Published online May 15, 2020. doi: 10.4251/wjgo.v12.i5.569
Peer-review started: December 29, 2019
First decision: February 20, 2020
Revised: March 24, 2020
Accepted: March 28, 2020
Article in press: March 28, 2020
Published online: May 15, 2020
Processing time: 136 Days and 11.3 Hours
Peritoneal metastasis (PM), arising from gastric cancer (GC), is the most common pattern of synchronous and metachronous dissemination and is generally associated with poor prognosis. New therapeutic modalities are being increasingly employed for such patients.
To develop more advanced methods, it becomes necessary to study the results of existing standard treatment methods in patients with PM in order to perform a comparative analysis of the strategies.
A retrospective analysis of the efficiency of standard treatment methods (i.e., palliative chemotherapy, palliative gastrectomy, and the best supportive care) was performed on 200 GC patients with synchronous PM.
The overall survival (OS) rate in 200 GC patients with PM under standard treatment was 5.4 mo. One-year survival occurred in 18.4% of patients. In multivariate analysis, the survival rate was significantly influenced by the following factors: Presence of extraperitoneal metastases, and stage of PM according to both the Japanese Gastric Cancer Association (JGCA) and the peritoneal cancer index (PCI). The median OS and 1-year survival of patients with Р1, P2, and P3 (JGCA) carcinomatosis were 9.8 mo, 6.7 mo, and 4.0 mo, and 47.2%, 18.8%, and 5.1%, respectively. The application of the palliative gastrectomy resulted in an increase in the median OS by up to 17 mo compared to the conservative approach where the value was 8.5 mo (P = 0.05) in patients with Р1 РМ. In patients with Р3, palliative chemotherapy increased the OS by up to 5.6 mo compared to the OS of 3.2 mo (P = 0.0006) for best supportive care. The median OS and 1-year survival of patients with РCI of 1-6, 7-12 and 13+ points were 8.5 mo, 4.2 mo, and 4.1 mo, and 39.8%, 6.7%, and 5.5%, respectively. Palliative gastrectomy increased the median OS to 12.6 mo compared to conservative approach of 8.0 mo (P = 0.03) in patients with РCI of 1-6 points. In patients with РCI 13+ points, only palliative chemotherapy increased the OS to 6.0 mo compared to the OS of 3.4 mo for best supportive care (P = 0.0008).
GC patients with PM are characterized by extremely poor prognoses. Long-term survivors were found in the group with PCI of 1-6 points, and there was no survival difference in groups with PCI 7-12 vs PCI 13+ points. Palliative gastrectomy could prove effective in treating patients with early stage PM. The three standard treatment methods are equally effective for moderate stages of PM. In cases with advanced peritoneal carcinomatosis, a significant increase in prognosis was registered only after treatment with palliative chemotherapy.
Core tip: This is a retrospective study to evaluate the efficacy of standard treatment methods (i.e., palliative chemotherapy, palliative gastrectomy, and the best supportive care) in gastric cancer patients with peritoneal metastases (PM). Analysis was performed on 200 patients with synchronous PM. Palliative gastrectomy could prove effective in treating patients with early stage PM. The three standard treatment methods are equally effective for moderate stages of PM. In cases with advanced peritoneal carcinomatosis, a significant increase in prognosis was registered only after treatment with palliative chemotherapy.
- Citation: Yarema R, Оhorchak М, Hyrya P, Kovalchuk Y, Safiyan V, Karelin I, Ferneza S, Fetsych M, Matusyak M, Oliynyk Y, Fetsych Т. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J Gastrointest Oncol 2020; 12(5): 569-581
- URL: https://www.wjgnet.com/1948-5204/full/v12/i5/569.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i5.569
Gastric cancer (GC) continues to be one of the leading types of malignancies worldwide despite an ongoing decrease in incidence. It is the fifth most common type of cancer in the world and the third leading cause of cancer-related deaths[1]. The peritoneum is the most frequent site where metastases occur and is also the first site of recurrence after radical surgery in 60% of all recurrences[2,3]. Peritoneal metastases (PM) are associated with poor prognosis – the median overall survival (OS) is 3–4 mo[4,5], with no survival at the 5th year after primary tumor resection[6].
To date, GC patients with PM have been treated using standard methods, such as palliative chemotherapy, palliative gastrectomy, or the best supportive care.
Systemic palliative chemotherapy in patients with intraperitoneally-disseminated GC is characterized by low efficiency and a minor increase in OS to nearly 8 mo[5,7].
Detection of PM in patients with GC during medical intervention is regarded by most surgeons as an absolute contraindication to resection surgery in full volume. This decision is based on negative results from retrospective and randomized trials[8,9]. At present, however, palliative surgery is being increasingly used in view of its efficiency in GC patients in the early stages of peritoneal carcinomatosis[10,11].
In oncological practice today, the following new active multimodal methods of combined treatment are frequently applied in GC patients with PM: Hyperthermic intraperitoneal chemotherapy (HIPEC)[12], early postoperative intraperitoneal chemotherapy[13], pressured intraperitoneal chemotherapy[14], neoadjuvant intraperitoneal chemotherapy[15], and repeated intraperitoneal chemotherapy[16], among others. The application of these advanced methods of combined treatment makes it important to study the results of standard treatment for such patients in order to perform a comparative analysis of strategies. The vast majority of medical publications on this topic are focused on a broad category of patients suffering from disseminated GC with metastases of different forms, whereas very few publications highlight separate studies on the efficiency of standard treatment methods for specific GC patient groups with PM[4].
The objective of this study was to investigate the results of palliative chemotherapy, palliative gastrectomy, and the best supportive care for GC patients with synchronous PM based on the stage of peritoneal carcinomatosis in compliance with the Japanese Gastric Cancer Association (JGCA) classification[17] and the peritoneal cancer index (PCI)[18]. The end goal was to generate objective data for comparative analysis in subsequent studies of new combined treatments.
A retrospective treatment analysis was performed on 200 GC patients with synchronous peritoneal metastases. All patients received inpatient treatment at the Clinic of Oncology and Medical Radiology at the Danylo Halytsky Lviv National Medical University on the basis of the Lviv Oncology Regional Treatment and Diagnostic Center from 2008 to 2017. As part of the study, we made an analysis of clinical and morphological prognostic factors and evaluated the efficiency of standard treatment methods (i.e., palliative chemotherapy, palliative sub- or total gastrectomy, and the best supportive care) based on indicators of PM dissemination rate, namely PCI and stage of peritoneal carcinomatosis according to JGCA.
Ages of the patients under study ranged from 30 years to 87 years, with a median age of 59.5 ± 7.9 years. The diagnosis of GC in all patients was verified morphologically prior to treatment onset. The GC study was conducted based on criteria from the TNM 7th edition classification (2009). The primary clinical and pathological features of patients are presented in Table 1.
| Characteristic | n (%) | |
| Sex | Male | 121 (60.5) |
| Female | 79 (39.5) | |
| Primary gastric cancer location | Antral part | 56 (28) |
| Corpus | 44 (22) | |
| Proximal part | 7 (3.5) | |
| Antral part + corpus | 34 (17) | |
| Corpus + proximal part | 12 (6) | |
| Subtotal or total lesion | 47 (23.5) | |
| Borrmann’s type | Type I | 3 (1.5) |
| Type II | 19 (9.5) | |
| Type III | 106 (53) | |
| Type IV | 72 (36) | |
| Tumor histology | G1 | 5 (2.5) |
| G2 | 12 (6) | |
| G3 | 54 (27) | |
| G4 | 98 (49) | |
| Signet ring cell | 24 (12) | |
| Mucinous | 3 (1.5) | |
| Unknown | 4 (2) | |
| Stage of peritoneal carcinomatosis according to Japanese classification (JGCA) | Р0 (Cyt+) | 4 (2) |
| Р1 | 46 (23) | |
| Р2 | 40 (20) | |
| Р3 | 110 (55) | |
| PCI, points [Median = 13 (0-37)] | 0 (Cyt+) | 4 (2) |
| 1-6 | 64 (32) | |
| 7-12 | 25 (12.5) | |
| 13 and more | 104 (52) | |
| Unknown | 3 (1.5) | |
| Ascites | Present | 87 (43.5) |
| Absent | 113 (56.5) | |
| Extraperitoneal metastases | Present | 34 (17) |
| Absent | 166 (83) | |
| Site of extraperitoneal metastases | Liver | 10 (29) |
| Non-regional lymph node | 16 (47) | |
| Pleura | 4 (12) | |
| Lung + non-regional lymph node | 2 (6) | |
| Suprarenal gland | 1 (3) | |
| Bones | 1 (3) | |
Assessment of the extent of intraperitoneal metastatic dissemination and its staging was carried out using PCI and peritoneal carcinomatosis staging by JGCA. Staging was performed during diagnostic laparoscopy, attempted laparotomy, non-resectable, or resectable palliative surgery. In patients without surgical verification of the peritoneal cavity, peritoneal carcinomatosis was staged on the basis of data obtained from spiral computed tomography.
РСІ was calculated in the following way: The abdominal cavity was divided into 13 conditional pelvic-abdominal sections. In each section, we evaluated the degree of carcinomatosis based on the size of PM from 1 to 3 points. This was followed by a summation of the values acquired from all sections; thus, the maximum value of PCI was 39[18].
Another objective method of the peritoneal carcinomatosis evaluation in patients with GC is JGCA classification: P0 (CY1) means there is no implantation metastases on the peritoneum, but there are malignant cells in the washings from the peritoneum, P1 means single metastases in the upper floor of the abdomen (above colon), P2 means single metastases in all departments of the abdominal cavity, and P3 implies diffuse carcinomatosis of the abdominal cavity, including the presence of ascites[17].
Assessment of the residual intraperitoneal metastatic process after resectable palliative surgery was based on completeness of the cytoreduction score (CC), calculated as follows: CC-0 - macroscopic residual tumor lesions on the peritoneum after surgery were not found; CC-1 - residual PM of not more than 2.5 mm in diameter were detected; CC-2 - the size of residual nodules was larger than 2.5 mm[18].
Two hundred patients with GC under this study received one treatment out of three modalities: Palliative chemotherapy, palliative gastrectomy (total or subtotal), or best supportive care. A detailed description of the treatment modalities applied to patients in this study is given in Table 2.
| Treatment modality | n (%) | |
| BSC | Total | 105 (52.5) |
| Only BSC | 31 (29.5) | |
| Attempted or non-resectable palliative surgery + BSC | 74 (70.5) | |
| Stage of peritoneal carcinomatosis according to JGCA | ||
| P0 (Cyt+) | 0 | |
| P1 | 20 (19) | |
| P2 | 23 (21.9) | |
| P3 | 62 (59.1) | |
| PCI | ||
| 0 (Cyt+) | 0 | |
| 1-6 | 31 (29.5) | |
| 7-12 | 13 (12.4) | |
| 13 + | 60 (57.2) | |
| Unknown | 1 (0.9) | |
| Palliative chemotherapy | Total | 51 (25.5) |
| Only palliative chemotherapy | 24 (47) | |
| Attempted or non-resectable palliative surgery + palliative chemotherapy | 27 (53) | |
| Stage of peritoneal carcinomatosis according to JGCA | ||
| P0 (Cyt+) | 0 | |
| P1 | 6 (11.8) | |
| P2 | 9 (17.6) | |
| P3 | 36 (70.6) | |
| PCI | ||
| 0 (Cyt+) | 0 | |
| 1-6 | 9 (17.6) | |
| 7-12 | 8 (15.7) | |
| 13 + | 32 (62.7) | |
| Unknown | 2 (4) | |
| Chemotherapy regimen | ||
| CF | 19 (37.2) | |
| 5-FU | 11 (21.6) | |
| CAF | 10 (19.6) | |
| XELOX | 7 (13.7) | |
| ECF | 3 (5.9) | |
| Tegafur | 1 (2) | |
| Palliative gastrectomy | Total | 44 (22) |
| Only palliative gastrectomy | 40 (91) | |
| Palliative gastrectomy + palliative chemotherapy | 4 (9) | |
| Stage of peritoneal carcinomatosis according to JGCA | ||
| P0 (Cyt+) | 4 (9.1) | |
| P1 | 20 (45.4) | |
| P2 | 8 (18.2) | |
| P3 | 12 (27.3) | |
| PCI | ||
| 0 (Cyt+) | 4 (9.1) | |
| 1-6 | 24 (54.5) | |
| 7-12 | 4 (9.1) | |
| 13 + | 12 (27.3) | |
| Lymph node dissection | ||
| D0, 1 | 35 (79.5) | |
| D1+, 2 | 9 (20.5) | |
| Completeness of cytoreduction score | ||
| CC-0 | 9 (20.5) | |
| CC-1 | 19 (43.2) | |
| CC-2 | 16 (36.3) | |
In the “best supportive care” and “palliative chemotherapy” groups, 49 patients underwent attempted (exploratory) laparotomy and biopsy, while 52 patients received non-resectable palliative surgery. The latter group underwent gastroenterostomy (43 patients, 82.9%), jejunostomy, cardia’s stenting, and tumor perforation suturing of the stomach (2 patients each, 3.8% each), ileotransversostomy, sigmostomy, and stoppage of gastrointestinal bleeding (1 patient each, 1.9% each).
In 44 patients of the “palliative gastrectomy” group, the following procedures were performed: total gastrectomy in 19 patients (43.2%), distal subtotal gastrectomy in 21 patients (47.7%), and total gastrectomy with lower esophageal resection in 4 patients (9.1%). Of these 44 patients, macroscopically complete cytoreduction of peritoneal metastases was achieved in nine (20.5%): Four patients underwent palliative surgery with P0Cyt+ and five patients went through the removal of metastases implantation.
After treatment was completed, all patients were followed up on a regular basis. We performed physical check-ups and ultrasonography of the abdominal cavity every 3 mo, as well as computerized tomography and chest radiography every 6 mo. The overall survival was measured from the date of surgery or the date of the other therapy onset to the date of death or the last follow-up examination.
Statistical analysis of the primary data was performed using SPSS 22 and Statistical 6 software. The censored Kaplan-Meier method helped to evaluate the cumulative survival of patients, whereas the reliability of the survival difference in certain groups was determined using a log-rank coefficient. Multivariate analysis was performed using the Cox model, and Pearson’s linear correlation coefficient allowed the determination of statistical correlations.
Post-operative morbidity (Clavien-Dindo grade I-IV complications) developed in 15 patients (10.3%) after exploratory, palliative non-resectable, or palliative resectable surgery. The 60-d postoperative mortality rate was 3.5% (5 patients): 3 patients died after the palliative gastrectomy; 1 patient after gastroenterostomy as a result of surgical complications; and 1 patient after colostomy, due to complications of GC. Overall, complications from palliative chemotherapy were observed in 8 patients (14.5%).
The median follow-up time for the study was 45.2 mo. The OS rate for 200 GC patients with PM, who underwent standard treatment, was 5.4 mo [95% confidence interval (CI): 4.4–6.8]. The 1-year survival rate was 18.4% (95%CI: 13.0–24.6). Results of the univariate analysis of prognostic factors are presented in Table 3.
| n | 1-yr survival, % | Median survival in mo | P value | |
| Gender | ||||
| Male | 121 | 18.4 | 5.2 | 0.4 |
| Female | 79 | 18.5 | 6.5 | |
| Age | ||||
| < 60 | 100 | 21.1 | 6.5 | 0.26 |
| 60+ | 100 | 15.4 | 5.0 | |
| Borrmann’s type | ||||
| I-II | 22 | 34.3 | 6.8 | 0.13 |
| III-IV | 178 | 16.5 | 5.2 | |
| Ascites | ||||
| Absence | 113 | 29.5 | 6.9 | < 0.0001 |
| Presence | 87 | 1.7 | 4.0 | |
| Histology | ||||
| G1 | 5 | 0 | 3.6 | 0.64 |
| G2 | 12 | 40 | 6.8 | |
| G3 | 54 | 13.5 | 5.6 | |
| G4 | 98 | 21.2 | 5.2 | |
| Signet ring | 24 | 9.5 | 6.5 | |
| Mucinous | 3 | 0 | 3.7 | |
| Extraperitoneal metastases | ||||
| No | 165 | 19.7 | 6.4 | 0.031 |
| Yes | 34 | 7.7 | 4.2 | |
| JGCA classification | ||||
| P0 (Cyt+) | 4 | 0 | 4.0 | < 0.0001 |
| P1 | 46 | 47.2 | 9.8 | |
| P2 | 40 | 18.8 | 6.7 | |
| P3 | 110 | 5.1 | 4.0 | |
| PCI | ||||
| 0 (Cyt+) | 4 | 0 | 4.0 | < 0.0001 |
| 1-6 | 64 | 39.8 | 8.5 | |
| 7-12 | 25 | 6.7 | 4.2 | |
| 13+ | 104 | 5.5 | 4.1 | |
| Lymph node dissection | ||||
| D0, 1 | 35 | 31.4 | 7.5 | 0.61 |
| D1+, 2 | 9 | 50.0 | 8.9 | |
| Cytoreduction score | ||||
| CC-0 | 9 | 22.2 | 7.5 | 0.065 |
| CC-1 | 19 | 58.2 | 13.1 | |
| CC-2 | 16 | 18.8 | 4.7 | |
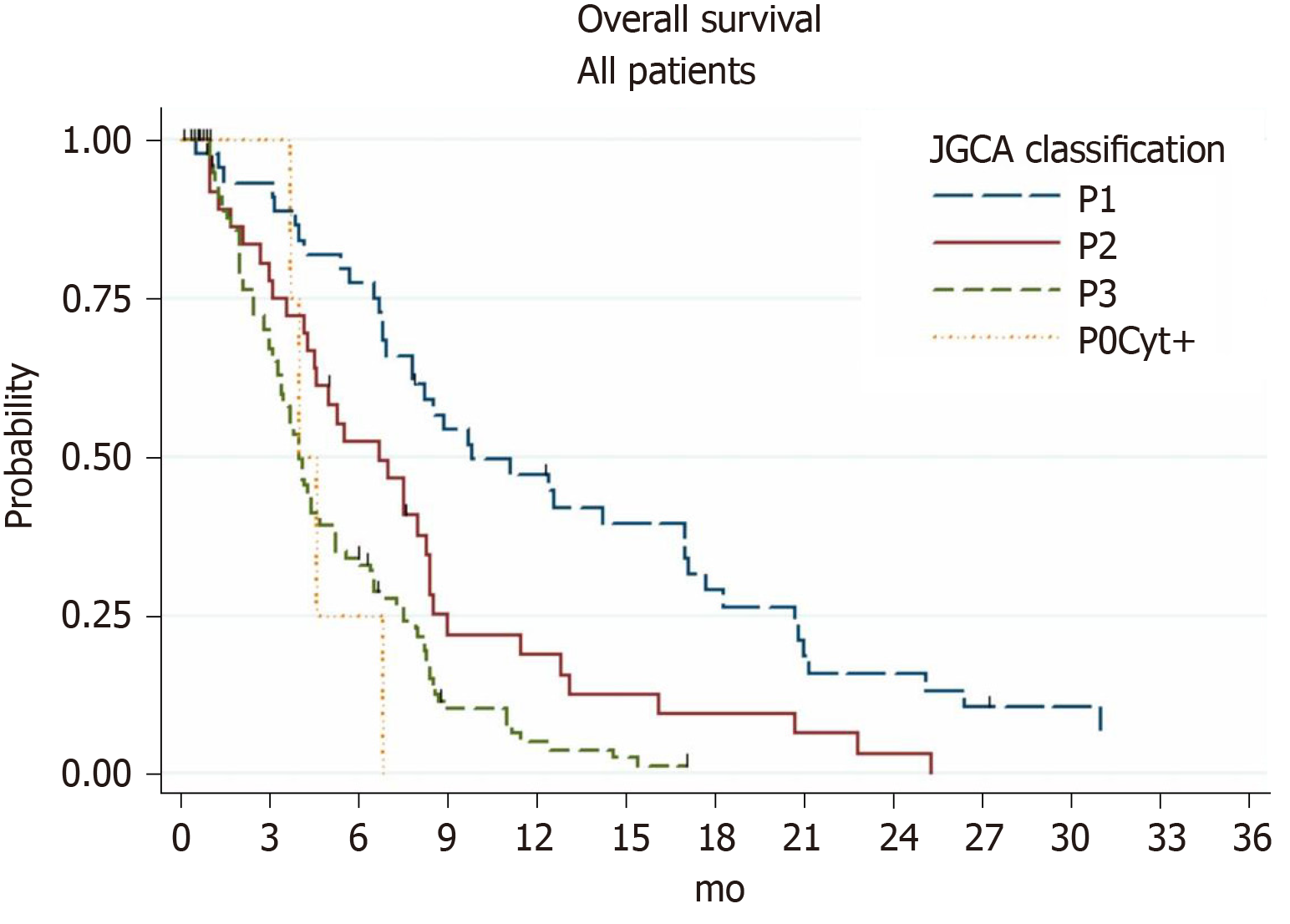
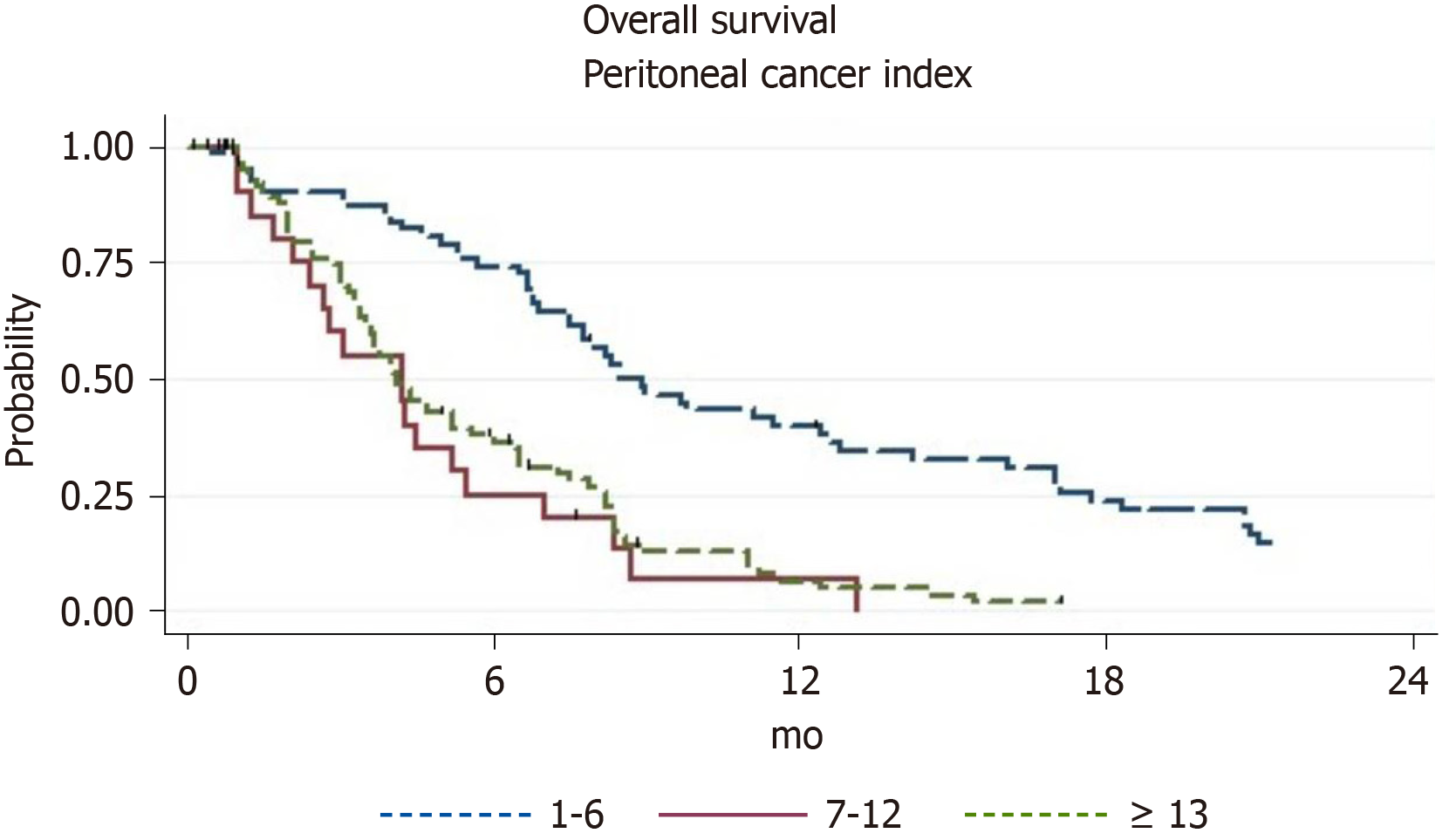
By means of the univariate analysis, we discovered that the following factors significantly influenced patient survival: Presence of ascites, presence of extraperitoneal metastases, stage of PM according to JGCA (Figure 1), and PCI (Figure 2). However, there was no statistical difference between the prognosis of patients with PCI 7-12 and patients with PCI 13+ points (P = 0.45).
In multivariate analysis, the following factors retained their statistical significance: the presence of extraperitoneal metastases [hazard ratio (HR) = 1.55, 95%CI: 0.95-2.42; P = 0.054], the stage of PM according to JGCA (HR = 3.41, 95%CI: 2.19-5.29; P = 0, 0001), and the PCI (HR = 1.78, 95%CI: 1.05-3.01; P = 0.032). The presence of ascites lost their statistical significance (HR = 1.32, 95%CI: 0.80-2.20; P = 0.28).
Table 2 shows a clear tendency to perform palliative gastrectomy in patients with early stage PM, and to use palliative chemotherapy and best supportive care for patients with diffuse peritoneal carcinomatosis. In this regard, further analysis of the efficiency of standard therapies was performed in those groups of patients, who were grouped on the basis of peritoneal carcinomatosis stages (PCI index and JGCA classification of GC).
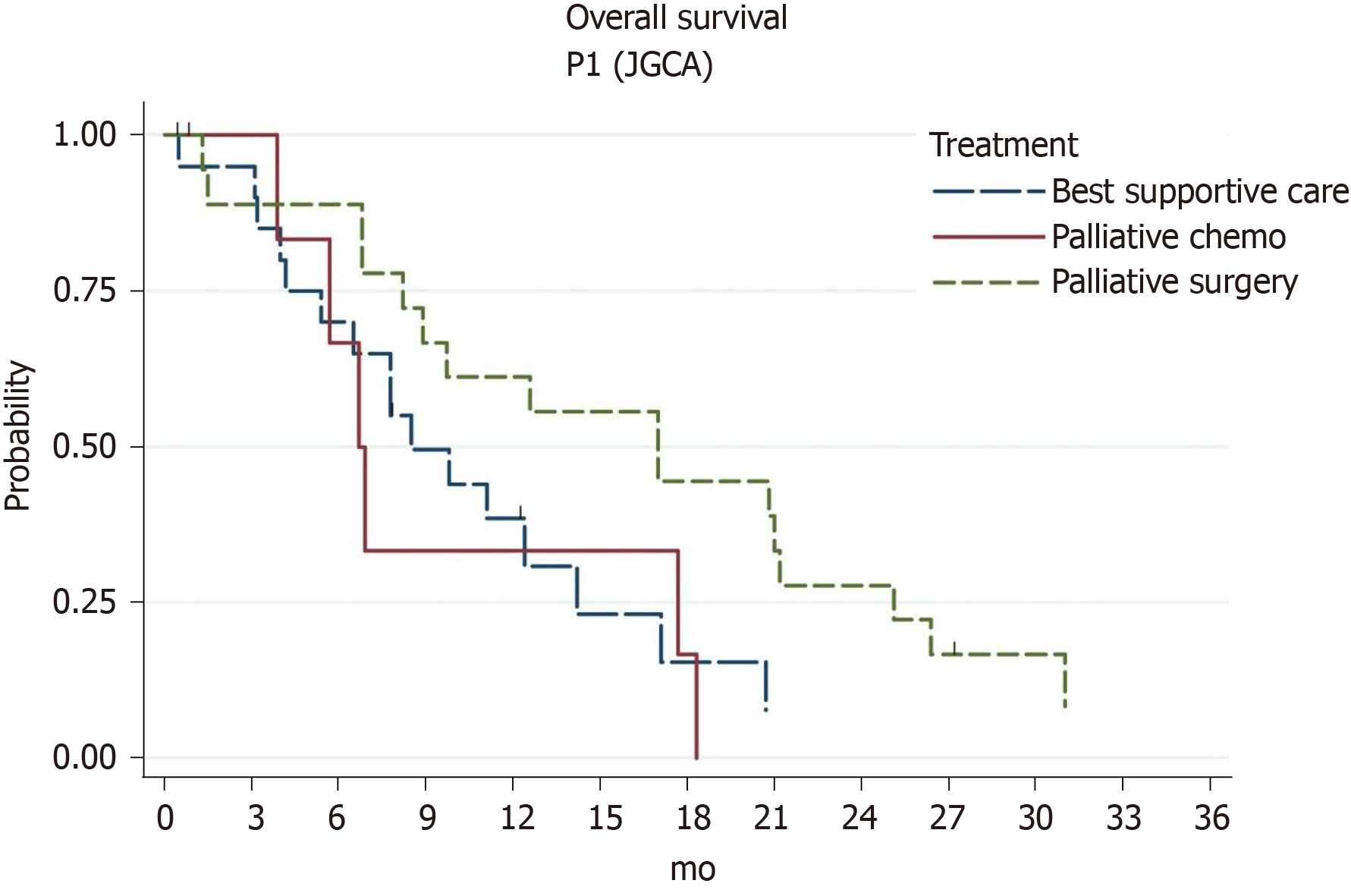
The median OS of 46 GC patients with P1 carcinomatosis was 9.8 mo (95%CI: 6.9-17.0), 1-year survival rate was 47.2% (95%CI: 31.9-61.1), and 2-year survival was 15.7% (95%CI: 6.5-28.9). Patients with extraperitoneal metastases had a median survival rate of 3.9 mo compared to 12.4 mo for patients without extraperitoneal metastases (P = 0.0001). Treatment with palliative gastrectomy resulted in a probable increase in survival compared to the conservative treatment approach (Table 4).
| n | 1-yr survival, % | Median survival in mo | P value | |
| P1 | ||||
| BSC | 20 | 38.5 | 8.5 | 0.12 |
| Palliative chemotherapy | 6 | 33.3 | 6.7 | |
| Palliative gastrectomy | 20 | 61.1 | 17 | |
| BSC | 20 | 38.5 | 8.5 | 0.71 |
| Palliative chemotherapy | 6 | 33.3 | 6.7 | |
| BSC | 20 | 38.5 | 8.5 | 0.032 |
| Palliative gastrectomy | 20 | 61.1 | 17 | |
| Conservative approach of BSC + palliative chemotherapy | 26 | 37.5 | 7.8 | 0.05 |
| Palliative gastrectomy | 20 | 61.1 | 17 | |
| P2 | ||||
| BSC | 23 | 11.7 | 5 | 0.56 |
| Palliative chemotherapy | 9 | 22.2 | 8.4 | |
| Palliative gastrectomy | 8 | 37.5 | 7.5 | |
| P3 | ||||
| BSC | 62 | 2.3 | 3.2 | 0.003 |
| Palliative chemotherapy | 36 | 7.8 | 5.6 | |
| Palliative gastrectomy | 12 | 8.3 | 4.7 | |
| BSC | 62 | 2.3 | 3.2 | 0.2 |
| Palliative gastrectomy | 12 | 8.3 | 4.7 | |
| BSC | 62 | 2.3 | 3.2 | 0.0006 |
| Palliative chemotherapy | 36 | 7.8 | 5.6 | |
The median OS of 40 GC patients with P2 carcinomatosis was 6.7 mo (95%CI: 4.3-8.4) and 1-year survival rate was 18.8% (95%CI: 7.8-33.5). Results of the standard treatment methods comparison did not show a statistical difference.
The median OS of 110 GC patients with P3 carcinomatosis was 4.0 mo (95%CI: 3.4-4.7) and 1-year survival rate was 5.1% (95%CI: 1.7-11.3). Only palliative chemotherapy showed a statistically probable increase in survival compared to best supportive care.
The median OS of 64 GC patients with PCI 1-6 points was 8.5 mo (95%CI: 7.5-12.4), 1-year survival rate was 39.8% (95%CI: 27.6-51.8), and 2-year survival rate was 12.7% (95%CI: 5.6-22.7). Patients with extraperitoneal metastases had a 1-year survival rate of 16.7% (95%CI: 0.7-51.7) compared to 41.2% (95%CI: 28.1-53.9) for patients without extraperitoneal metastases (P = 0.012). Patients who were classified as P1 in JGCA with a PCI of 1-6 points had a 1-year survival rate of 47.2% (95%CI: 31.9-61.1) compared to 22.2% (95%CI: 6.9-42.9) for P2 patients (P = 0.042). The use of palliative gastrectomy led to a probable increase in survival compared to the conservative treatment approach (Table 5).
| n | 1-year survival, % | Median survival, mo | P value | |
| PCI 1-6 | ||||
| BSC | 31 | 30.8 | 8.3 | 0.089 |
| Palliative chemotherapy | 9 | 33.3 | 6.9 | |
| Palliative gastrectomy | 24 | 54.5 | 12.6 | |
| BSC | 31 | 30.8 | 8.3 | 0.94 |
| Palliative chemotherapy | 9 | 33.3 | 6.9 | |
| BSC | 31 | 30.8 | 8.3 | 0.045 |
| Palliative gastrectomy | 24 | 54.5 | 12.6 | |
| Conservative approach of BSC + palliative chemotherapy | 40 | 31.5 | 8.0 | 0.03 |
| Palliative gastrectomy | 24 | 54.5 | 12.6 | |
| D0,1 lymph node dissection | 18 | 52.9 | 12.6 | 0.91 |
| D1+, 2 lymph node dissection | 6 | 60.0 | 17.0 | |
| PCI 7-12 | ||||
| BSC | 13 | 0 | 2.1 | 0.082 |
| Palliative chemotherapy | 8 | 0 | 4.3 | |
| Palliative gastrectomy | 4 | 50.0 | 4.2 | |
| PCI 13+ | ||||
| BSC | 60 | 2.5 | 3.4 | 0.004 |
| Palliative chemotherapy | 32 | 8.9 | 6.0 | |
| Palliative gastrectomy | 12 | 8.3 | 4.7 | |
| BSC | 60 | 2.5 | 3.4 | 0.26 |
| Palliative gastrectomy | 12 | 8.3 | 4.7 | |
| BSC | 60 | 2.5 | 3.4 | 0.0008 |
| Palliative chemotherapy | 32 | 8.9 | 6.0 | |
The median OS of 25 GC patients with PCI 7-12 points was 4.2 mo (95%CI: 2.1-5.5) and 1-year survival was 6.7% (95%CI: 0.49-25.2). Comparison of standard treatment methods did not show a statistically probable difference.
The median OS of 104 GC patients with PCI 13+ points was 4.1 mo (95%CI: 3.6-5.2), with a 1-year survival of 5.5% (95%CI: 1.9-12.2). Only palliative chemotherapy showed a statistically probable increase in survival rate compared to the best supportive care.
In view of a large clinical study currently being conducted to evaluate aggressive multimodal therapeutic approaches to treating GC patients, there is a need to have additional knowledge of the natural history of GC with PM. The results of our study confirmed an extremely poor prognosis (median OS of 5.4 mo) in GC patients, which depends on the extent of PM dissemination, as previously reported. Thus, the French multicenter study EVOCAPE1 demonstrated that the median OS of GC patients with sporadic PM was 7.9 mo, whereas it was 1.9 mo in those with diffuse peritoneal carcinomatosis[4]. Our study also discovered a probable prognosis dependence on the extent of PM dissemination. Thus, patients with early stage PM (according to JGCA) had an OS of 9.8 mo, whereas patients with diffuse peritoneal carcinomatosis had an OS of only 4 mo.
Research on HIPEC efficiency for GC patients, as previously reported, shows that prognosis is directly dependent on the level of PCI[19]. However, our study of standard treatment methods does not show any significant difference in the survival of patients with PCI of 7-12 points vs those with PCI of 13 or more. Long-term survival was observed only in patients with PCI of 1-6 points; median OS was 8.2 mo vs 4.1 mo in patients with PCI > 6. Similar unexpected results were obtained in other reports[20].
Discussion of the reasonability of palliative surgery in the resection volume of patients with intraperitoneally-disseminated GC remains open. Thus, in a randomized REGATTA trial[8], the use of gastrectomy with systemic palliative chemotherapy did not result in a significant survival advantage over chemotherapy alone. However, according to a number of retrospective studies, palliative gastrectomy feasibly increases the survival in patients at early stages of PM (P1, P2 dissemination according to JGCA), but at the same time does not affect the prognosis of patients with diffuse peritoneal carcinomatosis (P3 dissemination)[10,11,21]. In addition, according to recent data, survival rates in patients with good responsiveness to initial chemotherapy increase after subsequent palliative gastrectomy (conversion surgery)[22].
The results of our study also indicate a significant increase in survival after palliative gastrectomy in patients with early stage PM (Figure 3). Thus, at P1 stage of carcinomatosis (JGCA), a double increase in the median survival rate was recorded compared to the conservative approach (7.8-17 mo). In patients with PCI of 1-6 points, palliative gastrectomy also feasibly increased prognosis (8-12.6 mo). However, in patients with diffuse peritoneal carcinomatosis (P3, PCI > 6), palliative gastrectomy did not increase survival rate, as expected.
The results of this study showed no probable influence on the survival of intraperitoneally-disseminated GC patients of lymph node dissection volume during palliative gastrectomy. Naturally, this is conditioned by the development of early intraperitoneal relapses, which determine prognosis to a large extent. Besides, there was no influence registered on the survival rate of such patients after complete cytoreduction (CC-0). This is obviously because intraperitoneal chemotherapy was not studied in the course of patient treatment. However, a number of studies on HIPEC efficiency demonstrated a probable influence of complete cytoreduction on survival rates[12,19,23].
The results of palliative chemotherapy application in this study are consistent with data from the literature, although the inefficiency of this method for patients with early stage PM requires further analysis.
In summary, the obtained results of standard treatment methods applied to GC patients with PM may form the basis for comparative analyses of the efficiencies of new combined therapies for such patients in subsequent studies.
GC patients with PM are characterized by extremely poor prognoses. In this study, long-term survivors were found in the group with PCI of 1-6 points with no survival difference in the group with PCI 7-12 vs the group with PCI 13+ points. Palliative gastrectomy could be an effective treatment method for patients with early stage PM. The three standard treatment methods are equally effective for moderate stages of PM. With advanced peritoneal carcinomatosis, a significant increase in prognosis is only recorded after palliative chemotherapy.
Peritoneal metastasis is the most common pattern of synchronous and metachronous dissemination of gastric cancer (GC). Such patients are characterized by poor prognoses. New therapeutic modalities are being increasingly employed. However, the results of existing standard treatment methods remain insufficiently reported in the literature.
A large number of reports of results from modern methods of combined treatment of GC patients with peritoneal metastases (hyperthermic intraperitoneal chemotherapy, early postoperative intraperitoneal chemotherapy, pressurized intraperitoneal aerosol chemotherapy, etc) are now available in the literature. Most of them relate to early stages of peritoneal carcinomatosis (according to classifications of the Japanese Gastric Cancer Association and peritoneal cancer index). However, medical publications on standard treatment topics are focused on a broad category of patients with intraperitoneally-disseminated GC or with metastases of different forms.
To develop more advanced methods, it becomes necessary to study the results of existing standard treatment methods (i.e., palliative chemotherapy, palliative gastrectomy, and best supportive care) in patients with intraperitoneally-disseminated GC in order to perform a comparative analysis of strategies.
A retrospective analysis of the efficiency of standard treatment methods was performed on 200 GC patients with synchronous peritoneal metastases.
The median overall survival and 1-year survival of patients with РCI of 1-6, 7-12, and 13+ points were 8.5 mo, 4.2 mo, and 4.1 mo, and 39.8%, 6.7%, and 5.5%, respectively. Long-term survivors were found in the group with PCI of 1-6 points and there was no survival difference in groups with PCI 7-12 vs PCI 13+ points. Palliative gastrectomy increased the median overall survival to 12.6 mo compared to conservative approach of 8.0 mo in patients with РCI of 1-6 points. In patients with РCI 13+ points, only palliative chemotherapy increased the overall survival to 6.0 mo compared to 3.4 mo for best supportive care.
GC patients with peritoneal metastases are characterized by extremely poor prognoses. Palliative gastrectomy could prove effective in treating patients with early stage peritoneal metastases. The three standard treatment methods are equally effective for moderate stages of peritoneal metastases. In cases with far advanced peritoneal carcinomatosis, a significant increase in prognosis was registered only after treatment with palliative chemotherapy.
Generated objective data of the study could be used for comparative analysis in subsequent studies of new combined treatments.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20720] [Article Influence: 1883.6] [Reference Citation Analysis (23)] |
| 2. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 375] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 3. | Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer. 2014;17:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 5. | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 448] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 6. | Bozzetti F, Yu W, Baratti D, Kusamura S, Deraco M. Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol. 2008;98:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 607] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 8. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T; REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 530] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 9. | Yoshikawa T, Kanari M, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Should gastric cancer with peritoneal metastasis be treated surgically? Hepatogastroenterology. 2003;50:1712-1715. [PubMed] |
| 10. | Molfino S, Ballarini Z, Gheza F, Portolani N, Baiocchi GL. Is there a role for treatment-oriented surgery in stage IV gastric cancer? A systematic review. Updates Surg. 2019;71:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Hioki M, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg. 2010;34:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O; FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 13. | Kim DW, Park DG, Song S, Jee YS. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Treatment Options for Peritoneal Metastasis of Advanced Gastric Cancer. J Gastric Cancer. 2018;18:296-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J Gastrointest Surg. 2016;20:367-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Yamaguchi H, Kitayama J, Ishigami H, Kazama S, Nozawa H, Kawai K, Hata K, Kiyomatsu T, Tanaka T, Tanaka J, Nishikawa T, Otani K, Yasuda K, Ishihara S, Sunami E, Watanabe T. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: Intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol. 2015;7:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kitayama J, Ishigami H, Yamaguchi H, Sakuma Y, Horie H, Hosoya Y, Lefor AK, Sata N. Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2018;2:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 18. | Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg. 1999;384:576-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 206] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Rihuete Caro C, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano Á, Pérez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44:1805-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Gretschel S, Siegel R, Estévez-Schwarz L, Hünerbein M, Schneider U, Schlag PM. Surgical strategies for gastric cancer with synchronous peritoneal carcinomatosis. Br J Surg. 2006;93:1530-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Beom SH, Choi YY, Baek SE, Li SX, Lim JS, Son T, Kim HI, Cheong JH, Hyung WJ, Choi SH, Jung M, Kim HS, Jeung HC, Chung HC, Rha SY, Noh SH. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Rau B, Brandl A, Thuss-Patience P, Bergner F, Raue W, Arnold A, Horst D, Pratschke J, Biebl M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer. 2019;22:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Surgical Oncology (ESSO)
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cao ZF, Matowicka-Karna J, Ieni A S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Qi LL