Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.109055
Revised: July 4, 2025
Accepted: August 4, 2025
Published online: September 15, 2025
Processing time: 138 Days and 7.6 Hours
Among all solid tumors, pancreatic ductal adenocarcinoma (PDAC) is characterized by markedly poor survival outcomes, reflecting its high lethality, primarily as a result of late-stage diagnosis and limited treatment options. Pancreatic adenocarcinoma upregulated factor (PAUF) displays elevated expression in PDAC compared to non-neoplastic pancreatic samples and is involved in promoting tumor development. However, its exact diagnostic and prognostic significance remains unclear. This study aimed to assess the clinical relevance of PAUF expression in PDAC. We hypothesized that higher PAUF expression is associated with more aggressive clinicopathological features and poorer patient outcomes.
To investigate the expression of PAUF in PDAC and its value as a diagnostic and prognostic biomarker.
PAUF expression levels were assessed using immunohistochemistry in tumor tissues from 93 patients with PDAC. Staining intensity and the proportion of tumor cells showing PAUF positivity were assessed to categorize patients into low and high PAUF expression groups. Associations between PAUF expression and clinicopathological characteristics or survival outcomes were analyzed. Public datasets (The Cancer Genome Atlas, Genotype-Tissue Expression, and Clinical Proteomic Tumor Analysis Consortium) were employed to validate differences in PAUF expression in PDAC at mRNA and protein levels.
PAUF expression was observed in 82.8% of samples, primarily localized within the cytoplasm of tumor cells. High PAUF expression showed a significant correlation with metastasis to lymph nodes (78.4%, P = 0.0019), indicating a strong association with advanced disease. Public datasets confirmed elevated PAUF levels at both transcript and protein levels in PDAC relative to normal tissue. Kaplan-Meier estimates indicated that higher PAUF levels were linked with shorter overall survival (18.4 months vs 32.7 months, P = 0.032). Multivariate Cox regression confirmed high PAUF expression as a prognostically significant variable contributing to poor clinical outcomes [hazard ratio (HR) = 2.05; P = 0.009]. Poor tumor differentiation (HR = 2.47; P = 0.004) and lack of adjuvant therapy (HR = 0.39; P = 0.001) were also independently associated with unfavorable outcomes.
PAUF is a promising biomarker for tumor progression and prognosis in PDAC, with potential clinical utility in early diagnosis and the development of targeted therapies.
Core Tip: This study reveals that pancreatic adenocarcinoma upregulated factor is overexpressed in pancreatic ductal adenocarcinoma and displays a significant relationship with metastasis to lymph nodes. High pancreatic adenocarcinoma upregulated factor expression correlates with shorter overall survival and independently predicts poor prognosis, supporting its role as a biomarker for patient stratification and targeted therapy development.
- Citation: Kim JH, Na HY, Jung K, Jang D, Youn Y, Kim DH, Han HD, Hwang JH. Quantitative immunohistochemistry analysis of pancreatic adenocarcinoma upregulated factor expression in pancreatic cancer and its prognostic significance. World J Gastrointest Oncol 2025; 17(9): 109055
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/109055.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.109055
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy marked by poor survival outcomes, primarily because reliable methods for timely detection are lacking, and the limited availability of effective treatment options. Globally, PDAC affects 1 to 10 individuals per 100000 and ranks seventh among the causes of cancer-associated mortality, placing it as the twelfth most frequently identified malignancy across the globe[1]. The rising incidence of pancreatic cancer in conjunction with limited therapeutic options highlights the pressing need for trustworthy early detection indicators[2,3]. Currently, carbohydrate antigen 19-9 (CA 19-9) continues to serve as a clinical biomarker in the detection of pancreatic cancer. However, an increase in CA 19-9 Levels may also appear in hepatic disorders, diabetes mellitus, and autoimmune diseases, requiring cautious interpretation[4-7]. Further, the ability of serum CA 19-9, which remains limited in both sensitivity and specificity, reduces its diagnostic utility[8].
Recent studies have identified pancreatic adenocarcinoma upregulated factor (PAUF) as a novel soluble protein implicated in the promotion of tumorigenesis and metastatic behavior[9]. PAUF enhances cancer cell invasiveness via signaling pathways such as Toll-like receptor 4/myeloid differentiation primary response 88[10-12]. It also promotes metastasis by upregulating C-X-C chemokine receptor type 4 expression, as demonstrated by Lee et al[13]. GeneChip analyses have further demonstrated that PAUF is specifically overexpressed in cancerous but not normal pancreatic tissue[14]. These results underscore the central involvement of PAUF in the development of pancreatic malignancy.
PAUF has also been identified as a transforming growth factor beta-induced factor that significantly promotes the motility and invasiveness of pancreatic cancer cells. It acts as an essential contributor to pancreatic cancer metastasis, and its inhibition has been shown to suppress malignant properties in cancer cells[15]. Beyond pancreatic cancer, PAUF is also overexpressed in breast cancer, where it promotes clonogenic growth and influences cell migration and proliferation via the phosphoinositide 3-kinase/protein kinase B pathway. Combining chemopreventive agents bexarotene and carvedilol has shown potential in cancer prevention and therapy by targeting zymogen granule protein 16B, effectively modulating the stromal network and metabolic pathways[16]. Recently, computational analyses of zymogen granule protein 16B mutations have suggested that specific non-synonymous variants may destabilize protein structure, impair lectin domain function, and disrupt molecular interactions, including those with lysozyme, which may contribute to pancreatic tumor progression[17]. These findings support PAUF as a promising therapeutic target, given its roles in cancer cell proliferation, migration, stromal interaction, and metabolic regulation across multiple cancer types.
Immunohistochemistry (IHC) studies targeting PAUF provide a robust diagnostic approach for the timely and precise detection of pancreatic cancer. Notably, the polyclonal PAUF antibody (PBP1510) is currently under investigation as a diagnostic biomarker, further highlighting its clinical relevance. Therefore, we conducted this research to investigate PAUF expression levels in pancreatic tumor specimens and assess their association with oncologic outcomes using the polyclonal antibody PBP1510. According to current evidence, this work is among the first to quantitatively examine PAUF levels within a clearly characterized PDAC cohort, applying an original IHC scoring approach and exploring how these levels relate to pathological features and clinical outcomes. Our findings provide additional insight into the prognostic significance of PAUF protein levels in pancreatic cancer.
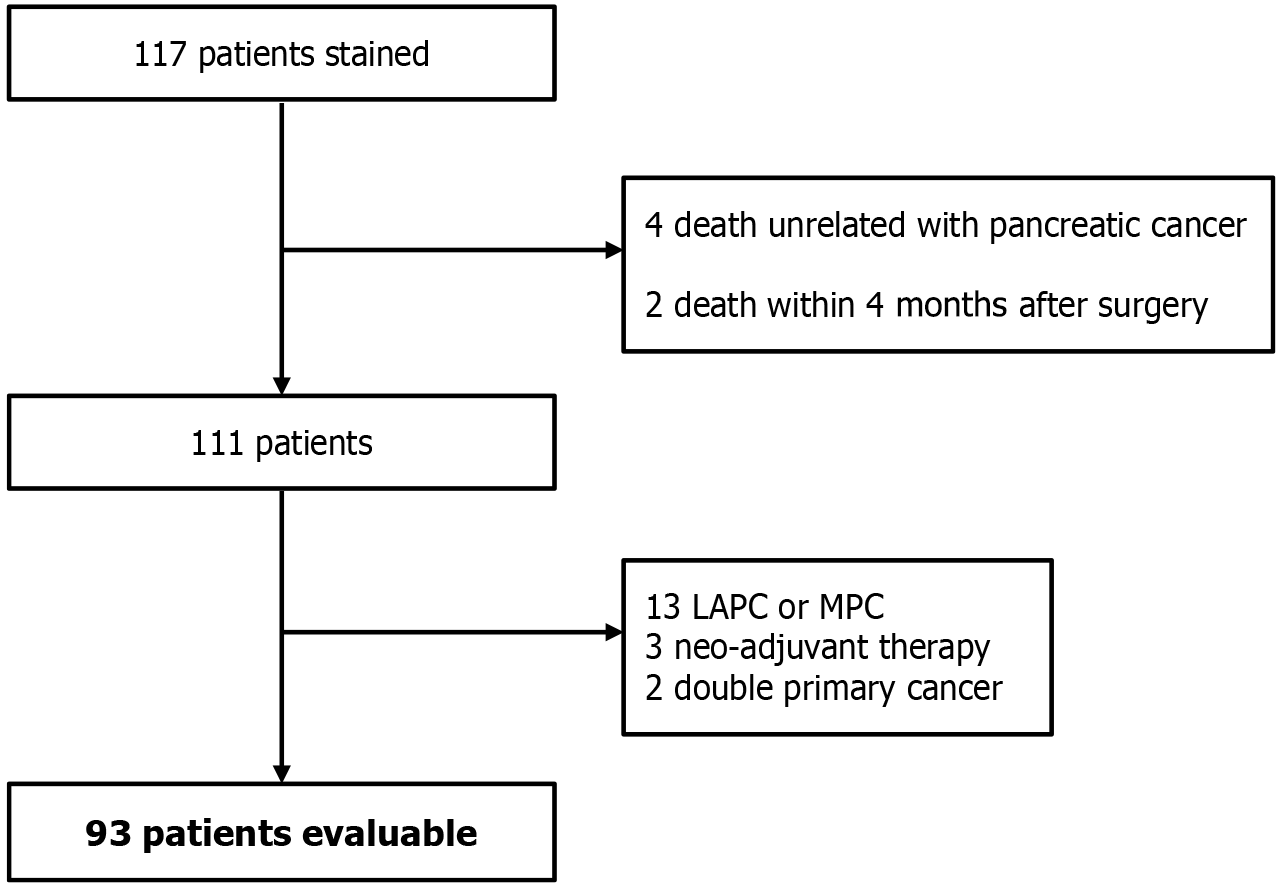
From 2003 to 2024, surgical specimens were collected from 117 individuals treated operatively for pancreatic malignancy at Seoul National University Bundang Hospital. Among them, 24 patients were excluded based on predefined criteria to minimize confounding effects on PAUF expression and survival outcomes, such as early postoperative mortality, neoadjuvant therapy, and advanced-stage disease, ensuring homogeneity of the study population. Specifically, four patients who died of causes unrelated to pancreatic cancer, two patients who died within 3 months post-surgery (early postoperative mortality), 13 individuals diagnosed with locally advanced pancreatic cancer or metastatic pancreatic cancer, three patients who received neoadjuvant therapy, and two patients with synchronous double primary malignancies were omitted from the dataset. Consequently, 93 cases were retained for final evaluation (Figure 1). All tissue collection was performed after obtaining informed consent, following Institutional Review Board guidelines, No. B-2103-675-106, Seoul National University Bundang Hospital. The study adhered to all ethical considerations in accordance with the Declaration of Helsinki, and was conducted following the principles of International Conference on Harmonization-Good Clinical Practice.
Pancreatic cancer tissue specimens preserved in 40 g/L formaldehyde and processed into paraffin blocks were sliced into thin sections measuring 4-5 μm, deparaffinized in xylene, and then rehydrated stepwise using graded ethanol. Tissue slides were immersed in 10 mmol/L sodium citrate buffer (pH 6.0), subjected to microwave heating for 10 minutes to retrieve antigens, and subsequently allowed to cool at room temperature for 30 minutes. Tissue margins were carefully outlined using a PAP pen. Endogenous peroxidase activity was blocked by exposure to 0.88 mol/L hydrogen peroxide diluted in methanol for 10 minutes. After blocking using 100 g/L normal animal serum for 30 minutes, the tissue sections underwent overnight incubation at 4 °C in the presence of a rabbit-derived polyclonal antibody against recombinant human PAUF, applied at a final concentration of 12 μg/mL. The antigen-antibody complex was visualized using a peroxidase-based chromogenic detection system (K5007, Dako, Carpinteria, CA, United States), which is specific for rabbit and mouse antibodies, combined with the EnVision+ HRP system, followed by a 10 minutes development 3,3′-diaminobenzidine. Histological slices underwent staining using hematoxylin, followed by mounting for microscopic evaluation. The slides were subsequently scanned and converted to digital images using a Pannoramic 250 Flash II scanner (3DHISTECH Ltd., Budapest, Hungary).
PAUF expression was evaluated by assessing the intensity of immunoreactivity and the percentage of tumor areas exhibiting detectable signal. Staining intensity was classified as 0 (no staining), 1+ (weak), or 2+ (moderate to strong), and the percentage of positively labeled tumor regions was documented as a value between 0 and 100%. An IHC evaluation was performed by a certified pathologist (Na HY) affiliated with the Department of Pathology at Seoul National University Bundang Hospital, who was blinded to all clinical information. Interobserver variability was not assessed. No missing data were reported for the IHC analysis, and all tissue samples included in the study had complete data for PAUF expression evaluation. Any missing clinical data were excluded from the statistical analyses; this exclusion did not significantly impact the results.
For statistical purposes, PAUF expression was dichotomized at a 5% cutoff: The PAUF-low group included samples with an intensity of 0 or 1%-4% staining. In comparison, the PAUF-high group included samples with an intensity of 1+ or 2+ and staining greater than 5%. This 5% cutoff was determined based on Kaplan-Meier analysis of survival data, which demonstrated that individuals exhibiting PAUF positivity > 5% experienced notably reduced survival following surgery relative to those showing PAUF expression of < 5%. This result highlights the clinical relevance of PAUF levels in relation to the prognosis of patients with pancreatic cancer, making the 5% cutoff an effective threshold for distinguishing clinically meaningful differences in patient survival outcomes.
Version 3.4.1 of the R statistical environment was employed to identify the optimal cutoff for PAUF expression via the maximum χ2 method and analyze its relationship with clinicopathologic characteristics. Kaplan-Meier survival plots were constructed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, United States), with statistical differences assessed via the log-rank Mantel-Cox test. Significance was defined as P < 0.05.
Transcriptomic profiles from the Genotype-Tissue Expression (GTEx) and The Cancer Genome Atlas (TCGA) projects were accessed through the Gene Expression Profiling Interactive Analysis platform (http://gepia.cancer-pku.cn/index.html), while proteomic data from the Clinical Proteomic Tumor Analysis Consortium were obtained via University of Alabama at Birmingham Cancer data analysis portal; https://ualcan.path.uab.edu/index.html). All essential evidence supporting the findings of this investigation is provided in the main text, its figures, and additional files. Further details are available from the corresponding author upon reasonable request.
PAUF expression was examined in tumor specimens from 93 patients with pancreatic cancer and analyzed in relation to clinicopathologic features. Table 1 presents baseline demographic and pathological characteristics. Participants had a median age of 68 years (28-88 years). The cohort included 43 males and 50 females. Tumor location was predominantly in the pancreatic head (n = 68), with the remainder in the body or tail (n = 25). Of the 93 patients, 67 had resectable pancreatic cancer (RPC), and 26 had borderline RPC. Lymph node metastasis was observed in 74 patients (80%), and positive resection margins (RM) were present in 32 (34%). Among the cohort, 52 patients received adjuvant therapy, while 41 did not. Elevated CA 19-9 serum concentrations (≥ 150 U/mL) were identified in 44 patients. Histological analysis revealed 16 poorly differentiated tumors and 77 of other subtypes: 8 well-differentiated, 62 moderately differentiated, and 7 classified as “other”. The “other” group comprised 1 intraductal papillary mucinous carcinoma, 3 adenocarcinoma not otherwise specified, 1 adenosquamous carcinoma, and 2 with unavailable histologic classification (Table 1). These features were used to evaluate the clinicopathological relevance of PAUF expression in PDAC.
| Characteristic | Total number (n = 93) | |
| Age (years) | < 60: 21 | ≥ 60: 72 |
| Sex | Male: 43 | Female: 50 |
| Tumor location | Head: 68 | Body or tail: 25 |
| Stage | RPC: 67 | BRPC: 26 |
| Lymph node | Negative: 19 | Positive: 74 |
| RM | Negative: 61 | Positive: 32 |
| Adjuvant therapy | None: 41 | Yes: 52 |
| CA 19-9 | < 150 U/mL: 49 | ≥ 150 U/mL: 44 |
| Histology | PD: 16 | WD/MD/others: 77 |
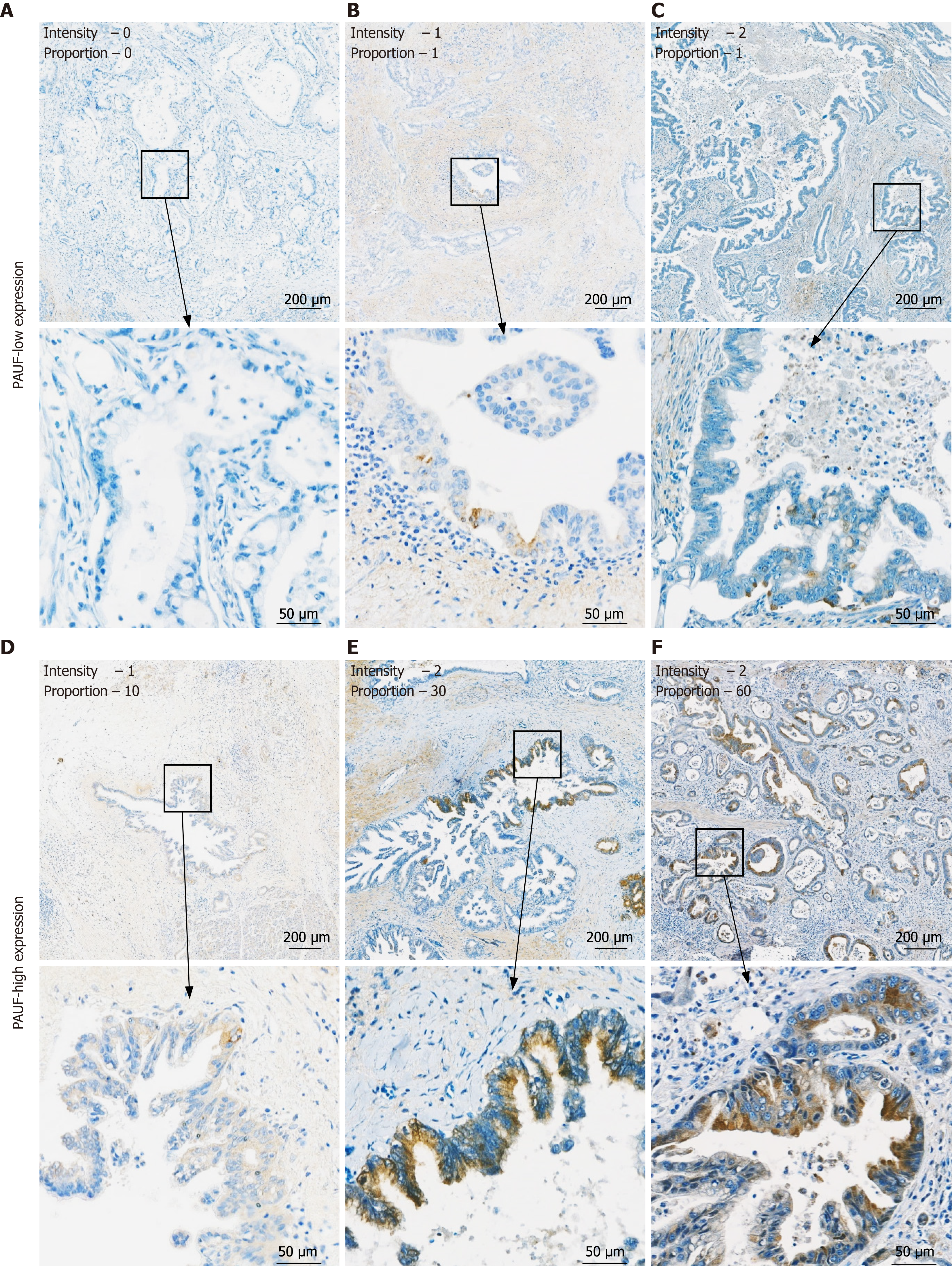
PAUF is predominantly overexpressed within the cytoplasmic compartment of pancreatic tumor cells and is also observed during early neoplastic changes[14]. In this study, PAUF expression was assessed by IHC in tumor tissues from 93 patients. Both staining intensity and the proportion of PAUF-positive tumor areas were evaluated. Staining strength was scored as 0 (no staining), 1+ (weak), or 2+ (moderate to strong), while the percentage of PAUF-positive area was calculated relative to the total tumor area. IHC analysis was performed by a board-certified pathologist who was unaware of any clinical information. Table 2 summarizes the frequency and intensity of PAUF positivity in PDAC tissues. Notably, 82.8% of patients had PAUF-positive tumor areas > 5%. Patients were categorized into PAUF-low and PAUF-high groups according to their IHC classification (Figure 2). Analysis did not reveal any meaningful link between PAUF expression and factors, such as age, sex, tumor location, tumor-nodes-metastasis stage, RM, adjuvant therapy, or histologic differentiation. However, lymph node metastasis was markedly higher in the PAUF-high group (78.4%) than in the PAUF-low group [21.6%; P = 0.0019; 95% confidence interval (CI): 1.7-14.0]. In contrast, elevated CA 19-9 Levels (≥ 150 U/mL) tended to appear more often in the PAUF-high group (79.5%) than in the PAUF-low group (20.5%), although the difference did not reach statistical significance (P = 0.084; 95%CI: 0.89-5.75; Table 3). These results imply that elevated PAUF expression correlates with more aggressive tumor behavior and may serve as a clinically meaningful marker of progression in PDAC.
| Intensity | Proportion of PAUF staining | Total | ||||||||||
| 0 | 1-4 | 5-10 | 11-20 | 21-30 | 31-40 | 41-50 | 51-60 | 61-70 | 71-80 | 81-90 | ||
| 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 |
| 1+ | 0 | 6 | 22 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 38 |
| 2+ | 0 | 5 | 22 | 2 | 4 | 1 | 1 | 3 | 0 | 0 | 1 | 39 |
| Total | 16 | 11 | 44 | 9 | 6 | 1 | 1 | 3 | 0 | 0 | 2 | 93 |
| Cumulative number (%) | 16 (17.2) | 27 (29.0) | 71 (76.3) | 80 (86.0) | 86 (92.5) | 87 (93.5) | 88 (94.6) | 91 (97.8) | 91 (97.8) | 91 (97.8) | 93 (100) | 93 |
| Clinicopathological features | Low group (%) | High group (%) | P value | 95%CI |
| Age (years) | 0.6217 | 0.46-3.7 | ||
| < 60 | 33.3 | 66.7 | ||
| ≥ 60 | 27.8 | 72.2 | ||
| Sex | 0.8245 | 0.37-2.2 | ||
| Male | 27.9 | 72.1 | ||
| Female | 30.0 | 70.0 | ||
| Tumor location | 0.8942 | 0.39-3.0 | ||
| Head | 29.4 | 70.6 | ||
| Body or tail | 28.0 | 72.0 | ||
| Stage | 0.46 | 0.26-1.8 | ||
| RPC | 26.9 | 73.1 | ||
| BRPC | 34.6 | 65.4 | ||
| Lymph node | 0.0019b | 1.7-14 | ||
| Negative | 57.9 | 42.1 | ||
| Positive | 21.6 | 78.4 | ||
| RM | 0.2707 | 0.64-4.71 | ||
| Negative | 32.8 | 67.2 | ||
| Positive | 21.9 | 78.1 | ||
| Adjuvant therapy | 0.3347 | 0.63-3.83 | ||
| None | 34.1 | 65.9 | ||
| Yes | 25.0 | 75.0 | ||
| CA 19-9 | 0.0842 | 0.89-5.75 | ||
| < 150 U/mL | 36.7 | 63.3 | ||
| ≥ 150 U/mL | 20.5 | 79.5 | ||
| Histology | 0.4122 | 0.20-1.93 | ||
| WD/MD/others | 37.5 | 62.5 | ||
| PD: 27.3 | 72.7 |
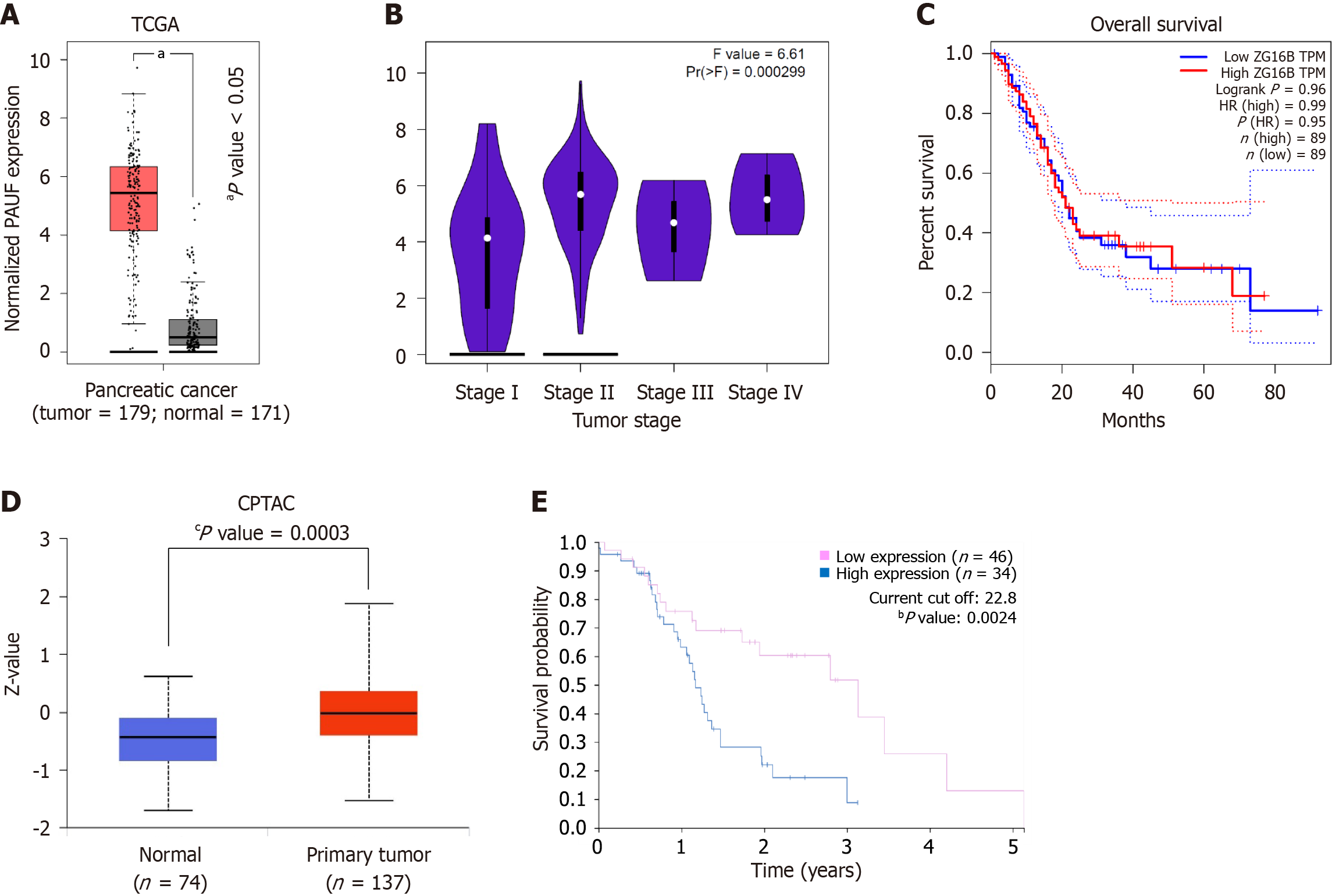
Analysis revealed elevated PAUF mRNA expression across 179 tumor samples from pancreatic cancer (TCGA), compared to 171 normal tissues (GTEx) (Figure 3A). The levels of mRNA varied by cancer stage and tended to increase in advanced stages (Figure 3B). However, mRNA expression did not show a meaningful correlation with overall survival (Figure 3C). By contrast, PAUF protein levels were markedly elevated in PDAC samples relative to normal tissues (Figure 3D) and correlated with poorer survival outcomes (Figure 3E). Overall, PAUF was overexpressed at both mRNA and protein levels, but only the protein component was associated with prognosis.
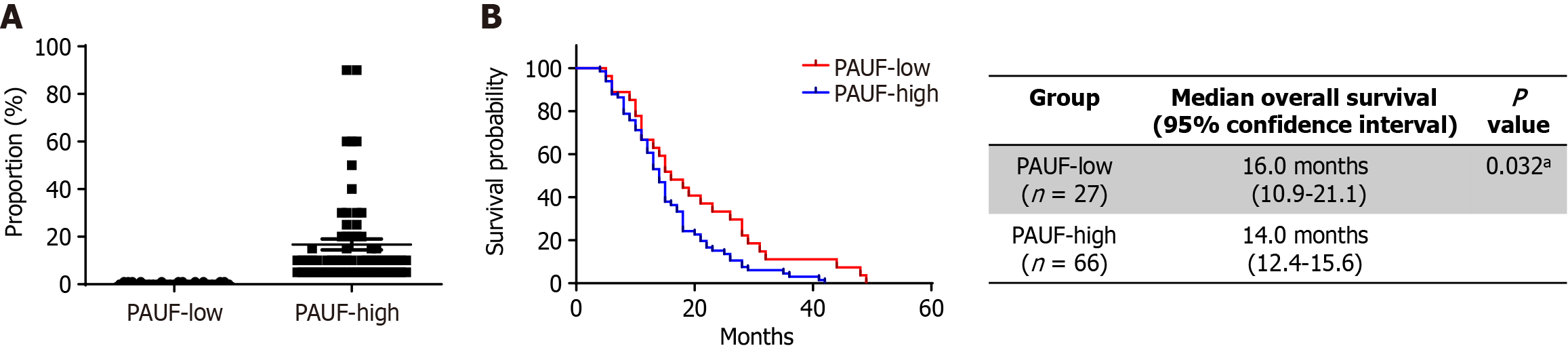
Patients were grouped into PAUF-low or PAUF-high expression groups according to their IHC classification (Figure 4A). Median overall survival rate was notably shorter among individuals belonging to the PAUF-high group relative to those classified as PAUF-low group (18.4 months vs 32.7 months, P = 0.032; Figure 4B). High PAUF expression emerged as a standalone factor linked to poor clinical prognosis [hazard ratio (HR) = 2.05; 95%CI: 1.19-3.53; P = 0.009; Table 4]. Low tumor differentiation was likewise linked to inferior survival compared to well- or moderately differentiated tumors (HR = 2.47; 95%CI: 1.34-4.58; P = 0.004), while adjuvant therapy was linked to better outcomes (HR = 0.39; 95%CI: 0.24-0.64; P = 0.001). Other variables, including age, tumor location, clinical stage (RPC vs borderline RPC), RM status, nodal status, and CA 19-9 concentration, were not significantly associated with overall survival rates.
| Factors | Overall survival, hazard ratio (95% confidence interval) | P value |
| PAUF expression, high vs low | 2.05 (1.19-3.53) | 0.009b |
| Age | 1.01 (0.98-1.03) | 0.921 |
| Location | ||
| Head | Reference | |
| Body | 1.85 (0.94-3.66) | 0.076 |
| Tail | 1.02 (0.53-1.96) | 0.959 |
| Stage | ||
| RPC | Reference | |
| BRPC | 1.07 (0.61-1.89) | 0.813 |
| Histology | ||
| WD or MD | Reference | |
| PD | 2.47 (1.34-4.58) | 0.004b |
| RM | ||
| Negative | Reference | |
| Positive | 1.00 (0.58-1.72) | 0.998 |
| Nodal status | ||
| Negative | Reference | |
| Positive | 1.24 (0.69-2.25) | 0.472 |
| Adjuvant therapy | 0.39 (0.24-0.64) | 0.001b |
| CA19-9 (< 150 vs ≥ 150 U/mL) | 1.12 (0.68-1.58) | 0.65 |
The current findings reveal that PAUF expression is upregulated in PDAC and associated with advanced disease and unfavorable prognosis, supporting its value as a prognostic indicator and potential candidate for targeted therapy. Further, our results support the utility of PAUF as a dual-purpose biomarker for both outcome prediction and treatment decision-making among individuals diagnosed with PDAC. Despite the widespread application of CA 19-9 for pancreatic cancer, its effectiveness is hindered by poor specificity due to elevations in other tumor types, including colorectal, hepatic, mammary, and pulmonary cancers, and benign conditions including obstructive jaundice, pancreatitis, cirrhosis, and pulmonary diseases[18-20]. The American Society of Clinical Oncology has also acknowledged that the CA 19-9 Level does not provide adequate diagnostic reliability for pancreatic cancer[21,22]. These limitations underscore the need for alternative biomarkers with greater diagnostic precision.
Emerging evidence highlights PAUF’s multifaceted role in pancreatic cancer biology, particularly its involvement in treatment, response, metastasis, and immune regulation. Mechanistically, PAUF contributes to tumor progression by activating β-catenin, thereby promoting proliferation and metastasis. It also supports cell adhesion and anoikis resistance through focal adhesion kinase signaling[14,23-26]. Additionally, PAUF has been implicated in immune modulation and chemokine regulation, suggesting a role in establishing an immunosuppressive tumor microenvironment[13]. The observed association with lymph node involvement and reduced survival reinforces the role of PAUF in PDAC progression. Although a trend was observed for elevated CA 19-9 Levels, it did not reach statistical significance. Notably, a subset of patients with high PAUF expression exhibited favorable outcomes, indicating the possibility that additional molecular modifiers or host-related factors may influence PAUF’s prognostic impact.
Public datasets (TCGA/GTEx and the Clinical Proteomic Tumor Analysis Consortium) were analyzed to validate these findings, confirming PAUF overexpression at both transcript and protein levels within tumors of the pancreas when evaluated against non-tumorous pancreatic samples. Although PAUF mRNA levels were not significantly associated with overall survival, high protein expression correlated with poor survival outcomes. This discrepancy highlights the role of post-transcriptional regulation and protein-level mechanisms in PDAC biology.
These results support findings from prior molecular studies, which revealed marked PAUF upregulation in PDAC. As quantitative reverse transcription-polymerase chain reaction shows, PAUF transcript levels were more than tenfold higher in tumor samples than in normal pancreatic tissues, highlighting its relevance as a tumor-associated gene[14]. Similarly, elevated PAUF expression was observed in 6 of 8 pancreatic cancer cell lines, further supporting its involvement in tumor biology[14].
Survival analysis demonstrated significantly shorter overall survival in the PAUF-high group relative to that in the PAUF-low group, underscoring the prognostic value of this stratification. Multivariate analysis confirmed that PAUF expression independently correlates with poor prognosis, reinforcing its value in patient risk stratification. These findings support its use in identifying high-risk patients who may benefit from closer monitoring or intensified treatment beyond conventional indicators such as tumor grade or CA 19-9. Additional IHC-based studies in ovarian cancer reported that high PAUF expression was significantly linked to tumors of higher histologic grade (P = 0.014) and chemoresistance (P = 0.017), supporting its role as a negative prognostic biomarker across epithelial malignancies[27].
There are a few limitations that warrant mention regarding this study. First, its single-institution, retrospective design and limited sample size may restrict the generalizability of the results. Second, the cohort primarily consisted of patients from a single geographic and ethnic background, which may not accurately reflect the global diversity of PDAC. Third, although internal validation was performed using public databases, external cohorts and functional studies were not conducted. Future studies should include larger, multi-center prospective cohorts and incorporate mechanistic experiments, such as in vitro knockdown and overexpression studies, as well as in vivo mouse models, to validate the functional relevance of PAUF in pancreatic cancer progression and metastasis. Collectively, these findings position PAUF as a clinically relevant biomarker with potential utility in prognosis and treatment stratification in pancreatic cancer.
The current findings showed that high PAUF protein expression exhibits a strong link with nodal involvement and shortened survival duration among individuals diagnosed with PDAC. Although PAUF was overexpressed at the transcript and protein levels, only the protein level demonstrated prognostic significance, supporting its role as both a clinical biomarker and a potential therapeutic candidate. Further research efforts should aim to clarify the molecular mechanisms underlying PAUF-driven tumor progression and investigate PAUF-targeted therapeutic strategies.
| 1. | National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. [cited 24 April 2025]. Available from: https://seer.cancer.gov/statfacts/html/pancreas.html. |
| 2. | Jamal MH, Porel P, Aran KR. Emerging biomarkers for pancreatic cancer: from early detection to personalized therapy. Clin Transl Oncol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Murray K, Oldfield L, Stefanova I, Gentiluomo M, Aretini P, O'Sullivan R, Greenhalf W, Paiella S, Aoki MN, Pastore A, Birch-Ford J, Rao BH, Uysal-Onganer P, Walsh CM, Hanna GB, Narang J, Sharma P, Campa D, Rizzato C, Turtoi A, Sever EA, Felici A, Sucularli C, Peduzzi G, Öz E, Sezerman OU, Van der Meer R, Thompson N, Costello E. Biomarkers, omics and artificial intelligence for early detection of pancreatic cancer. Semin Cancer Biol. 2025;111:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Kim S, Park BK, Seo JH, Choi J, Choi JW, Lee CK, Chung JB, Park Y, Kim DW. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci Rep. 2020;10:8820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (2)] |
| 5. | Kremser M, Weiss N, Kaufmann-Stoeck A, Vierbaum L, Schmitz A, Schellenberg I, Holdenrieder S. Longitudinal evaluation of external quality assessment results for CA 15-3, CA 19-9, and CA 125. Front Mol Biosci. 2024;11:1401619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Chang CY, Huang SP, Chiu HM, Lee YC, Chen MF, Lin JT. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology. 2006;53:1-4. [PubMed] |
| 7. | Tong Y, Song Z, Zhu W. Study of an elevated carbohydrate antigen 19-9 concentration in a large health check-up cohort in China. Clin Chem Lab Med. 2013;51:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, Grizzle WE, Huang Y, Lomakin A, Lokshin AE. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9:e94928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Choi CH, Chung JY, Park HS, Jun M, Lee YY, Kim BG, Hewitt SM. Pancreatic adenocarcinoma up-regulated factor expression is associated with disease-specific survival in cervical cancer patients. Hum Pathol. 2015;46:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Ikebe M, Kitaura Y, Nakamura M, Tanaka H, Yamasaki A, Nagai S, Wada J, Yanai K, Koga K, Sato N, Kubo M, Tanaka M, Onishi H, Katano M. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J Surg Oncol. 2009;100:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Youn SE, Jiang F, Won HY, Hong DE, Kang TH, Park YY, Koh SS. PAUF Induces Migration of Human Pancreatic Cancer Cells Exclusively via the TLR4/MyD88/NF-κB Signaling Pathway. Int J Mol Sci. 2022;23:11414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 12. | Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 714] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 13. | Lee Y, Kim SJ, Park HD, Park EH, Huang SM, Jeon SB, Kim JM, Lim DS, Koh SS. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene. 2010;29:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J, Jeon SB, Park EC, Kim YG, Lee B, Liu Q, Zeng W, Yeramilli S, Lee S, Koh SS, Song SY. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Lee M, Ham H, Lee J, Lee ES, Chung CH, Kong DH, Park JR, Lee DK. TGF-β-Induced PAUF Plays a Pivotal Role in the Migration and Invasion of Human Pancreatic Ductal Adenocarcinoma Cell Line Panc-1. Int J Mol Sci. 2024;25:11420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Lengyel M, Molnár Á, Nagy T, Jdeed S, Garai I, Horváth Z, Uray IP. Zymogen granule protein 16B (ZG16B) is a druggable epigenetic target to modulate the mammary extracellular matrix. Cancer Sci. 2025;116:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Namme JN, Reza HM, Bepari AK. Unraveling the impact of ZG16B missense mutations: computational prediction of structural and functional consequences. In Silico Pharmacol. 2025;13:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | George B, Kent M, Surinach A, Lamarre N, Cockrum P. The Association of Real-World CA 19-9 Level Monitoring Patterns and Clinical Outcomes Among Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Front Oncol. 2021;11:754687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Wu E, Zhou S, Bhat K, Ma Q. CA 19-9 and pancreatic cancer. Clin Adv Hematol Oncol. 2013;11:53-55. [PubMed] |
| 20. | Vitale F, Zileri Dal Verme L, Paratore M, Negri M, Nista EC, Ainora ME, Esposto G, Mignini I, Borriello R, Galasso L, Alfieri S, Gasbarrini A, Zocco MA, Nicoletti A. The Past, Present, and Future of Biomarkers for the Early Diagnosis of Pancreatic Cancer. Biomedicines. 2024;12:2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1133] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 22. | Benke M, Farkas N, Hegyi P, Tinusz B, Sarlós P, Erőss B, Szemes K, Vörhendi N, Szakács Z, Szücs Á. Preoperative Serum Carbohydrate Antigen 19-9 Levels Cannot Predict the Surgical Resectability of Pancreatic Cancer: A Meta-Analysis. Pathol Oncol Res. 2022;28:1610266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Cho IR, Koh SS, Min HJ, Kim SJ, Lee Y, Park EH, Ratakorn S, Jhun BH, Oh S, Johnston RN, Chung YH. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the expression of β-catenin, leading to a rapid proliferation of pancreatic cells. Exp Mol Med. 2011;43:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kim YJ, Nanda SS, Jiang F, Pyo SY, Han JY, Koh SS, Kang TH. Pancreatic Adenocarcinoma Up-Regulated Factor (PAUF) Transforms Human Monocytes into Alternative M2 Macrophages with Immunosuppressive Action. Int J Mol Sci. 2024;25:11545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Kim YJ, Jiang F, Park J, Jeong HH, Baek JE, Hong SM, Jeong SY, Koh SS. PAUF as a Target for Treatment of High PAUF-Expressing Ovarian Cancer. Front Pharmacol. 2022;13:890614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Lee YS, Kim SJ, Min HJ, Jo JY, Park EH, Koh SS. PAUF promotes adhesiveness of pancreatic cancer cells by modulating focal adhesion kinase. Exp Mol Med. 2011;43:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Choi CH, Kang TH, Song JS, Kim YS, Chung EJ, Ylaya K, Kim S, Koh SS, Chung JY, Kim JH, Hewitt SM. Elevated expression of pancreatic adenocarcinoma upregulated factor (PAUF) is associated with poor prognosis and chemoresistance in epithelial ovarian cancer. Sci Rep. 2018;8:12161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/