Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.113341
Revised: September 22, 2025
Accepted: October 13, 2025
Published online: December 15, 2025
Processing time: 111 Days and 10.2 Hours
Transarterial chemoembolization (TACE) is a widely accepted palliative therapy modality for unresectable hepatocellular carcinoma (HCC). Although it is rarely curative, complete radiological response can be achieved in selected patients, lea
Two patients with large, solitary HCCs (> 5 cm) were treated with DEB-TACE, and both achieved complete radiological response after two treatment sessions. Approximately 1 year after DEB-TACE, imaging demonstrated progressive peri
These two cases highlight the potential for complete remission and long-term survival in selected patients with large HCC following DEB-TACE. The appea
Core Tip: Tumoral calcification following transarterial chemoembolization (TACE) for hepatocellular carcinoma is a rare imaging finding, and its clinical significance remains unclear. This case report describes 2 patients with large hepatocellular carcinoma who developed complete radiological response after drug-eluting beads TACE, followed by progressive peripheral calcification and sustained disease-free survival for 6 years. The notable association between post-TACE calcification and long-term remission raises the possibility that calcification may serve as a late imaging biomarker of effective tumor necrosis and durable treatment response.
- Citation: Alharbi SR. Tumor calcification and sustained complete response after chemoembolization in hepatocellular carcinoma: Two case reports and review of literature. World J Gastrointest Oncol 2025; 17(12): 113341
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/113341.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.113341
Hepatocellular carcinoma (HCC) is one of the most prevalent solid tumors and the fourth-leading cause of cancer-related mortality worldwide[1-4]. Surgical resection, liver transplantation, and image-guided ablation remain the only widely accepted potentially curative options[5-8]. However, the majority of patients present at advanced stages or with impaired hepatic reserve, making them ineligible for these treatments[9-11].
For such patients transarterial chemoembolization (TACE), transarterial radioembolization, and systemic therapies are recommended, providing palliative benefit and improved survival[12,13]. Among these TACE is the most frequently employed locoregional therapy for unresectable HCC[14,15]. Its safety and efficacy have been validated in multiple randomized controlled trials, and it is endorsed by major clinical guidelines as the standard first-line treatment for intermediate-stage disease[15-17]. In routine practice, however, TACE is applied across the entire disease spectrum, from very early to advanced stages[18-20]. Outcomes remain heterogeneous, largely influenced by tumor burden, liver function, and procedural technique with reported median survivals ranging from 13 to 43 months[20-22].
The Barcelona Clinic Liver Cancer (BCLC) staging system is the most widely adopted framework for guiding HCC management, integrating tumor burden, liver function, and performance status into evidence-based treatment recom
Post-treatment tumoral calcification is a rare phenomenon that has been reported following HCC locoregional therapies. It has been described after radioembolization and very rarely after TACE. In conventional TACE, however, the detection of calcification is often obscured by lipiodol deposition[24-27]. To my knowledge progressive calcification following drug-eluting beads TACE (DEB-TACE), particularly in association with durable remission, has not been previously reported. Herein, I report 2 patients with large solitary HCCs who underwent DEB-TACE, achieved complete response (CR), and subsequently developed progressive peripheral calcification with sustained remission over 6 years.
Case 1: A 74-year-old male presented with a liver mass evident on abdominal computed tomography (CT).
Case 2: A 71-year-old male patient presented with a liver mass evident on abdominal magnetic resonance imaging (MRI).
Case 1 and Case 2 were asymptomatic and both underwent imaging as screening of high risk of HCC in patient liver cirrhosis.
Case 1: The patient had a known case of liver cirrhosis secondary to nonalcoholic steatohepatitis.
Case 2: The patient had a known case of liver cirrhosis secondary to hepatitis C virus.
Case 1 and Case 2 were unremarkable.
General and abdominal examinations were unremarkable for both cases.
Case 1: Liver function test results, complete blood count, and coagulation profile were all within normal limits apart from a low count of platelets (70/µL). Seum alpha fetoprotein was within normal limits.
Case 2: Complete blood count, coagulation profile, and liver function test results were all within normal limits. Seum alpha fetoprotein was elevated (420 ng/mL).
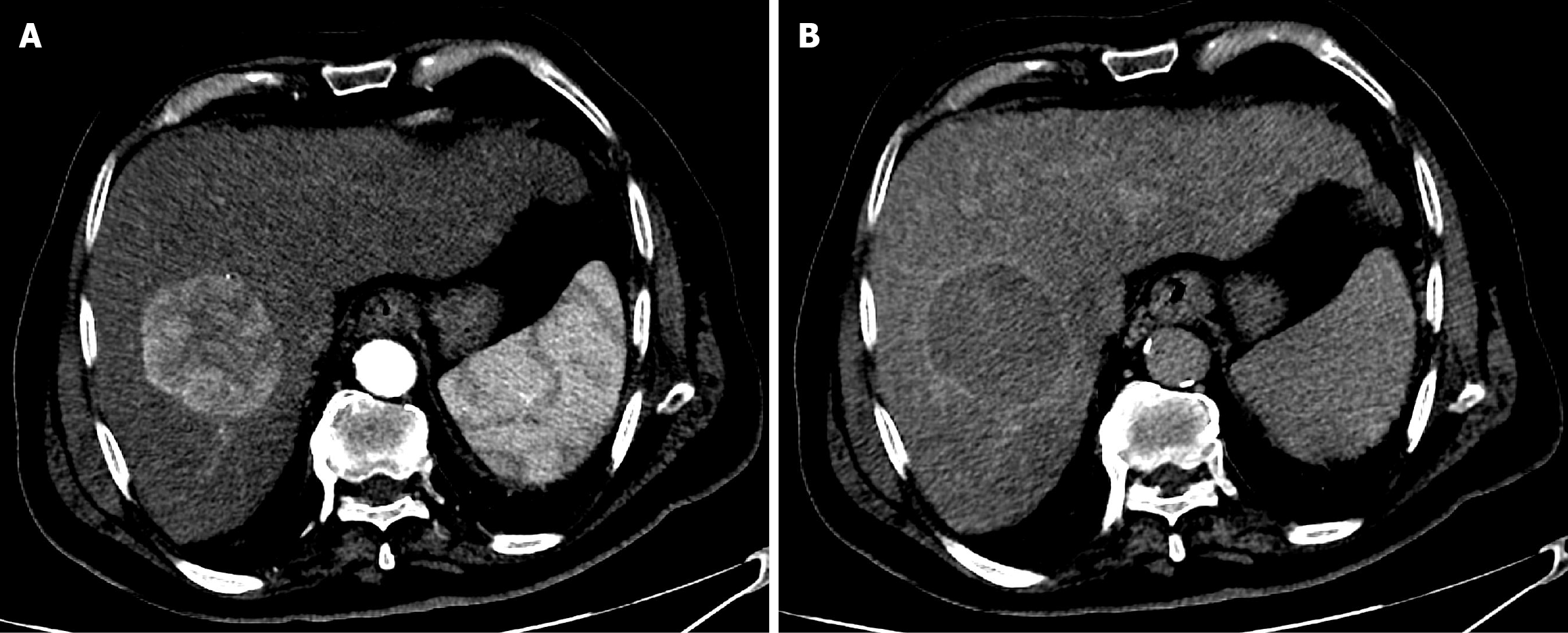
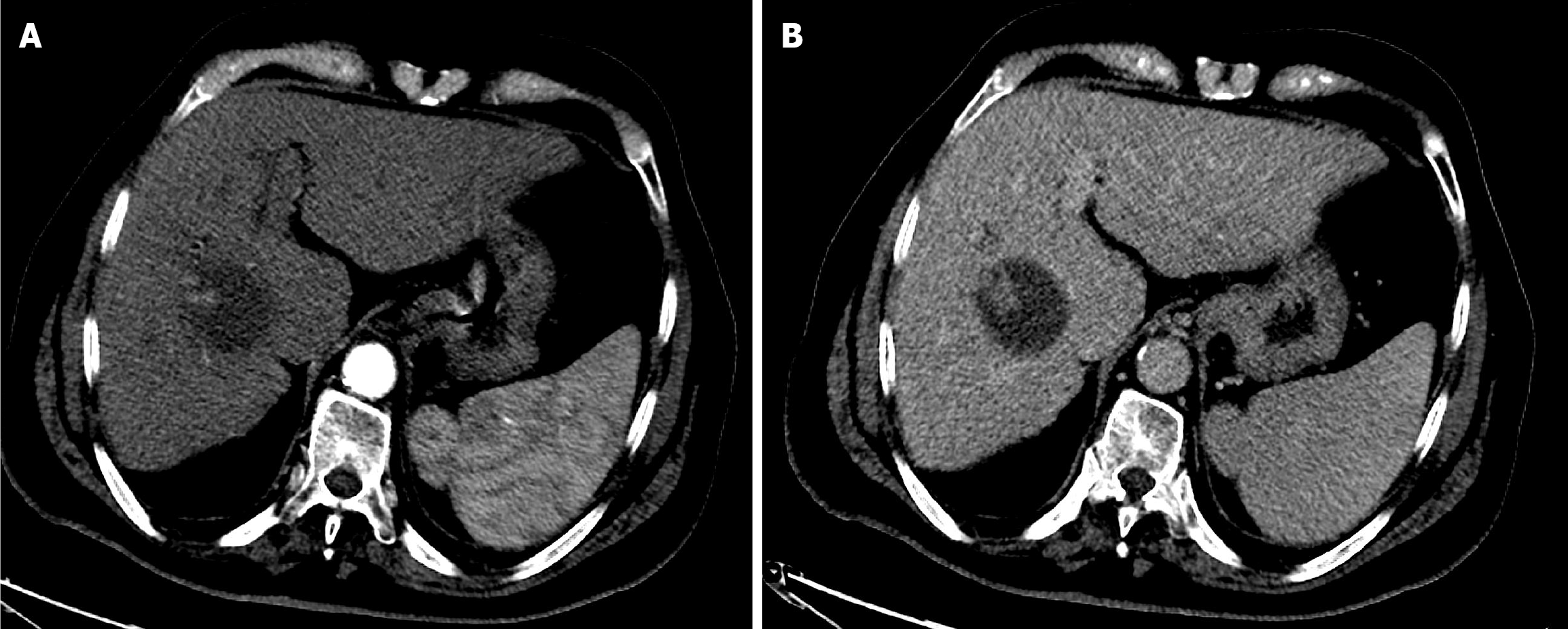
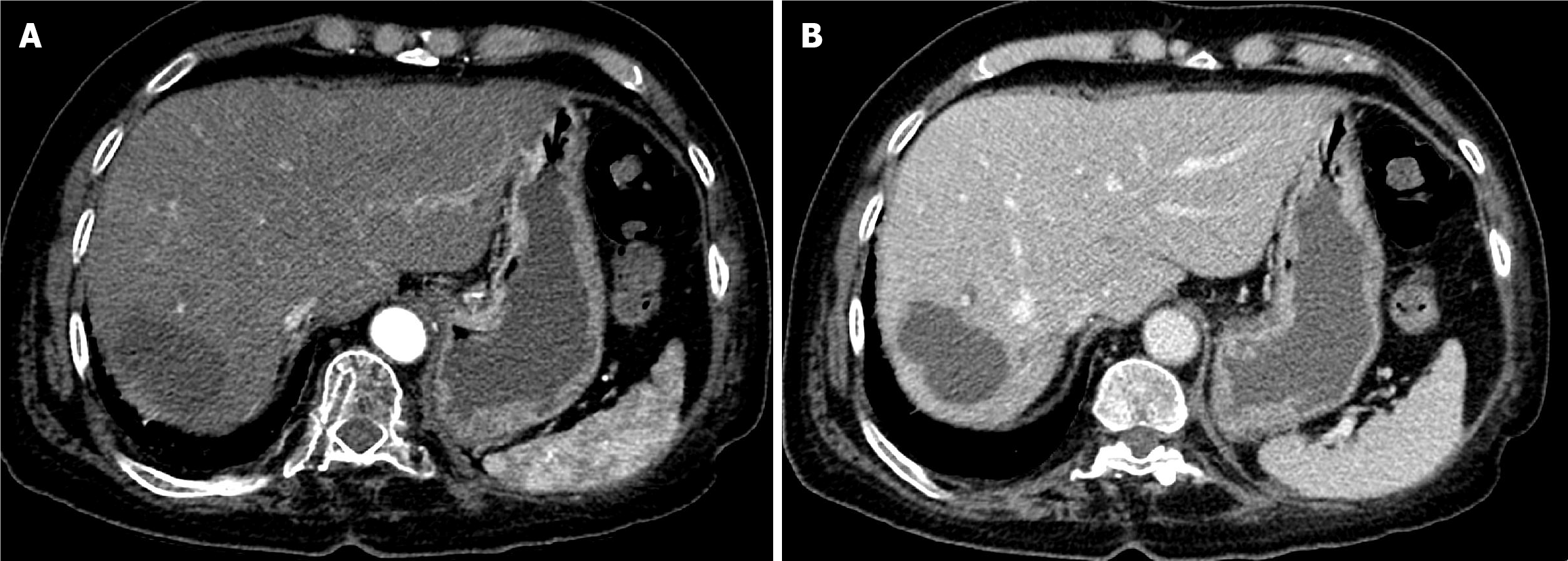
Case 1: The patient underwent CT with intravenous contrast. It showed a 6.5 cm segment 8 hepatic mass. The mass showed well defined delineate intense arterial phase enhancement with subsequent washout in the portal venous and delayed phases within the peripheral capsule. Findings revealed a liver imaging and report and data system (LI-RADS) category 5 lesion (Figure 1).
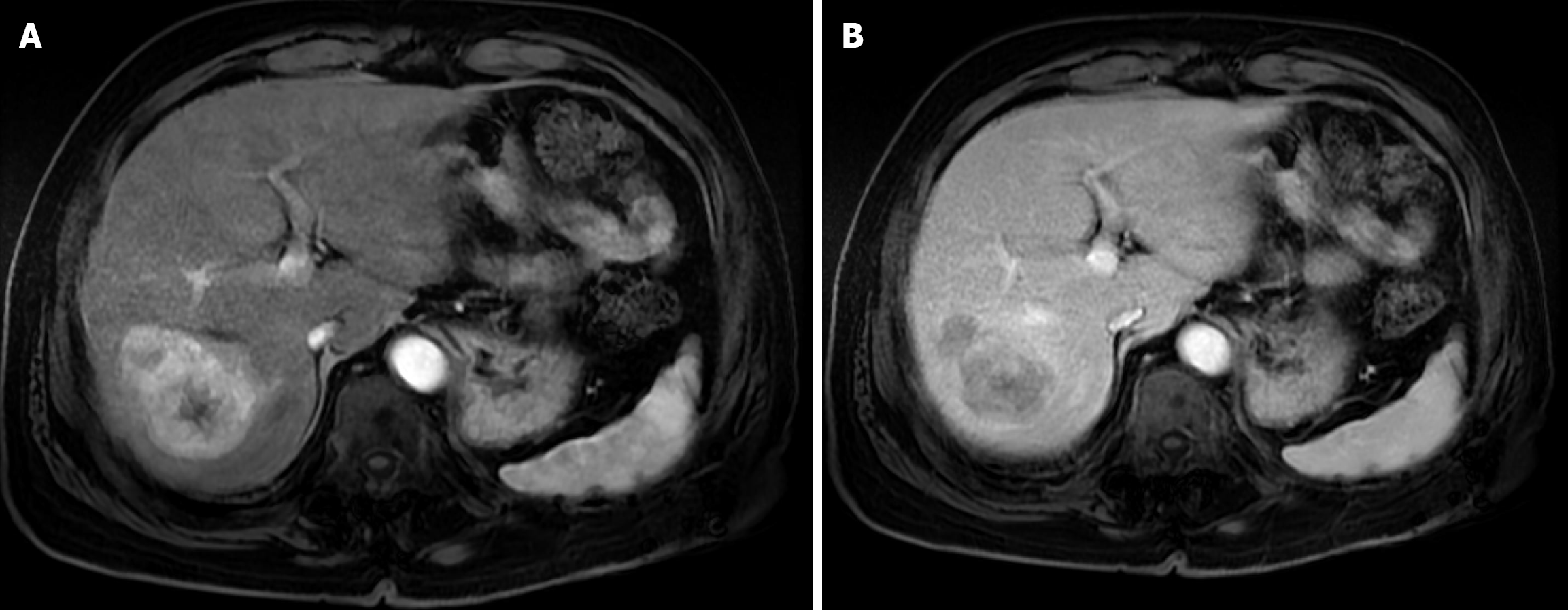
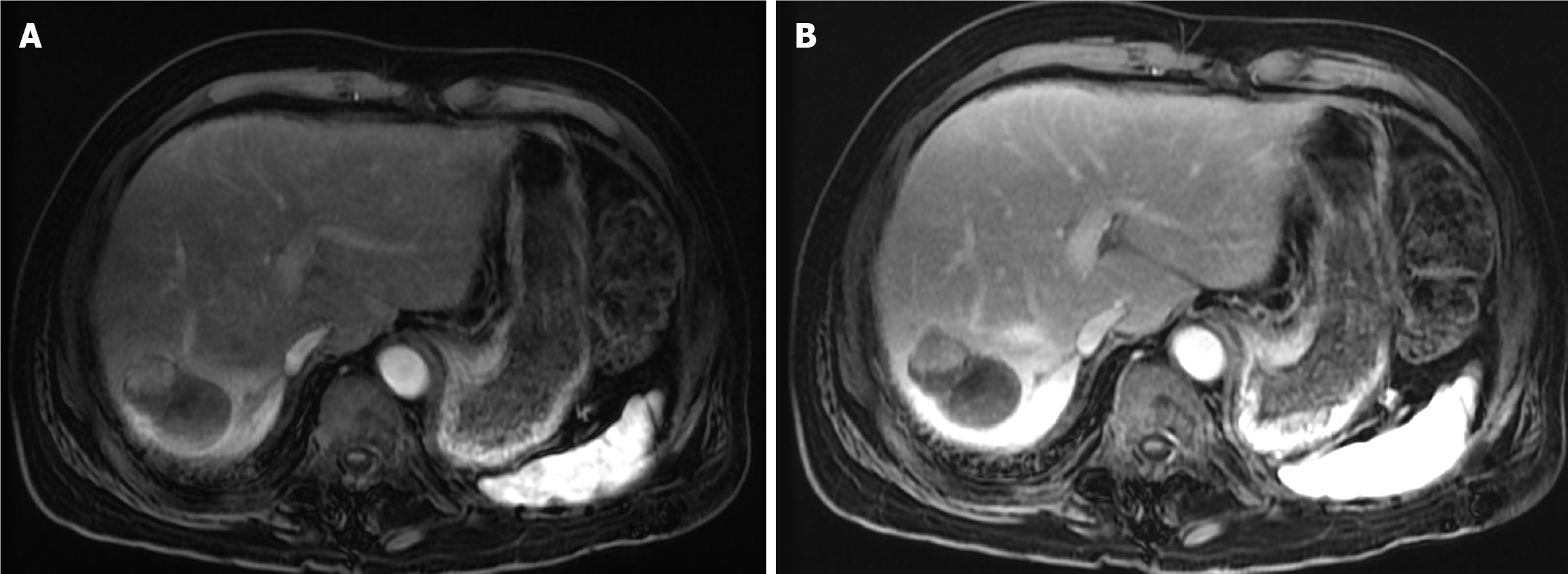
Case 2: The patient underwent abdominal MRI. It showed a 6 cm segment 7 hepatic mass. It showed intense arterial phase enhancement with subsequent washout in the portal venous and delayed phases. Findings revealed an LI-RADS category 5 lesion (Figure 2).
A case of large solitary HCC with preserved liver function and no vascular invasion or extrahepatic metastasis (early stage).
A case of large solitary HCC with preserved liver function and no vascular invasion or extrahepatic metastasis (early stage).
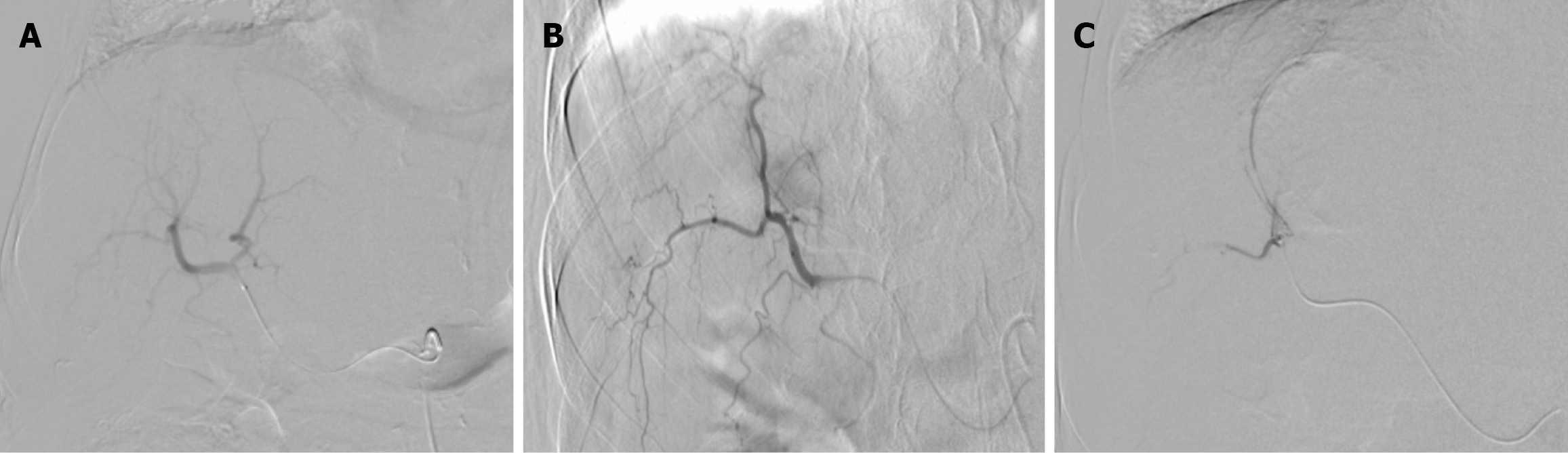
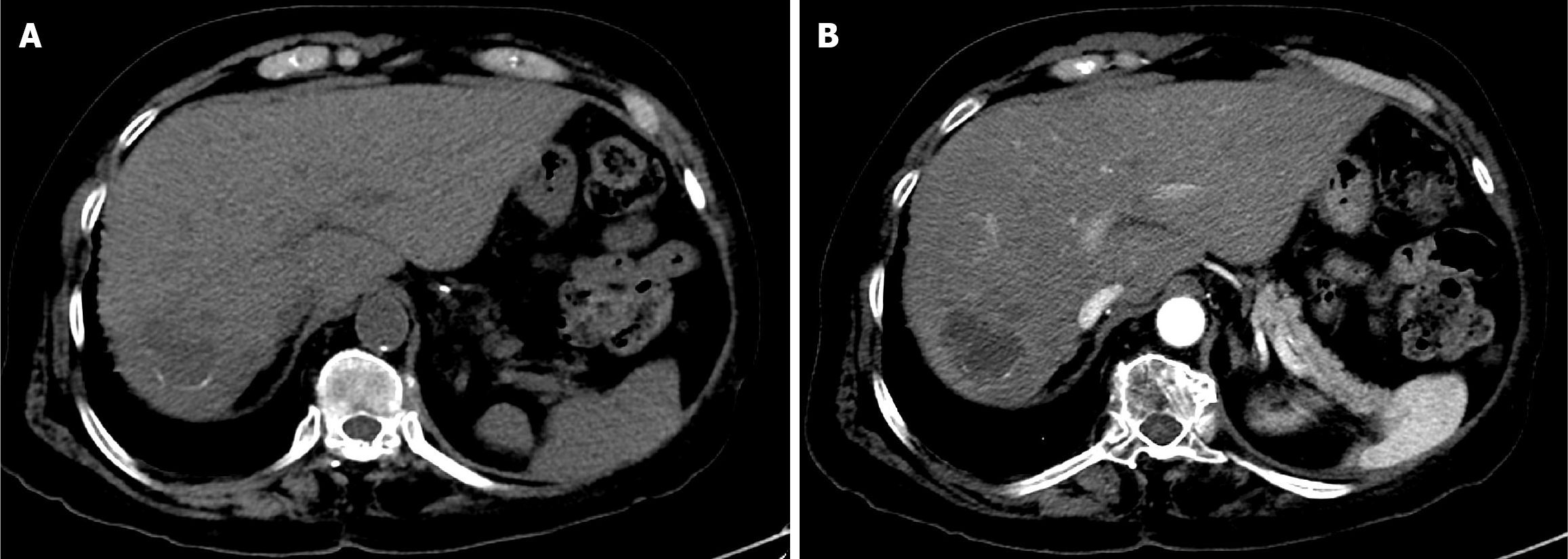
TACE, as a bridging and downstaging therapy prior to liver transplantation, was recommended after discussion with the HCC tumor board. The first session of DEB-TACE was performed using 150 mg doxorubicin loaded into two vials of 100-300 µm drug-eluting microspheres (DC Bead). Due to a partial response (PR), a second DEB-TACE session was performed using 75 mg doxorubicin loaded into one vial of 100-300 µm drug-eluting microspheres (Figure 3).
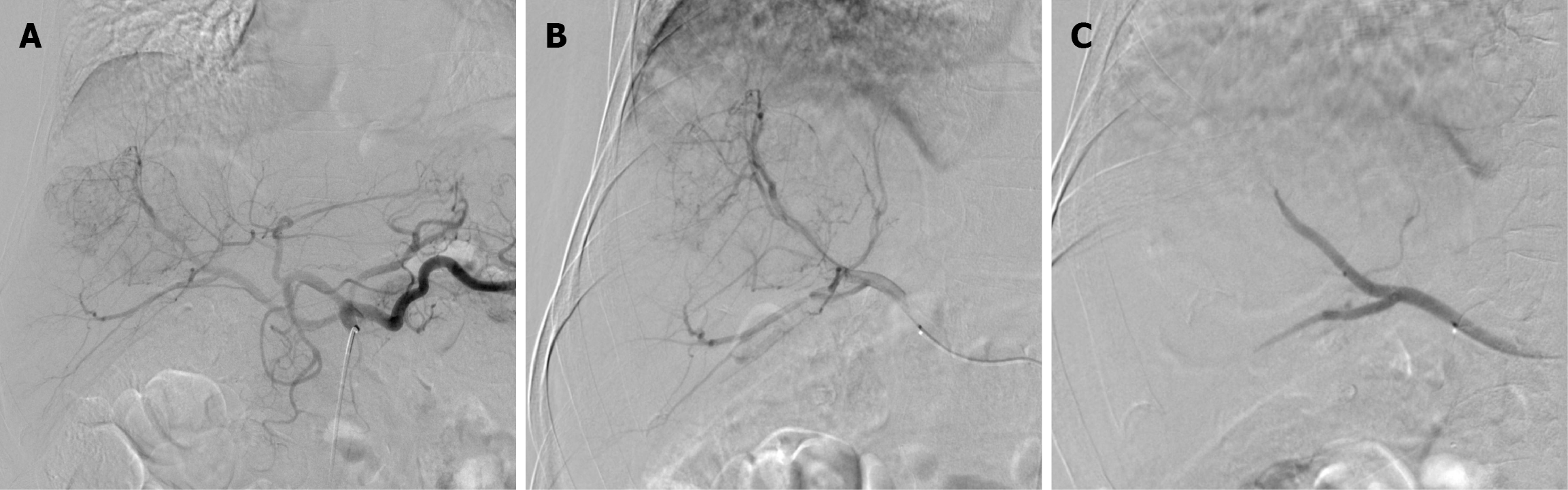
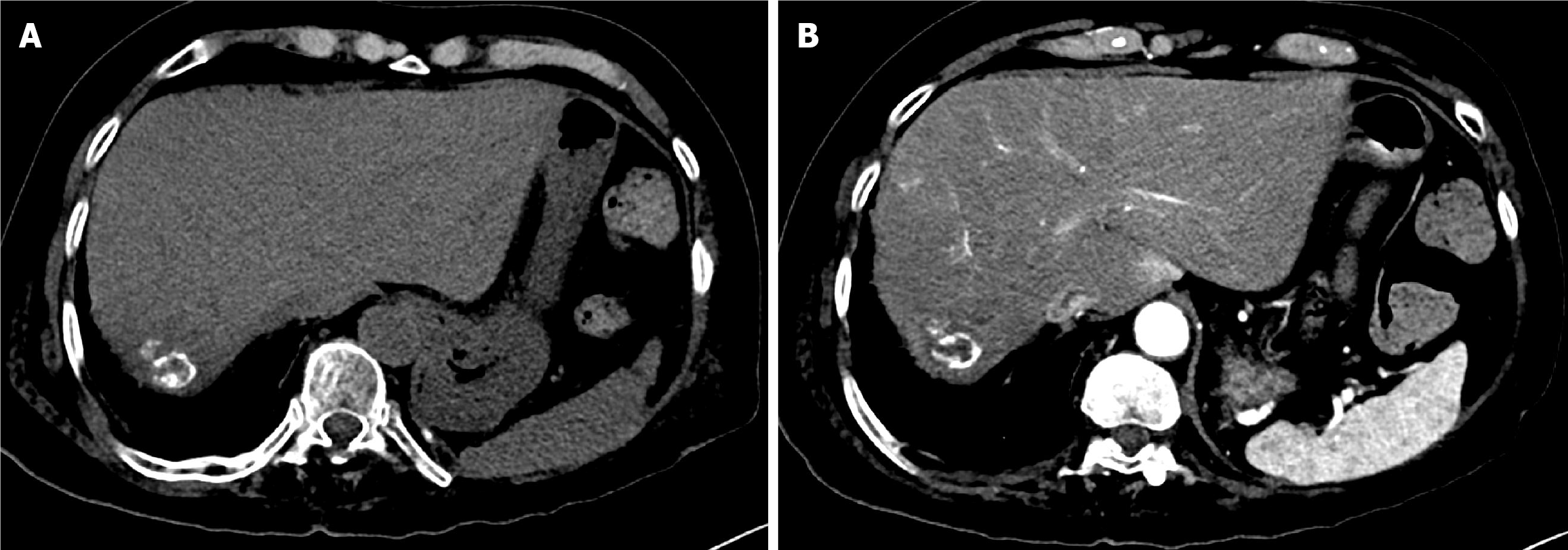
After discussion with the HCC tumor board, TACE was recommended as a bridging and downstaging therapy prior to liver transplantation. The first session of DEB-TACE was performed using 150 mg doxorubicin loaded into two vials of 100-300 µm drug-eluting microspheres (DC Bead). Due to a PR, a second DEB-TACE session was performed using 75 mg doxorubicin loaded into one vial of 100-300 µm drug-eluting microspheres (Figure 4).
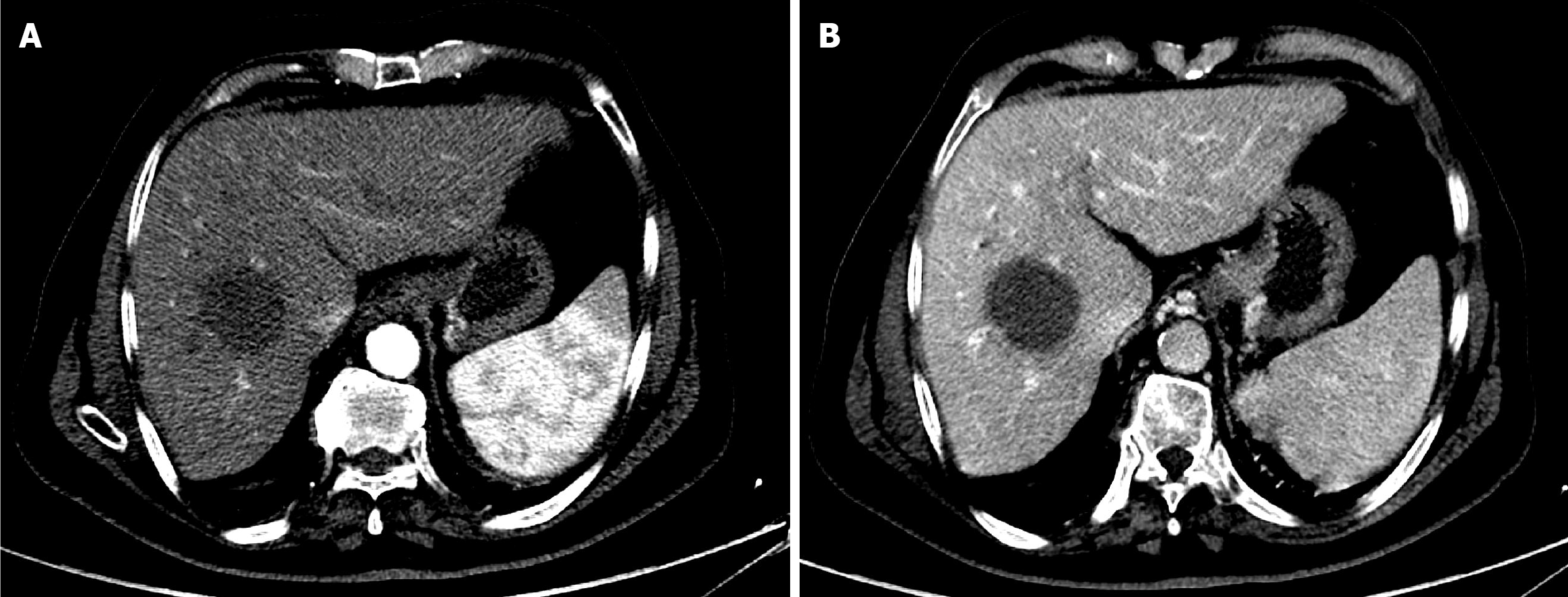
The first follow-up CT after 6 weeks of TACE revealed PR (Figure 5). The second follow-up CT after the second session revealed CR (Figure 6). The 1-year follow-up CT demonstrated CR with peripheral faint hyperdensities, suggesting early calcification (Figure 7). The patient was deemed ineligible for liver transplantation due to advanced age. He was actively monitored with clinical, laboratory, and imaging examinations. The 6-year follow-up CT demonstrated sustained CR with progressive peripheral hyperdensities encircling the tumor, consistent with concentric calcifications (Figure 8).
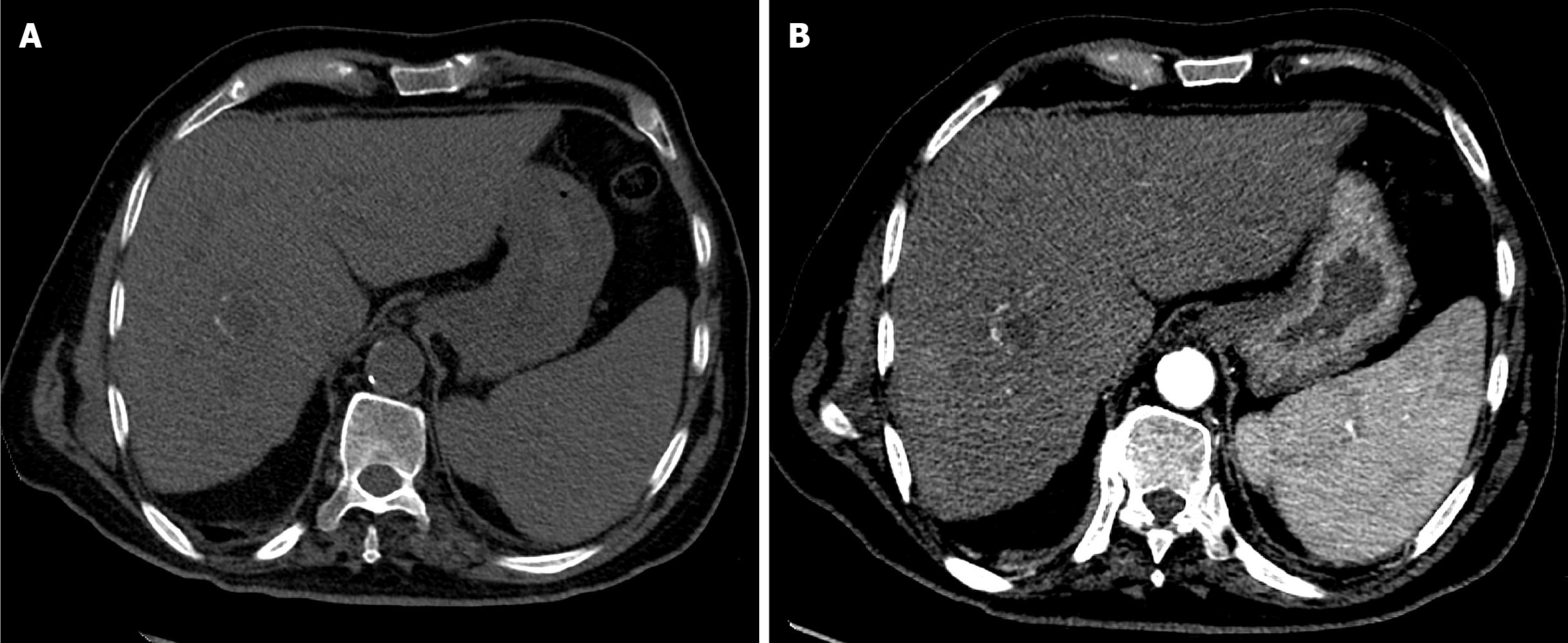
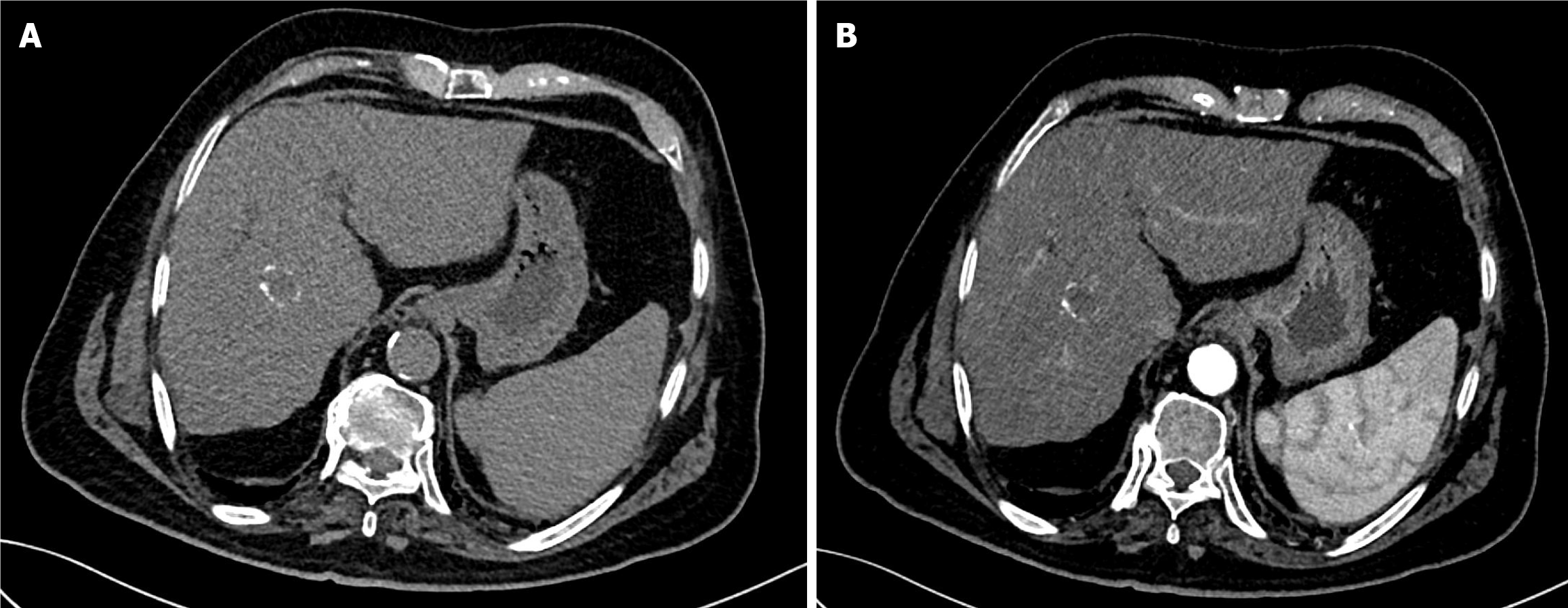
The first follow-up MRI, performed 8 weeks after TACE, demonstrated PR (Figure 9). The subsequent CT after the second TACE session revealed CR (Figure 10). The 1-year follow-up CT demonstrated CR with peripheral faint hyperdensities, consistent with early calcification (Figure 11). The patient declined liver transplantation as the tumor had achieved CR. He was enrolled in routine clinical, laboratory, and imaging follow-up. The 6-year follow-up CT demonstrated sustained CR with progressive peripheral concentric calcifications (Figure 12). Serum alpha-fetoprotein normalized after the second TACE session and remained within normal limits.
Although traditionally considered a palliative option, TACE can achieve curative intent in carefully selected patients. A prospective cohort demonstrated durable management of early-stage HCC using super-selective TACE[28], and several retrospective series have reported overall survival comparable with radiofrequency ablation in patients within the Milan criteria when ablation or surgery is contraindicated[29,30].
The optimal therapeutic objective of TACE is complete tumor necrosis[31,32]. Radiological CR, which is the strongest predictor of long-term survival[33,34], can even predict outcomes following subsequent curative treatments, such as transplantation when TACE is used as a bridge therapy[35]. Imaging characteristics of HCC can predict the likelihood of response: Well-circumscribed capsulated hypervascular HCCs ≤ 5 cm supplied by a dominant arterial feeder respond best, whereas ill-defined, infiltrative, hypovascular tumors > 5 cm seldom demonstrate CR[36,37].
Assessment of HCC treatment response using response evaluation criteria in solid tumor (RECIST) based on tumor shrinkage often underestimate response in HCC as they disregard tumor necrosis[31,32]. The modified RECIST (mRECIST) criteria based on contrast-enhanced CT or MRI to measure viable, enhancing tumor tissue are now the most widely accepted method and are endorsed by international guidelines[18,31]. The response is categorized as CR defined by the disappearance of all enhancing lesions or PR defined as at least a 30% reduction in enhancing tumor diameter[38]. Multiple studies show that objective response by mRECIST, particularly CR, is strongly associated with improved overall survival, making it a reliable prognostic factor and validated endpoint in HCC trials[34,35].
The prognosis, however, depends not only on controlling the tumor burden but also on preserving underlying liver function. Achieving high objective response rates and minimizing collateral hepatic injury are equally important for improving survival and maintaining quality of life[39-42]. Super-selective DEB-TACE fulfils both aims by delivering higher intratumoral drug concentration, enhancing better local control, and reducing systemic toxicity and non-target embolization of the healthy liver[43,44].
Post-treatment tumor calcification has been described in several malignancies, including colorectal liver metastases and ovarian cancers in which it often reflects dystrophic calcification of necrotic tissue and has been associated with favorable prognosis[45].
In HCC calcification following locoregional therapy is exceedingly rare. Calcification after transarterial radioembolization has been reported and was associated with better prognosis, suggesting its potential role as an early surrogate marker of CR[26]. Conversely, only a single case report has described dystrophic calcification after DEB-TACE, likely related to treatment-induced tissue injury[25]. In my cases DEB-TACE likely induced extensive coagulative necrosis leading to peripheral calcification that was unmasked by the absence of lipiodol.
Although the exact pathogenesis remains uncertain, tumoral calcification after TACE is generally considered a manifestation of dystrophic calcification within necrotic tissue rather than a process specific to DEB-TACE. Necrotic cells release phosphate ions that combine with calcium salts in an alkaline environment, and in the absence of normal blood supply and cellular inhibitory mechanisms, these salts precipitate and deposit. This mechanism explains the peripheral sickle-like or ring-like calcification pattern observed that may serve as a surrogate marker of effective therapy as demonstrated in my cases[25,46,47].
The association between calcification and long-term remission in my cases suggests that calcification may represent a late imaging marker of effective tumor necrosis. However, both patients had already achieved CR based on mRECIST prior to the appearance of calcification, and CR remains the most reliable predictor of outcome[33,34]. Thus, calcification should be considered a hypothesis-generating observation rather than a validated biomarker, underscoring the need for prospective studies to clarify whether it has independent prognostic significance.
This report has some limitations. It describes only 2 highly selected patients with favorable prognostic features, limiting its generalizability. As a case report it cannot establish causality between calcification and outcome. Never
While TACE is generally regarded as a palliative option, my cases demonstrated that with appropriate patient selection and technique CR and durable remission are achievable even in large HCCs. The appearance of peripheral tumoral calcification following DEB-TACE may represent a late imaging marker of effective tumor necrosis and durable disease control although this observation remains hypothesis-generating and requires validation in larger cohorts. These findings suggest that calcification after DEB-TACE could serve as a potential imaging biomarker of treatment success. Further prospective studies are warranted to clarify its prognostic value and validate its clinical significance.
The author thanks the interventional radiology nursing and technologist team at King Saud University Medical City for their assistance during patient procedures and follow-up care.
| 1. | Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993-3036. [PubMed] |
| 2. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3367] [Article Influence: 481.0] [Reference Citation Analysis (45)] |
| 3. | Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, Tsourouflis G, Garmpi A, Delladetsima I, Kontzoglou K, Kouraklis G. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282-5294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (4)] |
| 4. | Hatanaka T, Yata Y, Naganuma A, Kakizaki S. Treatment Strategy for Intermediate-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization, Systemic Therapy, and Conversion Therapy. Cancers (Basel). 2023;15:1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (3)] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6418] [Article Influence: 802.3] [Reference Citation Analysis (9)] |
| 7. | Agnello F, Salvaggio G, Cabibbo G, Maida M, Lagalla R, Midiri M, Brancatelli G. Imaging appearance of treated hepatocellular carcinoma. World J Hepatol. 2013;5:417-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Yacoub JH, Hsu CC, Fishbein TM, Mauro D, Moon A, He AR, Bashir MR, Burke LMB. Therapies for hepatocellular carcinoma: overview, clinical indications, and comparative outcome evaluation-part one: curative intention. Abdom Radiol (NY). 2021;46:3528-3539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Sun Z, Shi Z, Xin Y, Zhao S, Jiang H, Li J, Li J, Jiang H. Contrast-Enhanced CT Imaging Features Combined with Clinical Factors to Predict the Efficacy and Prognosis for Transarterial Chemoembolization of Hepatocellular Carcinoma. Acad Radiol. 2023;30 Suppl 1:S81-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3435] [Article Influence: 429.4] [Reference Citation Analysis (3)] |
| 11. | Park Y, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Yeon JE, Byun KS, Kim HS, Kim JH, Kim SU. Feasibility of dynamic risk assessment for patients with repeated trans-arterial chemoembolization for hepatocellular carcinoma. BMC Cancer. 2019;19:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol. 2023;11:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Yacoub JH, Mauro D, Moon A, He AR, Bashir MR, Hsu CC, Fishbein TM, Burke LMB. Therapies for hepatocellular carcinoma: overview, clinical indications, and comparative outcome evaluation. Part two: noncurative intention. Abdom Radiol (NY). 2021;46:3540-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Alan AM, Alan O, Asadov R, Demirtas CO, Kani HT, Yumuk PF, Ozdogan OC, Baltacioglu F, Gunduz F. Evaluation of the effectiveness of drug-eluting transarterial chemoebolization in hepatocellular carcinoma. Hepatol Forum. 2023;4:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:8165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 16. | Liu YS, Lin CY, Chuang MT, Lin CY, Tsai YS, Wang CK, Ou MC. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Wang ZX, Wang EX, Bai W, Xia DD, Mu W, Li J, Yang QY, Huang M, Xu GH, Sun JH, Li HL, Zhao H, Wu JB, Yang SF, Li JP, Li ZX, Zhang CQ, Zhu XL, Zheng YB, Wang QH, Li J, Yuan J, Li XM, Niu J, Yin ZX, Xia JL, Fan DM, Han GH; On Behalf Of China Hcc-Tace Study Group. Validation and evaluation of clinical prediction systems for first and repeated transarterial chemoembolization in unresectable hepatocellular carcinoma: A Chinese multicenter retrospective study. World J Gastroenterol. 2020;26:657-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 844] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 19. | Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int. 2016;36 Suppl 1:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Wang S, Zhang X, Chen Q, Jin ZC, Lu J, Guo J. A Novel Neutrophil-to-Lymphocyte Ratio and Sarcopenia Based TACE-Predict Model of Hepatocellular Carcinoma Patients. J Hepatocell Carcinoma. 2023;10:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 1013] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 22. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3122] [Article Influence: 780.5] [Reference Citation Analysis (61)] |
| 24. | Patnana M, Menias CO, Pickhardt PJ, Elshikh M, Javadi S, Gaballah A, Shaaban AM, Korivi BR, Garg N, Elsayes KM. Liver Calcifications and Calcified Liver Masses: Pattern Recognition Approach on CT. AJR Am J Roentgenol. 2018;211:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Kumar V, Shah M, Gala D, Singh MK, Jeanty H, Thomas R, Forlemu AN, Gayam VR, Etienne D. Hepatic Dystrophic Calcification Secondary to Transarterial Chemoembolization: Case Report and Review of Literature. Cureus. 2023;15:e35765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Kim HC, Joo I, Lee M, Kim YJ, Paeng JC, Chung JW. Radioembolization-induced Tumor Calcifications as a Surrogate Marker of Tumor Response in Patients With Hepatocellular Carcinoma. Anticancer Res. 2020;40:4191-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Dioguardi Burgio M, Sartoris R, Libotean C, Zappa M, Sibert A, Vilgrain V, Ronot M. Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging. 2019;19:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Zane KE, Nagib PB, Jalil S, Mumtaz K, Makary MS. Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol. 2022;14:885-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 29. | Lee M, Shin HP. Efficacy of Transarterial Chemoembolization (TACE) for Early-Stage Hepatocellular Carcinoma. Medicina (Kaunas). 2023;59:2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Hashem E, Sait S, Thomas DN, Watson C, Moeen S, Peddu P. Transarterial chemoembolisation for very early and early stage hepatocellular carcinoma: single-centre experience. Clin Radiol. 2023;78:e113-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Nicolini D, Agostini A, Montalti R, Mocchegiani F, Mincarelli C, Mandolesi A, Robertson NL, Candelari R, Giovagnoni A, Vivarelli M. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol. 2017;23:3690-3701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Müller L, Stoehr F, Mähringer-Kunz A, Hahn F, Weinmann A, Kloeckner R. Current Strategies to Identify Patients That Will Benefit from TACE Treatment and Future Directions a Practical Step-by-Step Guide. J Hepatocell Carcinoma. 2021;8:403-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Yun BY, Lee HW, Min IK, Kim SU, Park JY, Kim DY, Ahn SH, Kim BK. Prognosis of Early-Stage Hepatocellular Carcinoma: Comparison between Trans-Arterial Chemoembolization and Radiofrequency Ablation. Cancers (Basel). 2020;12:2527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, Park SI, Won JY, Lee DY, Han KH. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Lei J, Zhong J, Luo Y, Yan L, Zhu J, Wang W, Li B, Wen T, Yang J; Liver Surgery Group. Response to transarterial chemoembolization may serve as selection criteria for hepatocellular carcinoma liver transplantation. Oncotarget. 2017;8:91328-91342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Zhang W, Xu AH, Wang W, Wu YH, Sun QL, Shu C. Radiological appearance of hepatocellular carcinoma predicts the response to trans-arterial chemoembolization in patients undergoing liver transplantation. BMC Cancer. 2019;19:1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Alharbi SR. Sultan's Score: A Novel Predictive Score to Predict Complete Response Following Drug-Eluting Bead Chemoembolization. Cureus. 2025;17:e76822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, Charlton MR. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant. 2014;14:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | An H, Bhatia I, Cao F, Huang Z, Xie C. CT texture analysis in predicting treatment response and survival in patients with hepatocellular carcinoma treated with transarterial chemoembolization using random forest models. BMC Cancer. 2023;23:201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 40. | D'Avola D, Granito A, Torre-Aláez M, Piscaglia F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. 2022;76:1185-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 41. | Yin C, Armstrong S, Shin R, Geng X, Wang H, Satoskar RS, Fishbein T, Smith C, Banovac F, Kim AY, He AR. Bridging and downstaging with TACE in early and intermediate stage hepatocellular carcinoma: Predictors of receiving a liver transplant. Ann Gastroenterol Surg. 2023;7:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Wang T, Du YN, Sun J, Song H, Jiang Y, Liu F, Lv X. Drug-eluting bead transarterial chemoembolization could improve the hepatic hemodynamics of patients with unresectable hepatocellular carcinoma: a retrospective cohort study. J Gastrointest Oncol. 2023;14:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 44. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1721] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 45. | Zhou Y, Zhang J, Dan Pu, Bi F, Chen Y, Liu J, Li Q, Gou H, Wu B, Qiu M. Tumor calcification as a prognostic factor in cetuximab plus chemotherapy-treated patients with metastatic colorectal cancer. Anticancer Drugs. 2019;30:195-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Zimmermann A. Secondary Alterations of Hepatocellular Carcinoma. In: Tumors and Tumor-Like Lesions of the Hepatobiliary Tract. Cham: Springer, 2017: 121-149. [DOI] [Full Text] |
| 47. | Ortega MA, De Leon-Oliva D, Gimeno-Longas MJ, Boaru DL, Fraile-Martinez O, García-Montero C, de Castro AV, Barrena-Blázquez S, López-González L, Amor S, García-Honduvilla N, Buján J, Guijarro LG, Castillo-Ruiz E, Álvarez-Mon MÁ, Albillos A, Álvarez-Mon M, Diaz R, Saez MA. Vascular Calcification: Molecular Networking, Pathological Implications and Translational Opportunities. Biomolecules. 2024;14:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/