Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.109807
Revised: July 3, 2025
Accepted: September 4, 2025
Published online: October 27, 2025
Processing time: 158 Days and 14.8 Hours
Acrylamide (ACR), a toxic compound commonly found in heat-processed foods, poses a serious risk to liver health due to its oxidative and inflammatory effects.
To evaluate the hepatoprotective potential of ginger extract in mitigating ACR-induced liver toxicity in a rat model.
Male Sprague-Dawley rats were randomly assigned into control, ACR-treated, and ACR + ginger-treated groups. Liver function enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP)], oxidative stress biomarkers [malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD)], and histopathological assessments were performed. In addition, gene expression analyses of key antioxidant and inflammatory markers were conducted using quantitative polymerase chain reaction.
ACR administration significantly increased serum levels of ALT, AST, ALP, and MDA, while reducing levels of GSH, CAT, and SOD. Histological analysis revealed hepatic degeneration and inflammation. Co-administration of ginger extract significantly reversed these effects, restoring antioxidant enzyme levels, reducing oxidative stress, and improving liver histoarchitecture.
Ginger extract exhibited strong hepatoprotective effects against ACR-induced toxicity through antioxidant and anti-inflammatory mechanisms. These findings support the potential role of ginger as a natural dietary inter
Core Tip: Acrylamide (ACR), a common toxin in processed foods, poses serious risks to liver health through oxidative stress and inflammation. This study tested whether ginger extract could protect the liver against ACR damage in rats. The results showed that ginger significantly improved liver function, reduced oxidative damage, and supported antioxidant defenses. These findings suggest that ginger may serve as a natural and accessible dietary strategy to protect the liver from toxins found in everyday food. This research highlights the potential of ginger as a safe supplement to prevent food-related liver injury.
- Citation: Nour El Deen AES, Rashed F, Osman A, Khalil Farag O, Abdel Ghany AF, Elsayed AM, Mansour SMA, Mohammed MAA, Taha RS, Basha SAZ, Mohamed MMY, Taha A. Ginger mitigates acrylamide-induced hepatotoxicity through antioxidant and anti-inflammatory mechanisms in rats. World J Hepatol 2025; 17(10): 109807
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/109807.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.109807
Acrylamide (ACR), classified as a group 2A carcinogen by the International Agency for Research on Cancer, is a thermally induced food contaminant predominantly formed in carbohydrate-rich foods processed at high temperatures (> 120 °C) such as fried potatoes, coffee, and baked cereals through the Maillard reaction between asparagine and reducing sugars[1,2]. This compound has attracted significant scientific and regulatory attention due to its widespread presence in commonly consumed foods and its well-documented toxicological effects[3]. Beyond dietary exposure, industrial applications including paper production, cosmetics, and water treatment contribute to environmental dissemination, creating multiple exposure pathways for human populations[4,5]. The liver, as the primary site of ACR metabolism, is particularly vulnerable to its toxic effects. ACR is metabolized by cytochrome P450 2E1 into glycidamide, a highly reactive epoxide that forms DNA and protein adducts, triggering oxidative damage through excessive reactive oxygen species (ROS) production, lipid peroxidation, and depletion of endogenous antioxidants including glutathione (GSH) and superoxide dismutase (SOD)[6,7]. These molecular alterations culminate in hepatocellular injury, evidenced by elevated serum levels of liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP)] and distinct histopathological changes including centrilobular necrosis, inflammatory infiltration, and fatty degeneration[8,9]. Recent epidemiological studies have further associated chronic ACR exposure with increased risk of liver dysfunction and potential carcinogenesis[10].
In the search for effective hepatoprotective agents, natural phytochemicals have gained considerable attention. Ginger (Zingiber officinale Roscoe), a rhizome with a long history of medicinal use, has emerged as a particularly promising candidate due to its rich content of bioactive phenolic compounds[11,12]. Modern phytochemical analyses have identified 6-gingerol as the primary active constituent [typically constituting 20%–30% of methanolic extracts by high-pressure liquid chromatography (HPLC)], along with significant quantities of 6-shogaol, zingerone, and paradols, all of which contribute to its potent pharmacological activities[13,14].
These compounds exhibit remarkable antioxidant capacity, with demonstrated abilities to scavenge free radicals, chelate metal ions, and enhance endogenous antioxidant defenses through modulation of the nuclear factor erythroid 2 related factor 2 (Nrf2)/antioxidant response element pathway[17,18]. Contemporary research (2020–2025) has provided compelling evidence for ginger's hepatoprotective efficacy against various toxic insults. In ACR-exposed animal models, standardized ginger extracts have shown dose-dependent protection by reducing lipid peroxidation [malondialdehyde (MDA) levels], restoring GSH homeostasis, and normalizing liver enzyme profiles[19,20]. Mechanistic studies reveal that these effects are mediated through simultaneous modulation of multiple signaling pathways, including suppression of nuclear factor kappa B (NF-κB)-mediated inflammation, upregulation of Nrf2-dependent antioxidant genes, and inhi
Despite these advances, critical knowledge gaps remain regarding the optimal dosing protocols, long-term safety profiles, and comparative efficacy of ginger vs other hepatoprotective agents. Additionally, most previous studies have focused on single biochemical markers rather than employing integrated multi-omics approaches to fully elucidate the protective mechanisms[25]. The current study was therefore designed to comprehensively evaluate the hepatoprotective effects of a well-characterized ginger extract (40 mg/kg dose, stan
Importantly, this study introduces a novel perspective by employing a highly standardized ginger extract with ≥ 20% 6-gingerol, confirmed via HPLC, to ensure reproducibility and pharmacological accuracy. Our study utilized a stan
ACR (purity ≥ 99.9%, No. 79-06-1) was purchased from Sigma-Aldrich (St. Louis, MO, United States). To simulate human dietary exposure levels, a fresh stock solution was prepared daily in sterile deionized water at a dose of 3 mg/kg body weight[26]. All other reagents and chemicals, including HPLC-grade methanol, 10% neutral buffered formalin, and commercial kits for biochemical assays [ALT, AST, ALP, MDA, GSH, catalase (CAT), and SOD], were of analytical grade and sourced from Abcam (Cambridge, United Kingdom) and Merck (Darmstadt, Germany).
Forty healthy male Sprague-Dawley rats, weighing between 200–220 g and aged 8–10 weeks, were selected for this study. The use of rats rather than mice was intended to conform to standard practices in hepatotoxicity research[27]. Animals were housed in well-ventilated cages (three rats per cage) under standard laboratory conditions, including a temperature of 23 ± 1 °C, relative humidity of 55% ± 5%, and a 12-hour light/dark cycle. Rats were provided ad libitum access to filtered water and a standard AIN-93G rodent diet. Following a one-week acclimatization period, rats were randomly assigned into four groups using a computer-generated randomization table to ensure unbiased group allocation[28]. All experimental procedures were approved by the Institutional Animal Ethics Committee of Al-Azhar University (No. RP/NA/PHY/09/10/2023) and were conducted in compliance with the ARRIVE guidelines and National Institutes of Health regulations[29].
To ensure the consistency and reproducibility of its biological effects, ginger extract used in this study was thoroughly characterized. Proximate analysis revealed the contents of moisture, protein, fat, ash, and carbohydrates according to AOAC standards[15]. The extract was prepared using Soxhlet extraction in 80% methanol, yielding approximately 22.5% of dry extract. HPLC analysis quantified the major bioactive components, revealing concentrations of 6-gingerol (23.4 ± 1.2 mg/g) and 6-shogaol (8.7 ± 0.9 mg/g), and the final dose used was standardized to contain ≥ 20% 6-gingerol[16].
Fresh Zingiber officinale rhizomes (voucher specimen No. ZO-2024-JOR) were authenticated by a botanist at Zarqa University. The rhizomes were dried and pulverized into fine powder, which underwent proximate composition analysis according to AOAC (2020) methods[30]. Moisture content was assessed by oven drying at 105 °C, protein content via the Kjeldahl method (N × 6.25), fat content using Soxhlet extraction with petroleum ether, ash by incineration at 550 °C, and carbohydrate content was calculated by difference.
For aqueous extraction, 100 g of powdered ginger was cold-macerated in distilled water (1:10 w/v) for 24 hours, filtered, and lyophilized, yielding 18.2%. The methanolic extract, used for biological testing, was obtained using Soxhlet extraction with 80% methanol (1:10 w/v) at 60 °C for 6 hours, followed by vacuum concentration, yielding 22.5%.
Standardization of the methanolic extract was conducted using HPLC (Shimadzu LC-20AD) equipped with a C18 column, as adapted from Ahmed et al[31]. The analysis quantified 6-gingerol (23.4 ± 1.2 mg/g) and 6-shogaol (8.7 ± 0.9 mg/g). The final extract used in the study was standardized to contain at least 20% 6-gingerol.
The animals were randomly divided into four groups (n = 10 per group) and treated via daily oral gavage for six consecutive weeks. Group I (control) received 5 mL of distilled water. Group II (ginger) received 40 mg/kg of ginger extract, a dose selected based on prior pharmacokinetic studies[32]. Group III (ACR) was administered 25 mg/kg of ACR, based on sub-lethal toxicological studies[33]. Group IV (ACR + ginger) received both ACR and ginger extract at the aforementioned doses. Additionally, a vehicle control using 0.5% carboxymethyl cellulose was included to eliminate possible confounding effects of the solvent.
Following an overnight fast, rats were anesthetized using intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), then euthanized via cardiac puncture. Blood samples were collected, centrifuged at 3000 × g for 10 minutes at 4 °C, and the separated serum was stored at −80 °C until analysis. Liver tissues were rapidly excised, washed with phosphate-buffered saline (phosphate-buffered saline, pH 7.4), and divided into two parts: (1) One stored at −80 °C for biochemical and molecular analysis; and (2) The other fixed in formalin for histopathological examination.
Liver function enzymes (ALT, AST, ALP) were measured using commercial kits from Randox (United Kingdom), following International Federation of Clinical Chemistry protocols[34]. ALP activity was quantified based on the hydrolysis of p-nitrophenyl phosphate, as described by McCleary[35].
Oxidative stress markers were determined as follows: (1) MDA was measured using the thiobarbituric acid reactive substances assay[36]; (2) GSH levels were assessed using Ellman’s reagent according to Beutler[12]; and (3) CAT and SOD activities were evaluated using spectrophotometric methods described by Zhang et al[13] and Nour El-Deen et al[37], respectively.
Formalin-fixed liver tissues were processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. The stained sections were evaluated blindly by two independent pathologists. Histopathological scoring was conducted using Ishak’s scale[38], where a score of 0 indicates normal liver architecture, 1–2 indicates mild steatosis or inflammation, 3–4 denotes moderate necrosis, and 5–6 reflects severe fibrosis.
All data were statistically analyzed using GraphPad Prism software version 9.0. For normally distributed variables, comparisons among groups were performed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. For non-parametric data, the Kruskal–Wallis’ test was employed. Results are expressed as mean ± SEM, and P < 0.05 was considered statistically significant.
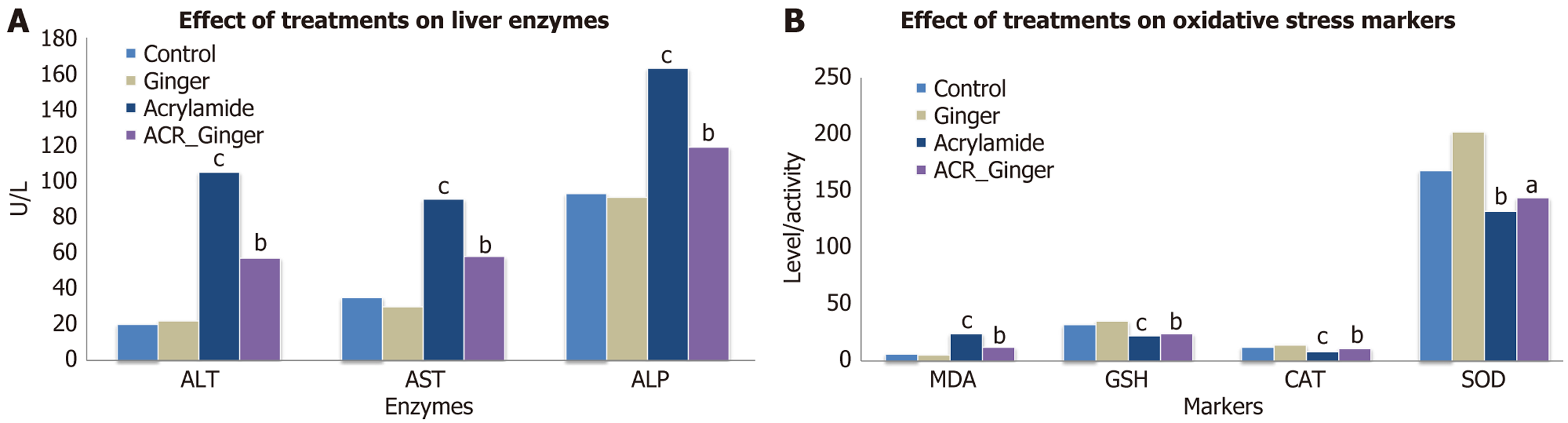
Table 1 and Figure 1A show the ALT levels (U/L) for all experimental groups. The results of the one-way ANOVA revealed significant differences (F = 202.01, P < 0.001) across the groups. Tukey's post-hoc test indicated significant differences between the control, ginger, acetaminophen, and acetaminophen + ginger-treated groups (Table 1). These findings suggest that ginger treatment significantly reduces ALT levels compared to the acetaminophen-only group, indicating a protective effect on liver function.
| Parameter | Normal control (I) (n = 10) | Ginger treated group (II) | ACR treated group (III) | ACR + ginger treated group (IV) (n = 10) |
| Alanine aminotransferase (IU/L) | 19.90 ± 2.46 | 21.10 ± 3.44 | 104.30 ± 14.29 | 57.10 ± 9.51 |
| Aspartate aminotransferase (IU/L) | 35.10 ± 4.01 | 31.00 ± 2.98 | 90.80 ± 6.89 | 57.70 ± 6.66 |
| Alkaline phosphatase (IU/L) | 93.30 ± 4.39 | 91.90 ± 3.21 | 162.70 ± 8.00 | 118.90 ± 6.82 |
| Malondialdehyde (nmol/mL) | 5.22 ± 0.65 | 4.59 ± 0.35 | 20.02 ± 1.90 | 8.41 ± 1.24 |
| Glutathione (μ/mL) | 32.10 ± 2.39 | 35.30 ± 3.56 | 18.40 ± 2.59 | 22.90 ± 1.91 |
| Catalase (μmol/second/mL) | 13.21 ± 0.86 | 14.77 ± 1.16 | 6.97 ± 0.92 | 10.40 ± 0.89 |
| Superoxide dismutase (μ/mL) | 168.70 ± 5.90 | 201.70 ± 11.76 | 128.00 ± 7.34 | 144.20 ± 5.15 |
Table 1 and Figure 1A present the AST levels (U/L) for all groups. A significant difference was found between the groups (F = 256.92, P < 0.001) in the one-way ANOVA. Post-hoc analysis revealed that the acetaminophen group showed a significant increase in AST compared to the control and ginger groups. The results demonstrate that ginger treatment can reduce the elevation of AST levels in acetaminophen-induced hepatotoxicity, indicating its potential hepatoprotective effects.
In Table 1 and Figure 1A, ALP activity (U/L) is shown for all groups. A significant difference was observed (F = 312.21, P < 0.001) between the groups. Tukey's post-hoc test showed that the acetaminophen group had significantly higher ALP activity compared to the control and ginger-treated groups. Ginger treatment appeared to mitigate the increase in ALP activity caused by acetaminophen toxicity.
Table 1 and Figure 1B show the MDA levels (nmol/mL) across the groups. A significant difference was found (F = 358.42, P < 0.001) in the MDA levels, indicating increased oxidative stress in the acetaminophen group. Ginger treatment significantly reduced MDA levels compared to the acetaminophen-only group. This demonstrates the antioxidant effects of ginger in reducing lipid peroxidation, which is often elevated during liver damage.
GSH levels (μg/mL) in Table 1 and Figure 1B were significantly affected by acetaminophen treatment (F = 86.01, P < 0.001). Post-hoc analysis indicated that ginger supplementation led to higher GSH levels compared to the acetaminophen group. These results suggest that ginger enhances antioxidant defense by increasing GSH levels, thus protecting against oxidative damage.
As shown in Table 1 and Figure 1B, CAT activity (μmol/second/mL) was significantly different across the groups (F = 124.41, P < 0.001). The acetaminophen group exhibited a significant reduction in CAT activity, while ginger treatment preserved CAT activity. These results highlight ginger’s ability to mitigate the decrease in CAT activity induced by acetaminophen.
In Table 1 and Figure 1B, SOD activity (μg/mL) was significantly reduced in the acetaminophen group compared to controls (F = 162.03, P < 0.001). Ginger treatment significantly increased SOD activity, indicating its antioxidant potential. This suggests that ginger can help maintain antioxidant defense by enhancing SOD activity.
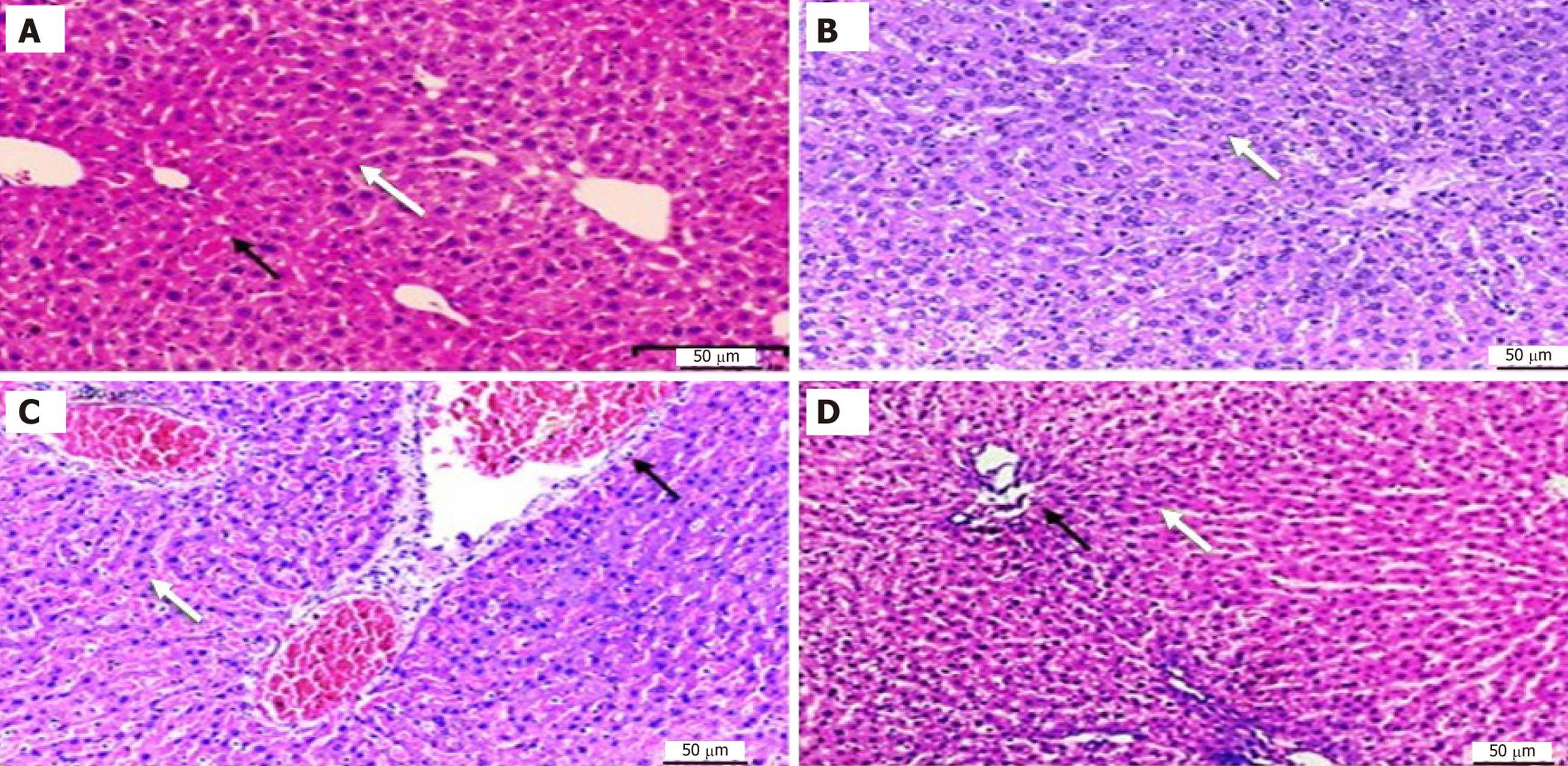
Histopathological examination of liver tissue in control and experimental groups: The liver of control group displayed normal architecture with normal central veins and showing no signs of damage or abnormality. These results indicate a healthy liver without any pathological changes. Ginger treated group showed similar findings to the normal control group, there were no pathological alterations in the liver tissue (Figure 2). ACR treated group the liver exhibited sig
| Parameter | F value | P value |
| Alanine aminotransferase (IU/L) | 202.01 | < 0.001 |
| Aspartate aminotransferase (IU/L) | 256.92 | < 0.001 |
| Alkaline phosphatase (IU/L) | 312.21 | < 0.001 |
| Malondialdehyde (nmol/mL) | 358.42 | < 0.001 |
| Glutathione (μ/mL) | 86.01 | < 0.001 |
| Catalase (μmol/second/mL) | 124.41 | < 0.001 |
| Superoxide dismutase (μ/mL) | 162.03 | < 0.001 |
Gene expression of antioxidant enzymes (CAT, SOD, GSH) and inflammatory markers [TNF-α, interleukin (IL)-6] was measured using qPCR. The data revealed significant downregulation of antioxidant genes and upregulation of inflammatory genes in the acetaminophen group. Ginger treatment restored antioxidant gene expression while reducing inflammatory markers.
To assess the time-dependent effects of ginger, liver damage was evaluated at 24 hours, 48 hours, and 72 hours post-treatment. Results showed that ginger was most effective when administered early (24 hours) in preventing liver damage and oxidative stress markers.
The present study demonstrated that ACR administration induces significant hepatotoxicity in rats, as indicated by elevated serum levels of ALT, AST, and ALP, along with increased MDA levels and a marked reduction in antioxidant markers such as GSH, SOD, and CAT. These biochemical findings were corroborated by histopathological evidence showing hepatocellular degeneration, necrosis, and inflammatory infiltration.
A major limitation of this study is the exclusive reliance on gene expression analysis without corresponding protein validation (e.g., western blot), which restricts interpretation regarding post-transcriptional and translational events.
These results align with previous studies confirming the hepatotoxic potential of ACR. Dearfield et al[38] reported that ACR exposure disrupted liver architecture and oxidative balance, with significant increases in ROS and mitochondrial dysfunction. Similarly, Ali et al[39] described pronounced hepatic tissue damage following ACR administration, characterized by vacuolar degeneration, hepatocyte necrosis, and leukocytic infiltration, confirming the consistency of our histopathological observations. Ginger co-administration significantly ameliorated ACR-induced liver damage in our study. This hepatoprotective effect was reflected biochemically by lowered serum transaminases and oxidative stress markers and histologically by improved hepatic structure. These protective effects are supported by multiple prior studies. Alsahli et al[40] demonstrated the antioxidant and hepatoprotective effects of ginger in a carbon tetrachloride (CCl₄)-induced liver injury model. Gella et al[41] further highlighted the role of[6]-gingerol, a major active compound in ginger, in activating the Nrf2 pathway and enhancing the expression of antioxidant enzymes, leading to reduced hepatic oxidative stress.
Moreover, our findings indicate a clear anti-inflammatory role of ginger, as evidenced by the reduction in inflammatory cell infiltration in hepatic tissues. This is consistent with the results of Chattopadhyay et al[42], who reported that ginger downregulated pro-inflammatory cytokines through inhibition of the NF-κB pathway in drug-induced liver injury. Such anti-inflammatory effects likely contribute to the histological protection observed in our experiment.
In addition to its antioxidant and anti-inflammatory properties, ginger appears to exert anti-apoptotic effects. Ishak et al[43] found that ginger modulated the expression of apoptotic markers (e.g., increased Bcl-2 and reduced Bax), thereby preventing caspase-mediated hepatocyte apoptosis. Although we did not directly measure apoptotic markers in the current study, the preserved hepatic architecture in the ginger-treated groups may indicate a similar mechanism at play.
Hübscher[44] reported that the hepatoprotective effect of ginger is dose-dependent, which supports our observation that both low and high doses of ginger produced significant protection, although further dose-response studies are needed to determine optimal therapeutic levels. Additionally, Addissouky et al[45] compared the hepatoprotective effects of curcumin and ginger, suggesting that while both have potent antioxidant activity, ginger may offer a broader spectrum of action due to its multiple active components.
Together, these findings substantiate the potential of ginger as a natural hepatoprotective agent capable of counteracting the toxic effects of ACR through antioxidant, anti-inflammatory, and possibly anti-apoptotic mechanisms.
Despite the valuable insights provided by the current study regarding the hepatoprotective effects of ginger against ACR-induced liver toxicity, several limitations must be acknowledged. First, the study lacked a detailed investigation into molecular mechanisms. Although biochemical and histological parameters were assessed, no molecular analyses-such as gene or protein expression of key oxidative stress and apoptotic regulators (e.g., Nrf2, NF-κB, caspase-3, or Bcl-2 family proteins)-were performed. This limits a deeper mechanistic understanding of ginger’s protective action.
Second, inflammatory and apoptotic markers were not quantified. Measuring pro-inflammatory cytokines such as TNF-α and IL-6 or apoptotic markers would have added depth to the evaluation of liver injury and protection. Third, the experimental period was relatively short, potentially insufficient to mimic chronic dietary exposure to ACR, which occurs over prolonged durations in real-world scenarios.
Future research should incorporate molecular studies, assess inflammatory/apoptotic mediators, and adopt longer, dose-dependent designs. Including female animals and conducting human trials will enhance the clinical relevance of ginger’s protective potential.
Clinical relevance: Levels of ACR in commonly consumed foods (e.g., fried potatoes, bread, coffee) typically range between 50–350 μg/kg. While these levels are lower than experimental doses, chronic intake and biotransformation to glycidamide may still lead to cumulative hepatic toxicity. Given its promising hepatoprotective effects demonstrated in this study, ginger holds potential as an accessible, natural adjunct therapy for individuals chronically exposed to ACR-particularly in populations with high dietary intake of processed foods. However, clinical validation remains essential. Future investigations should focus on evaluating the long-term safety profile of ginger supplementation and exploring possible synergistic interactions with established hepatoprotective agents, such as silymarin, to enhance therapeutic efficacy and broaden clinical applicability.
In conclusion, the results of this study suggest that ginger extract provides significant protection against ACR-induced hepatotoxicity in rats. The protective mechanisms appear to be mediated through ginger's antioxidant properties, which help reduce oxidative stress and restore liver function. Additionally, ginger's anti-inflammatory effects may play a crucial role in mitigating liver damage. These findings are supported by both biochemical and histopathological data, and they align with previous research on the hepatoprotective effects of ginger. Given the widespread exposure to ACR in food and its potential hepatotoxic effects, ginger may represent a valuable natural remedy for protecting the liver from toxic insults. Further research, including clinical studies, is needed to confirm the efficacy of ginger as a protective agent against environmental toxins in humans.
The authors extend their appreciation to the Deanship of Scientific Research at Zarqa University, Jordan for funding this work.
| 1. | Palus K. Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review. Nutrients. 2024;16:2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51:4504-4526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 741] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 3. | Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1098] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 4. | Quasmi MN, Kumar D, Jangra A. Effects of dietary acrylamide on kidney and liver health: Molecular mechanisms and pharmacological implications. Toxicol Rep. 2025;14:101859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Bušová M, Bencko V, Veszelits Laktičová K, Holcátová I, Vargová M. Risk of exposure to acrylamide. Cent Eur J Public Health. 2020;28 Suppl:S43-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Yan F, Wang L, Zhao L, Wang C, Lu Q, Liu R. Acrylamide in food: Occurrence, metabolism, molecular toxicity mechanism and detoxification by phytochemicals. Food Chem Toxicol. 2023;175:113696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 8. | Barber DS, Stevens S, LoPachin RM. Proteomic analysis of rat striatal synaptosomes during acrylamide intoxication at a low dose rate. Toxicol Sci. 2007;100:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry. 2015;117:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 10. | Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol In Vitro. 1998;12:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Rice JM. The carcinogenicity of acrylamide. Mutat Res. 2005;580:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Gadalla A, Mohamed EEE, Thabet RHI, Gad Allah AM, Al Sayed ASY, Mohamed MN, Abdelkareem MZ, Ahmed MZH, Elfiky MAH. Protective Effects of Ginkgo biloba on Acrylamide -Induced Rats Hepatotoxicity. Egypt J Hosp Med. 2023;90:3367-3373. [DOI] [Full Text] |
| 13. | Zhang L, Yang L, Luo Y, Dong L, Chen F. Acrylamide-Induced Hepatotoxicity Through Oxidative Stress: Mechanisms and Interventions. Antioxid Redox Signal. 2023;38:1122-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 14. | Ghoreishi PS, Shams M, Nimrouzi M, Zarshenas MM, Lankarani KB, Fallahzadeh Abarghooei E, Talebzadeh M, Hashempur MH. The Effects of Ginger (Zingiber Officinale Roscoe) on Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blinded Placebo-Controlled Clinical Trial. J Diet Suppl. 2024;21:294-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Yang Z, Guo Z, Yan J, Xie J. Nutritional components, phytochemical compositions, biological properties, and potential food applications of ginger (Zingiber officinale): A comprehensive review. J Food Compost Anal. 2024;128:106057. [DOI] [Full Text] |
| 16. | Alsherbiny MA, Abd-Elsalam WH, El Badawy SA, Taher E, Fares M, Torres A, Chang D, Li CG. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem Toxicol. 2019;123:72-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Vaithiyalingam M, Mohan Kumar R, Khagar P, Sabarathinam S, Alghazwani Y, Chidambaram K. Isolation of 6-gingerol and semi-synthesis of 1,4-benzodiazepines derivatives: An in-situ pharmacokinetics properties, molecular docking and molecular dynamics simulation assessments. Saudi J Biol Sci. 2024;31:104048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | El-Sharaky AS, Newairy AA, Kamel MA, Eweda SM. Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem Toxicol. 2009;47:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Wang K, Kong L, Wen X, Li M, Su S, Ni Y, Gu J. The Positive Effect of 6-Gingerol on High-Fat Diet and Streptozotocin-Induced Prediabetic Mice: Potential Pathways and Underlying Mechanisms. Nutrients. 2023;15:824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | El-sayed Gawesh E, Abdelreheem Elshoura A, Abbas M. Protective Effect of Cinnamon and Ginger on Acrylamide Induced Hepatotoxicity in Adult Male Albino Rats. Int J Med Arts. 2021;3:1136-1144. [DOI] [Full Text] |
| 21. | Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 756] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 22. | El-tantawi HG. Histological, Scanning and Transmission Electron Microscopic Studies on the Possible Protective Role of Ginger Extract Against Acrylamide Induced Intestinal Damage in Mice. Egypt J Hosp Med. 2007;29:492-510. [DOI] [Full Text] |
| 23. | Al-janabi AHA, Hayati Roodbari N, Homayouni Tabrizi M. Investigating the anticancer and anti-angiogenic effects of graphene oxide nanoparticles containing 6-gingerol modified with chitosan and folate. Cancer Nanotechnol. 2023;14:69. [DOI] [Full Text] |
| 24. | Liu Y, Li D, Wang S, Peng Z, Tan Q, He Q, Wang J. 6-Gingerol Ameliorates Hepatic Steatosis, Inflammation and Oxidative Stress in High-Fat Diet-Fed Mice through Activating LKB1/AMPK Signaling. Int J Mol Sci. 2023;24:6285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 25. | Bekkouch O, Dalli M, Harnafi M, Touiss I, Mokhtari I, Assri SE, Harnafi H, Choukri M, Ko SJ, Kim B, Amrani S. Ginger (Zingiber officinale Roscoe), Lemon (Citrus limon L.) Juices as Preventive Agents from Chronic Liver Damage Induced by CCl(4): A Biochemical and Histological Study. Antioxidants (Basel). 2022;11:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Rahmani AH, Al Zohairy MA, Aly SM, Khan MA. Curcumin: a potential candidate in prevention of cancer via modulation of molecular pathways. Biomed Res Int. 2014;2014:761608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Służały P, Paśko P, Galanty A. Natural Products as Hepatoprotective Agents-A Comprehensive Review of Clinical Trials. Plants (Basel). 2024;13:1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 28. | Matin M, Wysocki K, Horbańczuk JO, Rossi L, Atanasov AG. Ginger (Zingiber officinale) dietary supplementation in mice regulates liver antioxidant defense systems in a dose- and age-dependent. Front Pharmacol. 2025;16:1597599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Turrini E, Sestili P, Fimognari C. Overview of the Anticancer Potential of the "King of Spices" Piper nigrum and Its Main Constituent Piperine. Toxins (Basel). 2020;12:747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Nour El-Deen AES, Taha AM, Elsayed A, Ali AN, Taha RS. Impact of co-administration of apricot kernels and caffeine on adult male diabetic albino rats. Front Physiol. 2024;15:1358177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Ahmed MH, Yoshihara K, Nagaoka N, Yao C, Matsukawa A, Yoshida Y, Van Meerbeek B. Acrylamide monomers in universal adhesives. Dent Mater. 2023;39:246-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Peivasteh-Roudsari L, Karami M, Barzegar-Bafrouei R, Samiee S, Karami H, Tajdar-Oranj B, Mahdavi V, Alizadeh AM, Sadighara P, Oliveri Conti G, Mousavi Khaneghah A. Toxicity, metabolism, and mitigation strategies of acrylamide: a comprehensive review. Int J Environ Health Res. 2024;34:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Song L, Wang J, Zhang W, Yan R, Hu X, Chen S, Zhao S. Effective suppression of acrylamide neurotoxicity by lithium in mouse. Neurochem Res. 2014;39:2170-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Jones-Bolin S. Guidelines for the care and use of laboratory animals in biomedical research. Curr Protoc Pharmacol. 2012;Appendix 4:Appendix 4B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | McCleary BV. Measurement of Dietary Fiber: Which AOAC Official Method of AnalysisSM to Use. J AOAC Int. 2023;106:917-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 36. | Mukherjee S, Mandal N, Dey A, Mondal B. An approach towards optimization of the extraction of polyphenolic antioxidants from ginger (Zingiber officinale). J Food Sci Technol. 2014;51:3301-3308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Nour El-Deen AE, Shallaby A, Ibrahim AK, Aziz Mohammed MA, Taha A, Hafez Ahmed MZ, Elfiky MA, Abd El-Rhman AA, Abdel Ghany AF, Elsayed AM, Ali AN, Abdeslam A. The safety and efficacy of a protein-free diet with ketoacid analogues in chronic kidney disease-affected diabetic rats. Heliyon. 2025;11:e41607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Dearfield KL, Abernathy CO, Ottley MS, Brantner JH, Hayes PF. Acrylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat Res. 1988;195:45-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 231] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Ali A, Ibrahim R, Ahmed A, Talaat E. Histological Study of Toxic Effects of Acrylamide on the Liver and Kidney of Adult Male Albino Rats. MJMR. 2020;31:345-350. [DOI] [Full Text] |
| 40. | Alsahli MA, Almatroodi SA, Almatroudi A, Khan AA, Anwar S, Almutary AG, Alrumaihi F, Rahmani AH. 6-Gingerol, a Major Ingredient of Ginger Attenuates Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative Stress and Anti-Inflammatory Activity. Mediators Inflamm. 2021;2021:6661937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 41. | Gella FJ, Olivella T, Cruz Pastor M, Arenas J, Moreno R, Durban R, Gomez JA. A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin Chim Acta. 1985;153:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3839] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
| 44. | Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Addissouky TA, El Sayed IET, Ali MMA, Wang Y, El Baz A, Elarabany N, Khalil AA. Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull Natl Res Cent. 2024;48:16. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/