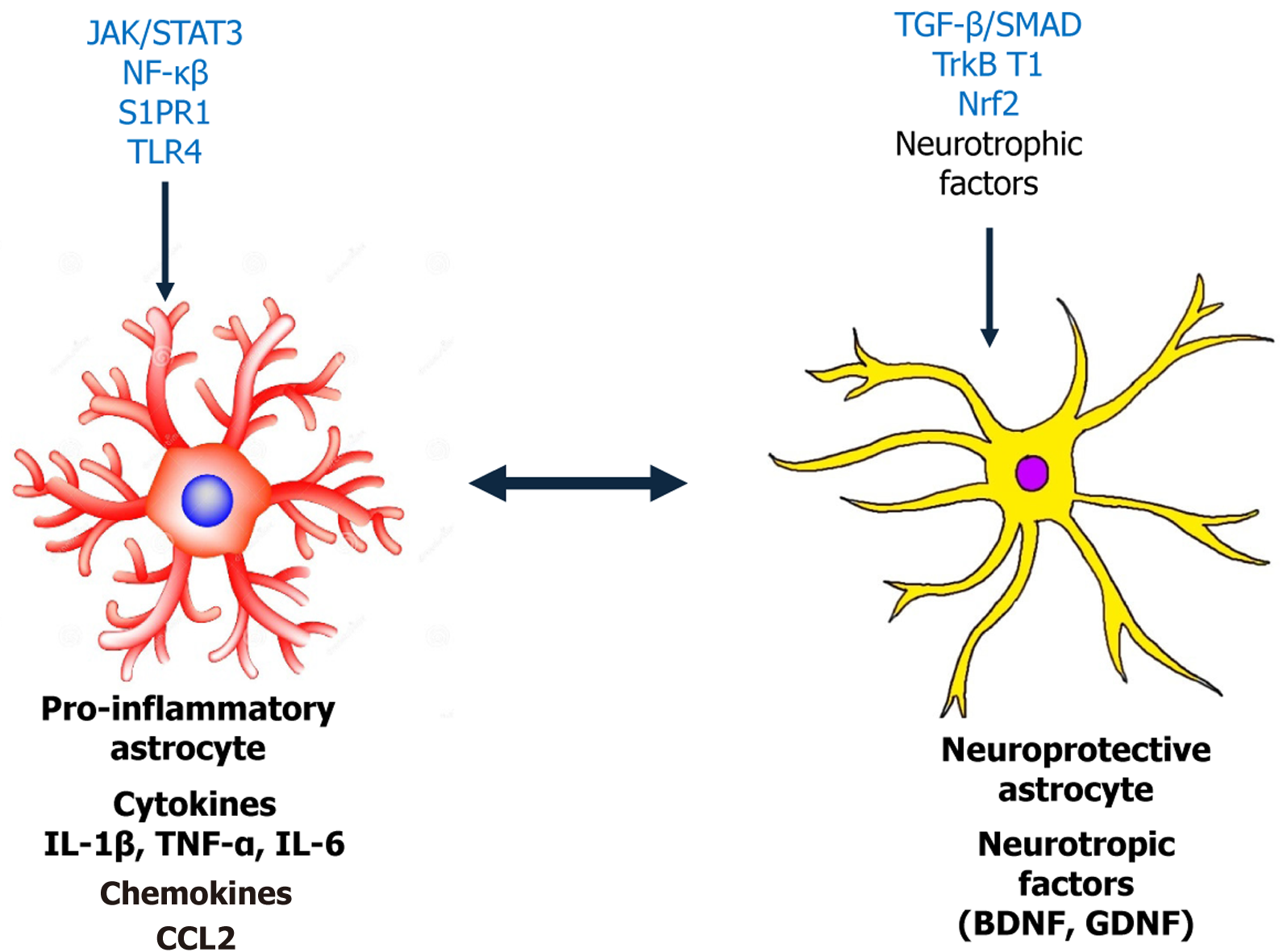
Figure 1 Functional dichotomy of glial phenotypes and signaling cascades in diabetic neuropathy.
The left panel shows pro-inflammatory astrocytes, which become reactive in response to diabetic stressors. Activation is mediated by pathways such as Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), nuclear factor kappa B (NF-κB), Toll-like receptor 4 (TLR4), and sphingosine-1-phosphate receptor 1 (S1PR1), leading to the release of cytokines [e.g., interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6)], chemokines [e.g., C-C motif chemokine ligand 2 (CCL2)], and neurotoxic factors that promote central sensitization and neuropathic pain. The right panel illustrates neuroprotective astrocytes, driven by signaling through nuclear factor erythroid 2-related factor 2 (Nrf2), transforming growth factor beta (TGF-β)/SMAD, and tyrosine receptor kinase B (TrkB.T1), which enhance the release of neurotrophic factors [e.g., brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF)] and antioxidants. These cells contribute to synaptic maintenance, neuronal survival, and the resolution of inflammation.
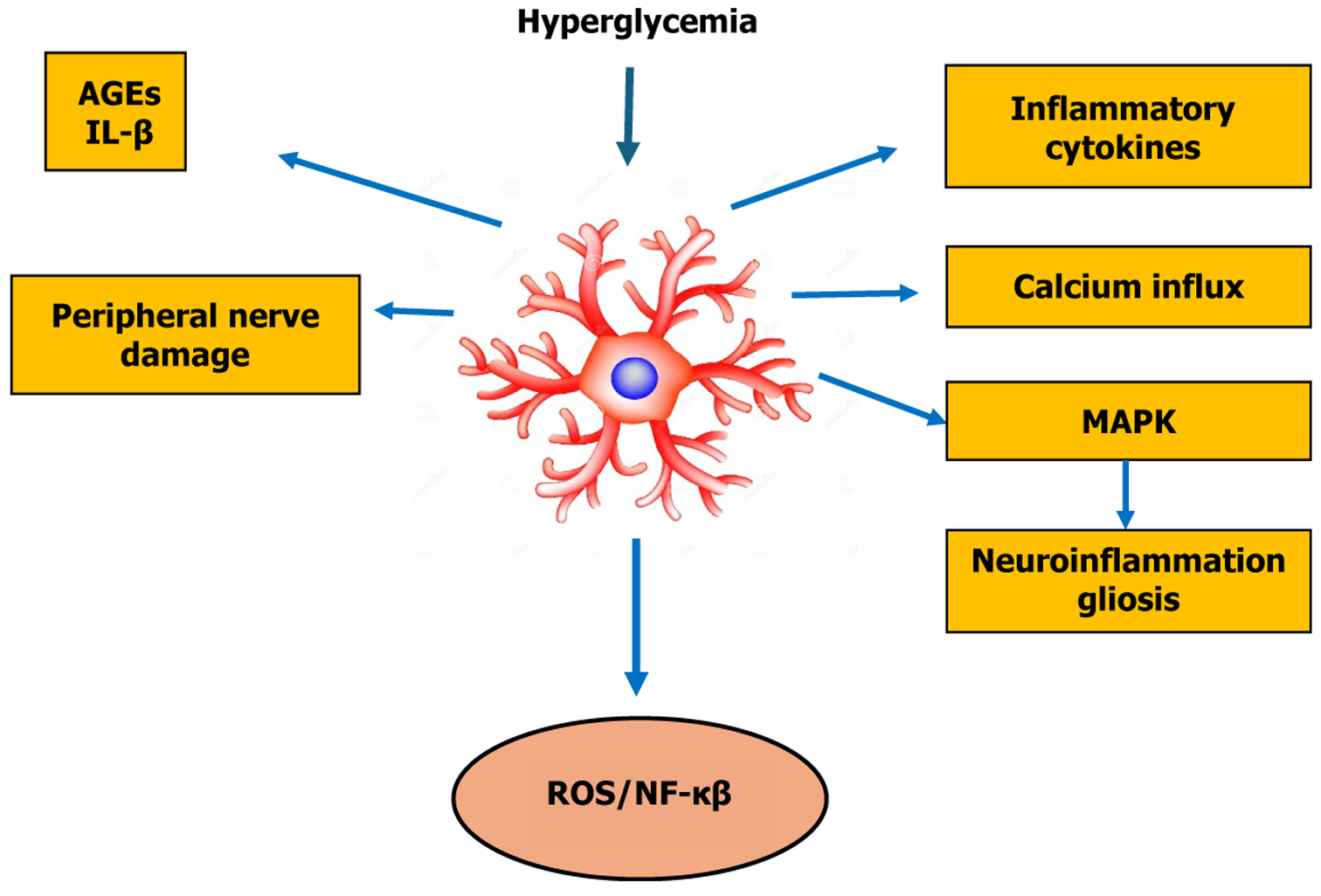
Figure 2 Specific molecular and signaling mechanisms of astrocytes involved in the pathogenesis and progression of diabetic neuropathy.
Under diabetic conditions, hyperglycemia activates astrocytes leading to increased reactive oxygen species (ROS) and nuclear factor kappa B (NF-κB) signaling. This cascade triggers the release of inflammatory cytokines, calcium influx, and mitogen-activated protein kinase (MAPK) activation, culminating in neuroinflammation and astrogliosis. The accumulation of advanced glycation end products (AGEs) and pro-inflammatory cytokines such as interleukin 1 beta (IL-1β) exacerbate peripheral nerve damage, creating a feedback loop that perpetuates astrocyte reactivity and neuronal dysfunction.
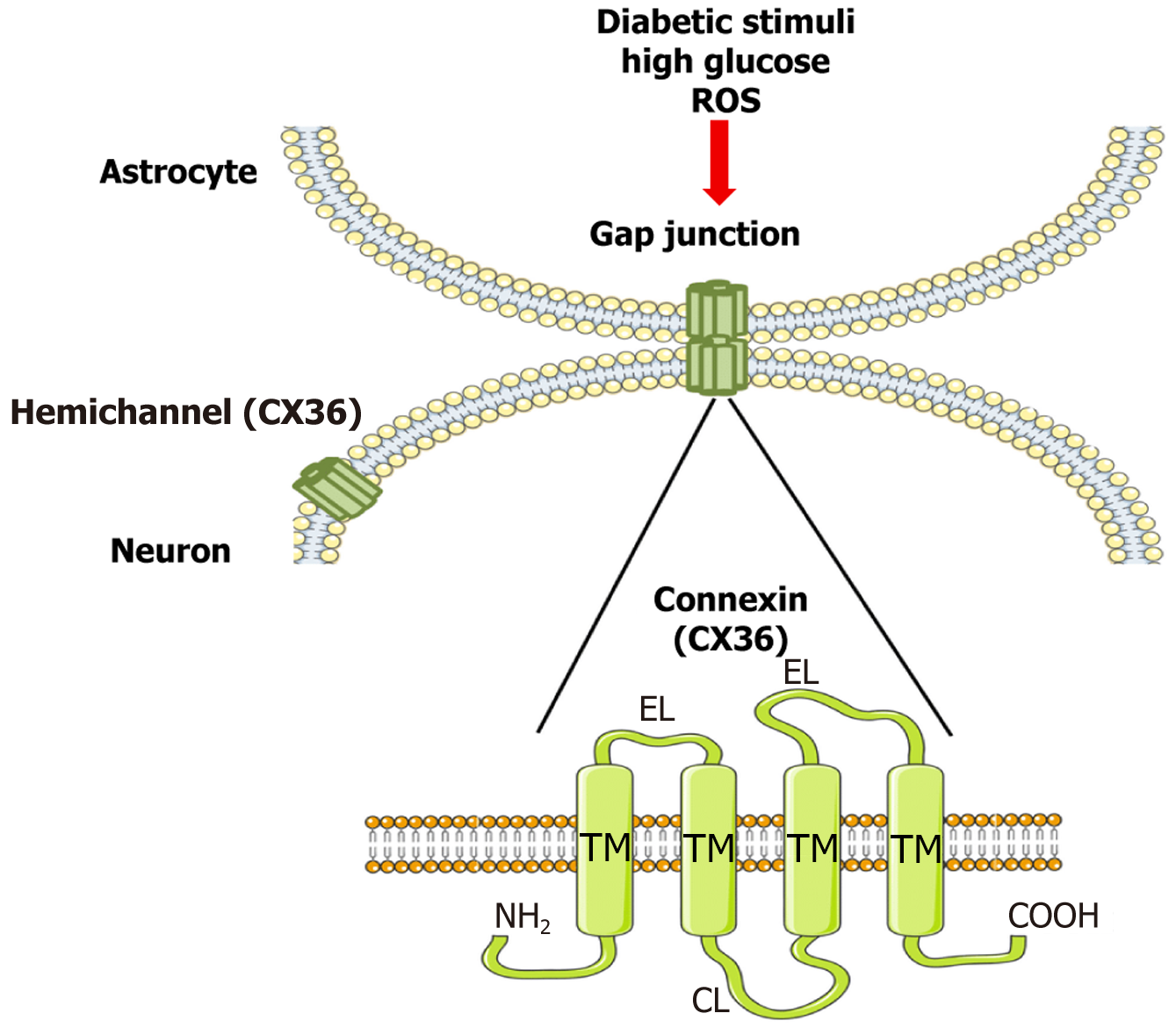
Figure 3 Connexin 36 oligomerization and astrocyte-neuron coupling in diabetic neuropathy.
Under physiological conditions, connexin 36 (CX36) forms hexameric hemichannels that align to create gap junctions, enabling synchronized glial-neuronal signaling. Diabetic stressors alter CX36 expression and function, leading to disrupted intercellular communication and central sensitization. EL: Extracellular loop; ROS: Reactive oxygen species; TM: Transmembrane domain.
- Citation: Haris K, Long I. Role of astrocytes in diabetic neuropathy: Review of their involvement in disease mechanisms. World J Diabetes 2025; 16(10): 108714
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/108714.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.108714