Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.111425
Revised: August 20, 2025
Accepted: November 13, 2025
Published online: December 27, 2025
Processing time: 180 Days and 13.6 Hours
Recurrence prediction of hepatocellular carcinoma (HCC) after thermal ablation represents a challenge that can impact patients' quality of life. Artificial intel
To evaluate the effectiveness of AI-driven predictive models in predicting HCC recurrence.
A systematic literature search in PubMed and Scopus was performed, and a total of ten studies were included in this systematic review. All studies included res
The developed models demonstrated high accuracy in predicting local pro
AI-driven predictive models based on multimodal radiomic analyses integrated with clinical data represent promising tools for predicting tumor recurrence after thermal ablation in HCC patients.
Core Tip: Artificial intelligence can aid the prediction of post-thermal ablation treatment recurrence of hepatocellular carcinoma, improving patient's quality of life, tailoring the follow-up and avoiding unnecessary treatments while providing an early recognition of tumor recurrence.
- Citation: Posa A, Lippi M, Barbieri P, Andreani EV, Iezzi R. Performance of artificial intelligence in predicting hepatocellular carcinoma recurrence after thermal ablation: A systematic review. World J Hepatol 2025; 17(12): 111425
- URL: https://www.wjgnet.com/1948-5182/full/v17/i12/111425.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i12.111425
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide, most commonly affecting patients with underlying liver cirrhosis and significantly reducing life expectancy[1].
In early-stage HCC, defined by the Barcelona Clinic Liver Cancer guidelines as up to three lesions, each measuring no more than 3 cm, the treatment of choice is liver transplantation, which is considered the only curative option[2]. When transplantation is not feasible, surgical resection may be considered[3]. However, not all patients are eligible for surgery due to factors such as lesion location, comorbidities, or patient refusal. In such cases, heat-based thermal ablation plays a key role[4]. These techniques, including radiofrequency ablation (RFA) and microwave ablation (MWA), provide lo
Despite their minimally invasive nature, these techniques have limitations, especially in case of larger or multiple lesions, where complete response rates may be suboptimal. Moreover, even after an initial complete response, recurrence-free survival may be suboptimal due to early recurrence, often driven by undetected microsatellite nodules, thereby wor
Predicting early recurrence after thermal ablation is a major focus of research since the introduction of these techniques. The ability to anticipate treatment response and recurrence risk would allow clinicians to better tailor the
In recent years, the rise of radiomics and artificial intelligence (AI)-based models [deep learning (DL) models and machine learning (ML) ones] has transformed predictive oncology, showing promise in forecasting treatment response and tumor recurrence across a wide range of malignancies[9].
The aim of this systematic review is to evaluate the performance of AI-based models (both DL and ML) in predicting early recurrence of HCC following thermal ablation, with a particular focus on metrics such as the area under the curve (AUC), sensitivity, specificity, positive and negative predictive values, concordance index (C-index), and accuracy.
This systematic review was registered in PROSPERO (CRD420251120913) and was designed using the Population, Intervention, Comparator, Outcome framework to define the clinical question and inclusion criteria[10]. The framework was constructed as follows:
Population: Adult patients diagnosed with HCC treated with curative intent using heat-based thermal ablation tech
Intervention: Application of AI, ML, or DL models to predict local recurrence following thermal ablation, integrating imaging and clinical information or using imaging alone.
Comparator: Conventional prediction models, follow-up examinations, or no comparator in cases where the study was purely predictive without controls.
Outcome: Diagnostic performance of AI-based models in predicting recurrence, evaluated using metrics such as AUC, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), C-index, and accuracy.
Studies eligible for inclusion comprised randomized controlled trials (RCTs) and prospective and retrospective cohort studies.
The PRISMA standards were used to conduct this systematic review[11]. A comprehensive literature search was performed in three electronic databases: PubMed, Scopus, and Web of Science. The search aimed to identify all relevant studies evaluating the use of AI, ML, or DL models to predict local recurrence after heat-based thermal ablation (RFA or MWA) in patients with HCC.
The following keywords and medical subject headings terms were used alone and in multiple combinations: “HCC” “Hepatocellular carcinoma” “Recurrence” “Ablation” “RFA” “MWA” “AI” “Artificial Intelligence” “Radiomic” “Machine learning” “Deep learning” “Prediction”; in particular, the combinations used were: HCC recurrence ablation AI; AI recurrence HCC ablation; recurrence RFA HCC radiomic; recurrence ablation HCC radiomic; prediction ablation HCC AI; prediction ML model ablation HCC. The search was designed to maximize sensitivity and database coverage. No restrictions on the year of publication were applied, up to June 2025, and only articles published in English were considered. The following exclusion criteria were applied: Case reports, conference abstracts, reviews, technical notes, editorials, book chapters, surveys, and letters to the editor. Only peer-reviewed original articles were considered eligible.
Two authors (Lippi M, Andreani EV) independently screened the titles and abstracts of all identified records to assess their eligibility for inclusion. Any disagreements regarding inclusion were resolved through a discussion with a third expert author (Barbieri P). Final inclusion decision and methodological consistency were independently verified and app
Studies were excluded if they focused on patients with malignancies other than HCC, included treatments other than heat-based thermal ablation, or assessed only clinical prediction models without AI or radiomics components.
The primary outcomes extracted from the included studies were: AUC, sensitivity, specificity, PPV, NPV, C-Index, and accuracy of the models in the context of predicting local recurrence of HCC after RFA or MWA.
The GRADEpro Guideline Development Tool (GDT) was used to create table summaries of results in Cochrane sy
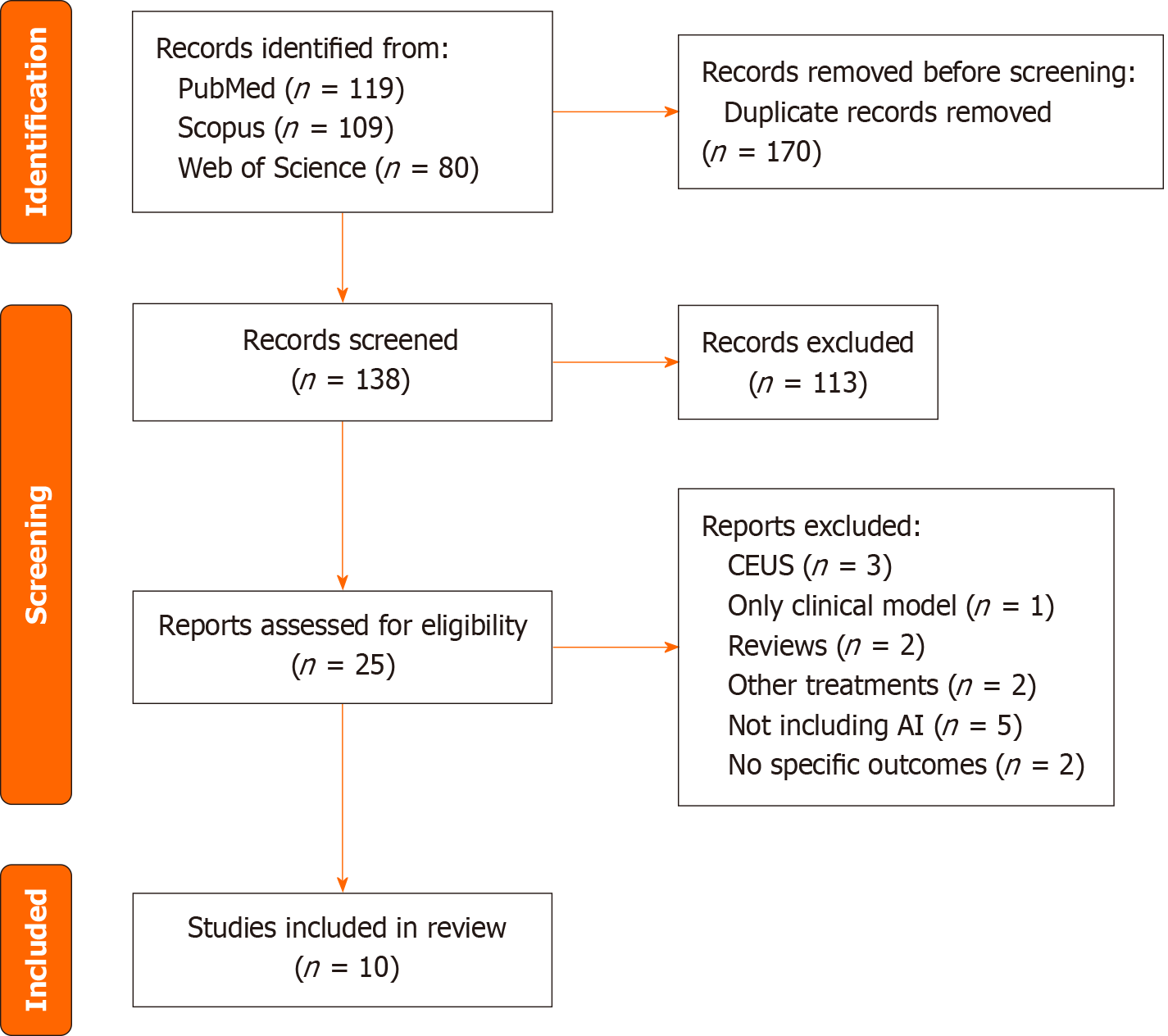
The literature search initially yielded 308 articles. After removal of duplicates, 138 articles remained for title and abstract screening. Based on the predefined inclusion and exclusion criteria, 113 records were excluded. A total of 25 full-text articles were assessed for eligibility. Among these, 3 studies were excluded for using contrast-enhanced ultrasound as imaging evaluation modality, 1 study was excluded for focusing exclusively on a clinical (non-imaging based) predictive model, 2 were reviews and thus excluded, 2 studies investigated treatment approaches other than RFA or MWA, 5 did not employ ML or DL models, 1 article included outcomes related to secondary liver lesions, 1 focused on post-treatment outcomes of other therapies. Ultimately, 10 studies met the inclusion criteria and were included in the final synthesis[12-21] (Table 1). The study selection process is illustrated in the PRISMA flow diagram (Figure 1).
| Ref. | Study type | Patient number | Model | AUC | Sensitivity | Specificity | C-index | PPV | NPV | Accuracy |
| Lim et al[12] | Retro | 74 | DL | 0.99 | 96% | 97% | N/A | 0.91 | N/A | 97.6% |
| Sato et al[13] | Retro | 1778 | ML + C | N/A | N/A | N/A | 0.67 | N/A | N/A | N/A |
| Zhang et al[14] | Retro | 90 | ML + C | 0.86 | N/A | N/A | N/A | N/A | N/A | N/A |
| Tabari et al[15] | Retro | 97 | ML + R + C | 0.83 | 82% | 67% | N/A | 0.69 | 0.80 | N/A |
| Peng et al[16] | Retro | 149 | ML + R + C | N/A | N/A | N/A | 0.72 | N/A | N/A | N/A |
| Ren et al[17] | Retro | 607 | ML + C | 0.89 | N/A | 85% | N/A | N/A | N/A | N/A |
| Chen et al[18] | Retro | 417 | DL + R + C | 0.87 | 86% | 91% | N/A | 0.79 | 0.90 | 88% |
| Wang et al[19] | Retro | 535 | DL + R + C | 0.79 | 72% | 86% | N/A | N/A | N/A | 78% |
| Kong and Li[20] | Retro | 289 | DL + R + C | 0.74 | 71% | 71% | N/A | 0.39 | 0.91 | 71% |
| Li et al[21] | Retro | 288 | DL + R + C | 0.98 | N/A | N/A | N/A | 0.94 | 0.87 | 91.6% |
The ten included studies, published between 2022 and 2025, involved a total of 4324 patients with HCC treated with heat-based thermal ablation techniques, either RFA or MWA. Specifically, all patients underwent RFA in 2 studies[13,14], all patients were treated with MWA in 1 study[17], both RFA and MWA were used in 4 studies[15,16,18,19], while the ablation modality was not specified in 3 studies[12,20,21]. All studies had a retrospective design, and 3/10 of them (30%) were multicentric[19-21]. Pre-procedural imaging (CT or MRI) was explicitly required in 6 studies, with imaging performed between 2 weeks and 3 months prior to treatment[14-16,18,19,21]. Follow-up was performed using CT or MRI. Five studies reported on post-treatment follow-up duration, with a minimum of 24 months[14,16-18,21].
All ten studies (100%) reported on the performance of AI-based models, either DL or ML, for predicting HCC recurrence following thermal ablation. The reporting of specific outcome metrics was as follows: 8/10 (80%) studies reported the models’ AUC, 5/10 their sensitivity (50%) and 6/10 their specificity (60%), 2/10 their C-index (20%), 5/10 their PPV (50%), 4/10 their NPV (40%), and 5/10 their accuracy (50%).
The overall median and mean AUC were 0.865 (range 0.74-0.99) and 0.869, respectively. The overall median and mean sensitivity were 82% (range 71%-96%) and 81.4%, respectively. The overall median and mean specificity were 85.5% (range 67%-97%) and 82.83%, respectively. The overall median and mean C-index were 0.735 (range 0.67-0.8) and 0.735, respectively. The overall median and mean PPV were 0.79 (range 0.39-0.94) and 0.744, respectively. The overall median and mean NPV were 0.885 (range 0.80-0.91) and 0.87, respectively. The overall median and mean accuracy were 88% (range 71%-97.6%) and 85.24%, respectively. When considering only studies on DL models, the median and mean AUC, sensitivity, specificity, PPV, NPV, and accuracy were 0.87 and 0.87, 79% and 81.3%, 88.5% and 86.3%, 0.85 and 0.76, 0.90 and 0.89, and 88% and 85.2%, respectively[12,18-21]. None of the studies investigated the C-index.
When considering only studies on ML models, the median and mean AUC, specificity, and C-index were 0.86 and 0.86, 76% and 76%, and 0.70 and 0.70, respectively[13-17]. Only one study reported sensitivity, PPV, and NPV[15]. None of the studies investigated the accuracy of their models[13-17].
As all studies had a retrospective design and the majority were monocentric, quality assessment using the GradePRO GDT revealed a high degree of clinical and methodological heterogeneity among the included studies, such that quan
| Outcome | Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Certainty of evidence |
| AUC | 8 | Retro | Moderate | Low | Low | Moderate | Low |
| Accuracy | 5 | Retro | Moderate | High | Moderate | High | Very low |
| C-Index | 2 | Retro | Moderate | Moderate | Low | Moderate | Low |
| Sens/Spec | 5 | Retro | Moderate | Moderate | Low | Moderate | Low |
| PPV/NPV | 4 | Retro | Moderate-High | High | Moderate | High | Very low |
A key objective in oncologic care is to accurately predict treatment outcomes based on pre-treatment data or imaging. This is particularly important for HCC, where early recurrence significantly compromises prognosis. Being able to stratify patients based on recurrence risk is essential for tailoring the most effective and individualized therapeutic strategies, as well as for optimizing follow-up protocols and resource allocation. Recent advances in AI have introduced the possibility of enhancing predictive accuracy beyond traditional clinical judgement. For example, Iseke et al[22] trained an ML model on predicting tumor recurrence after thermal ablation, surgical resection, and liver transplantation from pretreatment clinical characteristics, laboratory data, and imaging features from MRI examinations, obtaining fair to good perfor
This systematic review highlights the growing interest in and application of AI-based models for predicting early recurrence of HCC following RFA or MWA. The systematic review focuses on clinical relevance of reported AI per
Sato et al[13] reported a C-index of 0.67 using a gradient boosting decision tree ML model, which is indicative of a poor performance, while Peng et al[16] reported a C-index of 0.72 using a random survival forest ML model[23].
In their retrospective monocentric study, Yin et al[24] further extended the potential utility of AI models by dem
Despite these promising findings, several limitations affect the overall strength of evidence, as all studies were retrospective, with only 30% being multicentric. Moreover, sample size and characteristics varied significantly between studies, and there was heterogeneity in AI model types, as well as in the proposed treatment (RFA, MWA, or both), and in the pre-procedural diagnostic imaging techniques used (MRI, CT, or both). In addition, the time gap between pre-treatment imaging and ablation procedure was not specified, although 60% of studies specified a pre-treatment imaging window ranging from 2 weeks to 3 months; however, this time-gap is too broad and could be a source of bias. This interval should ideally be minimized to avoid tumor growth or disease progression in the interval, which could affect model accuracy. Moreover, only 5/10 studies (50%) reported a minimum follow-up of 24 months post-treatment, which also limits the comparability of the studies.
The heterogeneity of reported outcome measures limits the clinical application of these AI-based approaches; in particular, the absence of clearly defined performance thresholds that are clinically meaningful hinders clinical adoption: While many studies report high AUC values, these do not directly translate into patient-centered outcomes unless accompanied by high sensitivity and specificity levels that could be sufficient to guide decision-making in real-world practice. In a diagnostic setting, a sensitivity threshold above 90% may be necessary to minimize false negatives, whereas in prognostic models, specificity and predictive values may carry greater weight. This variability in model performance across various studies, together with incomplete reporting of key outcome metrics, underscores the need for uniform reporting guidelines in AI-related clinical research and for standardized benchmarks to evaluate AI tools.
Moreover, some AI-based models could be better suited for specific clinical contexts. These limitations underline the need for standardized, prospective, multicenter validation trials with harmonized methodology, defined endpoints, and external validation to confirm the generalizability and utility of AI-based prediction models in this setting.
This systematic review suggests that AI-based models show promising performance in predicting early tumor recurrence following heat-based thermal ablation treatments with RFA or MWA in patients with HCC. These models have the potential to inform clinical decision-making by identifying non-responders before treatment allocation, supporting per
| 1. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 535] [Article Influence: 178.3] [Reference Citation Analysis (2)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3141] [Article Influence: 785.3] [Reference Citation Analysis (61)] |
| 3. | Vitale A, Cabibbo G, Iavarone M, Viganò L, Pinato DJ, Ponziani FR, Lai Q, Casadei-Gardini A, Celsa C, Galati G, Gambato M, Crocetti L, Renzulli M, Giannini EG, Farinati F, Trevisani F, Cillo U; HCC Special Interest Group of the Italian Association for the Study of the Liver. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023;24:e312-e322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 4. | Iezzi R, Pompili M, Posa A, Carchesio F, Siciliano M, Annicchiarico BE, Agnes S, Giuliante F, Garcovich M, Cerrito L, Ponziani FR, Basso M, Cassano A, Rapaccini GL, De Gaetano AM, Gasbarrini A, Manfredi R; HepatoCATT Study Group for the Multidisciplinary Management of HCC. Interventional oncology treatments for unresectable early stage HCC in patients with a high risk for intraprocedural bleeding: Is a single-step combined therapy safe and feasible? Eur J Radiol. 2019;114:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol. 2019;25:4614-4628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Lee MW, Han S, Gu K, Rhim H. Local Ablation Therapy for Hepatocellular Carcinoma: Clinical Significance of Tumor Size, Location, and Biology. Invest Radiol. 2025;60:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 8. | Iezzi R, Casà C, Posa A, Cornacchione P, Carchesio F, Boldrini L, Tanzilli A, Cerrito L, Fionda B, Longo V, Miele L, Lancellotta V, Cellini F, Tran HE, Ponziani FR, Giuliante F, Rapaccini GL, Grieco A, Pompili M, Gasbarrini A, Valentini V, Gambacorta MA, Tagliaferri L, Manfredi R. Project for interventional Oncology LArge-database in liveR Hepatocellular carcinoma - Preliminary CT-based radiomic analysis (POLAR Liver 1.1). Eur Rev Med Pharmacol Sci. 2022;26:2891-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 9. | Wu L, Lai Q, Li S, Wu S, Li Y, Huang J, Zeng Q, Wei D. Artificial intelligence in predicting recurrence after first-line treatment of liver cancer: a systematic review and meta-analysis. BMC Med Imaging. 2024;24:263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1070] [Cited by in RCA: 1893] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51936] [Article Influence: 10387.2] [Reference Citation Analysis (2)] |
| 12. | Lim S, Shin Y, Lee YH. Arterial enhancing local tumor progression detection on CT images using convolutional neural network after hepatocellular carcinoma ablation: a preliminary study. Sci Rep. 2022;12:1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Sato M, Tateishi R, Moriyama M, Fukumoto T, Yamada T, Nakagomi R, Kinoshita MN, Nakatsuka T, Minami T, Uchino K, Enooku K, Nakagawa H, Shiina S, Ninomiya K, Kodera S, Yatomi Y, Koike K. Machine Learning-Based Personalized Prediction of Hepatocellular Carcinoma Recurrence After Radiofrequency Ablation. Gastro Hep Adv. 2022;1:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Wang C, Zheng D, Liao Y, Wang X, Huang Z, Zhong Q. Radiomics nomogram based on multi-parametric magnetic resonance imaging for predicting early recurrence in small hepatocellular carcinoma after radiofrequency ablation. Front Oncol. 2022;12:1013770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tabari A, D'Amore B, Cox M, Brito S, Gee MS, Wehrenberg-Klee E, Uppot RN, Daye D. Machine Learning-Based Radiomic Features on Pre-Ablation MRI as Predictors of Pathologic Response in Patients with Hepatocellular Carcinoma Who Underwent Hepatic Transplant. Cancers (Basel). 2023;15:2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Peng W, Jiang X, Zhang W, Hu J, Zhang Y, Zhang L. A radiomics-based model can predict recurrence-free survival of hepatocellular carcinoma after curative ablation. Asian J Surg. 2023;46:2689-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Ren H, An C, Fu W, Wu J, Yao W, Yu J, Liang P. Prediction of local tumor progression after microwave ablation for early-stage hepatocellular carcinoma with machine learning. J Cancer Res Ther. 2023;19:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Chen C, Han Q, Ren H, Wu S, Li Y, Guo J, Li X, Liu X, Li C, Tian Y. Multiparametric MRI-based model for prediction of local progression of hepatocellular carcinoma after thermal ablation. Cancer Med. 2023;12:17529-17540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Zhang Y, Xiao J, Geng X, Han L, Luo J. Multicenter Integration of MR Radiomics, Deep Learning, and Clinical Indicators for Predicting Hepatocellular Carcinoma Recurrence After Thermal Ablation. J Hepatocell Carcinoma. 2024;11:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Kong Q, Li K. Predicting early recurrence of hepatocellular carcinoma after thermal ablation based on longitudinal MRI with a deep learning approach. Oncologist. 2025;30:oyaf013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Li YH, Qian GX, Zhu Y, Lei XD, Tang L, Bu XY, Wei MT, Jia WD. An Integrated Model Combined Conventional Radiomics and Deep Learning Features to Predict Early Recurrence of Hepatocellular Carcinoma Eligible for Curative Ablation: A Multicenter Cohort Study. J Comput Assist Tomogr. 2025;49:860-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Iseke S, Zeevi T, Kucukkaya AS, Raju R, Gross M, Haider SP, Petukhova-Greenstein A, Kuhn TN, Lin M, Nowak M, Cooper K, Thomas E, Weber MA, Madoff DC, Staib L, Batra R, Chapiro J. Machine Learning Models for Prediction of Posttreatment Recurrence in Early-Stage Hepatocellular Carcinoma Using Pretreatment Clinical and MRI Features: A Proof-of-Concept Study. AJR Am J Roentgenol. 2023;220:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 696] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 24. | Yin Y, de Haas RJ, Alves N, Pennings JP, Ruiter SJS, Kwee TC, Yakar D. Machine learning-based radiomic analysis and growth visualization for ablation site recurrence diagnosis in follow-up CT. Abdom Radiol (NY). 2024;49:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/