Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.108960
Revised: June 16, 2025
Accepted: August 7, 2025
Published online: September 15, 2025
Processing time: 142 Days and 2.5 Hours
Steroid-refractory immune-related cholangitis, characterized by biliary obstru
A 60-year-old female patient with biliary tract carcinoma and peritoneal metastasis developed elevated liver enzymes following four cycles of combined therapy with anti-PD-1 (Pembrolizumab) and a tyrosine kinase inhibitor. Magne
Stent placement offers prompt alleviation of cholestasis and constitutes an effective therapeutic strategy for managing immune-related cholangitis.
Core Tip: Steroid-refractory immune-related cholangitis, characterized by biliary obstruction, can be caused by drugs such as immune checkpoint inhibitors (ICIs). While there a few reports of sclerosing cholangitis after ICI administration, the therapeutic importance of local relief of obstruction has not been reported. Stent implantation provides rapid relief from cholestasis and is an effective approach to the treatment of immune-related cholangitis.
- Citation: Gao Z, Zhang JX, Tian XD, Wu SK, Jin X. Rapid cholestasis improvement as key strategy for steroid-refractory immune-related cholangitis: A case report. World J Gastrointest Oncol 2025; 17(9): 108960
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/108960.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.108960
Currently, immunotherapy has become an effective and safe treatment option for patients with malignant tumors[1]. After binding with receptors, immune checkpoint inhibitors would activate the T cells and exert antitumor activity[2]. Although immunotherapy is effective, its use is frequently associated with immune-related adverse events (irAEs). Immune-related cholangitis is a relatively rare adverse reaction associated with immunotherapy[3]. It differs from immune-related hepatic injury in terms of pathogenesis and treatment priorities, and differential diagnosis mainly relies on pathological biopsy[4]. In 2017, two different patterns of nivolumab-related cholangitis were reported for the first time[5,6]. Steroid therapy in patients with immune-related cholangitis has been less effective[7]. Recent studies demonstrated that ursodeoxycholic acid (UDCA) may achieve a better therapeutic effect[8,9]. A case report has also emerged on successfully using UDCA and tocilizumab to manage immune-related cholangitis[10]. Herein we report a case of steroid-refractory immune-related cholangitis caused by immune combination therapy. UDCA and immunosuppressive therapy were less favorable, and responses were brief. However, liver function returned to normal after the patient underwent local stent implantation.
A 60-year-old female patient with biliary tract carcinoma and peritoneal metastasis developed elevated liver enzymes following four cycles of combined therapy with anti-PD-1 (Pembrolizumab) and a tyrosine kinase inhibitor (TKI).
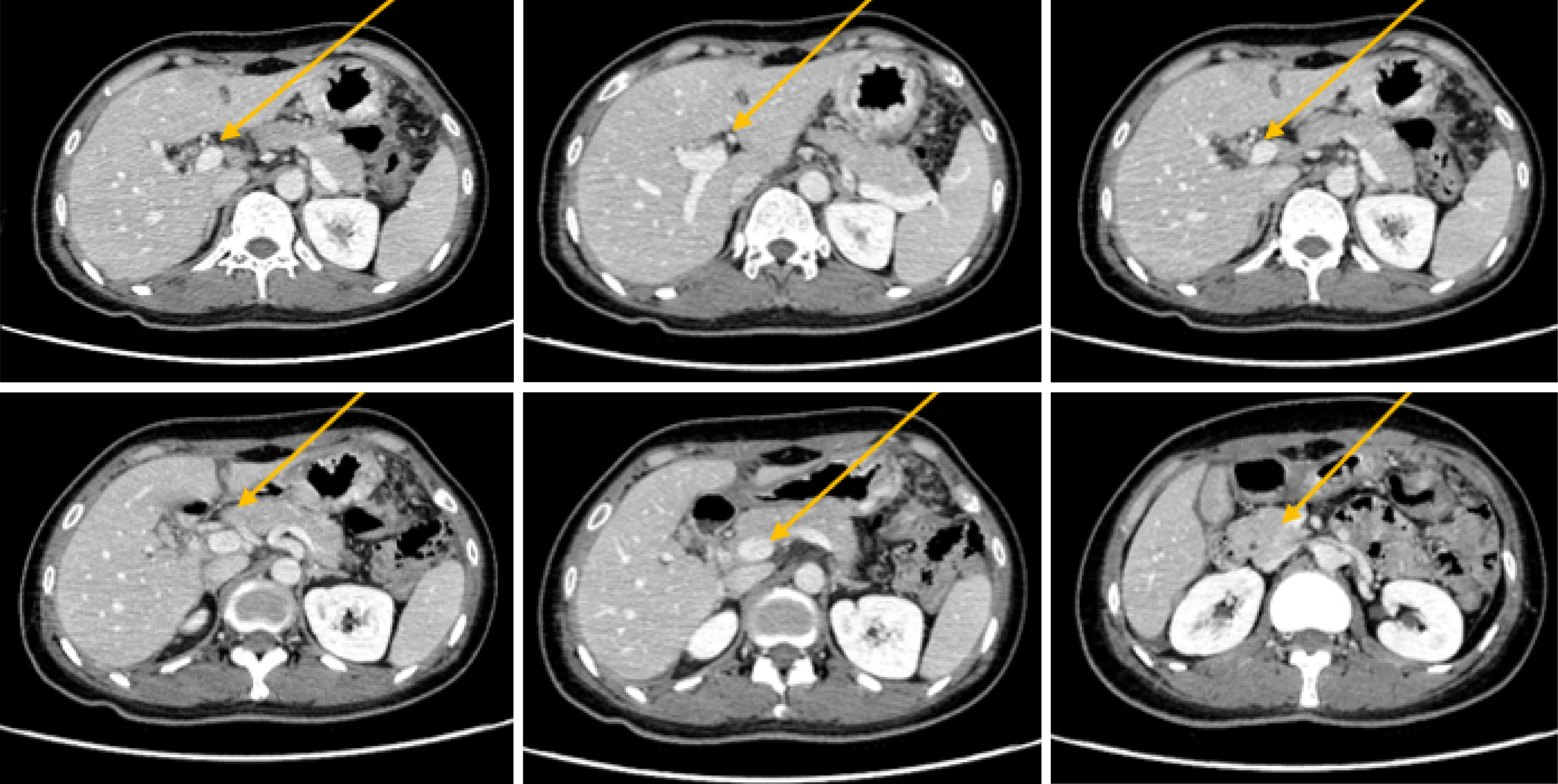
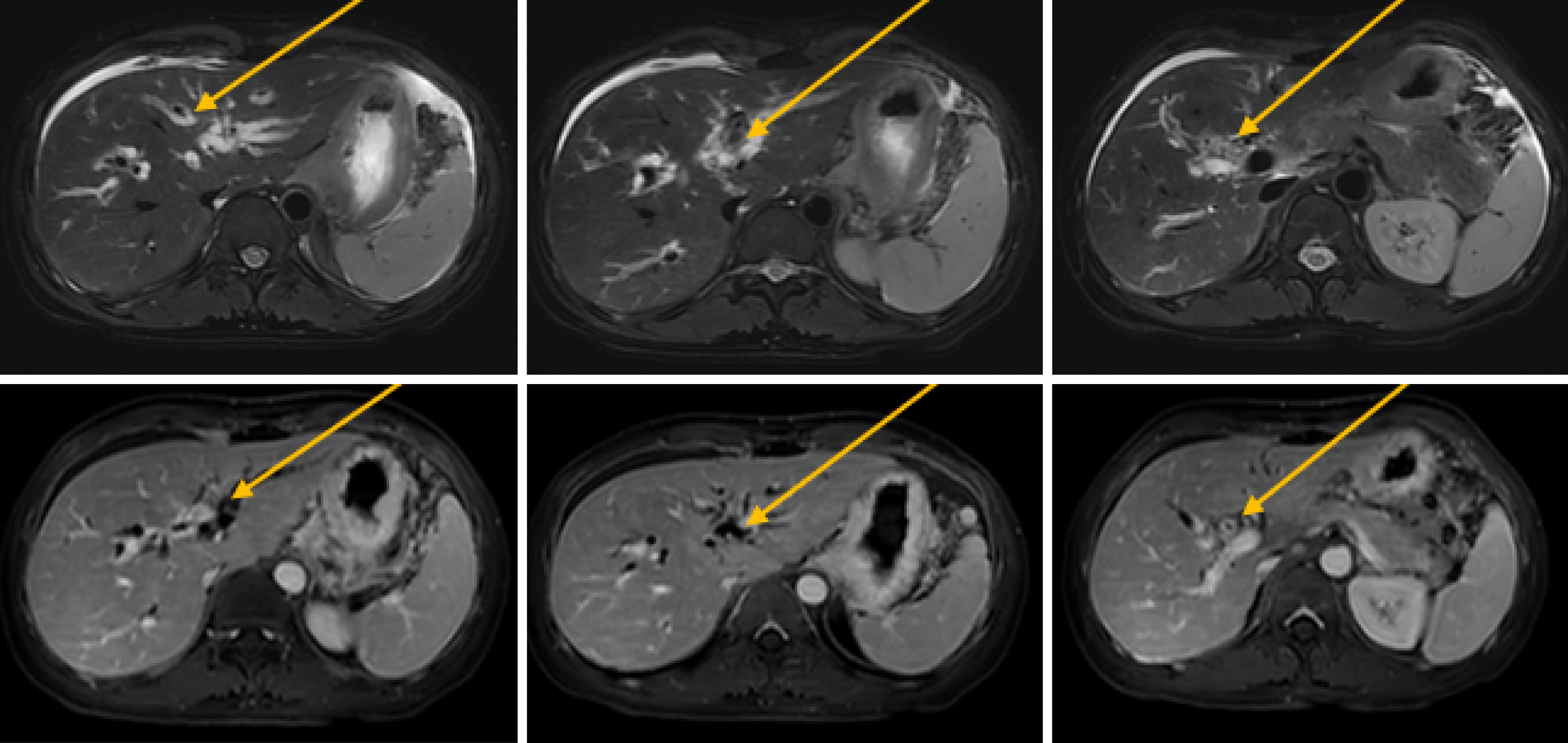
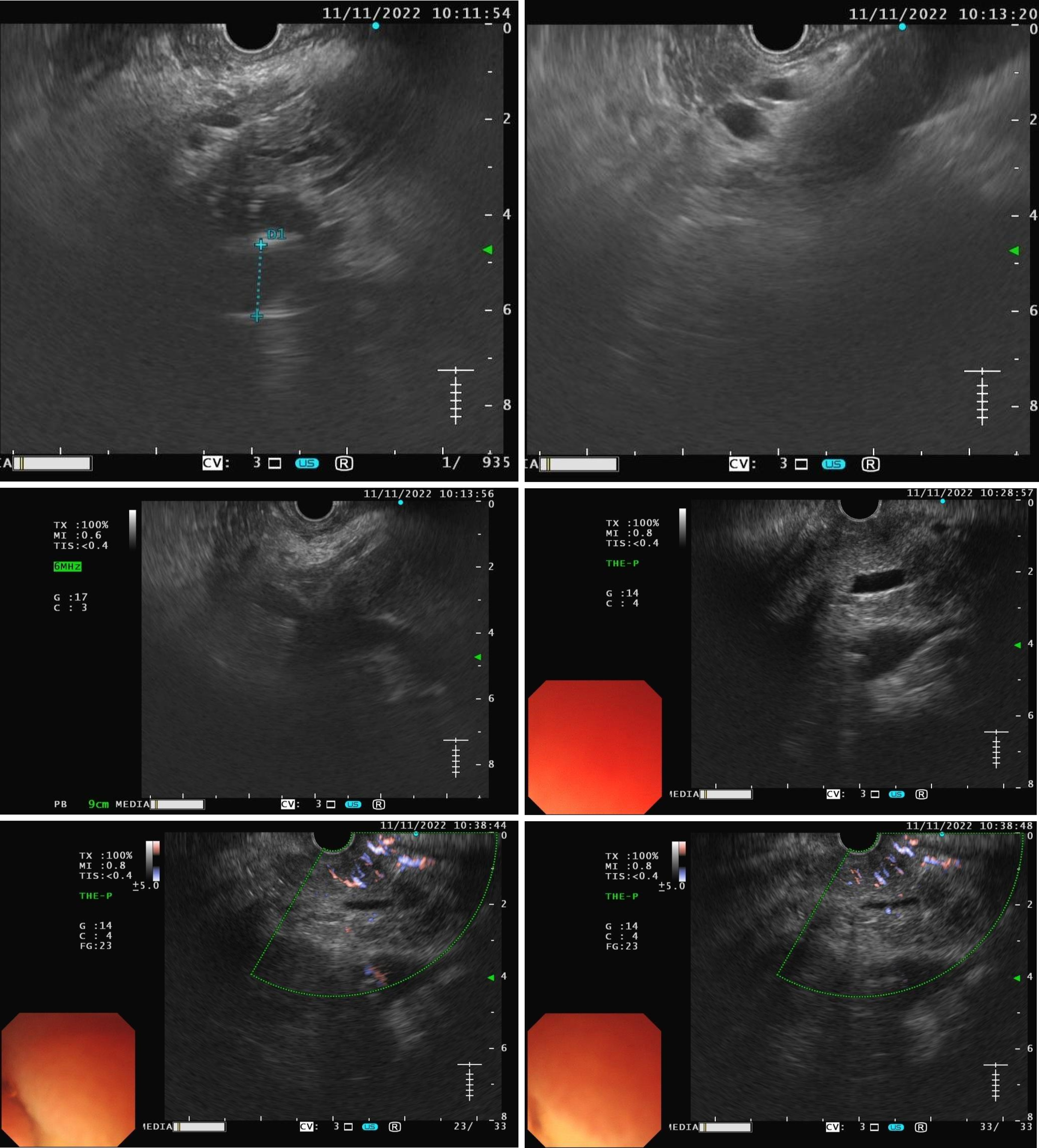
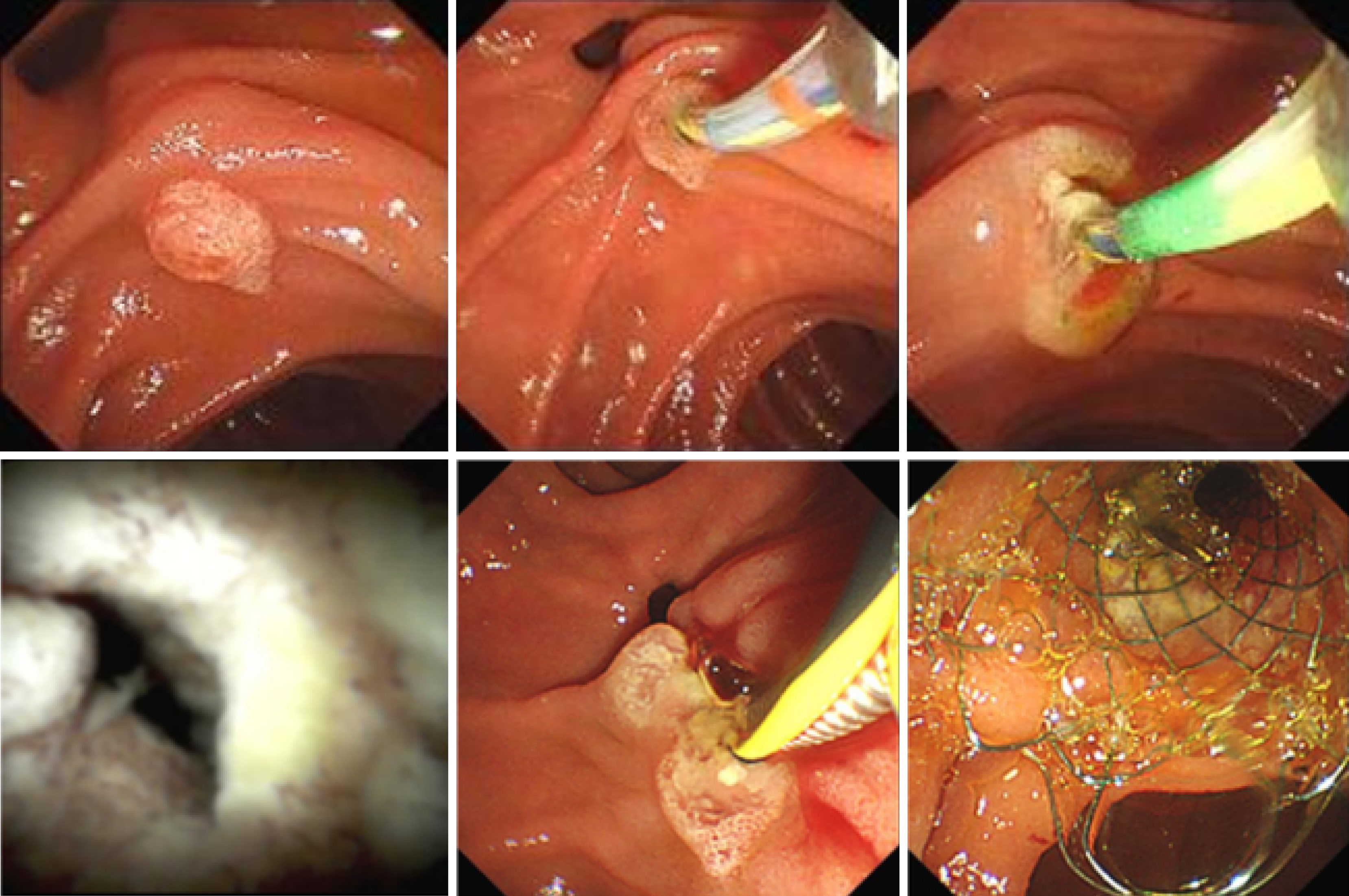
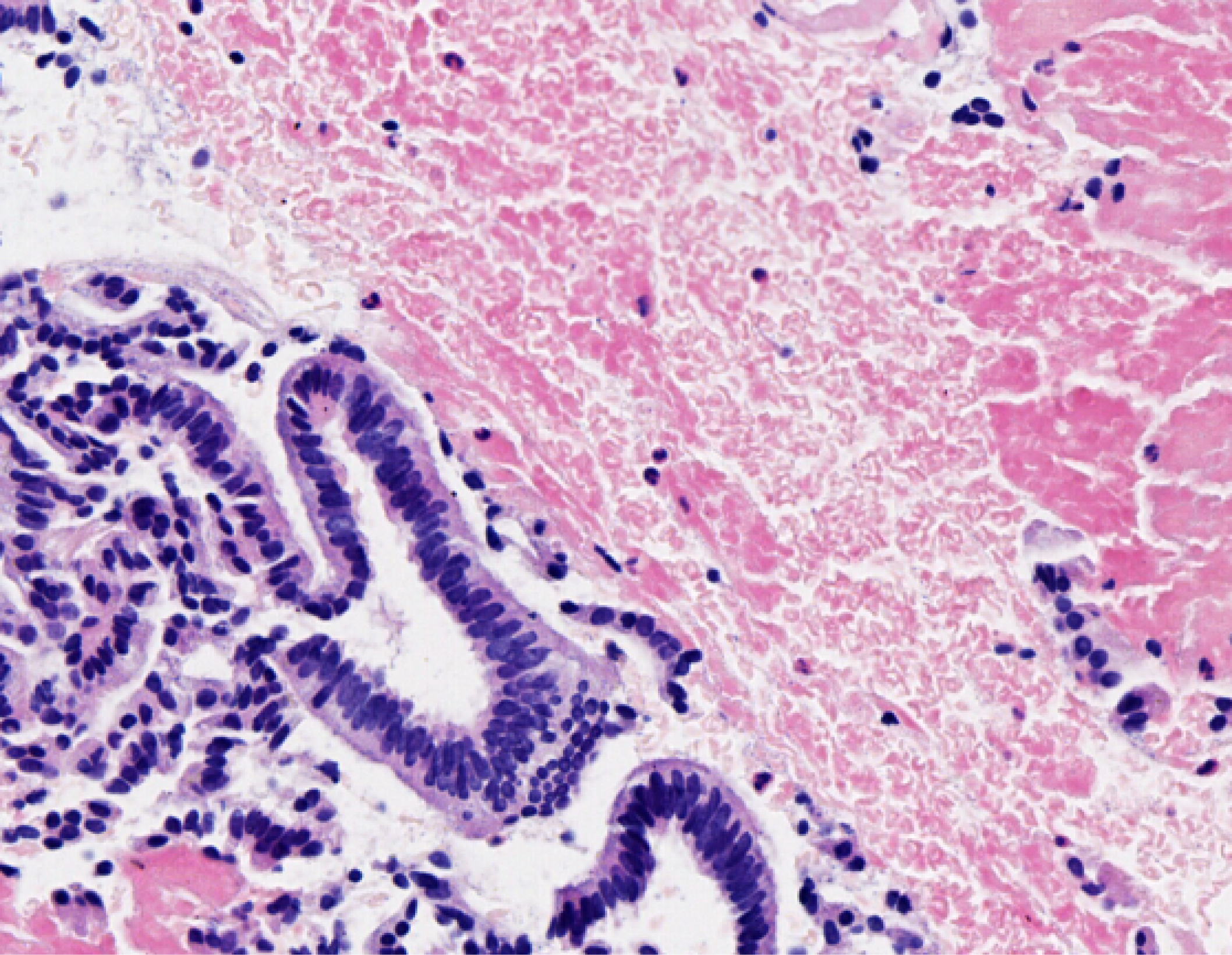
The patient is experiencing loss of appetite, accompanied by decreased urine output and reduced frequency of bowel movements. The patient's Eastern Cooperative Oncology Group (ECOG) performance status is grade 3. Computed tomography (CT) showed common bile duct wall thickening (Figure 1). Magnetic resonance cholangiopancreatography (MRCP) indicated the thickening of the upper and the pancreatic sections of the bile duct with narrow lumens. Dilatation of the intrahepatic bile ducts was observed (Figure 2). As endoscopic ultrasound (EUS), the middle section of the lumen was interrupted, and the wall structure was unclear (Figure 3). Endoscopic retrograde cholangiopancreatography (ERCP) showed several mucosal irregularities and tortuous surface vessels on the common bile duct, irregular angiographic defects in the middle and lower part of the common bile duct, and multifocal stenosis and dilatation in the upper part of the bile duct (Figure 4). Biopsy specimens were taken through the ERCP endoscope from the raised lesion area. Pathologic examination of the biopsy specimen revealed numerous inflammatory cells in the epithelium, suggesting that cholangitis was associated with irAEs (Figure 5).
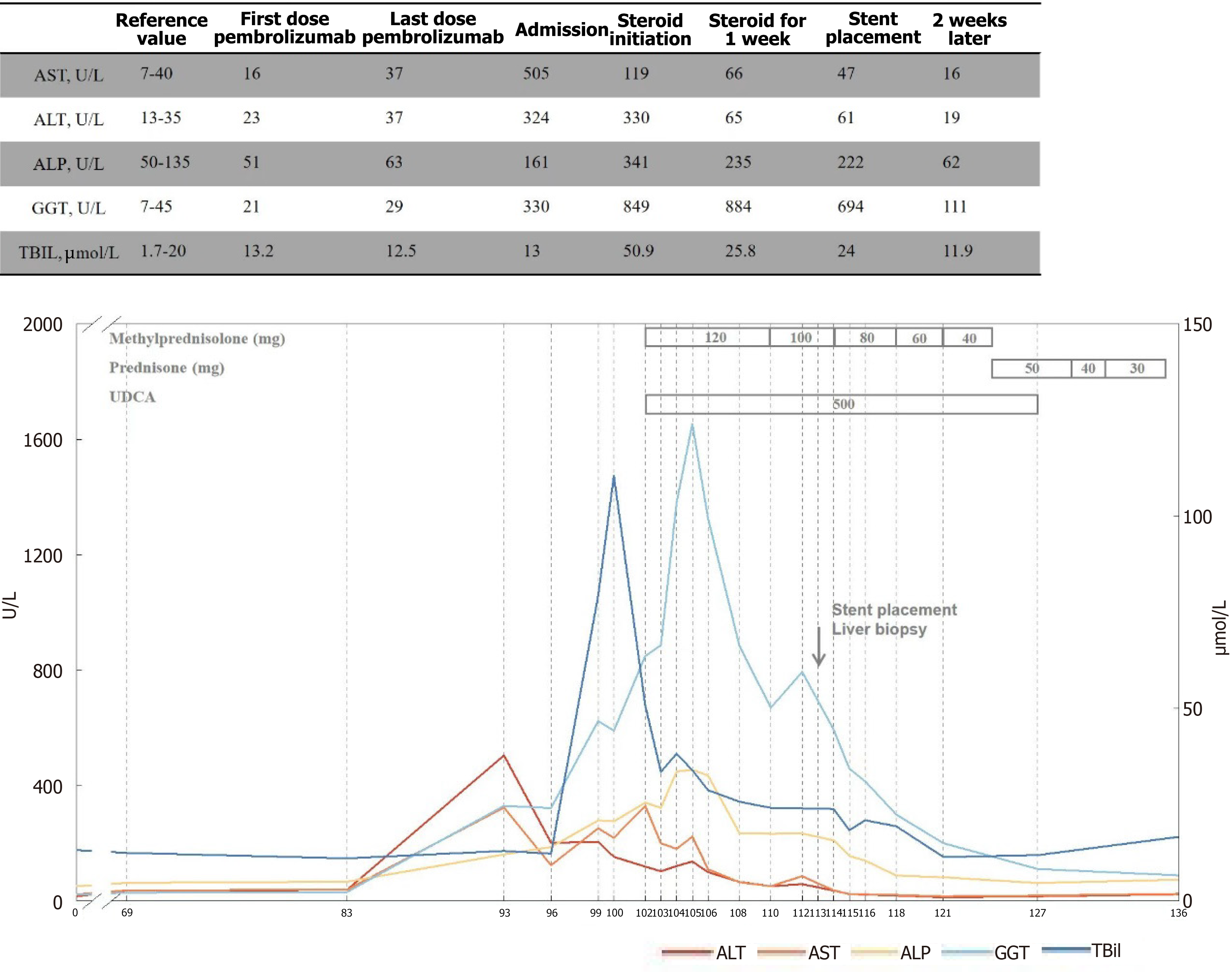
The aspartate aminotransferase (AST) and bilirubin levels decreased significantly after steroid therapy. In contrast, there was no typical imaging remission and Gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) levels were recurrent repeatedly, considering steroid insensitive immune-related cholangitis. Meanwhile, no improvement was seen in liver function after undergoing UDCA and hormone therapy, and it remained elevated. Symptoms gradually improved after stents being placed at ERCP (Figure 6), ECOG status has now improved to grade 1.
The patient had no significant past medical history.
There was no personal or family history of cancer treatment in the patient.
Biopsy specimens were taken through the ERCP endoscope from the raised lesion area.
Elevated liver enzymes after 4 cycles of the combined treatment of anti-PD-1 (Pembrolizumab) and TKI (Lenvatinib).
CT showed common bile duct wall thickening (Figure 1). MRCP indicated the thickening of the upper and the pancreatic sections of the bile duct with narrow lumens. Dilatation of the intrahepatic bile ducts was observed (Figure 2). EUS revealed disruption of the middle lumen segment, with poorly defined wall structures (Figure 3). ERCP demonstrated mucosal irregularities with tortuous surface vessels along the common bile duct. Angiographic imaging revealed irregular defects in the middle and lower common bile duct segments, while the proximal duct exhibited multifocal stenosis alternating with dilatation (Figure 4).
Biopsy samples obtained via ERCP from the elevated mucosal lesions showed dense epithelial inflammatory cell infiltration, consistent with immune-related cholangitis (Figure 5).
The AST and bilirubin levels decreased significantly after steroid therapy. In contrast, there was no typical imaging remission and GGT and ALP levels were recurrent repeatedly, considering steroid insensitive immune-related cholangitis. Meanwhile, no improvement was seen in liver function after undergoing UDCA and hormone therapy, and it remained elevated.
Symptoms gradually improved after stents being placed at ERCP (Figure 6), ECOG status has now improved to grade 1.
In the majority of cases, liver enzyme levels fail to normalize despite prolonged corticosteroid therapy for immune-related cholangitis[11-13]. Although hepatocellular injury usually exists, biliary tract injury is the primary pathogenesis for immune-related cholangitis. This kind of liver injury is not sensitive to steroid and immunosuppressant treatment, and the prognosis is poor[7]. There have been cases of UDCA treatment[14]. Our cases was treated with UDCA when steroid therapy was ineffective, but no improvement remains. While the liver function gradually returned to normal following the removal of biliary obstruction and cholestasis.
Although long-term immunosuppression is given during treatment for immune-related cholangitis, recovery is a slow intrinsic repair process rather than the result of our immunosuppressive intervention in terms of treatment time. Gourari et al[15] explored that, in one case of immune-related cholangitis without any treatment, liver function returned to normal after 16 weeks, as did a patient receiving steroids and UDCA. Hormones and immunosuppressants may not be necessary for all patients with immune-related cholangitis, and there are heterogeneity and self-repair processes. Anti-tumor immunotherapy has caused severe damage to the liver, which subsequent hormones and immunosuppressants cannot effectively reverse. It suggests that immunosuppressive therapy is ineffective in severe cholangitis and that the body's self-repair function matters. Biliary involvement may serve as a critical indicator of this severe injury. Complete resolution of hepatic irAEs often necessitates prolonged immunosuppressive therapy, which carries the risk of significant complications and may compromise the efficacy of primary tumor immunotherapy. It seems to be a good strategy for immunotherapy-related bile duct injury to choose the best symptomatic and supportive treatment after steroid and immunosuppressive therapy are ineffective.
Stent implantation has the advantages of suitable tolerance, less trauma, immediate effect, and high safety for the symptomatic treatment of severe cholangitis. For patients with ineffective medical treatment or who need long-term treatment, or a long course of the disease, it could improve hyperbilirubinemia and liver failure. Stent drainage can improve cholestasis through bile secretion and reduce liver cell damage. The surgical trauma is relatively tiny, and simultaneously relieves acute obstructive symptoms, which is especially suitable for inflammatory lesions of the hilar bile duct and common bile duct. The hypothesized mechanisms by which stent placement may ameliorate inflammation include biliary decompression, modulation of local immune activity, or other potential pathways. However, the specific mechanism requires further investigation. Removing cholestasis through drainage is considered the preferred treatment strategy for future cases of immune-related cholangitis, as opposed to relying solely on hormones or immunosuppressants, which could present greater challenges. Currently, there is no definitive theoretical basis for determining the optimal timing of drainage intervention. Nonetheless, we propose that drainage should be promptly initiated when obstructive symptoms manifest. Combining drainage with immunosuppressive therapy may potentially yield better therapeutic outcomes. Future research endeavors could focus on further elucidating the pathogenesis, treatment strategies, and prognostic factors associated with immune-related cholangitis. Such investigations would facilitate the optimization of treatment plans and ultimately improve clinical outcomes for affected patients.
Stent placement offers prompt alleviation of cholestasis and constitutes an effective therapeutic strategy for managing immune-related cholangitis.
| 1. | Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, Rugo HS, de Braud F, Varga AI, Hansen A, Wang H, Krishnan S, Norwood KG, Doi T. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 2. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3476] [Article Influence: 434.5] [Reference Citation Analysis (0)] |
| 3. | Huang W, Zhang Y, Liu Y, Sun Z. Immune cholangitis related to anti-programmed cell death and programmed cell death ligand agents for the treatment of intrahepatic cholangiocarcinoma. Rev Esp Enferm Dig. 2025;117:168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gelsomino F, Vitale G, D'Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28:671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, Kamata K, Takenaka M, Kimura M, Chikugo T, Sato T, Kudo M, Ito A, Nakagawa K. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs. 2017;35:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Bik E, Piluch P, Kośnik A, Stadnik A, Grzybowski J, Raszeja-Wyszomirska J. Dual pattern of immune-related hepatotoxicity induced by pembrolizumab: hepatitis and sclerosing cholangitis. Pol Arch Intern Med. 2025;135:17008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Pi B, Wang J, Tong Y, Yang Q, Lv F, Yu Y. Immune-related cholangitis induced by immune checkpoint inhibitors: a systematic review of clinical features and management. Eur J Gastroenterol Hepatol. 2021;33:e858-e867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Sato K, Hayashi M, Abe K, Fujita M, Takahashi A, Ohira H. Pembrolizumab-induced sclerosing cholangitis in a lung adenocarcinoma patient with a remarkable response to chemotherapy: a case report. Clin J Gastroenterol. 2020;13:1310-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Fouchard M, Jantzem H, Quere G, Descourt R, Robinet G, Poureau PG. Three cases of immune cholangitis related to anti-programmed cell death and programmed cell death ligand agents for the treatment of non-small cell lung cancer. Eur J Cancer. 2019;115:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Reddy CA, Schneider BJ, Brackett LM, Tai AW. Nivolumab-induced large-duct cholangiopathy treated with ursodeoxycholic acid and tocilizumab. Immunotherapy. 2019;11:1527-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, Parkinson CA, Corrie PG. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Li Y, Stewart L, Tang S, McWhirter E. Pembrolizumab-induced immune-related sclerosing cholangitis. BMJ Case Rep. 2023;16:e256125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, Kawata S, Matsumoto K, Isomoto H. Programmed cell death-1 inhibitor-related sclerosing cholangitis: A systematic review. World J Gastroenterol. 2020;26:353-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 14. | Zhu GQ, Shi KQ, Huang GQ, Wang LR, Lin YQ, Braddock M, Chen YP, Zhou MT, Zheng MH. A network meta-analysis of the efficacy and side effects of UDCA-based therapies for primary sclerosing cholangitis. Oncotarget. 2015;6:26757-26769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Gourari K, Catherine J, Garaud S, Kerger J, Lepida A, Georgala A, Lebrun F, Gomez Galdon M, Gil T, Willard-Gallo K, Langouo Fontsa M. A Rare Case of Hepatic Vanishing Bile Duct Syndrome Occurring after Combination Therapy with Nivolumab and Cabozantinib in a Patient with Renal Carcinoma. Diagnostics (Basel). 2022;12:539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/