Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.113853
Revised: September 28, 2025
Accepted: November 3, 2025
Published online: December 15, 2025
Processing time: 97 Days and 19.8 Hours
Inflammatory cytokines are associated with cancer prognosis, but their specific role in cholangiocarcinoma remains poorly understood. The lymphocyte to C-re
To validate the LCR as an independent prognostic factor for overall survival (OS), surgical site infection (SSI), and length of hospital stay in patients with resectable cholangiocarcinoma.
We conducted a retrospective analysis of 76 patients with cholangiocarcinoma who underwent radical surgery between 2008 and 2013. The preoperative LCR was calculated as the lymphocyte count divided by C-reactive protein level, using a cutoff value of 180. Univariate and multivariate logistic regression analyses were performed to evaluate factors associated with SSI and hospitalization duration, while Kaplan-Meier survival curves and Cox proportional hazards models were used to assess predictors of OS.
Patients in the low LCR group was significantly associated with several adverse clinical outcomes: A shorter median OS (14.93 months vs 46.67 months; P = 0.022); a 4.5-fold increased risk of prolonged hospitalization (P = 0.007); and a higher incidence of SSI (odds ratio = 4.41, P = 0.045). Multivariate analysis confirmed that LCR was an independent predictor of OS [hazard ratio (HR) = 3.204, P = 0.002], SSI, and hospitalization duration. Additionally, R0 resection (HR = 3.546, P = 0.002) and advanced tumor-node-metastasis stage (HR = 2.016, P = 0.035) were identified as independent prognostic factors for OS.
In this retrospective study, preoperative LCR is a cost-effective and practical biomarker that independently predicts OS, postoperative complications, and hospitalization duration in patients with resectable cholangiocarcinoma, thereby facilitating more precise patient stratification.
Core Tip: This pioneering study establishes the lymphocyte to C-reactive protein ratio (LCR) as the first validated inflammatory biomarker for predicting survival and postoperative outcomes in cholangiocarcinoma. We demonstrate that a low preoperative LCR (< 180) independently correlates with a 4.5-fold increase in the risk of prolonged hospitalization, elevated risk of surgical site infections (odds ratio = 4.41), and significantly reduced median survival (14.93 months vs 46.67 months). As a cost-effective prognostic tool, LCR facilitates refined patient stratification, thereby supporting optimized surgical decision-making and postoperative management.
- Citation: Xiao F, Zhou DH, Liu GW, Lin CW, Wu ZY, Yu H, Gong W, Tan WF. Lymphocyte to C-reactive protein ratio as a novel inflammatory biomarker: Validation and clinical relevance as an independent prognostic factor in cholangiocarcinoma. World J Gastrointest Oncol 2025; 17(12): 113853
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/113853.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.113853
Cholangiocarcinoma is a malignant neoplasm arising from the epithelial cells of the bile ducts[1]. Its incidence has been steadily increasing in recent years[2]. Due to the absence of specific clinical symptoms in the early stages, diagnosis remains challenging, and most patients are already at an advanced stage upon detection, thereby missing the window for curative surgical intervention[3]. Furthermore, cholangiocarcinoma exhibits limited responsiveness to conventional radiotherapy and chemotherapy, while emerging therapeutic strategies such as targeted therapy and immunotherapy remain under development[4-7]. Consequently, the long-term prognosis for patients remains poor, and mortality rates remain high. Although advances in clinical diagnostic techniques (e.g., preoperative staging) and surgical approaches (e.g., technical refinements) have significantly improved treatment outcomes, the overall survival (OS) rate continues to be suboptimal.
The relationship between inflammation and cancer has long been a focus of research. Accumulating evidence indicates that disease progression in cancer patients is not solely dictated by the biological features of the tumor, but is also significantly influenced by the host’s inflammatory response[8]. Chronic inflammatory stimulation can promote the selective proliferation of tumor cells and contribute to tumorigenesis[9]. For example, zinc oxide nanoparticles can exacerbate chronic inflammatory responses and promote tumorigenesis through the activation of specific signaling pathways[10]. In cholangiocarcinoma, the pathological origin in a subset of patients is associated with primary sclerosing cholangitis, characterized by a persistent chronic inflammatory response in bile duct epithelial cells[11,12]. Further studies have demonstrated that inflammatory responses and the infiltration of inflammatory cells can disrupt the local microenvironment, thereby facilitating tumor progression and metastasis, which ultimately leads to a poorer long-term prognosis[13]. The complex and paradoxical role of inflammation in both antagonizing and promoting tumorigenesis, particularly in the context of modern immunotherapies, has been extensively discussed in recent authoritative reviews[8].
In recent years, preoperative systemic inflammation and nutritional status have garnered increasing attention in surgical research. Patients with malignant tumors, particularly those with gastrointestinal malignancies, commonly exhibit a state of systemic inflammation and nutritional deficiency. According to the European Society for Parenteral and Enteral Nutrition, patients at nutritional risk during the perioperative period are more likely to experience postoperative complications such as impaired wound healing, surgical site infection (SSI), and pulmonary infections, all of which may prolong hospitalization and elevate healthcare expenditures[14]. Prognostic scoring systems based on systemic inflammatory response markers, such as C-reactive protein (CRP), the Glasgow Prognostic Score (GPS), and the neutrophil-to-lymphocyte ratio (NLR), have emerged as key areas of interest in cancer research[15-17].
Lymphocyte count serves as an indicator of immune function and nutritional status in patients, while CRP is a well-established biomarker of systemic inflammation. Therefore, the preoperative lymphocyte to CRP ratio (LCR) may serve as a comprehensive marker reflecting both the systemic immune-inflammatory status and nutritional condition in patients with malignant tumors, thereby predicting postoperative recovery and the long-term biological behavior of tumors. Emerging evidence indicates that LCR demonstrates significant predictive value for long-term prognosis in patients with gastrointestinal cancers[18]; indeed, the ongoing exploration of novel prognostic biomarkers, including inflammatory scores, remains a high priority in cholangiocarcinoma research[4]. However, its prognostic role in cholangiocarcinoma remains to be elucidated[18].
Building on the aforementioned background, this study proposes, for the first time, that preoperative LCR may serve as a valuable indicator for assessing systemic inflammation and nutritional status in patients with cholangiocarcinoma, and is associated with postoperative SSI, length of hospital stay, and long-term survival outcomes. By conducting a retrospective analysis of data from 76 cholangiocarcinoma patients who underwent curative-intent surgery, this study aims to validate the clinical significance of LCR as an independent prognostic marker and to provide evidence for optimizing patient stratification and surgical decision-making.
The diagnosis was confirmed by pathological examination. Patients who had received preoperative adjuvant therapy, those with biliary tract infection, hematological disorders, or other concomitant malignancies were excluded. Inclusion criteria were as follows: Patients diagnosed with cholangiocarcinoma and who underwent radical surgical resection at Shanghai Fourth People’ Hospital or Shanghai Xinhua Hospital between 2008 and 2013 (n = 76). Exclusion criteria included: Preoperative receipt of neoadjuvant chemotherapy, radiotherapy, or targeted therapy; presence of biliary tract infection or uncontrolled systemic infection prior to surgery; comorbid hematological diseases (e.g., leukemia, lymphoma) or other malignant tumors; absence of key preoperative laboratory data (e.g., CRP, lymphocyte count); loss to follow-up within one year postoperatively; non-radical resection (R2 resection); emergency surgery; or combined multi-organ resection (e.g., combined liver and pancreatic resection). Patients with obstructive jaundice were not excluded, provided they met the following conditions: (1) Adequate biliary drainage with total bilirubin levels < 100 μmol/L; and (2) Absence of signs of infection following biliary drainage. Ethical approval was obtained from the Ethics Committee of Shanghai Fourth People’s Hospital (Approval No. 2025097-001). The requirement for written informed consent was waived by the Ethics Committee for this retrospective study.
Clinical variables included gender, age, preoperative peripheral blood parameters (white blood cell count, lymphocyte count, CRP, albumin, hemoglobin), tumor markers [carbohydrate antigen 199 (CA199), carcinoembryonic antigen, CA125], and total bilirubin. Tumor characteristics encompassed anatomical location (intrahepatic vs extrahepatic), tumor size, tumor-node-metastasis (TNM) stage (7th edition of the American Joint Committee on Cancer staging system), presence of vascular invasion, lymph node metastasis, and surgical margin status (R0/R1/R2). Outcome measures consisted of OS (defined as the time from surgery to death or last follow-up), SSI (including bile leakage, pancreatic fistula, and peritonitis), and length of hospital stay. Blood samples were collected within one week prior to surgery. For patients with obstructive jaundice, blood sampling was performed after adequate biliary decompression and resolution of infection.
LCR was calculated as the ratio of lymphocyte count (cells/μL) to CRP level (mg/dL). The optimal cutoff value of 180 was determined using the maxstat package in R, which maximizes rank statistics for prognostic stratification[19]. SSI was defined as the presence of bile leakage, pancreatic fistula, cholangitic peritonitis, or intra-abdominal infection. Prolonged hospital stay was defined as a postoperative hospitalization duration exceeding 31.17 days, which corresponds to the mean length of stay in the study cohort.
Statistical analysis was performed using SPSS version 17.0 and R software. For comparative analysis, Student’s t-test or one-way analysis of variance (ANOVA) was applied for continuous variables. Univariate and multivariate logistic regression models were employed to identify risk factors associated with SSI and prolonged hospital stay. All variables included in the multivariate model were selected based on a prespecified threshold of P < 0.1 in univariate analysis, ensuring adequate adjustment for potential confounders. Survival analysis was conducted using Kaplan-Meier curves and the log-rank test to assess OS. Independent prognostic factors were determined through multivariate Cox proportional hazards regression analysis, with adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) reported. Cut-off values for laboratory parameters (e.g., albumin 35 g/L, CA199 39 U/mL) were determined based on established clinical thresholds or the median value of the study cohort.
This study enrolled 76 patients with cholangiocarcinoma who underwent curative-intent surgery (Table 1). The cohort had a mean age of 62.48 years (range: 36-84 years), with a male-to-female ratio of 46:30. Patients were categorized based on the predefined LCR threshold of 180: The low LCR group (< 180) included 54 patients (71.05%), with a mean LCR of 64.12 ± 49.41; the high LCR group (≥ 180) included 22 patients (28.95%), with a mean LCR of 301.51 ± 94.35. No significant differences were observed in baseline characteristics, including sex, age, tumor location (intrahepatic vs extrahepatic), and surgical margin status, between the two groups (all P > 0.05), indicating adequate intergroup comparability. Notably, the low LCR group exhibited significantly higher rates of preoperative CA199 abnormalities (69.74% vs 30.26%) and elevated CRP levels (56.58% vs 43.42%) compared to the high LCR group, suggesting a potential link between systemic inflammation and nutritional status.
| Variables | Number of patients | Low LCR | High LCR | P value |
| Age | 0.287 | |||
| ≤ 70 | 58 (76.32) | 43 (56.58) | 15 (19.74) | |
| > 70 | 18 (23.68) | 11 (14.47) | 7 (9.21) | |
| Sex | 0.870 | |||
| Male | 46 (60.53) | 33 (43.42) | 13 (17.11) | |
| Female | 30 (39.47) | 21 (27.63) | 9 (11.84) | |
| Obstructive jaundice | 0.432 | |||
| Yes | 50 (65.79) | 37 (48.7) | 13 (17.1) | |
| No | 26 (34.21) | 17 (22.4) | 9 (11.8) | |
| CEA | 0.989 | |||
| Normal | 45 (59.21) | 32 (42.1) | 13 (17.1) | |
| Abnormal | 31 (40.79) | 22 (28.9) | 9 (11.8) | |
| CA199 | ||||
| Normal | 23 (30.26) | 17 (22.4) | 6 (7.9) | 0.717 |
| Abnormal | 53 (69.74) | 37 (48.7) | 16 (21.1) | |
| CA125 | 0.606 | |||
| Normal | 52 (68.42) | 36 (47.4) | 16 (21.1) | |
| Abnormal | 24 (31.58) | 18 (23.7) | 6 (7.9) | |
| CRP | < 0.001a | |||
| Normal | 33 (43.42) | 11 (14.5) | 22 (28.9) | |
| Abnormal | 43 (56.58) | 43 (56.6) | 0 (0.0) | |
| WBC count (109/L) | 0.343 | |||
| Normal | 35 (46.05) | 23 (30.3) | 12 (15.8) | |
| Abnormal | 41 (53.95) | 31 (40.8) | 10 (13.2) | |
| Albumin (g/L) | 0.471 | |||
| Normal | 58 (76.32) | 40 (52.6) | 18 (23.7) | |
| Abnormal | 18 (23.68) | 14 (18.4) | 4 (5.3) | |
| Hemoglobin (g/L) | 0.851 | |||
| Normal | 23 (30.26) | 16 (21.1) | 7 (9.2) | |
| Abnormal | 53 (69.74) | 38 (50.0) | 15 (19.7) | |
| Tumor location | 0.277 | |||
| Intrahepatic | 21 (27.63) | 13 (17.1) | 8 (10.5) | |
| Extrahepatic | 55 (72.37) | 41 (53.9) | 14 (18.4) | |
| Number of tumor | ||||
| 1 | 71 (93.42) | 51 (67.1) | 20 (26.3) | 0.573 |
| ≥ 2 | 5 (6.58) | 3 (3.9) | 2 (2.6) | |
| Tumor size | 0.900 | |||
| Largest diameter ≥ 5 cm | 18 (23.68) | 13 (17.1) | 5 (6.6) | |
| Largest diameter < 5 cm | 58 (76.32) | 41 (53.9) | 17 (22.4) | |
| Surrounding vascular invasion | 0.188 | |||
| Yes | 18 (23.68) | 15 (19.7) | 3 (3.9) | |
| No | 58 (76.32) | 39 (51.3) | 19 (25.0) | |
| Surrounding tissues invasion | 0.307 | |||
| Yes | 64 (84.21) | 44 (57.9) | 20 (26.3) | |
| No | 12 (15.79) | 10 (13.2) | 2 (2.6) | |
| Lymph node metastasis | 0.666 | |||
| Yes | 27 (35.52) | 20 (26.3) | 7 (9.2) | |
| No | 49 (64.48) | 34 (44.7) | 15 (19.7) | |
| Tumor stage | ||||
| II | 38 (50.00) | 27 (34.5) | 11 (14.5) | 1.0 |
| III | 38 (50.00) | 27 (34.5) | 11 (14.5) | |
| Tumor grade | 0.404 | |||
| I | 26 (34.21) | 16 (21.1) | 10 (13.2) | |
| II | 39 (51.32) | 30 (39.5) | 9 (11.8) | |
| III | 11 (14.47) | 8 (10.5) | 3 (3.9) | |
| R0 resection | 0.115 | |||
| Yes | 66 (86.84) | 49 (64.5) | 17 (22.3) | |
| No | 10 (13.16) | 5 (6.6) | 5 (6.6) |
The incidence of SSI was significantly higher in the low LCR group (Table 2). Univariate analysis demonstrated that a low LCR was associated with a 3.4-fold increased risk of SSI [odds ratio (OR) = 3.438, 95%CI: 0.901-13.125, P = 0.071]. A multivariate logistic regression model further confirmed that LCR was an independent predictor of SSI (OR = 4.412, 95%CI: 1.035-18.806, P = 0.045). The specific manifestations of SSI included bile leakage (12 cases), pancreatic fistula (6 cases), and intra-abdominal infection (3 cases), with the low LCR group accounting for 85.7% (18/21 cases) of all SSI complications.
| Variables | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | ||||||
| > 70 | 2.436 | 0.628-9.448 | 0.098 | 2.520 | 0.592-10.734 | 0.211 |
| Sex | ||||||
| Female | 2.400 | 0.872-6.603 | 0.190 | |||
| Obstructive jaundice | ||||||
| Yes | 2.600 | 0.932-7.255 | 0.068 | 2.437 | 0.707-8.394 | 0.158 |
| CEA | ||||||
| Abnormal | 1.007 | 0.367-2.761 | 0.989 | |||
| CA199 | ||||||
| Abnormal | 1.978 | 0.695-5.626 | 0.201 | |||
| CA125 | ||||||
| Abnormal | 1.800 | 0.638-5.081 | 0.267 | |||
| CRP | ||||||
| Abnormal | 1.156 | 0.423-3.158 | 0.778 | |||
| WBC count (109/L) | ||||||
| Abnormal | 1.034 | 0.382-2.799 | 0.947 | |||
| Albumin (g/L) | ||||||
| Abnormal | 1.824 | 0.598-5.564 | 0.291 | |||
| Hemoglobin (g/L) | ||||||
| Abnormal | 2.443 | 0.722-8.265 | 0.151 | |||
| Tumor location | ||||||
| Intrahepatic | 2.423 | 0.835-7.028 | 0.099 | 2.094 | 0.568-7.726 | 0.267 |
| Number of tumors | ||||||
| ≥ 2 | 1.700 | 0.264-10.947 | 0.577 | |||
| Tumor size | ||||||
| Largest diameter ≥ 5 cm | 1.312 | 0.421-4.090 | 0.639 | |||
| Surrounding vascular invasion | ||||||
| Yes | 1.575 | 0.455-5.457 | 0.474 | |||
| Surrounding tissues invasion | ||||||
| Yes | 1.975 | 0.552-7.065 | 0.295 | |||
| Lymph node metastasis | ||||||
| Yes | 1.697 | 0.573-5.028 | 0.340 | |||
| Tumor stage | ||||||
| III | 1.292 | 0.478-3.494 | 0.613 | |||
| Tumor grade | ||||||
| III vs II vs I | 1.050 | 0.501-2.2 | 0.897 | |||
| R0 resection | ||||||
| Yes | 1.739 | 0.339-8.390 | 0.507 | |||
| LCR | ||||||
| Low | 3.438 | 0.901-13.125 | 0.071 | 4.412 | 1.035-18.806 | 0.045 |
The average hospital stay was significantly longer in the low LCR group (Table 3). Using the cohort’s mean hospital stay of 31.17 days as the cutoff, low LCR was associated with a 4.5-fold increased risk of prolonged hospitalization (univariate OR = 4.533, 95%CI: 1.526-13.467, P = 0.007). Multivariate analysis further confirmed that LCR independently predicted prolonged hospital stay, with an even stronger effect (OR = 8.511, 95%CI: 2.403-30.149, P = 0.001). Notably, SSI complications themselves were significantly associated with extended hospitalization (OR = 8.392, P = 0.002), and hypoalbuminemia (< 35 g/L) also independently predicted prolonged hospital stay (OR = 4.306, P = 0.041), highlighting the synergistic impact of nutritional and inflammatory factors on postoperative recovery.
| Variables | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | ||||||
| > 70 | 1.538 | 0.531-4.458 | 0.427 | |||
| Sex | ||||||
| Male | 1.190 | 0.474-2.991 | 0.711 | |||
| Obstructive jaundice | ||||||
| Yes | 1.174 | 0.455-3.032 | 0.740 | |||
| CEA | ||||||
| Abnormal | 1.161 | 0.464-2.908 | 0.749 | |||
| CA199 | ||||||
| Normal | 1.252 | 0.468-3.351 | 0.655 | |||
| CA125 | ||||||
| Abnormal | 1.094 | 0.415-2.886 | 0.856 | |||
| CRP | ||||||
| Abnormal | 3.267 | 1.267-8.419 | 0.414 | |||
| WBC count (109/L) | ||||||
| Abnormal | 1.353 | 0.547-3.346 | 0.513 | |||
| Albumin (g/L) | ||||||
| Abnormal | 2.985 | 0.942-9.458 | 0.063 | 4.306 | 1.062-17.454 | 0.041 |
| Hemoglobin (g/L) | ||||||
| Abnormal | 1.696 | 0.632-4.551 | 0.294 | |||
| Tumor location | ||||||
| Extrahepatic | 1.014 | 0.371-2.775 | 0.978 | |||
| Number of tumors | ||||||
| 1 | 4.875 | 0.519-45.821 | 0.166 | |||
| Tumor size | ||||||
| Largest diameter ≥ 5 cm | 2.143 | 0.708-6.483 | 0.177 | |||
| Surrounding vascular invasion | ||||||
| Yes | 1.167 | 0.403-3.377 | 0.766 | |||
| Surrounding tissues invasion | ||||||
| No | 2.000 | 0.547-7.312 | 0.295 | |||
| Lymph node metastasis | ||||||
| Yes | 1.515 | 0.586-3.919 | 0.391 | |||
| Tumor stage | ||||||
| III | 1.894 | 0.761-4.716 | 0.070 | 1.695 | 0.574-5.004 | 0.339 |
| Tumor grade | ||||||
| III vs II vs I | 1.412 | 0.713-2.797 | 0.323 | |||
| R0 resection | ||||||
| Yes | 1.800 | 0.464-6.976 | 0.395 | |||
| LCR | ||||||
| Low | 4.533 | 1.526-13.467 | 0.007 | 8.511 | 2.403-30.149 | 0.001 |
| Complication | ||||||
| Yes | 3.367 | 1.177-9.631 | 0.024 | 8.392 | 2.246-31.359 | 0.002 |
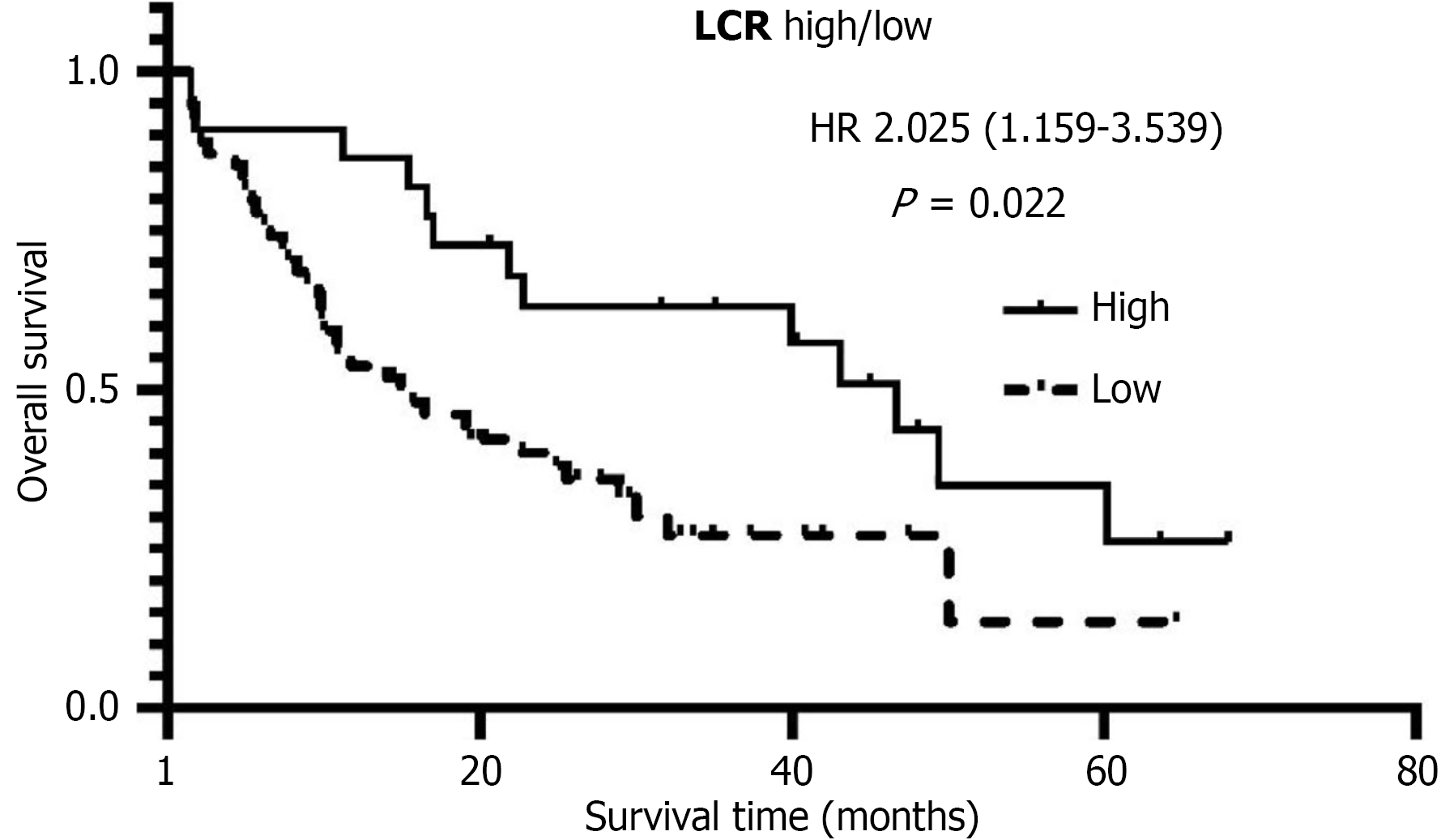
Survival analysis (Figure 1) revealed that the median OS in the low LCR group was only 14.93 months, significantly shorter than the 46.67 months observed in the high LCR group (P = 0.022). The multivariate Cox proportional hazards model (Table 4) confirmed that low LCR was an independent risk factor for reduced OS (HR = 3.204, 95%CI: 1.159-3.539, P = 0.002). Additionally, both R0 resection (HR = 3.547, P = 0.002) and advanced TNM stage (HR = 2.017, P = 0.035) were significantly associated with poorer prognosis.
| Variables | Median OS | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age | |||||||
| ≤ 70/> 70 | 21.86/17.03 | 0.919 | 0.489-1.728 | 0.793 | |||
| Sex | |||||||
| Male/female | 22.80/17.03 | 0.799 | 0.447-1.359 | 0.379 | |||
| Obstructive jaundice | |||||||
| Yes/no | 16.47/22.70 | 1.129 | 0.635-2.010 | 0.679 | |||
| CEA | |||||||
| Abnormal/normal | 21.86/22.80 | 1.02 | 0.575-1.811 | 0.946 | |||
| CA199 | |||||||
| Abnormal/normal | 17.33/28.90 | 1.434 | 0.774-2.658 | 0.252 | |||
| CA125 | |||||||
| Abnormal/normal | 19.43/22.80 | 1.118 | 0.593-2.108 | 0.729 | |||
| CRP | |||||||
| Abnormal/normal | 11.73/39.93 | 1.695 | 0.962-2.987 | 0.068 | |||
| WBC count (109/L) | |||||||
| Abnormal/normal | 19.43/22.80 | 1.09 | 0.626-1.899 | 0.760 | |||
| Albumin (g/L) | |||||||
| Abnormal/normal | 10.03/22.70 | 1.104 | 0.577-2.111 | 0.766 | |||
| Hemoglobin (g/L) | |||||||
| Abnormal/normal | 19.43/22.70 | 1.2 | 0.675-2.133 | 0.535 | |||
| Tumor location | |||||||
| Extrahepatic/intrahepatic | 25.53/19.43 | 0.787 | 0.551-1.125 | 0.189 | |||
| Number of tumors | |||||||
| ≥ 2/1 | 19.43/22.80 | 1.552 | 0.555-4.339 | 0.402 | |||
| Tumor size | |||||||
| Largest diameter ≥ 5 cm/< 5 cm | 8.27/22.80 | 1.528 | 0.813-2.873 | 0.188 | |||
| Surrounding vascular invasion | |||||||
| Yes/no | 7.37/24.87 | 2.07 | 1.130-3.793 | 0.019 | 1.292 | 0.629-2.655 | 0.486 |
| Surrounding tissues invasion | |||||||
| Yes/no | 19.10/32.03 | 1.565 | 0.667-3.674 | 0.304 | |||
| Lymph node metastasis | |||||||
| Yes/no | 17.03/24.87 | 1.605 | 0.913-2.820 | 0.100 | |||
| Tumor stage | |||||||
| III/II | 13.93/32.03 | 1.728 | 0.992-3.008 | 0.053 | 2.017 | 1.051-3.871 | 0.035 |
| Tumor grade | |||||||
| III/II/I | 15.70/22.70/46.51 | 1.497 | 1.003-2.234 | 0.094 | |||
| R0 resection | |||||||
| No/ yes | 7.37/24.87 | 2.156 | 1.042-4.461 | 0.038 | 3.547 | 1.567-8.029 | 0.002 |
| LCR | |||||||
| Low/high | 14.93/46.67 | 2.025 | 1.159-3.539 | 0.022 | 3.204 | 1.546-6.643 | 0.002 |
| Complication | |||||||
| Yes/no | 17.03/21.86 | 1.406 | 0.563-1.941 | 0.024 | 1.261 | 0.663-2.395 | 0.480 |
| Postoperative adjuvant therapy | |||||||
| Yes/no | 22.80/15.40 | 0.721 | 0.405-1.284 | 0.267 | |||
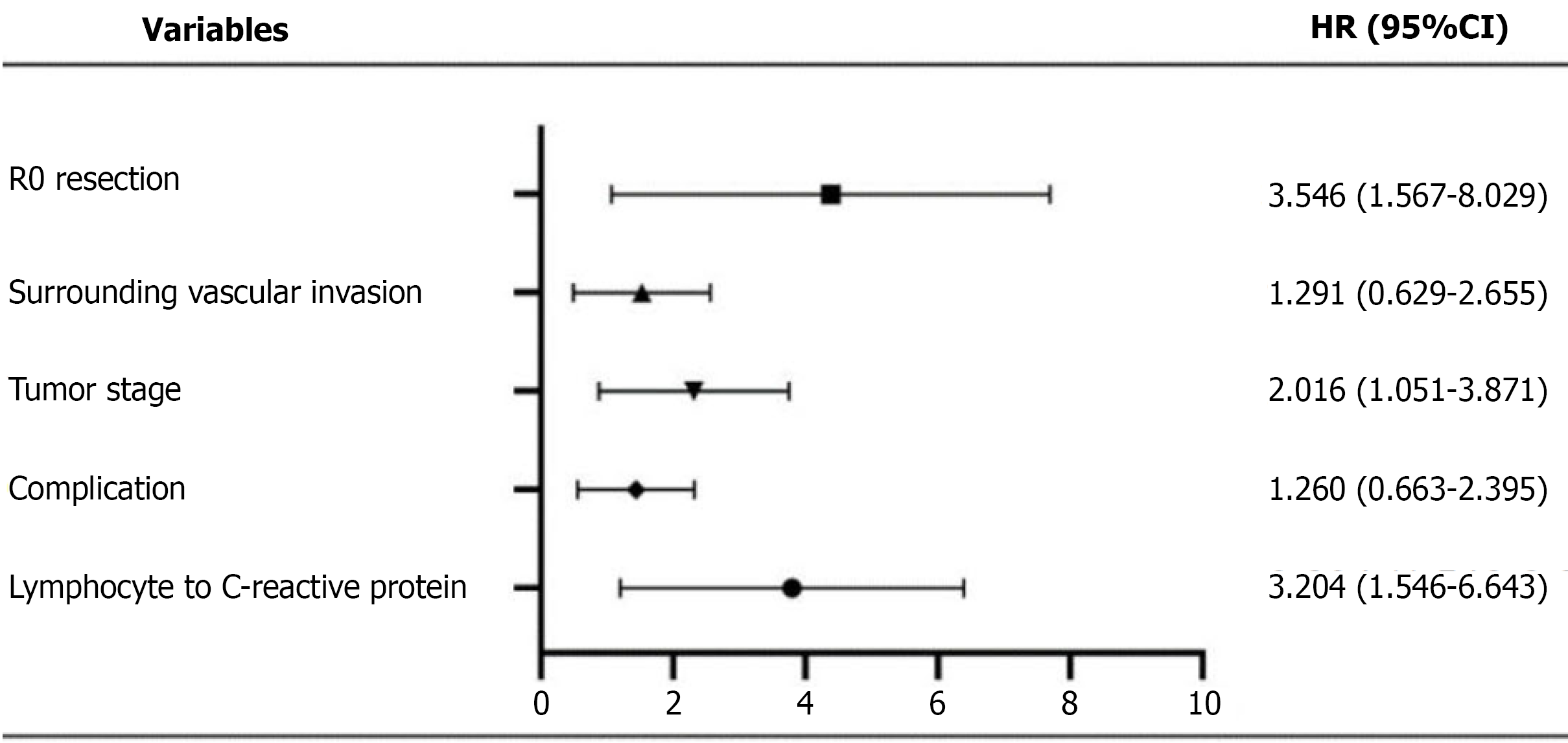
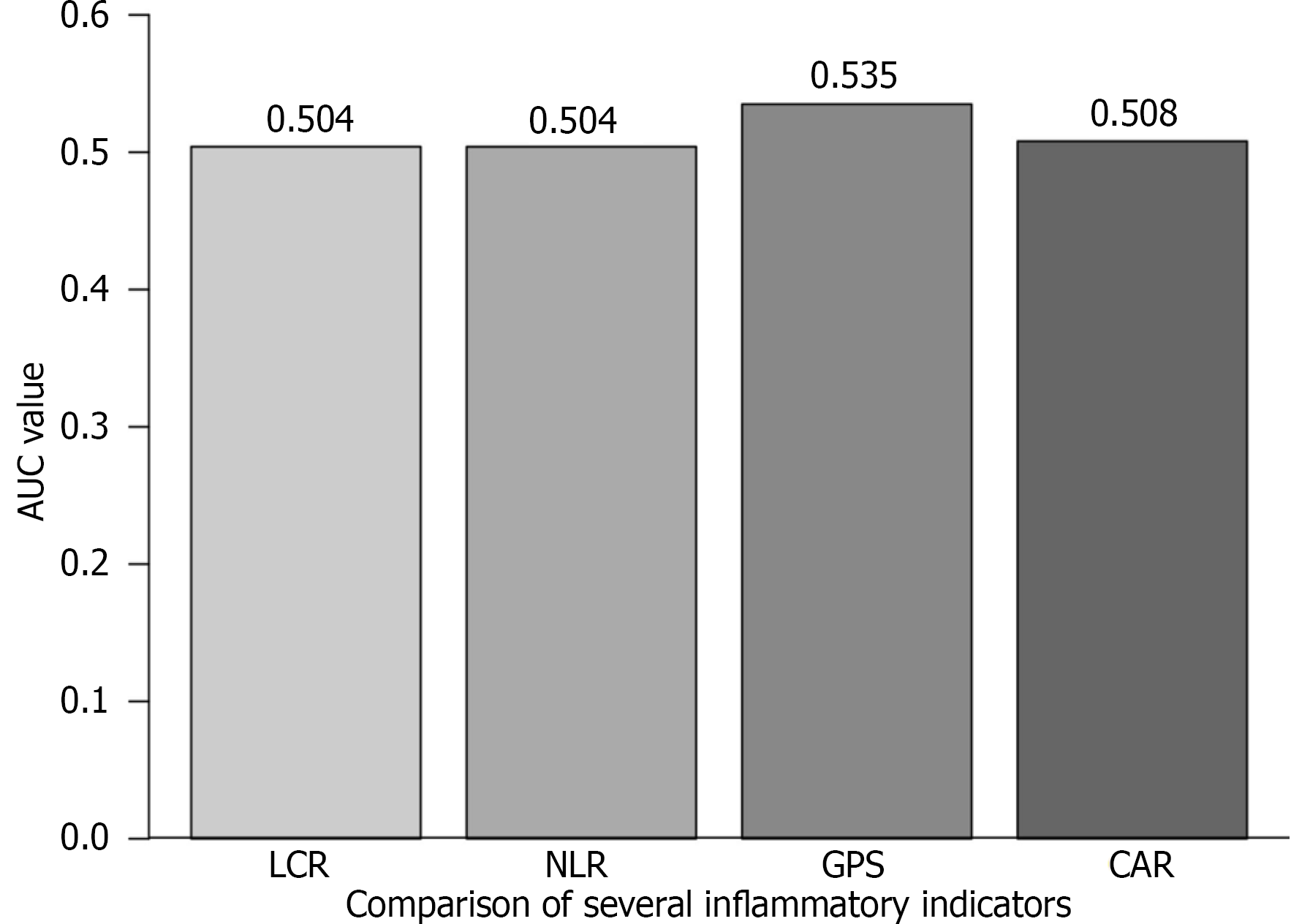
The comprehensive prognostic model generated through Cox regression (Figure 2) demonstrates that the predictive power of LCR (HR = 3.204) is second only to R0 resection status (HR = 3.546). To further evaluate the predictive performance of LCR relative to established inflammatory scores, we performed receiver operating characteristic curve analysis for OS. The area under the curve (AUC) for the LCR was 0.504, comparable to that of the NLR (AUC = 0.504) and CRP-albumin ratio (AUC = 0.507), and marginally lower than the GPS (AUC = 0.535). Collectively, these results suggest limited discriminative performance among these inflammation-based prognostic markers in predicting OS (Figure 3). Notably, LCR, as a modifiable parameter, exhibits greater clinical utility compared to the fixed TNM stage. The forest plot clearly illustrates the HRs of each variable: The 95%CI for low LCR (1.159-3.539) lies entirely to the right of the null-effect line and does not overlap with the CI of R0 resection, confirming the independent prognostic contributions of these two factors.
This study represents the first comprehensive investigation into the clinical significance of preoperative LCR as a prognostic inflammatory marker in patients with cholangiocarcinoma. Notably, patients with low preoperative LCR levels exhibited significantly shorter median survival times, a finding consistent with evidence from prior studies on gastrointestinal malignancies[18]. Du et al[20] reported that inflammatory markers are associated with improved prognosis in gastric cancer, potentially through the mitigation of immune-nutritional deterioration. Similarly, chronic inflammatory conditions such as primary sclerosing cholangitis in cholangiocarcinoma contribute to tumor development by compromising the integrity of the local tumor microenvironment (TME)[7]. The Cox proportional hazards model confirmed that LCR functions as an independent prognostic factor beyond TNM staging, highlighting its potential to refine risk stratification in cholangiocarcinoma patients.
Patients with low preoperative LCR demonstrated a 4.5-fold increased risk of prolonged hospitalization and a significantly higher incidence of SSIs, underscoring the impact of systemic inflammation on impaired postoperative recovery. Mitra et al[21] emphasized the central role of inflammation in the wound healing process, demonstrating that persistent inflammatory responses not only delay tissue repair but also significantly increase the risk of infection[14]. This adverse effect is attributed to the disruption of key biological processes, including impaired cell migration and compromised angiogenesis during the prolonged inflammatory phase. The observed divergence between univariate and multivariate results may be attributed to collinearity between low LCR and established risk factors such as malnutrition and systemic inflammation. Low LCR frequently coexists with hypoalbuminemia and high CA199 levels, which collectively contribute to increased surgical risk. By adjusting for these interrelated variables, multivariate analysis isolates the unique contribution of LCR, thereby revealing its distinct predictive role. This interpretation aligns with the immune-inflammation-nutrition composite scoring system proposed by Zhu et al[22], reinforcing the importance of integrating multidimensional biomarkers for improved risk prediction in complex clinical settings.
Compared with traditional biomarkers such as NLR and GPS, LCR offers a stronger theoretical foundation by inte
Although the data were collected between 2008 and 2013, it is important to note that LCR, as a baseline inflammatory and nutritional marker, reflects the patient’s fundamental immune-nutritional status prior to any intervention. While treatment strategies have evolved, the prognostic value of such host-related factors may still hold relevance, particularly in resource-limited settings or as a complement to emerging biomarkers. Nevertheless, we acknowledge that contemporary treatment paradigms might influence postoperative outcomes and long-term survival, and therefore, the predictive power of LCR should be further validated in cohorts receiving modern therapies. Future studies should prioritize prospective validation of LCR in patients receiving contemporary treatment protocols, including immunotherapy and targeted agents, to ascertain its relevance in the current therapeutic landscape.
The receiver operating characteristic analysis in this study demonstrates that the discriminatory capacity of LCR for predicting OS is comparable to that of the NLR and slightly lower than that of the GPS. The limited sample size may compromise the stability of model discrimination and could result in underestimation of the true predictive potential of all inflammatory markers, including LCR, NLR, and GPS. In cholangiocarcinoma, no single inflammatory marker may fully capture the complexity of prognostic information. Nevertheless, LCR offers a distinct advantage through its integrative nature, combining CRP - a marker of systemic inflammation - with lymphocyte count, which reflects immune function and nutritional status. This study has several limitations, including its retrospective design and the use of a single-center cohort, which may render the threshold susceptible to overfitting and potentially compromise its stability and broader applicability. Future multicenter prospective trials are warranted to validate the performance of LCR in comparison with other scoring systems, such as the GPS and PLR. With the advancement of cancer immunotherapy, particularly programmed cell death protein 1 inhibitors, further investigation into the potential of LCR as a predictive biomarker for immunotherapy response may reveal novel clinical applications.
LCR serves as a practical risk stratification tool in cholangiocarcinoma, enabling the preoperative identification of high-risk patients (those with low LCR) and facilitating enhanced perioperative nutritional support and anti-inflammatory management. It aids in optimizing surgical decision-making and tailoring individualized adjuvant treatment strategies for patients with low LCR. As a core component of the prognostic model, LCR significantly improves outcome prediction accuracy when integrated with TNM staging and R0 resection status.
This study demonstrates a significant association between low LCR and adverse prognosis, with the underlying mechanism potentially rooted in the reprogramming of the TME[24]. Cholangiocarcinoma is characterized by a highly immunosuppressive TME, wherein lymphopenia and CRP-mediated chronic inflammation - both reflected by low LCR - synergistically amplify this immunosuppressive state: (1) Immune cell dysfunction: Reduced lymphocyte counts directly impair the anti-tumor activity of CD8+ T cells and promote the expansion of regulatory T cells, thereby fostering an environment conducive to immune escape[7,25]; and (2) Inflammation-tumor positive feedback loop: Elevated CRP activates the nuclear factor-kappa B signaling pathway, leading to increased secretion of pro-inflammatory cytokines such as interleukin-6, which further suppress lymphocyte function and drive the proliferation of tumor-initiating cells[26-28].
This mechanism is indirectly supported by the findings of this study: The low LCR group exhibited a significantly higher rate of CA199 abnormalities, and CA199 has been well-established as positively correlated with the extent of immune suppression in the TME of cholangiocarcinoma[29]. Notably, the dynamic nature of LCR offers promising avenues for clinical intervention: (1) Preoperative immunonutrition support: Supplementing with ω-3 fatty acids may reduce CRP levels while increasing lymphocyte counts, thereby normalizing LCR[30]; (2) Targeting inflammatory pathways: Interleukin-6 inhibitors, such as tocilizumab, have the potential to disrupt the inflammation-tumor vicious cycle[31]; and (3) Combination immunotherapy: Programmed cell death protein 1 inhibitors demonstrate greater efficacy in tumors with high inflammatory burden, and low LCR may serve as a predictive biomarker for immunotherapy response[32].
LCR serves as a cost-effective and clinically practical biomarker, capable of independently predicting OS, the risk of SSI, and length of hospital stay in patients with resectable cholangiocarcinoma, thereby enabling more precise patient stratification.
We would like to express our sincere gratitude to the medical team and staff at Shanghai Xinhua Hospital for their exceptional care and support. This work would not have been possible without their expertise and dedication.
| 1. | Rodrigues PM, Olaizola P, Paiva NA, Olaizola I, Agirre-Lizaso A, Landa A, Bujanda L, Perugorria MJ, Banales JM. Pathogenesis of Cholangiocarcinoma. Annu Rev Pathol. 2021;16:433-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Warren EAK, Maithel SK. Molecular pathology for cholangiocarcinoma: a review of actionable genetic targets and their relevance to adjuvant & neoadjuvant therapy, staging, follow-up, and determination of minimal residual disease. Hepatobiliary Surg Nutr. 2024;13:29-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Munugala N, Maithel SK, Shroff RT. Novel biomarkers and the future of targeted therapies in cholangiocarcinoma: a narrative review. Hepatobiliary Surg Nutr. 2022;11:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Khorsandi SE, Dokal AD, Rajeeve V, Britton DJ, Illingworth MS, Heaton N, Cutillas PR. Computational Analysis of Cholangiocarcinoma Phosphoproteomes Identifies Patient-Specific Drug Targets. Cancer Res. 2021;81:5765-5776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Song J, Cui D, Wang J, Qin J, Wang S, Wang Z, Zhai X, Ma H, Ma D, Liu Y, Jin B, Liu Z. Overexpression of HMGA1 confers radioresistance by transactivating RAD51 in cholangiocarcinoma. Cell Death Discov. 2021;7:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Xia T, Li K, Niu N, Shao Y, Ding D, Thomas DL, Jing H, Fujiwara K, Hu H, Osipov A, Yuan C, Wolfgang CL, Thompson ED, Anders RA, He J, Mou Y, Murphy AG, Zheng L. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. J Hematol Oncol. 2022;15:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Liu X, Yin L, Shen S, Hou Y. Inflammation and cancer: paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023;10:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Yu W, Tu Y, Long Z, Liu J, Kong D, Peng J, Wu H, Zheng G, Zhao J, Chen Y, Liu R, Li W, Hai C. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxid Med Cell Longev. 2022;2022:2606928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Meng J, Yang J, Pan T, Qu X, Cui S. ZnO nanoparticles promote the malignant transformation of colorectal epithelial cells in APC(min/+) mice. Environ Int. 2022;158:106923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Villard C, Friis-Liby I, Rorsman F, Said K, Warnqvist A, Cornillet M, Kechagias S, Nyhlin N, Werner M, Janczewska I, Hagström T, Nilsson E, Bergquist A. Prospective surveillance for cholangiocarcinoma in unselected individuals with primary sclerosing cholangitis. J Hepatol. 2023;78:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Kano Y, Ishikawa T, Yamao K, Mizutani Y, Iida T, Uetsuki K, Yamamura T, Furukawa K, Nakamura M, Kawashima H. What is the appropriate method of pathological specimen collection for cholangiocarcinoma detection in primary sclerosing cholangitis? J Gastroenterol. 2024;59:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Mehla K, Hollingsworth MA. Inflammatory and immune effects on tumor progression. Trends Immunol. 2022;43:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale RG, Waitzberg D, Bischoff SC, Singer P. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40:4745-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 440] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 15. | Mikkelsen MK, Lindblom NAF, Dyhl-Polk A, Juhl CB, Johansen JS, Nielsen D. Systematic review and meta-analysis of C-reactive protein as a biomarker in breast cancer. Crit Rev Clin Lab Sci. 2022;59:480-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 16. | Noormohammadpour P, Hueniken K, Pienkowski M, Huang SH, Yuan B, Grant B, Yao C, Hope A, Tsai J, McPartlin A, Goldstein D, Hosni A, de Almeida JR, Grant RC, Liu G, Li Y. Differential prognostic association of systemic inflammatory biomarkers on survival outcomes in head and neck squamous cell carcinoma patients by human papillomavirus status. Neoplasia. 2025;66:101178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Golder AM, McMillan DC, Park JH, Mansouri D, Horgan PG, Roxburgh CS. The prognostic value of combined measures of the systemic inflammatory response in patients with colon cancer: an analysis of 1700 patients. Br J Cancer. 2021;124:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Ruan GT, Xie HL, Yuan KT, Lin SQ, Zhang HY, Liu CA, Shi JY, Ge YZ, Song MM, Hu CL, Zhang XW, Liu XY, Yang M, Wang KH, Zheng X, Chen Y, Hu W, Cong MH, Zhu LC, Deng L, Shi HP. Prognostic value of systemic inflammation and for patients with colorectal cancer cachexia. J Cachexia Sarcopenia Muscle. 2023;14:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 20. | Du Y, Li Y, Tan Z, Song J, Jiang Y, Liu S, Guo Y, Qiao Y, Zhu J, Li S, Li J. Prognostic value of combining preoperative immune-inflammatory-nutritional index and tumor biomarkers in gastric cancer patients undergoing radical resection. Front Nutr. 2025;12:1562202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Mitra A, Shahid A, Kumari S, Mukherjee T, Pramanick S, Mohanty S, Ansari MA, Adhikary K, Prabhakar PK, Kesari KK. Optimizing wound healing: insights from phytochemicals and advanced therapies. Inflammopharmacology. 2025;33:4009-4035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Zhu J, Wang D, Liu C, Huang R, Gao F, Feng X, Lan T, Li H, Wu H. Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: A multicenter study. Front Immunol. 2023;14:1165510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 23. | Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci Trends. 2021;14:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Yu X, Zhu L, Wang T, Chen J. Immune microenvironment of cholangiocarcinoma: Biological concepts and treatment strategies. Front Immunol. 2023;14:1037945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 25. | Gao Y, Guan J, Zhou R, Li Z, Chen T, Yang K, Huang R, Huang Y, Zheng S, Huang Z, Rong X. Single cell RNA sequencing reveals the reshaping effect of perfluorooctanoic acid on the intestinal microenvironment of mice. Ecotoxicol Environ Saf. 2025;302:118540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Feng JR, Li X, Han C, Chang Y, Fu Y, Feng GC, Lei Y, Li HY, Tang PM, Ji SR, Hou Y, Wu Y. C-Reactive Protein Induces Immunosuppression by Activating FcγR2B in Pulmonary Macrophages to Promote Lung Metastasis. Cancer Res. 2024;84:4184-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Socha MW, Malinowski B, Puk O, Wartęga M, Bernard P, Nowaczyk M, Wolski B, Wiciński M. C-reactive protein as a diagnostic and prognostic factor of endometrial cancer. Crit Rev Oncol Hematol. 2021;164:103419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Naqash AR, McCallen JD, Mi E, Iivanainen S, Marie MA, Gramenitskaya D, Clark J, Koivunen JP, Macherla S, Jonnalagadda S, Polsani S, Jiwani RA, Hafiz M, Muzaffar M, Brunetti L, Stroud CRG, Walker PR, Wang K, Chung Y, Ruppin E, Lee SH, Yang LV, Pinato DJ, Lee JS, Cortellini A. Increased interleukin-6/C-reactive protein levels are associated with the upregulation of the adenosine pathway and serve as potential markers of therapeutic resistance to immune checkpoint inhibitor-based therapies in non-small cell lung cancer. J Immunother Cancer. 2023;11:e007310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Ma D, Wei P, Liu H, Hao J, Chen Z, Chu Y, Li Z, Shi W, Yuan Z, Cheng Q, Gao J, Zhu J, Li Z. Multi-omics-driven discovery of invasive patterns and treatment strategies in CA19-9 positive intrahepatic cholangiocarcinoma. J Transl Med. 2024;22:1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Liang X, Luo L, Lu J, Xie X. Dietary Omega-3 fatty acids inversely associated with systemic inflammatory response index (SIRI): a population-based analysis of NHANES 2005-2018. Front Nutr. 2025;12:1614427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Sehgal PB. Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells. Cells. 2022;11:1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Herbst RS, Arkenau HT, Bendell J, Arrowsmith E, Wermke M, Soriano A, Penel N, Santana-Davila R, Bischoff H, Chau I, Mi G, Wang H, Rasmussen E, Ferry D, Chao BH, Paz-Ares L. Phase 1 Expansion Cohort of Ramucirumab Plus Pembrolizumab in Advanced Treatment-Naive NSCLC. J Thorac Oncol. 2021;16:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the ori