Published online May 15, 2020. doi: 10.4251/wjgo.v12.i5.582
Peer-review started: December 29, 2019
First decision: January 19, 2020
Revised: March 13, 2020
Accepted: March 24, 2020
Article in press: March 24, 2020
Published online: May 15, 2020
Processing time: 136 Days and 19.3 Hours
Gastric Helicobacter pylori (H. pylori) infection is related to chronic gastritis, gastroduodenal ulcer, and gastric malignancies; whether this infection is related to colorectal polyps and colorectal cancer (CRC), remains debatable.
To investigate the relationship between gastric H. pylori infection and the risk of colorectal polyps and CRC.
We retrospectively analyzed 3872 patients with colorectal polyps who underwent colonoscopy and pathological diagnosis. We also analyzed 304 patients with primary CRC. The characteristics of these patients were compared with those of the control group, which included 2362 patients with the normal intestinal mucosa. All subjects completed a 14C-urea breath test, bidirectional gastrointestinal endoscopy, and a biopsy on the same day. Data on the number, size, location, and pathology of the polyps, the location, and pathology of the CRC, the detection of H. pylori, and the incidence of H. pylori-associated atrophic gastritis or intestinal metaplasia were obtained. A logistic regression model was used to analyze the relationship between gastric infection due to H. pylori, and the incidence of colorectal polyps and CRC.
The prevalence of H. pylori infection was higher in the multiple polyps group than in the solitary polyp group and the control group [95% confidence interval (CI) = 1.02-1.31, P = 0.03; 95%CI: 2.12-2.74, P < 0.001]. The patients with adenomatous polyps had a higher incidence of H. pylori infection than patients with non-adenomatous polyps [59.95% vs 51.75%, adjusted odds ratio (OR) = 1.41, 95%CI: 1.24-1.60, P < 0.01]. Patients with H. pylori-associated atrophic gastritis or intestinal metaplasia were at high risk of CRC (adjusted OR = 3.46, 95%CI: 2.63-4.55, P < 0.01; adjusted OR = 4.86, 95%CI: 3.22-7.34, P < 0.01, respectively). The size and location of the polyps, the histopathological characteristics and the location of CRC were not related to H. pylori infection.
Our study demonstrates that the incidence of gastric H. pylori infection and H. pylori-associated atrophic gastritis or intestinal metaplasia elevates the risk of colorectal polyps and CRC.
Core tip: This study investigated the association of gastric Helicobacter pylori (H. pylori) infection with the risk of colorectal polyps and colorectal cancer (CRC). The results indicated that patients with H. pylori infection were 2.19 and 3.05 times more likely to develop colorectal polyps and CRC, respectively, than those without H. pylori infection. The prevalence of H. pylori infection was higher in the patient group with multiple polyps and colorectal adenomas than in those with a solitary polyp and non-adenomatous polyps, respectively. Gastric H. pylori infection and H. pylori-associated atrophic gastritis or intestinal metaplasia elevated the risk of colorectal polyps and CRC. Therefore, earlier and frequent colonoscopy is necessary.
- Citation: Wang M, Kong WJ, Zhang JZ, Lu JJ, Hui WJ, Liu WD, Kang XJ, Gao F. Association of Helicobacter pylori infection with colorectal polyps and malignancy in China. World J Gastrointest Oncol 2020; 12(5): 582-591
- URL: https://www.wjgnet.com/1948-5204/full/v12/i5/582.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i5.582
Colorectal cancer (CRC) accounted for 881000 deaths in 2018, representing 9.8% of deaths worldwide. The incidence rate of CRC has increased to the extent that 1.8 million new CRC cases were diagnosed in 2018, according to recent data by the World Health Organization[1]. However, it is difficult to detect CRC early due to the lack of typical clinical symptoms, and 20% of patients who are asymptomatic at diagnosis may manifest with aggressive metastases[2]. The appearance of colorectal polyps and malignancy is a complex process that involves a combination of dietary habits, smoking, drinking and other environmental factors, and genetic susceptibility[3,4]. Colorectal polyps are abnormal protrusions on the surface of the large intestine[5] that are either non-adenomatous or adenomatous polyps. Colorectal adenomatous polyps are considered to be the most important precancerous lesions, developing into CRC through the adenomatous cancer sequence[6]. Therefore, early screening and detection of precancerous lesions can prevent the occurrence and development of CRC[7].
Gastric Helicobacter pylori (H. pylori) infection is a global public health concern, which has infected approximately[8] 50% of the world population, according to recent epidemiological studies[9]. The chronic inflammatory state caused by H. pylori infection is closely related to the occurrence of gastric cancer. H. pylori is listed as a class I carcinogen by the World Health Organization International Agency for Cancer Research[10] and is also involved in the tumorigenesis of extragastric target organs, such as lung cancer, and hepatocellular carcinoma[11]. Previous studies have indicated that gastric H. pylori infection increased the risk of colorectal tumors[12-16]. In contrast, however, several other reports have concluded that gastric H. pylori infection was not correlated with colorectal polyps or CRC[17-21]. Thus, the results of different research studies in different regions vary significantly. Therefore, the present study investigated the relationship between gastric H. pylori infection, colorectal polyps, and CRC in northwestern China. In addition, the study provides answers to questions regarding the necessity for colonoscopy screening in patients with gastric H. pylori infection.
We reviewed the consecutive electronic medical records of patients who underwent gastroscopy and colonoscopy at a regional institution from January 2014 to January 2019. The inclusion criteria included: (1) Complete general information (including gender, age, ethnicity, past history, family history, etc.); (2) Age ≥ 18 years; (3) Patients who underwent bidirectional endoscopy (colonoscopy performed immediately after gastroscopy); (4) A clear pathological diagnosis of colorectal polyps or CRC; and (5) Detection of H. pylori infection. Exclusion criteria were as follows: (1) History of gastric cancer, peptic ulcer, and other malignant tumors; (2) Received antibiotics, NSAIDs, proton pump inhibitors or glucocorticoids in the past month; (3) Patients who underwent H. pylori eradication therapy previously, or radiation therapy, chemotherapy, and other specific treatment for tumors; (4) No total colonoscopy or biopsy; (5) Previous history of gastrointestinal surgery; (6) Presence of inflammatory bowel disease, familial adenoma, Gardner’s syndrome (a disease that affects the incidence of CRC); (7) A history of severe systemic disease; (8) A family history of polyposis, and (9) Patients who underwent repeated hospitalizations and a history of endoscopic polyp therapy. All patients provided consent for the study.
The diagnosis of normal intestinal mucosa, colorectal polyps, CRC, atrophic gastritis, and intestinal metaplasia was mainly based on endoscopic manifestations and histopathological examinations. Pathological diagnoses required confirmation by two pathologists. Referring to the fourth national consensus report on H. pylori infection treatment in 2012[22], H. pylori infection was defined as follows: Positive 14C-urea breath test and/or positive hematoxylin and eosin staining on gastric biopsies.
All subjects underwent complete colonoscopy and data regarding the location, size, shape, and number of polypoid lesions and the location of tumors were recorded. According to the size of the largest polyp, the patients with colorectal polyps were divided into those with a maximum diameter ≥ 1 cm and those with a maximum diameter ≤ 1 cm. According to the number of polyps, patients with a single polyp were included in the solitary polyp group, and those with ≥ 2 polyps were included in the multiple polyps group. Colorectal polyps and CRC were classified according to the location. The distal colorectum was defined as the anus to the splenic flexure, while the proximal colon was defined as the cecum to the splenic flexure, and patients with multiple lesions on both sides were defined as the whole colon. We divided H. pylori-related gastric disease into atrophy and intestinal metaplasia according to a method described previously[23]. Colorectal polyps including non-adenomatous and adenomatous polyps were considered. The prevalence of gastric H. pylori infection in CRC patients, patients with colorectal polyps, and the control group were compared. In addition, the prevalence of atrophic gastritis or intestinal metaplasia with gastric H. pylori infection among CRC patients, colorectal polyp patients, and the control group was also compared.
SPSS 17.0 was used for statistical analysis. Data for continuous variables were expressed as mean ± SD, and categorical data as a ratio or percentage. The t-test was used for data with a normal distribution. For comparisons among multiple groups of means (e.g., sex, BMI), one-way ANOVA was used. If the hypothesis of homogeneity of variance was not satisfied, Welch’s ANOVA was used. In addition, we used the χ2 test to compare categorical variables. A logistic regression model was applied to estimate the correlation between H. pylori infection, colorectal polyps, and CRC. After adjusting for gender and age, we calculated the odds ratio (OR) value and 95% confidence interval (95%CI). All variables with P < 0.05 were considered statistically significant and remained in the final models.
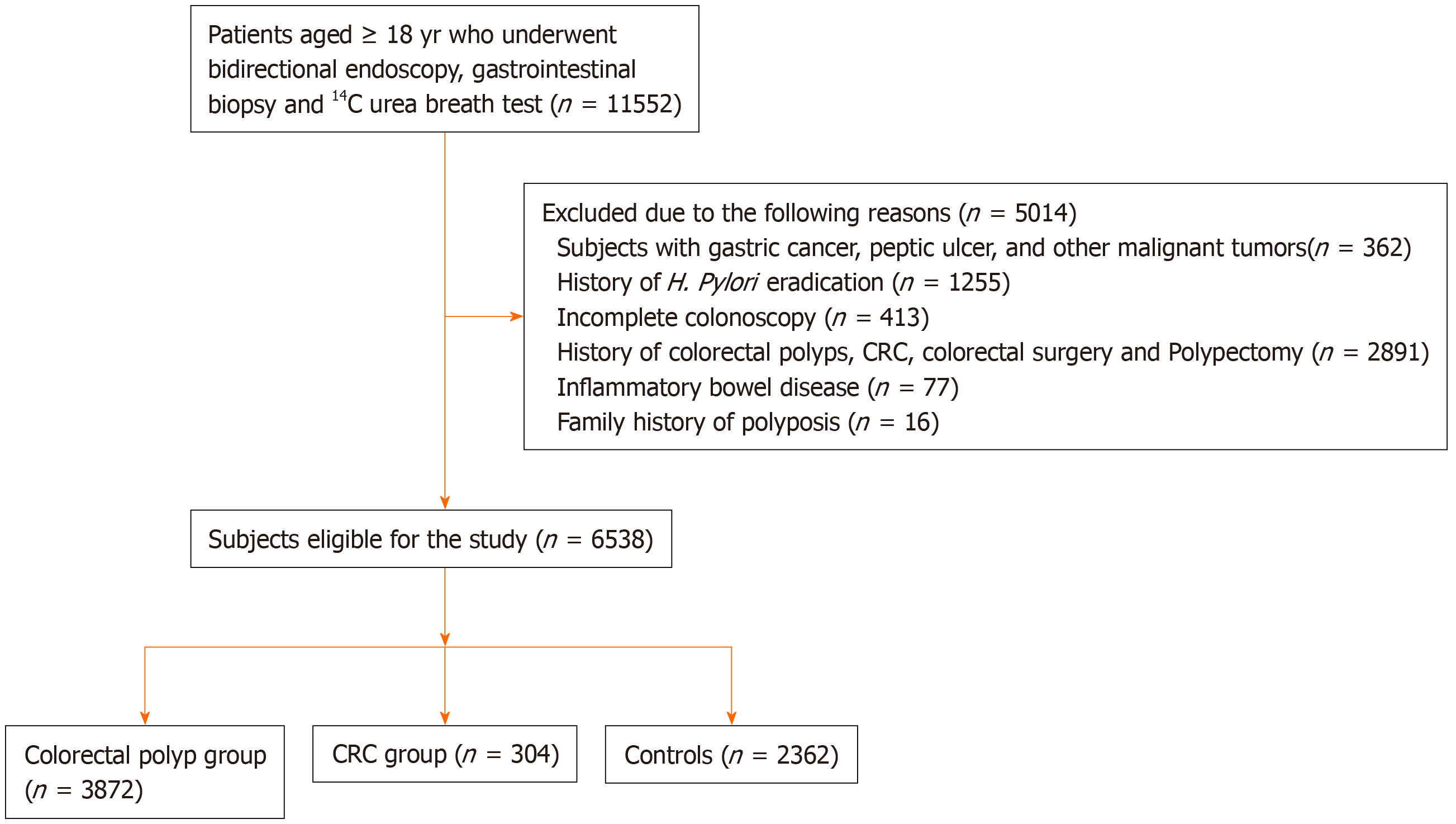
In total, 6538 patients were enrolled in our study (Figure 1). The characteristics of the participants are outlined in Table 1. Of these patients, 3872 were confirmed to have colorectal polyps and 304 had CRC by colorectal biopsy, and the remaining 2362 patients who had no abnormalities on colonoscopy, no history of polypectomy, and no clinical symptoms were classified as controls. The patient group with colorectal polyps included 2189 men and 1683 women. Their mean age was 53.01 ± 12.60 years. The patient group with CRC comprised 167 men and 137 women with an average age of 61.26 ± 12.94 years. The control group comprised 1355 men and 1007 women with a mean age of 45.81 ± 13.44 years. There were significant differences in age among the three groups (Welch F = 320.48, P < 0.001). However, there were no significant differences in gender, BMI, smoking, drinking, or family history of CRC among the three groups (P = 0.66; P = 0.06; P = 0.39; P = 0.28; P = 0.16, respectively). All patients underwent gastroscopy on the day of colonoscopy. The main indications for performing gastroscopy and colonoscopy are shown in Table 2.
| Parameter | Control group (n = 2362) | Colorectal polyps group (n = 3872) | CRC group (n = 304) | F / χ2 | P value |
| Age, mean ± SD (yr) | 45.81 ± 13.44 | 53.01 ± 12.60 | 61.26 ± 12.94 | 320.48 | < 0.001 |
| Male/female | 1355/1007 | 2189/1683 | 167/137 | 0.85 | 0.66 |
| BMI, mean ± SD (kg/m²) | 24.78 ± 3.83 | 24.85 ± 3.82 | 25.34 ± 3.66 | 2.87 | 0.06 |
| Smoking | 677 (28.66) | 1048 (27.07) | 94 (30.92) | 1.89 | 0.39 |
| Drinking | 271 (11.47) | 501 (12.94) | 41 (13.49) | 2.52 | 0.28 |
| Family history of CRC | 124 (5.25) | 243 (6.28) | 23 (7.57) | 3.73 | 0.16 |
| Indication for gastroscopy and colonoscopy1 | Gastroscopy (%) | Colonoscopy (%) |
| Abdominal pain | 35.23 | 27.95 |
| Diarrhea | 10.12 | 20.03 |
| Constipation | 8.05 | |
| Gastrointestinal bleeding | 12.07 | 14.43 |
| Gastroesophageal reflux disease | 16.51 | |
| Dyspepsia | 19.22 | |
| Nausea/vomiting | 10.16 | |
| Dysphagia | 6.2 | |
| Weight loss | 7.38 | 5.24 |
| Anemia | 6.47 | 6.32 |
| Other | 6.35 | 3.26 |
| Gastric cancer/CRC screening | 28.19 | 26.92 |
| Polyp surveillance | 7.94 | 9.21 |
The colonoscopy findings and pathological characteristics of colorectal polyps are shown in Table 3. The prevalence of H. pylori infection in the colorectal polyp group (2134/3872, 55.11%) was higher than that in the control group (890/2362, 37.68%; P < 0.001). Multivariate logistic regression demonstrated that the patients with positive H. pylori infection in the colorectal polyp group had a higher OR after age and gender adjustment (adjusted OR = 2.19, 95%CI: 1.96-2.44, P < 0.001). The prevalence of H. pylori infection was higher in the multiple polyps group than that in the solitary polyp group and the control group (adjusted OR = 1.15, 95%CI: 1.02-1.31, P = 0.03; adjusted OR = 2.41, 95%CI: 2.12-2.74, P < 0.001). The positive rate of H. pylori infection in the adenomatous polyp group (952/1588, 59.95%) was higher than that in the non-adenomatous polyp group (1182/2284, 51.75%, adjusted OR = 1.41, 95%CI = 1.24-1.60, P < 0.001). The same association was found between the adenomatous polyps and control groups (adjusted OR = 2.53, 95%CI: 2.20-2.89, P < 0.001). However, the polyp size and locations were not associated with H. pylori (P = 0.26; P = 0.08). According to the polyp location, the P value of the proximal colon was 0.18, the distal colorectal was 0.23, and the whole colon was 0.51. Furthermore, based on the status of H. pylori infection and histopathological findings of the gastroscopy specimens, we found that the incidence of H. pylori-related atrophic gastritis in the colorectal polyp group was 34.99% (1355/3872), which was higher than that in the control group (541/2362, 22.90%, P < 0.001) (Table 4). H. pylori-associated atrophic gastritis was significantly associated with colorectal polyps compared to that in the control group (adjusted OR = 5.42, 95%CI: 4.67-6.30, P < 0.001). The prevalence of H. pylori-related intestinal metaplasia in the patient group with colorectal polyps was 8.96% (347/3872), which was higher than that in the control group (105/2362, 4.45%) (P < 0.001). Overall, H. pylori-associated intestinal metaplasia status was positively associated with colorectal polyps (adjusted OR = 5.88, 95%CI: 4.60-7.52, P < 0.001).
| Parameter | H. pylori positive [n (%)] | H. pylori negative [n (%)] | Adjusted OR (95%CI) | P value |
| Control group | 890 (37.68) | 1472 (62.32) | 1 | |
| Colorectal polyp group | 2134 (55.11) | 1738 (44.89) | 2.19 (1.96-2.44) | < 0.01 |
| Polyp size | ||||
| ≥ 1 cm | 529 (56.70) | 404 (43.30) | 2.33 (1.98-2.74) | < 0.01 |
| < 1 cm | 1605 (54.61) | 1334 (45.39) | 2.15 (1.92-2.41) | < 0.01 |
| Polyp number | ||||
| Solitary | 942 (53.34) | 824 (46.66) | 1.98 (1.74-2.25) | < 0.01 |
| Multiple | 1192 (56.60) | 914 (43.40) | 2.41 (2.12-2.74) | < 0.01 |
| Polyp histology | ||||
| Adenomatous polyps | 952 (59.95) | 636 (40.05) | 2.53 (2.20-2.89) | < 0.01 |
| Non-adenomatous polyps | 1182 (51.75) | 1102 (48.25) | 2.00 (1.77-2.26) | < 0.01 |
| Polyp location | ||||
| Proximal colon | 732 (57.28) | 546 (42.72) | 2.49 (2.15-2.88) | < 0.01 |
| Distal colorectal | 1160 (53.51) | 1008 (46.49) | 1.98 (1.75-2.24) | < 0.01 |
| Whole colon | 242 (56.81) | 184 (43.19) | 2.37 (1.92-2.94) | < 0.01 |
| CRC group | 189 (62.17) | 115 (37.83) | 3.05 (2.33-3.99) | < 0.01 |
| CRC location | ||||
| Proximal colon | 65 (67.01) | 32 (32.99) | 3.73 (2.39-5.82) | < 0.01 |
| Distal colorectal | 124 (59.90) | 83 (40.10) | 2.79 (2.04-3.81) | < 0.01 |
| H. pylori-associated atrophic gastritis [n (%)] | Adjusted OR (95%CI) | P value | H. pylori-associated intestinal metaplasia [n (%)] | Adjusted OR (95%CI) | P value | |
| Control group | 541 (22.90) | 1 | 105 (4.45) | 1 | ||
| Colorectal polyp group | 1355 (34.99) | 5.42 (4.67-6.30) | < 0.01 | 347 (8.96) | 5.88 (4.60-7.52) | < 0.01 |
| CRC group | 144 (47.37) | 3.46 (2.63-4.55) | < 0.01 | 55 (18.09) | 4.86 (3.22-7.34) | < 0.01 |
The prevalence of H. pylori infection in the CRC group (189/304, 62.17%) was higher than that in the control group (890/2362, 37.68%) (P < 0.001). After adjustment for age and gender, multivariate logistic regression demonstrated that the patients with positive H. pylori infection in the CRC group had a higher OR (adjusted OR = 3.05, 95%CI: 2.33-3.99, P < 0.001) and among 304 patients with CRC, 270 cases had adenocarcinoma, including 167 cases with H. pylori infection (61.85%); 11 cases had neuroendocrine tumors, including 7 cases with H. pylori infection (63.64%); 14 cases had intramucosal cancer, including 9 cases with H. pylori infection (64.29%); 9 cases had signet ring cell cancer, including 6 cases with H. pylori infection (66.67%). The histopathological findings of CRC were not related to H. pylori (P > 0.05). Moreover, the χ2 test showed that CRC location was not correlated with H. pylori infection (P = 0.62). Compared to the control group, both H. pylori-related atrophic gastritis and intestinal metaplasia significantly increased the risk of CRC (adjusted OR = 3.46, 95%CI: 2.63-4.55, P < 0.001; adjusted OR = 4.86, 95%CI: 3.22-7.34, P < 0.001) (Table 2).
H. pylori infection plays an important role in the pathogenesis of gastrointestinal diseases[24]. However, whether gastric H. pylori infection increases the risk of colorectal polyps and CRC has been debated in various studies. This could be related to differences in the dietary habits of the study population, the patient population susceptible H. pylori, study sample size, and other factors. The H. pylori infection rate in China is more than 50%[25]. The relationship between gastric H. pylori infection and colorectal polyps and CRC in Northwest China is unclear; thus, we conducted this study. Some studies have shown that gender and age are related to H. pylori infection, colorectal polyps, and CRC[12,26]. Therefore, we conducted a multifactorial logistic regression analysis after adjusting for those factors. No significant differences in BMI, smoking, drinking, or family history of CRC among the subjects were found.
The results showed that the increase in H. pylori infection rate was positively correlated with the increase in colonic polyp incidence, polyp number, and malignancy, suggesting that H. pylori infection might be a risk factor for colorectal polyps and tumors. Patients with H. pylori infection are 2.19 times more likely to develop colorectal polyps and 3.05 times more likely to develop CRC than those who do not have H. pylori infection. Additionally, we found that the incidence of H. pylori infection coexisting with atrophic gastritis or intestinal metaplasia was higher in patients with colorectal polyps and CRC than in the control group. There was a significant correlation between H. pylori-associated gastropathy and colorectal adenomatous polyps or CRC. These results are consistent with those of previous studies[26,27]. A large-scale population-based study by Sonnenberg et al[28] also supports this argument. However, another study found no significant relationship between H. pylori infection and CRC. This may have been due to the broader age range and the limited number of patients[29].
There are a few plausible theories to explain the distribution of colorectal polyps and CRC and its association with H. pylori infection. Hong et al[30] found that gastric H. pylori infection was positively associated with an increased risk of proximal colorectal adenomatous polyps. Zhang et al[31] found that H. pylori infection mainly increased the risk of distal CRC. However, our study illustrates that gastric H. pylori infection could increase the risk in both proximal and distal colorectal neoplasms, which was consistent with the study by Inoue et al[32,33]. H. pylori infection causes microbiological changes in the digestive tract, increases the production of bile acids, causes DNA damage and activation, plays an important role in the proximal colonic mucosa, and increases the risk of proximal colonic polyps and malignancy[34]. Preclinical models have demonstrated that increased gastrin secretion caused by H. pylori infection has a mitogenic effect and selectively acts on the distal colon, thereby increasing the risk of distal colon polyps and malignancy[35]. These mechanisms may work synergistically. In addition, in our study, no significant differences were found among CRC patients with different pathological types and the prevalence of H. pylori infection. This may be because the most prevalent type of CRC is adenocarcinoma; however, studies with a larger sample size are needed to confirm this hypothesis.
The mechanism by which gastric H. pylori infection increases the incidence of colorectal polyps and CRC is not clear. Some studies have shown that gastrin gene expression is up-regulated in both colorectal polyps and CRC[36,37], and H. pylori infection can cause hypergastrinemia. Gastrin acts on gastrointestinal epithelial cells and can promote the formation of COX-2, which affects the occurrence, development, invasion, and metastasis of colorectal neoplasia[38]. Gastrin can also induce colonic mucosal cell proliferation to promote the development of CRC[39]. Moreover, from the perspective of gastrointestinal microecology, chronic gastritis caused by long-term H. pylori infection can lead to massive glandular atrophy and decreased gastric acid secretion. Low gastric acid may adversely affect the intestinal flora, cause bacterial overgrowth, colonic disorders, and colorectal carcinogenesis[40,41]. In addition, H. pylori infection may cause damage to colorectal epithelium through the chronic inflammatory response mediated by inflammatory factors such as interleukin-8[42].
The early diagnosis of CRC is relatively difficult. During the development of CRC, the normal mucosa develops into an adenoma and then to adenocarcinoma. This process provides opportunities for early detection and intervention of CRC. Early diagnosis and resection of colonic polyps can reduce the morbidity and mortality of CRC[43]. Further studies with regard to pathogenic mechanisms should be continued, which can help to develop relevant prevention and early detection strategies.
Some studies found that only the current situation of H. pylori infection could stimulate the immune response, thus inducing or perpetuating chronic inflammation of the gastrointestinal tract[44,45]. The strength of our study was that histopathological results and the 14C-urea breath test were used to determine H. pylori. A histopathological examination has high specificity and sensitivity in the diagnosis of gastric pathological changes and H. pylori infection. However, histopathological examination and the 14C-urea breath test can only diagnose the current infection of H. pylori, compared with the serological tests, which do not distinguish current or past infections. Therefore, our study explains the current relationship between H. pylori infection, colorectal polyps, and CRC more accurately. In addition, our sample size was relatively large, which is an advantage of this study.
Our study also has several limitations. First, we did not consider the possible effect of the duration of H. pylori infection on colorectal polyps and CRC. Secondly, we did not consider other confounding factors, such as constipation, eating habits, and metabolic syndrome. Thirdly, this was a single-center study. More investigation through prospective multicenter studies with large sample sizes should be conducted.
In conclusion, this study showed that gastric H. pylori infection and H. pylori-related gastric atrophic or intestinal metaplasia increased the risk of colorectal polyps and CRC. Early colonoscopy screening and surveillance is necessary to reduce the risk of colonic polyps and CRC in patients with H. pylori infection. Further investigation is required to understand whether the eradication of gastric H. pylori can reduce the occurrence of colorectal polyps and CRC.
Gastric Helicobacter pylori (H. pylori) infection is a global public health problem. It is associated with chronic gastritis, gastroduodenal ulcer and gastric malignancies. The relationship between H. pylori infection and the risk of colorectal polyps and colorectal cancer (CRC) has also received extensive attention in recent years.
There is still no clear conclusion regarding the relationship between gastric H. pylori infection and the risk of colorectal polyps and CRC.
Our main purpose was to investigate the correlation between gastric H. pylori infection and the risk of colorectal polyps and CRC, which is essential for the early screening and detection of colorectal precancerous lesions.
A retrospective analysis of 6538 patients who underwent colonoscopy was conducted. The patients were divided into three groups: The CRC group, colorectal polyps group, and the control group. All subjects completed a 14C-urea breath test, bidirectional gastrointestinal endoscopy, and a biopsy on the same day. The characteristics of gastrointestinal endoscopy, pathology of gastritis, polyps and CRC, and the detection of H. pylori in the three groups were analyzed.
Patients with H. pylori infection were 2.19 times more likely to develop colorectal polyps and 3.05 times more likely to develop CRC than those who did not have H. pylori infection. The prevalence of H. pylori infection was higher in the multiple polyps group than in the solitary polyp group, and was also higher in the adenomatous polyps group than in the non-adenomatous polyps group. Additionally, we found that the incidence of H. pylori infection coexisting with atrophic gastritis or intestinal metaplasia was higher in patients with colorectal polyps and CRC than in the control group. The size and location of polyps, the histopathological characteristics and the location of CRC were not related to H. pylori infection.
The incidence of colonic polyps and CRC in patients with gastric H. pylori infection and H. pylori-associated atrophic gastritis or intestinal metaplasia was significantly higher than that in the normal population. Early and frequent colonoscopy is necessary to reduce the risk of colonic polyps and CRC in patients with H. pylori infection. The mechanism by which gastric H. pylori infection increases the incidence of colorectal polyps and CRC should be further studied.
This study demonstrates that early colonoscopy screening and surveillance are necessary to reduce the risk of colonic polyps and CRC in patients with H. pylori infection. The future direction of research is to evaluate whether the eradication of gastric H. pylori can reduce the occurrence of colorectal polyps and CRC. Large-scale and long-term follow-up investigations are needed.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56678] [Article Influence: 7084.8] [Reference Citation Analysis (135)] |
| 2. | ChangxiChen, Mao Y, Du J, Xu Y, Zhu Z, Cao H. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol. 2019;19:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Bailie L, Loughrey MB, Coleman HG. Lifestyle Risk Factors for Serrated Colorectal Polyps: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 4. | Syed AR, Thakkar P, Horne ZD, Abdul-Baki H, Kochhar G, Farah K, Thakkar S. Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol. 2019;11:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Sun M, Sun M, Zhang L, Shi S. Colorectal polyp risk is linked to an elevated level of homocysteine. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Senore C, Giovo I, Ribaldone DG, Ciancio A, Cassoni P, Arrigoni A, Fracchia M, Silvani M, Segnan N, Saracco GM. Management of Pt1 tumours removed by endoscopy during colorectal cancer screening: Outcome and treatment quality indicators. Eur J Surg Oncol. 2018;44:1873-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ansari S, Yamaoka Y. Current understanding and management of Helicobacter pylori infection: an updated appraisal. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (7)] |
| 10. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (7)] |
| 11. | Franceschi F, Covino M, Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24 Suppl 1:e12636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Nam JH, Hong CW, Kim BC, Shin A, Ryu KH, Park BJ, Kim B, Sohn DK, Han KS, Kim J, Lee CW. Helicobacter pylori infection is an independent risk factor for colonic adenomatous neoplasms. Cancer Causes Control. 2017;28:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Lee JY, Park HW, Choi JY, Lee JS, Koo JE, Chung EJ, Chang HS, Choe J, Yang DH, Myung SJ, Jung HY, Yang SK, Byeon JS. Helicobacter pylori Infection with Atrophic Gastritis Is an Independent Risk Factor for Advanced Colonic Neoplasm. Gut Liver. 2016;10:902-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Kim TJ, Kim ER, Chang DK, Kim YH, Baek SY, Kim K, Hong SN. Helicobacter pylori infection is an independent risk factor of early and advanced colorectal neoplasm. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 15. | Papastergiou V, Karatapanis S, Georgopoulos SD. Helicobacter pylori and colorectal neoplasia: Is there a causal link? World J Gastroenterol. 2016;22:649-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Hu KC, Wu MS, Chu CH, Wang HY, Lin SC, Liu CC, Su TH, Liao WC, Chen CL, Liu CJ, Shih SC. Decreased Colorectal Adenoma Risk After Helicobacter pylori Eradication: A Retrospective Cohort Study. Clin Infect Dis. 2019;68:2105-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Patel S, Lipka S, Shen H, Barnowsky A, Silpe J, Mosdale J, Pan Q, Fridlyand S, Bhavsar A, Abraham A, Viswanathan P, Mustacchia P, Krishnamachari B. The association of H. pylori and colorectal adenoma: does it exist in the US Hispanic population? J Gastrointest Oncol. 2014;5:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 18. | Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, Perez-Perez GI, Blaser MJ, Taylor PR, Virtamo J, Albanes D. Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:1095-1099. [PubMed] |
| 19. | Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Atrophic gastritis, Helicobacter pylori, and colorectal cancer risk: a case-control study. Helicobacter. 2007;12:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. 2001;96:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Liou JM, Lin JW, Huang SP, Lin JT, Wu MS. Helicobacter pylori infection is not associated with increased risk of colorectal polyps in Taiwanese. Int J Cancer. 2006;119:1999-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Liu WZ, Xie Y, Cheng H, Lv NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW, Xiao SD, Pan GZ, Hu PJ. The fourth national consensus report on the treatment of Helicobacter pylori infection. Zhonghua Xiaohua Zazhi. 2012;32:655-661. [DOI] [Full Text] |
| 23. | Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504-509. [PubMed] |
| 24. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 25. | Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 26. | Yan Y, Chen YN, Zhao Q, Chen C, Lin CJ, Jin Y, Pan S, Wu JS. Helicobacter pylori infection with intestinal metaplasia: An independent risk factor for colorectal adenomas. World J Gastroenterol. 2017;23:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 27. | Qing Y, Wang M, Lin YM, Wu D, Zhu JY, Gao L, Liu YY, Yin TF. Correlation between Helicobacter pylori-associated gastric diseases and colorectal neoplasia. World J Gastroenterol. 2016;22:4576-4584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Boyuk B, Ozgur A, Atalay H, Celebi A, Ekizoglu I, Aykurt E. Helicobacter pylori infection coexisting with intestinal metaplasia is not associated with colorectal neoplasms. Prz Gastroenterol. 2019;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 30. | Hong SN, Lee SM, Kim JH, Lee TY, Kim JH, Choe WH, Lee SY, Cheon YK, Sung IK, Park HS, Shim CS. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci. 2012;57:2184-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 31. | Zhang Y, Hoffmeister M, Weck MN, Chang-Claude J, Brenner H. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol. 2012;175:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 32. | Inoue I, Mukoubayashi C, Yoshimura N, Niwa T, Deguchi H, Watanabe M, Enomoto S, Maekita T, Ueda K, Iguchi M, Yanaoka K, Tamai H, Arii K, Oka M, Fujishiro M, Takeshita T, Iwane M, Mohara O, Ichinose M. Elevated risk of colorectal adenoma with Helicobacter pylori-related chronic gastritis: a population-based case-control study. Int J Cancer. 2011;129:2704-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Inoue I, Kato J, Tamai H, Iguchi M, Maekita T, Yoshimura N, Ichinose M. Helicobacter pylori-related chronic gastritis as a risk factor for colonic neoplasms. World J Gastroenterol. 2014;20:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 474] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 35. | Houli N, Loh SW, Giraud AS, Baldwin GS, Shulkes A. Mitogenic effects of both amidated and glycine-extended gastrin-releasing peptide in defunctioned and azoxymethane-treated rat colon in vivo. Regul Pept. 2006;134:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 37. | Finley GG, Koski RA, Melhem MF, Pipas JM, Meisler AI. Expression of the gastrin gene in the normal human colon and colorectal adenocarcinoma. Cancer Res. 1993;53:2919-2926. [PubMed] |
| 38. | Chao C, Hellmich MR. Gastrin, inflammation, and carcinogenesis. Curr Opin Endocrinol Diabetes Obes. 2010;17:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Horie H, Kanazawa K, Okada M, Narushima S, Itoh K, Terada A. Effects of intestinal bacteria on the development of colonic neoplasm: an experimental study. Eur J Cancer Prev. 1999;8:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Kanno T, Matsuki T, Oka M, Utsunomiya H, Inada K, Magari H, Inoue I, Maekita T, Ueda K, Enomoto S, Iguchi M, Yanaoka K, Tamai H, Akimoto S, Nomoto K, Tanaka R, Ichinose M. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun. 2009;381:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (3)] |
| 42. | Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770-5; quiz 711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 44. | Kountouras J, Kapetanakis N, Zavos C, Polyzos SA, Kouklakis G, Venizelos I, Nikolaidou C, Tzilves D, Paikos D, Katsinelos P, Giouleme O, Soufleris K. Active Helicobacter pylori infection is associated with colorectal mucosa-adenomatous polyp--early and advanced adenocarcinoma sequence. Scand J Gastroenterol. 2014;49:381-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Pilpilidis I, Kountouras J, Zavos C, Katsinelos P. Upper gastrointestinal carcinogenesis: H. pylori and stem cell cross-talk. J Surg Res. 2011;166:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Buzas G, Islek A, Karatapanis S, Lombardo L, Ribaldone DG S-Editor: Zhang L L-Editor: Webster JR E-Editor: Qi LL