Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.113198
Revised: September 18, 2025
Accepted: October 30, 2025
Published online: December 15, 2025
Processing time: 114 Days and 7.3 Hours
Pancreatic neuroendocrine microtumors (PNEMTs) are small (< 5 mm), non-functioning, well-differentiated neuroendocrine neoplasms. Although they are rare, they are not invariably benign. PNEMTs are typically discovered inciden
To investigate the prevalence and histopathological characteristics of PNEMTs in elderly individuals by analyzing cadaveric pancreatic tissues.
We conducted a retrospective analysis of 85 pancreatic specimens (age range: 58-109 years) obtained from cadavers for anatomical education and research at the Department of Life Dentistry, Nippon Dental University. Paraffin sections of the pancreatic head, body, and tail were prepared for histological and immunohistochemical analysis.
Five cases with PNEMTs (5/85, 5.9%; male, n = 33; female, n = 52; mean age: 85.8 ± 12.1 years) were identified. The tumors were solitary, well circumscribed, and located within the pancreatic parenchyma (body: n = 4; tail: n = 1), and all were < 5 mm (range: 0.54-2.20 mm) in size. All tumors showed strong chromogranin A and synaptophysin positivity, and were predominantly glucagon (GLU)-positive. Ki-67 immunostaining indicated minimal proliferative activity; therefore, these tumors were considered non-functioning, GLU-producing, well-differentiated grade 1 PNEMTs.
Small, predominantly low-grade, GLU-secreting PNEMTs were present in 5.9% of elderly individuals, highlighting the prevalence of subclinical PNEMTs and the need for careful follow-up.
Core Tip: This cadaveric study identified pancreatic neuroendocrine microtumors (PNEMTs) in 5.9% of predominantly elderly individuals (mean age: 88.0 ± 9.3 years) that were small (≤ 2.20 mm), secreted glucagon, had minimal proliferative activity (Ki-67 near negative), and had well-differentiated grade 1 lesions. These findings emphasize that biologically indolent PNEMTs are significantly more prevalent in the aged population than are clinically recognized. Given that tumor enlargement may be associated with malignant potential, early detection is of critical clinical importance for managing incidental lesions.
- Citation: Yang T, Ren K, Chen XQ, Toriumi T, Natsuyama Y, Li J, Sukeda A, Nagao T, Yi SQ. Pancreatic neuroendocrine microtumors in the elderly: A retrospective study using cadaveric pancreatic tissue. World J Gastrointest Oncol 2025; 17(12): 113198
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/113198.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.113198
Non-functioning pancreatic neuroendocrine neoplasms measuring < 5 mm were termed pancreatic neuroendocrine microadenomas and are considered benign entities[1,2]. However, in the 2022 WHO classification, the term was changed to pancreatic neuroendocrine microtumors (PNEMTs), which are now recognized as potentially malignant regardless of size, as they may exhibit lymph node metastases or undergo high-grade transformation[3-5]. PNEMTs are also regarded as an early developmental stage within the neoplastic phase of pancreatic neuroendocrine neoplasms[6,7].
Typically, most PNEMTs are undetectable using current imaging modalities owing to their small size and lack of significant clinical symptoms. Consequently, they are almost exclusively discovered incidentally during pathological examinations or in pancreatic resection specimens removed for other diseases[7-9]. Histologically, PNEMTs are typically well circumscribed and exhibit an organoid architecture characterized by nests and trabeculae of uniform cells. Tumor cells typically have round to oval centrally located nuclei. Significant atypia and mitotic figures are exceedingly uncommon[10,11]. Immunohistochemically, these cells express markers of endocrine differentiation and may produce a single type of hormonal peptide, the most frequently observed being glucagon (GLU)[5,11].
PNEMTs have been predominantly reported in case studies, with limited data on their prevalence (ranging from 1.4% to 10.0%) in autopsy series depending on the sampling extent[12-14]. However, little is known about their occurrence in elderly individuals, particularly in those identified through cadaveric studies. These lesions have been documented in association with other pancreatic neoplasms, including pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasms (IPMNs), or occur as a part of hereditary syndromes[15-17]. Furthermore, emerging evi
Therefore, the present study investigated the prevalence and histopathological characteristics of PNEMTs in elderly individuals using Japanese cadaveric pancreatic specimens along with histopathological alterations in the surrounding pancreatic tissue.
Eighty-five pancreatic tissue specimens [male, n = 33; female, n = 52; mean age at the time of death, 88.0 ± 9.3 (range, 58-109) years] were obtained from cadavers used for anatomical education and research at the School of Life Dentistry, Nippon Dental University in Tokyo, and Niigata in 2014, 2023, and 2024. Each cadaver was refrigerated immediately after death and fixed using systemic perfusion with 10% formalin on the same day. All cadavers were fixed and preserved in formalin for approximately six months. Intact whole pancreatic tissue was removed from the cadavers and all selected specimens were confirmed (by the naked eye) to be well fixed. Cadavers with intra-abdominal injury or gross intra-abdominal pathology, particularly diseases of the pancreas and surrounding organs, were excluded from this study. The age, sex, and cause of death are listed in Supplementary Table 1.
Tissue sample handling was performed as previously described[18]. To obtain well-preserved sections, pancreatic specimens obtained from cadavers were divided into head (including the uncinate process), body, and tail sections. The specimens were cut into 5-mm-thick tissue blocks, prepared in 20-27 blocks per pancreas, which collectively covered all three regions. The slides were then washed thoroughly for 4-5 hours under running tap water, embedded in paraffin, cut into a 5-μm-thick section, and placed on gelatin-coated glass slides. Hematoxylin and eosin (H&E) staining of each specimen was performed, and some samples were subjected to immunohistochemistry. To ensure comprehensive and objective sampling, at least five sections were obtained from each tissue block for systematic microscopic analysis (Supplementary Figure 1).
Immunohistochemical analyses were performed as previously described[18]. In brief, tissue sections were deparaffinized in xylene, rehydrated through a graded ethanol series, washed under running tap water, and rinsed in 0.01 M phosphate-buffered saline (PBS) buffer (pH 7.2). Antigen retrieval was performed by pretreatment at high temperature [pH 6 in citrate buffer (No. C9999; Sigma-Aldrich, St. Louis, MO, United States) or Tris-EDTA buffer (pH 9.0) (ab93684; Abcam, Cambridge, United Kingdom)]. Endogenous peroxidase activity was inhibited by incubating with methanol containing 0.3% (v/v) hydrogen peroxide for 15 minutes. To prevent nonspecific binding, sections were blocked with 5% goat serum in PBS containing 3% bovine serum albumin and 0.05% Tween-20 for 1 hour at room temperature (RT).
The sections were then incubated overnight at 4 °C in a humidified chamber with primary antibodies. The following day, the slides were washed 3 times with PBS for 5 minutes each at RT and incubated with the secondary antibody for 1 hour at RT. Subsequently, the sections were again washed 3 times with PBS for 5 minutes each. Immunoreactivity was visualized using 3,3’-diaminobenzidine as the chromogen. Nuclear counterstaining was performed using a hematoxylin solution for 10 seconds.
The primary antibodies used were polyclonal guinea pig anti-human insulin antibody (ready-to-use, IR00261-2J; Dako, Tokyo, Japan), rabbit anti-GLU antibody (1:100, ab92517; Abcam, Tokyo, Japan), mouse anti-human chromogranin A antibody (1:1000, AMAb90525; Atlas Antibodies AB, Stockholm, Sweden), rabbit anti-human synaptophysin (Syn) antibody (1:100, HPA002858; Atlas Antibodies AB, Stockholm, Sweden), and rabbit anti-Ki-67 antibody (1:100, ab16667; Abcam, Tokyo, Japan) diluted in PBS supplemented with 3% bovine serum albumin. The secondary antibodies were anti-rabbit IgG-HRP (H+L chain; No. 458; MBL, Tokyo, Japan), anti-guinea pig IgG-HRP (H+L chain; No. 106-065-003; FUJIFILM, Tokyo, Japan), and anti-mouse IgG-HRP (H+L chain; No. 330; MBL), diluted to 1:100 in PBS 0.01 M (pH 7.2) supplemented with 3% bovine serum albumin.
For double immunofluorescence staining, the initial steps, including deparaffinization, rehydration, methanol treatment, rinsing, and blocking, were performed as described for the Immunohistochemical staining. The sections were then incubated overnight at 4 °C with the primary antibody, polyclonal guinea pig anti-human insulin (ready-to-use, IR00261-2J; Dako, Tokyo, Japan).
The following day, the slides were washed 3 times with PBS for 5 minutes each at RT and subsequently incubated overnight at 4 °C with the second primary antibody, rabbit anti-GLU (1:100, ab92517; Abcam, Tokyo, Japan).
On the third day, appropriate secondary antibodies, Alexa Fluor 594-conjugated goat anti-guinea pig IgG (1:100, A11076; Invitrogen, Carlsbad, CA, United States) and Alexa Fluor 488-conjugated chicken anti-rabbit IgG (1:100, A21441; Invitrogen) were applied to the sections and incubated for 1 hour at RT.
Finally, sections were mounted using Fluoromount (K-024; Diagnostic BioSystems, CA, United States) and examined under a fluorescence microscope (Zeiss Axio Imager M1; Carl Zeiss AG, Oberkochen, Germany).
As negative controls, sections were incubated either without primary antibodies or with 0.05 M Tris-BSA buffer substituted for the primary antibody. Control sections were processed concurrently with experimental sections.
The age, sex, and causes of death at the time of death of the 85 pancreatic specimen cadavers used in this study are listed in Supplementary Table 1. Histological examinations revealed PNEMTs in 5 cases [5.9% (95% exact binomial CI: 2.0%-13.3%)]; their baseline characteristics, tumor sizes (measured on H&E-stained sections) and locations, and immunohistochemical profiles of these 5 cases with PNEMTs are summarized in Table 1.
| Characteristic | PNEMTs (n = 5) |
| Incidence (95%CI) | 5.9% (2.0%-13.3%) |
| Age (years), mean ± SD | 85.8 ± 12.1 |
| Sex (Male:Female) | 2:3 |
| Tumor size (mm) median (range) | 0.78 (0.54-2.2) |
| Location of pancreas (n) | |
| Head:Body:Tail | 0:4:1 |
| Pathology analysis | |
| Chromogranin A (+++, ++, +, -) | 5 (+++) |
| Synaptophysin (+++, ++, +, -) | 5 (+++) |
| Ki-67 index (%) | 5 (< 3%) |
| Insulin (+++, ++, +, -) | 5 (+) |
| Glucagon (+++, ++, +, -) | 5 (+++) |
| Tumor grade (2022 WHO classification) | |
| Grade 1, 2, 3 | 5 (G1) |
Among the 5 PNEMTs cases, there were 2 males (Cases 1 and 2) and 3 females (Cases 3-5), with ages ranging from 71 to 97 (mean age: 85.8 ± 12.1) years. The causes of death were aspiration pneumonia (Case 2), congestive heart failure (Case 5), and senility (Cases 1, 3, and 4). The PNEMTs were found in the pancreatic body in 4 cases (Cases 1-4) and the pancreatic tail in 1 case (Case 5). Microscopic examination revealed that the maximum diameter of the tumor foci ranged from 0.54 mm to 2.20 mm (Table 1, Supplementary Table 1).
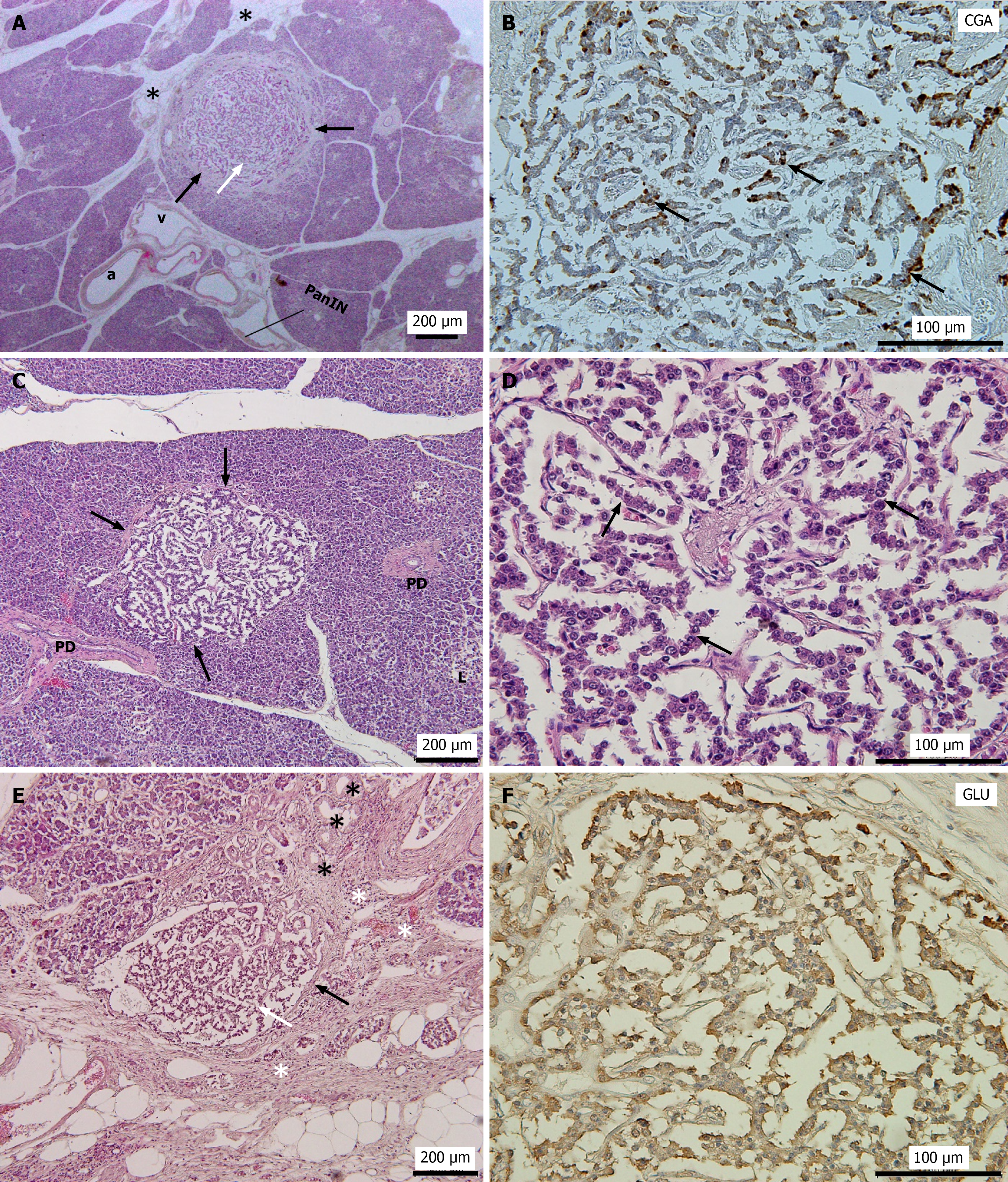
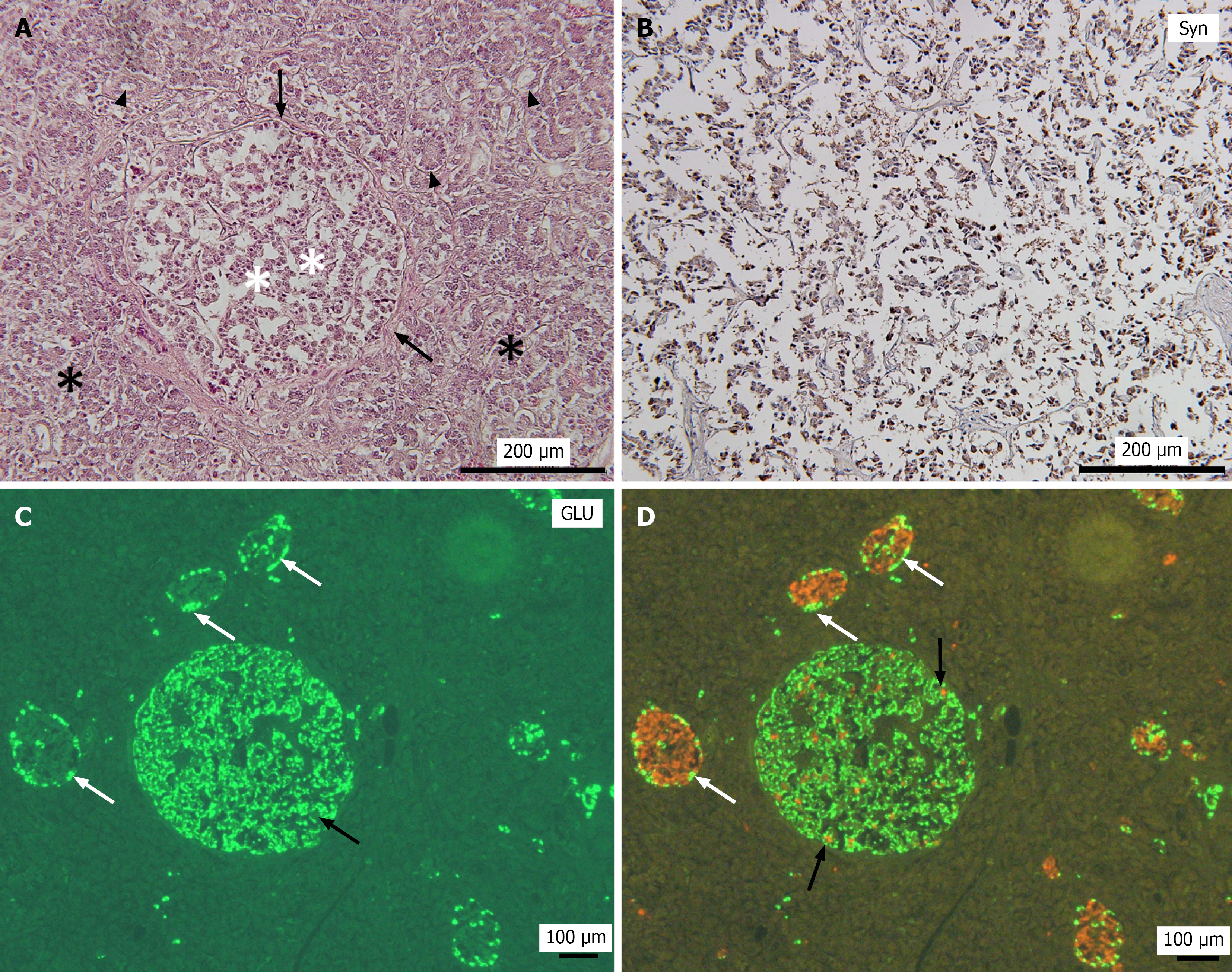
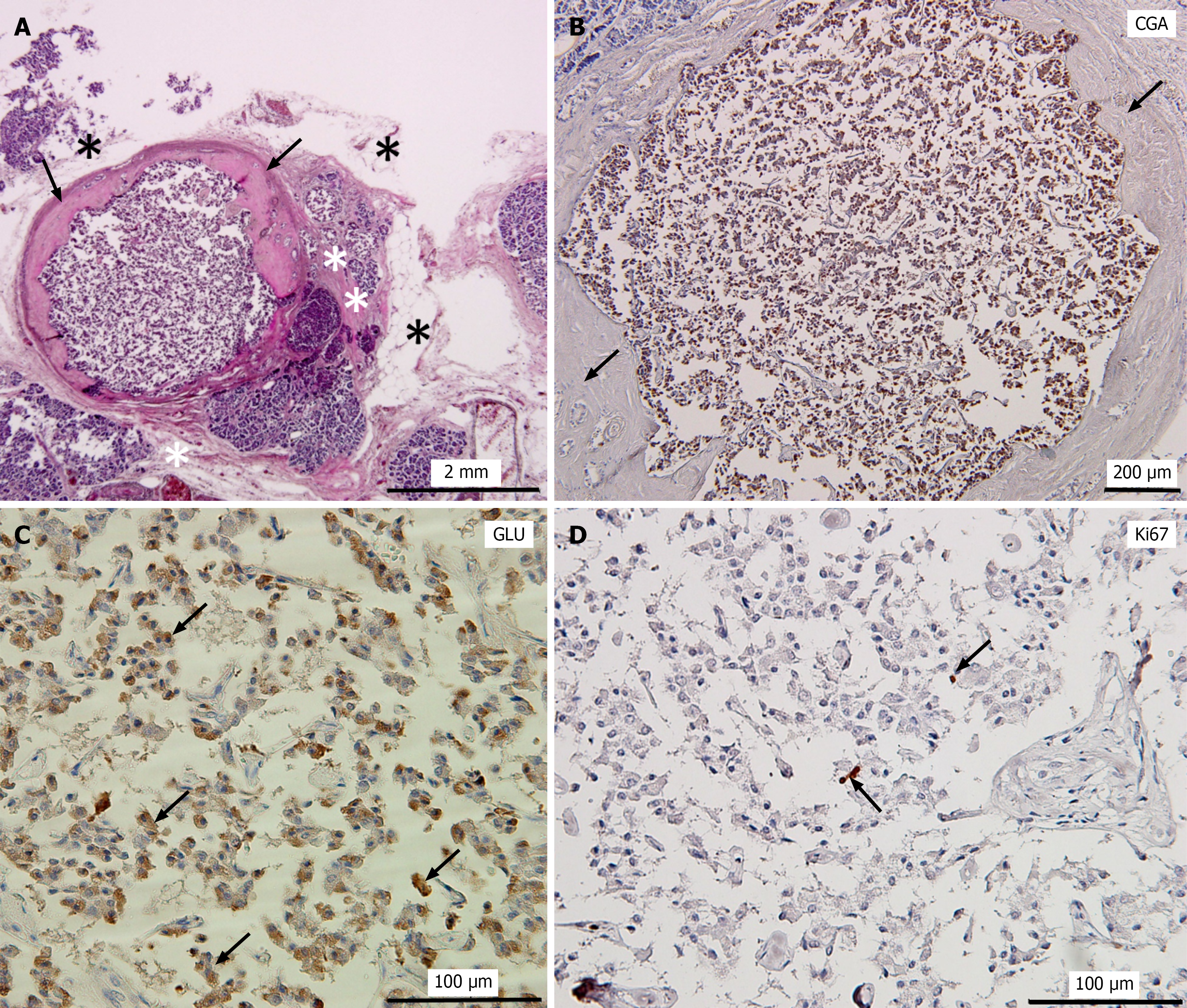
All lesions were solitary and well-circumscribed tumors located entirely within the pancreatic parenchyma (Figures 1, 2 and 3). Most tumor cells exhibited trabecular growth and organoid-like (pseudoglandular) architectural patterns, composed of monotonous tumor cells with round nuclei and finely granular (“salt-and-pepper”) chromatin (Figures 1D and 3A).
In Cases 1, 2, and 4, the pancreatic tissue surrounding the tumor appeared to be largely normal (Figures 1A, 1C, and 2A). In contrast, the tumor in Case 3 was surrounded by marked fatty degeneration and fibrosis accompanied by acinar-to-ductal metaplasia (ADM) (Figure 1E). In Case 5, a well-circumscribed round tumor nodule was observed. The tumor was partially encapsulated by a thick fibrotic wall, and the surrounding pancreatic tissue exhibited fibrosis and fatty degeneration (Figures 1E and 3A). Furthermore, islet cell hyperplasia was observed in the same sections as in the tumors in Cases 3 and 5, with a diffuse increase in islet tissue, forming islets of varying sizes (data not shown). No evidence of vascular or perineural invasion or metastasis was observed in any case.
Immunohistochemical analyses revealed strong and diffuse positivity for chromogranin A and Syn in all 5 PNEMTs, indicating neuroendocrine differentiation (Figures 1B, 2B, and 3B). All PNEMTs showed the presence of a majority of GLU-positive cells (Figures 1F, 2C, and 3C). Double immunofluorescence staining demonstrated strong positivity for GLU, but minimal insulin positivity in PNEMTs (Figure 2C and D). Ki-67 immunostaining demonstrated minimal proliferative activity and was nearly negative in all 5 PNEMTs cases (Figure 3D). No mitotic figures were identified; therefore, these results are consistent with the histopathological characteristics of well-differentiated grade 1 PNEMTs, and no multifocal incidental PNEMTs were observed in this study.
A total of 85 cadaveric specimens of the pancreas from elderly people (with a mean age, 85.8 ± 12.1 years) were collected in this study, among which 5 cases (5.9%) were identified as PNEMTs. All tumors were solitary, well-differentiated, < 5 mm (range: 0.54-2.20 mm), and without evidence of vascular or peripancreatic invasion. Histologically, some tumors exhibited well-demarcated margins with fibrous encapsulation, associated stromal fibrosis, and/or a trabecular/solid architecture. Immunohistochemical staining demonstrated that the PNEMTs were positive for chromogranin A, Syn and GLU. Some cases were accompanied by histological changes in the surrounding pancreatic parenchyma (e.g. islet cell hyperplasia, ADM, and fatty degeneration).
To our knowledge, the vast majority of PNEMTs are incidentally discovered in surgically resected specimens or during autopsy[4]; however, systematic studies investigating their prevalence based on autopsy data are scarce. The reported prevalence rates of PNEMTs vary considerably depending on the extent of pancreatic tissue examined[12,16,18], a prevalence which aligns with the upper range of previously reported rates (1.6%-10%) employed in meticulous whole-organ autopsy-based studies[12]. Our systematic analysis of 85 cadaveric pancreatic specimens identified PNEMTs in 5 cases (5.9%). This variation highlights the strong dependence of detection rates on the comprehensiveness of the pathological sampling. Unlike surgical series, which report a lower prevalence (1.4%-2.7%) and are subject to selection bias related to clinical indications[12], our findings reflect an unselected predominantly aged population, reducing clinical selection bias. This also confirms that PNEMTs are not rare in the elderly population.
PNEMTs can occur in any region of the pancreas[13,19], and our findings showed that 4 of the 5 cases were located in the pancreatic body and 1 in the pancreatic tail, which is consistent with reports indicating a predominance in the distal pancreas[16]. Similarly, PNEMTs are generally considered to show no distinct age or sex predilection[13,19]. However, the mean age of individuals with PNEMTs identified in our predominantly aged cohort (88.0 ± 9.3 years) was 85.8 ± 12.1 years. This suggested PNEMTs not only persist into advanced age without progression, but may also increase in prevalence, supporting their indolent biology. Although a slight female predominance was observed, our small sample size precluded definitive conclusions. Therefore, PNEMTs may be characterized as age-associated lesions, a profile uniquely revealed through autopsy studies in elderly populations.
In addition, all 5 lesions identified in our study were small (range 0.54-2.20 mm), grade 1, and immunohistochemically positive for GLU, consistent with several established autopsy-based studies that have shown that nonfunctioning tumors smaller than 5 mm may occasionally produce hormones without causing clinical symptoms, with the majority expressing GLU (87.5%)[1,2,20].
Moreover, we observed that some cases of PNEMTs were accompanied by islet cell hyperplasia. Although the coexistence of nesidioblastosis and PNEMTs has been reported in some cases, experimental models suggest a potential link between these two entities[21,22]. GLU receptor gene inactivation leads to GLU cell hyperplasia and subsequent neoplasia, illustrating a clear hyperplasia-to-neoplasia sequence within islet cells[23,24]. Similar processes are evident in hereditary syndromes such as MEN1 and von Hippel-Lindau disease, where a spectrum of lesions ranging from endocrine hyperplasia and dysplasia to overt neoplasia is frequently observed[17,23,25]. Rare clinical reports, including recurrent insulinomas arising in the setting of underlying nesidioblastosis[21,22], further support the concept that islet hyperplasia may act as a precursor state. Notably, all PNEMTs in our study were solitary lesions, in contrast to the multifocal tumors that are typical of genetic syndromes. This distinction underscores the need for further investigation into the role of islet hyperplasia in the pathogenesis of sporadic PNEMTs and larger neuroendocrine tumors, particularly the molecular mechanisms driving progression from hyperplasia to neoplasia.
Furthermore, several cases demonstrated PNEMTs surrounded by chronic pancreatic alterations, including fatty degeneration, fibrosis, and ADM. Although the causal relationship between these changes and PNEMTs development remains unclear, previous studies have reported that PNEMTs are incidentally identified in specimens with IPMNs[13,16] or adjacent to PanIN lesions[11,15], and chronic pancreatitis has been proposed as a potential factor for the development of PNEMTs[16,26]. While PanIN and IPMNs lesions were not observed in close proximity to PNEMTs in our cohort, the relatively high prevalence of PNEMTs in the very elderly, together with frequent age-related chronic pancreatic alterations, suggests a possible interplay between chronic pancreatic injury and remodeling and neuroendocrine microtumorigenesis, which merits further exploration.
From a clinical perspective, detecting small pancreatic neuroendocrine tumors is difficult: Over 68% of neuroendocrine tumors smaller than 10 mm cannot be detected by computed tomography, and endoscopic ultrasound sensitivity remains suboptimal (86%-89%)[27-29]. As PNEMTs are usually non-functional and mostly less than 5 mm in size, they are rarely detectable by current imaging modalities[1,4,7]. Our findings suggest that the actual prevalence of PNEMTs is likely underestimated and affirm that these small, well-differentiated, non-functioning PNEMTs are a common age-related phenomenon, especially in the very elderly. These results may help mitigate overly aggressive interventions for asymptomatic, minute lesions in elderly patients and support current consensus guidelines favoring surveillance over immediate resection[4,12,30].
This study has several limitations, including the small sample size owing to specimen availability and lack of control. Second, although we applied a standardized protocol and examined at least five serial sections from 20 to 27 blocks per pancreas, all tumors detected were less than 5 mm in size, and we cannot completely exclude the possibility that very small lesions were overlooked. Nonetheless, this study offers valuable insights into PNEMTs in an underrepresented elderly population. The limited number of cases restricted the microenvironment analysis to descriptive methods rather than statistical methods. Additionally, tumor sizes measured on histological sections without shrinkage correction may underestimate actual dimensions.
This systematic and retrospective study suggests that small, well-differentiated PNEMTs may be present in 5.9% of individuals, with an elderly predominance. These tumors are asymptomatic and small, making their clinical identification difficult. Although the examined cases showed indolent features, tumor enlargement may have increased the malignant potential. Assessing their natural course, prognosis, and optimal management, as well as recognizing their existence, early detection, and follow-up have important clinical value.
| 1. | Anlauf M, Schlenger R, Perren A, Bauersfeld J, Koch CA, Dralle H, Raffel A, Knoefel WT, Weihe E, Ruszniewski P, Couvelard A, Komminoth P, Heitz PU, Klöppel G. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Campbell F, Verbeke CS. Pathology of the Pancreas. 2013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 619] [Article Influence: 154.8] [Reference Citation Analysis (2)] |
| 4. | Chouchane A, Kirchner P, Marinoni I, Sticová E, Jirásek T, Perren A. Pancreatic Neuroendocrine Microtumors (WHO 2022) Are Not Always Low-Grade Neoplasms: A Case with a Highly Increased Proliferation Rate. Endocr Pathol. 2024;35:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Sipos B. [Multiple neuroendocrine tumors of the pancreas]. Pathologie (Heidelb). 2024;45:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Anlauf M, Perren A, Klöppel G. Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: criteria, molecular concepts and clinical significance. Pathobiology. 2007;74:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Tang LH. Pancreatic Neuroendocrine Neoplasms: Landscape and Horizon. Arch Pathol Lab Med. 2020;144:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kwon JH, Kim HJ, Park DH, Lee YJ, Heaphy CM, Klöppel G, Hruban RH, Hong SM. Incidentally detected pancreatic neuroendocrine microadenoma with lymph node metastasis. Virchows Arch. 2018;473:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Vo N, Cohen DW, Dillhoff ME, Jin M. Pancreatic neuroendocrine microadenomatosis: A case report of cytology and histology correlation. Diagn Cytopathol. 2017;45:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Lee J, Lee KJ, Hwang DW, Hong SM. Malignant potential of neuroendocrine microtumor of the pancreas harboring high-grade transformation: lesson learned from a patient with von Hippel-Lindau syndrome. J Pathol Transl Med. 2024;58:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Coulibaly B, Delage-Corre M, Durand-Fontanier S, Mathonnet M, Paraf F, Labrousse F. [Two cases reports of pancreatic endocrine microadenoma incidentally found]. Ann Pathol. 2013;33:406-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Okawa Y, Tsuchikawa T, Hatanaka KC, Matsui A, Tanaka K, Nakanishi Y, Asano T, Noji T, Nakamura T, Mitsuhashi T, Okamura K, Hatanaka Y, Hirano S. Clinical Features of Pancreatic Neuroendocrine Microadenoma: A Single-Center Experience and Literature Review. Pancreas. 2022;51:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Partelli S, Giannone F, Schiavo Lena M, Muffatti F, Andreasi V, Crippa S, Tamburrino D, Zamboni G, Rubini C, Doglioni C, Falconi M. Is the Real Prevalence of Pancreatic Neuroendocrine Tumors Underestimated? A Retrospective Study on a Large Series of Pancreatic Specimens. Neuroendocrinology. 2019;109:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci. 1991;36:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Chung MH, Chien HJ, Peng SJ, Chou YH, Chiang TC, Chang HP, Lee CY, Chen CC, Jeng YM, Tien YW, Tang SC. Multimodal 3-D/2-D human islet and duct imaging in exocrine and endocrine lesion environment: associated pancreas tissue remodeling. Am J Physiol Endocrinol Metab. 2022;323:E354-E365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Liszka Ł. Increased prevalence of pancreatic neuroendocrine microadenomas in patients with intraductal papillary mucinous neoplasms: yet another example of exocrine-neuroendocrine interaction? Nowotwory J Oncol. 2024;74:1-11. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Périgny M, Hammel P, Corcos O, Larochelle O, Giraud S, Richard S, Sauvanet A, Belghiti J, Ruszniewski P, Bedossa P, Couvelard A. Pancreatic endocrine microadenomatosis in patients with von Hippel-Lindau disease: characterization by VHL/HIF pathway proteins expression. Am J Surg Pathol. 2009;33:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Li R, Yang T, Zhang M, Ren K, Li J, Sato I, Yi SQ. A new histopathological phenomenon: Pancreatic islet cell loss in the elderly population. Dig Liver Dis. 2024;56:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Partelli S, Muffatti F, Rancoita PMV, Andreasi V, Balzano G, Crippa S, Doglioni C, Rubini C, Zamboni G, Falconi M. The size of well differentiated pancreatic neuroendocrine tumors correlates with Ki-67 proliferative index and is not associated with age. Dig Liver Dis. 2019;51:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Anlauf M, Enosawa T, Henopp T, Schmitt A, Gimm O, Brauckhoff M, Dralle H, Musil A, Hauptmann S, Perren A, Klöppel G. Primary lymph node gastrinoma or occult duodenal microgastrinoma with lymph node metastases in a MEN1 patient: the need for a systematic search for the primary tumor. Am J Surg Pathol. 2008;32:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Dauriz M, Maneschi C, Castelli C, Tomezzoli A, Fuini A, Landoni L, Malleo G, Ferdeghini M, Bonora E, Moghetti P. A Case Report of Insulinoma Relapse on Background Nesidioblastosis: A Rare Cause of Adult Hypoglycemia. J Clin Endocrinol Metab. 2019;104:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Orujov M, Lai KK, Forse CL. Concurrent Adult-Onset Diffuse β-Cell Nesidioblastosis and Pancreatic Neuroendocrine Tumor: A Case Report and Review of the Literature. Int J Surg Pathol. 2019;27:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Cidade-Rodrigues C, Santos AP, Calheiros R, Santos S, Matos C, Moreira AP, Inácio I, Souteiro P, Oliveira J, Jácome M, Pereira SS, Henrique R, Torres I, Monteiro MP. Non-functional alpha-cell hyperplasia with glucagon-producing NET: a case report. Front Endocrinol (Lausanne). 2024;15:1405835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Sipos B, Sperveslage J, Anlauf M, Hoffmeister M, Henopp T, Buch S, Hampe J, Weber A, Hammel P, Couvelard A, Höbling W, Lieb W, Boehm BO, Klöppel G. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab. 2015;100:E783-E788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | De Sousa SM, Haghighi KS, Qiu MR, Greenfield JR, Chen DL. Synchronous Nesidioblastosis, Endocrine Microadenoma, and Intraductal Papillary Mucinous Neoplasia in a Man Presenting With Hyperinsulinemic Hypoglycemia. Pancreas. 2016;45:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Capurso G, Falconi M, Panzuto F, Rinzivillo M, Boninsegna L, Bettini R, Corleto V, Borgia P, Pederzoli P, Scarpa A, Delle Fave G. Risk factors for sporadic pancreatic endocrine tumors: a case-control study of prospectively evaluated patients. Am J Gastroenterol. 2009;104:3034-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Manta R, Nardi E, Pagano N, Ricci C, Sica M, Castellani D, Bertani H, Piccoli M, Mullineris B, Tringali A, Marini F, Germani U, Villanacci V, Casadei R, Mutignani M, Conigliaro R, Bassotti G, Zullo A. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J Gastrointestin Liver Dis. 2016;25:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Huang F, Fan Y, Li D, Tao Q, Tang D, Deng L, Ma C. Diagnostic value of endoscopic ultrasound for detecting pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Am J Med Sci. 2022;363:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Xie Y, Abaydulla E, Zhang S, Liu H, Hang H, Li Q, Qiu Y, Cheng H. Preoperative prediction of pancreatic neuroendocrine tumors grade based on computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Abdom Radiol (NY). 2025;50:4553-4562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Partelli S, Massironi S, Zerbi A, Niccoli P, Kwon W, Landoni L, Panzuto F, Tomazic A, Bongiovanni A, Kaltsas G, Sauvanet A, Bertani E, Mazzaferro V, Caplin M, Armstrong T, Weickert MO, Ramage J, Segelov E, Butturini G, Staettner S, Cives M, Frilling A, Moulton CA, He J, Boesch F, Selberheer A, Twito O, Castaldi A, De Angelis CG, Gaujoux S, Holzer K, Wilson CH, Almeamar H, Vigia E, Muffatti F, Lucà M, Lania A, Ewald J, Kim H, Salvia R, Rinzivillo M, Smid A, Gardini A, Tsoli M, Hentic O, Colombo S, Citterio D, Toumpanakis C, Ramsey E, Randeva HS, Srirajaskanthan R, Croagh D, Regi P, Gasteiger S, Invernizzi P, Ridolfi C, Giovannini M, Jang JY, Bassi C, Falconi M. Management of asymptomatic sporadic non-functioning pancreatic neuroendocrine neoplasms no larger than 2 cm: interim analysis of prospective ASPEN trial. Br J Surg. 2022;109:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/