Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.109898
Revised: June 25, 2025
Accepted: September 11, 2025
Published online: October 27, 2025
Processing time: 156 Days and 1.3 Hours
Metabolic dysfunction-associated steatotic liver disease, characterized by pathological intracellular triglyceride (TG) accumulation, is mechanistically associated with the disrupted spatiotemporal regulation of hepatocyte nuclear factor (HNF)-dependent transcriptional programs. HNFs, including key members such as HNF-1α, HNF-4α, and HNF-6, constitute a liver-enriched family of transcription factors that govern hepatic lipid metabolism through hierarchical transcriptional regulatory networks. These networks critically regulate the dynamic equilibrium of TG metabolism, encompassing TG synthesis, storage, lipolysis, and lipoprotein-mediated export. This review comprehensively dec
Core Tip: Abnormal hepatic triglyceride deposition is the hallmark of metabolic dysfunction-associated steatotic liver disease. While this process may originate either as a hepatic manifestation of systemic metabolic dysfunction or direct local hepatic lipid metabolic imbalance, the two pathways dynamically interact to drive disease progression. Hepatocyte nuclear factors (HNFs) regulate hepatic triglyceride metabolism via transcriptional networks, and their dysfunction constitutes a key driver of metabolic dysfunction-associated steatotic liver disease pathogenesis. Targeting critical nodes in HNF regulatory networks presents new opportunities for precision therapy. This review systematically analyzes the clinical challenges involved in HNF-targeted therapies and explores potential solutions.
- Citation: Li SQ, Wu JH, Zhou Y, Wang CX, Xie L, Liu SY, Su YZ, He W, Chen H, Zhong WW, He YH. Hepatocyte nuclear factors dynamically regulate triglyceride metabolic reprogramming in metabolic dysfunction-associated steatotic liver disease: Mechanisms and implications. World J Hepatol 2025; 17(10): 109898
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/109898.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.109898
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as the predominant cause of chronic liver disease worldwide, with its prevalence paralleling the global epidemics of obesity and type 2 diabetes[1,2]. The hallmark pathological feature of MASLD is intrahepatocytic triglyceride (TG) accumulation as a result of dysregulated lipid metabolism. This development profoundly reflects the bidirectional interplay between systemic metabolic disturbances and localized hepatic pathology. MASLD was initially described in 1980 by Ludwig et al[3] as non-alcoholic fatty liver disease to characterize hepatic steatosis occurring in the absence of significant alcohol consumption or other definitive hepatotoxic factors. This diagnostic framework faced criticism for its exclusionary nature. In 2020, a multinational panel of 32 experts led by Professor Mohammed Eslam proposed that the condition be renamed to metabolic-associated fatty liver disease to emphasize its metabolic underpinnings[4,5]. However, the stigmatizing connotation of “fatty” raised concerns, which hindered the universal adoption of this terminology. This nomenclature debate culminated in June 2023 when the American Association for the Study of Liver Diseases, the Latin American Association for the Study of the Liver, and the European Association for the Study of the Liver jointly endorsed “MASLD” as the current standardized terminology[6]. Although the global disease burden of MASLD continues to escalate, the condition exhibits significant epidemiological heterogeneity with varying prevalence rates in different regions. Recent summary analyses estimate a global pooled prevalence of approximately 32.4%, with China demonstrating a slightly lower national prevalence of 29.6% that surges to over 60% in obese/diabetic populations[1,7]. The natural history of MASLD typically involves progression through sequential stages: Among those with the initial pathological condition of metabolic dysfunction-associated steatotic liver, approximately 12%-40% develop metabolic dysfunction-associated steatohepatitis (MASH); among the latter, 40.8% demonstrate fibrotic progression during follow-up[8]. The development of advanced cirrhosis in MASLD patients correlates with substantial elevations in the all-cause mortality rate and hepatocellular carcinoma incidence rate (0.5%-4.5%)[8,9]. Notably, MASLD exhibits significant extrahepatic manifestations, including increased risks of cardiovascular diseases and colorectal malignancies, which profoundly impact patients’ quality of life and longevity[10]. Given the substantial health burden imposed by MASLD and the current therapeutic paradigm, which is predominantly anchored in lifestyle interventions[11], there exists an imperative need to elucidate the molecular pathogenesis of MASLD and develop targeted therapeutic agents that address the core mechanisms of this disease. A deep understanding of the pathogenesis of MASLD will not only help to break through existing treatment bottlenecks but also have significant public health implications for optimizing the management strategies of chronic metabolic diseases.
Hepatocyte nuclear factors (HNFs) are a group of transcriptional factors that constitute the central regulatory axis of hepatic transcriptional programming, with principal members encompassing HNF-1α/β, forkhead box A (FOXA, historically designated as HNF-3 isoforms FOXA1/A2/A3), HNF-4α, and HNF-6. These master regulators establish a hierarchical transcriptional architecture that orchestrates the spatiotemporal dynamics of lipid metabolic machinery, thereby maintaining TG homeostasis through the coordinated activation/repression of lipogenic and lipolytic gene cascades[12]. Aberrant intrahepatic TG accumulation is the core pathological feature of MASLD, which is directly linked to HNF network dysfunction. This review systematically examines the molecular mechanisms of HNF-driven TG dysregulation in MASLD, focusing on the synergistic regulation of TG metabolism by HNF interactomes and the epigenetic modulation of HNF transcriptional activity. We further evaluate translational challenges in targeting HNF signaling nodes, including pharmacologic specificity and tissue-targeting limitations.
The abnormal deposition of TGs within the liver is not only the core pathological feature of MASLD but also the initial trigger driving inflammation and hepatic fibrosis. When hepatic lipid uptake and de novo lipogenesis (DNL) exceed the capacity of lipid catabolism and export, lipids accumulate abnormally in hepatocytes, ultimately leading to hepatic steatosis[13].
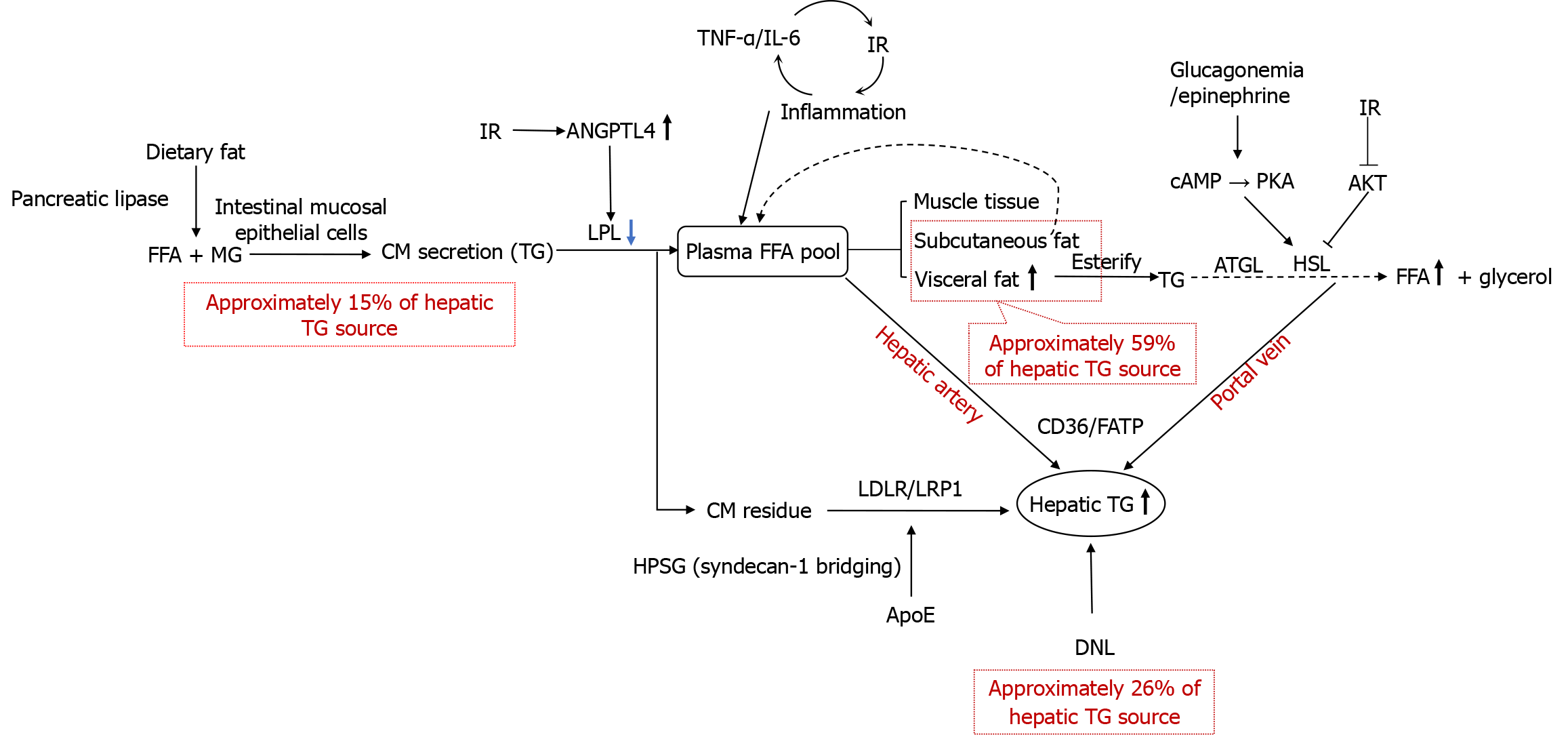
Lipid uptake into hepatocytes from the bloodstream primarily occurs through two molecular mechanisms: The first is the active transport of free fatty acids (FFAs) mediated by CD36 and fatty acid transporter protein; this serves as the main source through which hepatocytes acquire extracellular lipids. The second mechanism involves the receptor-mediated endocytosis of chylomicron (CM) remnants and very low-density lipoprotein (VLDL) remnants; this represents the primary and exclusive pathway through which hepatocytes obtain TGs. Additionally, when FFA concentrations rise, FFA uptake into hepatocytes can occur via passive diffusion[14] (Figure 1).
The origin of FFAs in the bloodstream primarily relies on two metabolic pathways: The transport of dietary fats after intestinal digestion and absorption, and the release of fatty acids from adipose tissue via lipolysis (the predominant source). In patients with MASLD, 59.0% of hepatic TGs originate from plasma FFAs, 26.1% from DNL, and 14.9% from dietary intake. The primary pathway for hepatic TG accumulation involves FFAs released into the plasma FFA pool via the lipolysis of stored TGs in adipose tissue. Notably, when dietary fat intake exceeds 30% of daily energy requirements, postprandial dietary fatty acids spill over into the plasma FFA pool, contributing approximately 15% to hepatic TG sources[15]. Dietary fats are emulsified by bile acids into micelles in the small intestine, and then hydrolyzed into FFAs and monoacylglycerol by pancreatic lipase. The hydrolyzed products are absorbed by intestinal mucosal epithelial cells, re-esterified into TGs through the monoacylglycerol pathway in the smooth endoplasmic reticulum (ER), and assembled into CMs by combining with apolipoproteins (Apo) such as ApoB-48, ApoA1, and ApoA4. The CMs are then secreted into the intestinal lymphatic vessels via exocytosis, and enter the bloodstream through the lymphatic system.
In the bloodstream, the core TG component of CMs undergoes gradual hydrolysis, catalyzed by lipoprotein lipase (LPL) on the surface of endothelial cells. LPL is primarily synthesized and secreted by capillary endothelial cells in adipose tissue, skeletal muscle, and cardiac muscle. It is anchored to the endothelial cell surface via heparan sulfate proteoglycan and requires ApoC2 as a cofactor for its activity. However, it can be inhibited by ApoC3[16]. LPL specifically hydrolyzes the ester bonds at the sn-1 and sn-3 positions of TG molecules, converting one molecule of TG into two molecules of FFAs and one molecule of 2-monoacylglycerol. The FFAs released from TG hydrolysis are taken up by adjacent skeletal muscle and adipose tissue for utilization, while a portion binds to albumin and enters the circulation to supply other tissues. Most of the TGs secreted by small intestinal mucosal epithelial cells are hydrolyzed, and the remaining TGs enter the circulation as CM remnants for uptake by the liver and other tissues. LPL plays a critical role in CM-TG hydrolysis, and LPL dysfunction prolongs the half-life of CM remnants[17]. Lipoprotein-associated phos
Following CM-TG hydrolysis, the reduction in CM size leads to the transfer of its surface components such as phospholipids and ApoA1 to high-density lipoprotein (HDL), forming CM remnants. Concurrently, key ligands like ApoE and ApoB-48 are exposed, facilitating the clearance of CM remnants. Heparan sulfate proteoglycans on hepatocyte surfaces capture ApoE-enriched CM remnants via electrostatic interactions, promoting their binding to LDL receptors and LDL receptor-related protein 1, thereby mediating hepatocyte-specific uptake[19]. Syndecan-1, a transmembrane heparan sulfate proteoglycan, directly binds to ApoE on the surface of CM remnants through its heparan sulfate chains, forming a “receptor-bridging complex” that assists the LDL receptor-related protein 1/LDL receptor-mediated endocytosis of CM remnants. After internalization by hepatocytes, CM remnants undergo hydrolysis of TGs and cholesteryl esters by lysosomal enzymes, releasing FFAs and cholesterol for hepatic utilization or storage.
After the uptake of FFAs from the bloodstream via adipose tissue-specific transport proteins, FFAs are re-esterified into TGs through the glycerol-3-phosphate pathway and stored in lipid droplets. During periods of increased energy demand, TGs are hydrolyzed into FFAs and glycerol via lipolysis mediated by adipose TG lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase, and FFAs are again released into the circulation[20]. ATGL catalyzes the hydrolysis of a TG molecule into diacylglycerol and one FFA, representing the first rate-limiting step of lipolysis. This process requires activation by the cofactor comparative gene identification-58 and dominates basal lipolysis. HSL primarily hydrolyzes diacylglycerol into monoacylglycerol and one FFA, serving as the second rate-limiting step of lipolysis[21]. HSL is anchored to lipid droplets via the cofactor perilipin 1 and remains inactive in the cytoplasm under basal conditions. Its activation depends on the cAMP/PKA signaling pathway[22,23]. Monoacylglycerol lipase completes the final step of lipolysis by hydrolyzing monoacylglycerol into glycerol and one FFA[24]. Under IR, reduced protein kinase B (AKT) signaling pathway activity alleviates the phosphorylation-mediated inhibition of HSL, enhancing HSL activity. Concurrently, hyperglucagonemia or elevated adrenaline levels activate PKA, which phosphorylates perilipin 1, enabling the translocation of HSL to lipid droplet surfaces and its activation. This accelerates the hydrolysis of TGs in adipose tissues into glycerol and FFAs, leading to significantly increased hepatic FFA uptake. Adipose tissue is primarily composed of subcutaneous adipose tissue and visceral adipose tissue (VAT). VAT (such as mesenteric, omental, and retroperitoneal fat) is located within the abdominal cavity, and surrounds the internal organs; it exhibits a direct anatomical connection to the portal venous system. Compared to subcutaneous adipose tissue, VAT shows a high expression of β3-adrenergic receptors, which enhance its sensitivity to catecholamines and result in a significantly higher lipolysis rate. Additionally, VAT demonstrates a greater proportion of macrophage infiltration, and these macrophages secrete pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). VAT, characterized by high metabolic activity and vascular perfusion, directly releases FFAs and pro-inflammatory factors into the liver via the portal vein. In contrast, subcutaneous adipose tissue has a lower lipolysis rate, and the FFAs it releases enter the systemic circulation, where they are predominantly taken up and oxidized for energy utilization by tissues such as the skeletal muscle.
Central obesity, characterized by abnormal fat deposition in the trunk and abdominal regions (quantified using waist circumference or waist-to-hip ratio), exhibits a positive and independent association with MASLD risk, as indicated by its specific metric, the Chinese visceral adiposity index (CVAI), which integrates waist circumference, age, body mass index, and lipid profiles[25,26]. VAT, functioning as a neuro-endocrine-immune-integrated metabolic organ, releases excessive FFAs into the portal venous system through heightened lipolytic activity. Compared to subcutaneous adipose tissue, VAT demonstrates elevated LPL activity, enhancing its efficiency in capturing FFAs derived from TG hydrolysis in CMs[27]. Concurrently, VAT-derived inflammatory cytokines (e.g., IL-6 and TNF-α) impair insulin signaling, exacerbating IR and visceral adiposity while promoting hepatic steatosis[28]. IR perpetuates adipose tissue inflammation via nutrient-redistribution mechanisms (e.g., glucose diversion to immune cells), establishing a vicious cycle of inflammation-IR[29]. This persistent disordered lipid metabolism constitutes the core pathological basis for central obesity-driven hepatic lipid accumulation.
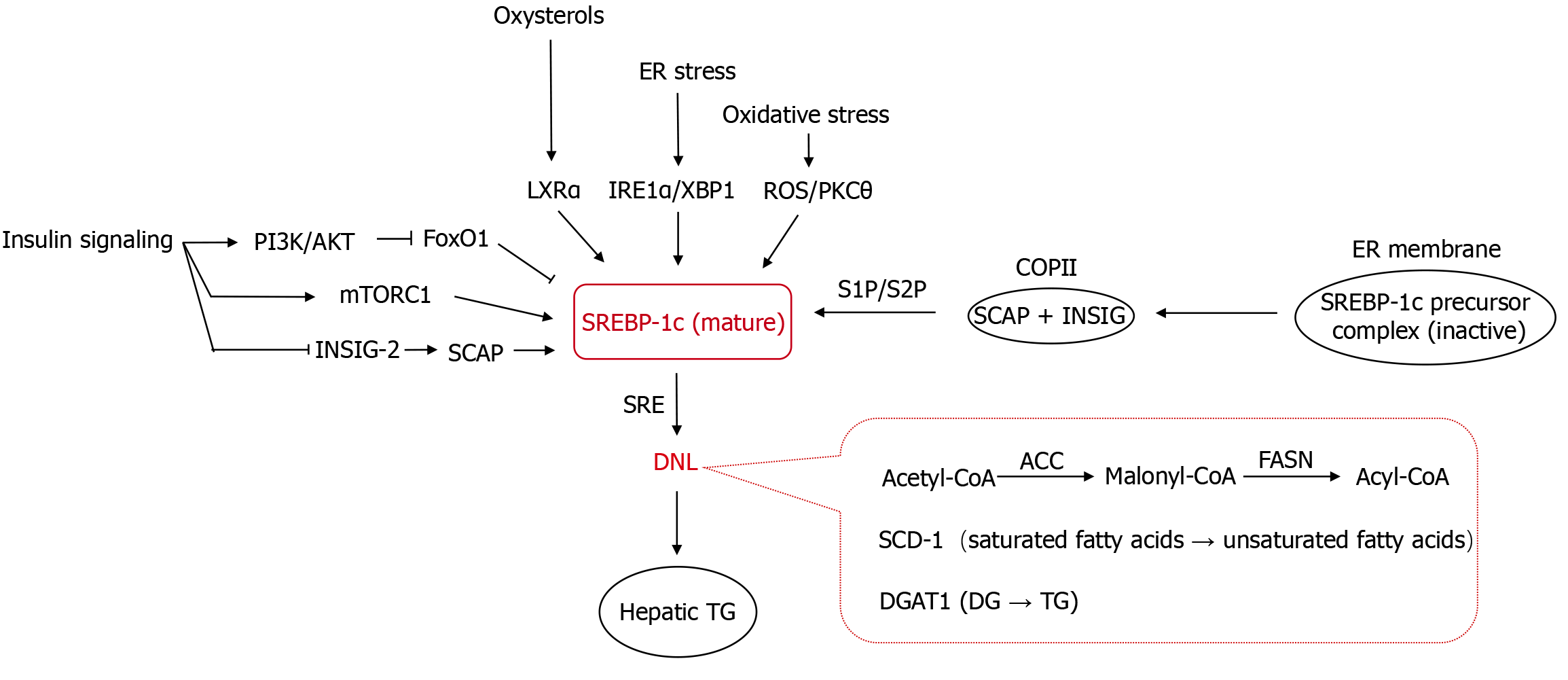
The core pathological alteration in MASLD is abnormal lipid accumulation in hepatocytes, predominantly TGs (60%-80%) followed by cholesterol (15%-20%)[30,31]. Under physiological conditions, approximately 5% of hepatic TGs originate from DNL via acetyl-coenzyme A (CoA)[32]. However, in the pathological state of MASLD, 25%-30% of hepatic TGs are derived from DNL[33]. This aberrant metabolic process is primarily driven by key transcription factors within the regulatory network of DNL: Sterol regulatory element-binding protein-lc (SREBP-1c), which is regulated by insulin signaling, and carbohydrate response element-binding protein (ChREBP), which is regulated by glucose signaling. These factors modulate lipid synthesis by regulating the transcription of key downstream metabolic enzymes, thereby altering enzyme protein levels. The rate-limiting enzyme in fatty acid synthesis, acetyl-CoA carboxylase (ACC), catalyzes the conversion of acetyl-CoA to malonyl-CoA. Fatty acid synthase (FASN) synthesizes long-chain fatty acids from malonyl-CoA. Stearoyl-CoA desaturase-1 (SCD-1) regulates fatty acid desaturation, influencing unsaturated fatty acid production. Diacylglycerol acyltransferase 1 (DGAT1), a key enzyme in TG synthesis, esterifies diacylglycerol with fatty acyl-CoA to generate TGs[13,34]. Studies confirm that in MASLD, the activities of hepatic ACC, FASN, SCD-1, and DGAT1 are pathologically enhanced[35-37]. In mouse models of MASLD with liver-specific knockout of Acc1 or Scd1, hepatic DNL and TG content are significantly reduced, and steatosis is attenuated[38], highlighting the critical role of upregulation of enzymes involved in DNL in MASLD pathogenesis. ChREBP is specifically activated under conditions of excessive fructose intake or energy surplus. Fructose enhances the nuclear translocation and DNA-binding capacity of ChREBP through its unique metabolic pathways (e.g., rapid generation of intermediates such as dihydroxyacetone phosphate), enabling its targeted binding to carbohydrate response elements in the promoters of lipogenic genes (e.g., ACC, FASN), thereby markedly elevating the efficiency of de novo FFA synthesis. Additionally, ChREBP regulates the transcription of enzymes like DGAT1, further promoting TG synthesis. However, under pathological states such as IR, the overexpression of forkhead box protein O1 (FoxO1), a downstream effector of insulin signaling, in hepatocytes reduces the activity of ChREBP by suppressing O-linked glycosylation and reducing protein stability, thereby establishing SREBP-1c–dominated DNL[39] (Figure 2).
SREBP-1c, a member of the basic helix-loop-helix leucine zipper transcription factor family, forms a ternary complex with SREBP cleavage-activating protein (SCAP) and insulin-induced gene (INSIG), anchoring on the ER membrane as an inactive precursor. Under physiological conditions, stable binding between INSIG and SCAP effectively prevents the transport of the SCAP-SREBP-1c complex to the Golgi apparatus. However, in pathological states (e.g., reduced cellular sterol levels, inflammatory responses, or ER stress), the downregulation of INSIG protein expression relieves its suppressive effect on the SREBP-1c/SCAP complex. This complex is then transported to the Golgi apparatus via coat protein II-mediated vesicular trafficking, where it undergoes sequential proteolytic cleavage by site-1 protease and site-2 protease. This releases the transcriptionally active N-terminal fragment-mature SREBP-1c (cleaved SREBP-1c)[40,41]. Mature SREBP-1c specifically recognizes and binds to sterol regulatory elements in the promoter regions of target genes, upregulating the expression of key enzymes such as ACC, FASN, and SCD-1, thereby promoting hepatic DNL[42].
The abnormal activation of SREBP-1c is one of the core mechanisms underlying increased hepatic lipid synthesis in MASLD. Both insulin signaling and IR can activate SREBP-1c, with full induction of the mature transcriptionally active SREBP-1c protein requiring insulin. Insulin binds to its receptor, activating the phosphatidylinositol 3-kinase/AKT signaling pathway. AKT phosphorylates and inhibits the transcription factor FoxO1, thereby relieving the FoxO1-mediated suppression of SREBP-1c promoter, leading to SREBP-1c transcription. The insulin-mediated activation of SREBP-1c promoter also involves the combinatorial effects of cis-acting elements such as liver X receptor alpha (LXRα), SREBP-1c, Sp1, and nuclear factor-Y[43,44]. Additionally, insulin activates mechanistic target of rapamycin complex 1 (mTORC1) to enhance the cleavage and nuclear localization of the SREBP-1c precursor, strengthening its ability to regulate lipid synthesis genes (e.g., FASN and ACC). Insulin also specifically downregulates hepatic Insig-2 mRNA expression to promote fatty acid synthesis[41,45]. Under physiological conditions, insulin activates SREBP-1c to stimulate hepatic fatty acid and TG synthesis for energy storage. In IR, hepatocyte oxidative stress and inflammation activate LXRα, which directly binds to the SREBP-1c promoter and drives its expression. LXRα, a nuclear hormone receptor highly expressed in the liver, induces genes involved in cholesterol efflux and clearance, and is activated by oxysterols (cholesterol metabolites). In vivo studies highlight the role of LXRα in regulating SREBP-1c expression and its lipogenic target genes[46]. Animals lacking LXRα exhibit reduced basal expression of SREBP-1c, FASN, ACC, and SCD-1. Under pathological conditions such as hyperglycemia or stress, elevated SREBP-1c expression and enhanced nuclear translocation efficiency activate hepatic DNL, increasing the synthesis of FFAs and TGs, thereby exacerbating abnormal lipid accumulation in hepatocytes. Pro-inflammatory cytokines like TNF-α and IL-6 upregulate SREBP-1c precursor processing through activation of the nuclear factor-kappa B (NF-κB), c-Jun N-terminal kinase (JNK), or activator protein 1 pathways, increasing the nuclear translocation of mature SREBP-1c. In oxidative stress, reactive oxygen species (ROS) may enhance SREBP-1c stability and activity via pathways such as protein kinase C (PKC) θ activation. The inositol-requiring enzyme 1 alpha-X-box-binding protein 1 pathway in the unfolded protein response of the ER upregulates SREBP-1c expression to promote lipid synthesis and alleviate ER stress, as validated in mouse models with hepatic Srebp1c overexpression driving enhanced DNL[47].
Although suppressing SREBP-1c activity can reduce TG accumulation, the pathological effects of SREBP-1c-targeting intervention strategies are complex. On the one hand, SREBP-1c inhibitors (e.g., 25-hydroxycholesterol, methyl protodioscin) effectively reduce the expression of DNL-related genes in MASLD models and alleviate hepatic steatosis by blocking the ER-Golgi transport of the SREBP-1c/SCAP complex or inhibiting SREBP-1c gene expression[48,49]. On the other hand, the functional inhibition of the SREBP-1c/SCAP pathway may trigger compensatory lipid metabolic imbalance. For instance, liver-specific Scap knockout inhibits SREBP-1c activation but exacerbates liver injury, fibrosis, and carcinogenesis. This mechanism involves SREBP-1 suppression-induced impairment of fatty acid synthesis and abnormal phosphatidylcholine remodeling (associated with the downregulation of lysophosphatidylcholine acyltransferase 3), leading to ER stress, hepatocyte damage, and fibrosis[50], suggesting that SREBP-1c exerts dual regulatory effects on cellular homeostasis. Notably, interventions at different nodes may produce heterogeneous effects: The nuclear translocation inhibitor nobiletin (a bioactive component in the traditional Chinese medicine Chenpi) blocks the nuclear translocation of SREBP-1 and its binding to the ATP-citrate lyase promoter, thereby inhibiting phosphatidylinositol 3-kinase/AKT/mechanistic target of rapamycin pathway activation and inducing autophagy-dependent cell death[51]. The Aurora kinase inhibitor TAK901 suppresses glioblastoma proliferation and induces apoptosis by downregulating SREBP-1c expression and activation, an effect reversible by SREBP-1c overexpression[52]. Additionally, the metabolic pathway inhibitor MSI-1 enhances chemotherapy sensitivity in lung squamous cell carcinoma by antagonizing the SREBP-1-mediated Warburg effect (reduced glucose uptake and glycolysis)[53]. However, drugs specifically inhibiting SREBP-1 cleavage and activation (e.g., fatostatin), lower TG content in MASLD model hepatocytes, but aggravate apoptosis and programmed necrosis[54], likely due to the loss of the anti-cell death function of SREBP-1[55,56]. Collectively, these findings highlight the necessity to balance lipid metabolic regulation and cellular survival when designing SREBP-1-targeted therapies.
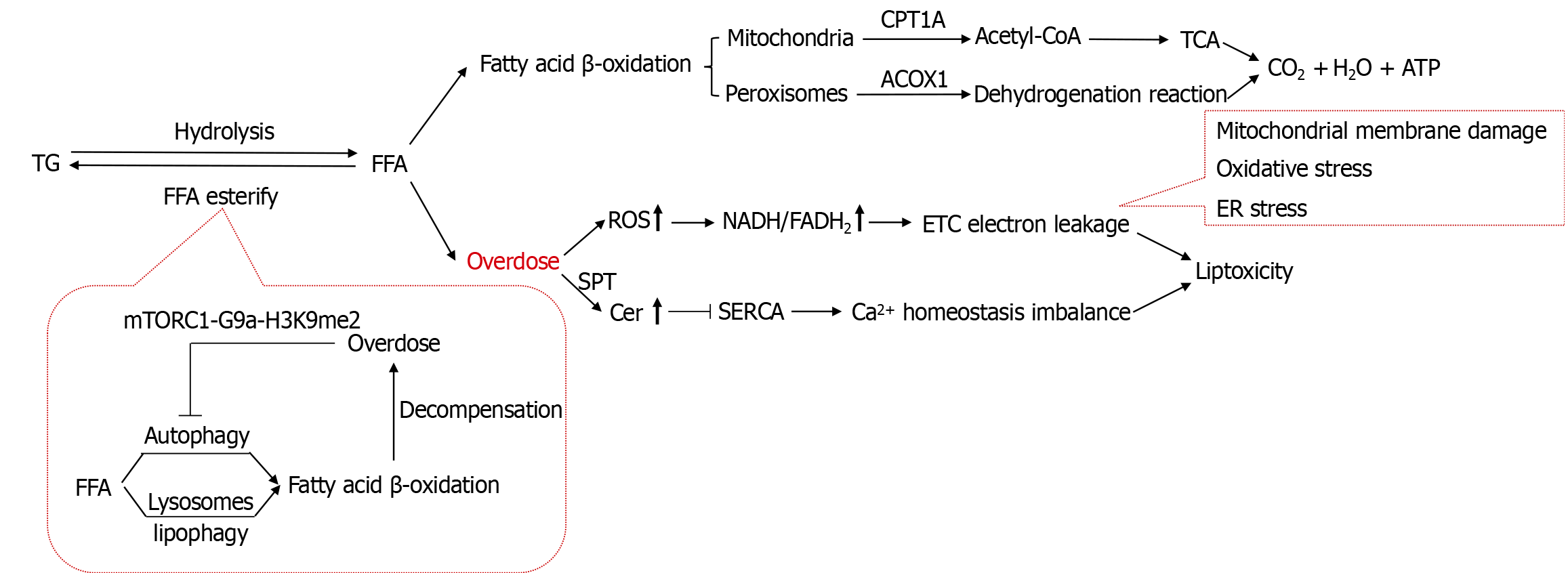
The catabolism of TGs in hepatocytes is a critical process for maintaining lipid homeostasis. Under the catalysis of enzymes such as ATGL, HSL, and monoacylglycerol lipase, intracellular TGs are hydrolyzed into one molecule of glycerol and three molecules of FFAs[57]. Glycerol subsequently enters the gluconeogenesis pathway, while FFAs are oxidized via fatty acid β-oxidation to generate ATP, re-esterified into TGs for storage, or released into the bloodstream for energy utilization (Figure 3).
Mitochondrial β-oxidation, a critical biochemical pathway for ATP synthesis and ketone body production during FFA metabolism, is rate-limited by the carnitine palmitoyltransferase 1A (CPT1A)-catalyzed formation of long-chain acylcarnitines. Upregulating CPT1A expression accelerates FFA β-oxidation, reduces their esterification into TGs, and thereby suppresses lipid deposition. Pharmacological or genetic strategies to enhance CPT1A expression and catalytic activity not only significantly boost fatty acid β-oxidation but also inhibit the phosphorylation cascade of the JNK signaling pathway, exerting anti-inflammatory and anti-apoptotic effects[58]. Furthermore, these interventions improve insulin signaling sensitivity and alleviate IR[59]. Conversely, reduced CPT1A activity suppresses hepatic fatty acid β-oxidation, driving lipid reprogramming that promotes excessive FFA esterification into TGs, ultimately leading to progressive pathological lipid accumulation in hepatocytes[60]. CPT1A overexpression ameliorates obesity-related metabolic disorders through multifaceted mechanisms: Enhancing insulin sensitivity and glucose homeostasis in carbohydrate metabolism, reducing hepatic TG accumulation in lipid metabolism, and exerting antioxidant/anti-inflammatory effects by suppressing ROS production and pro-inflammatory cytokine expression[61]. Enhanced DNL elevates malonyl-CoA levels, which inhibit CPT1A and redirect FFAs toward esterification, exacerbating TG deposition.
Peroxisome proliferator-activated receptors (PPARs), including PPARα, PPARβ, and PPARγ, are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily. PPARα is predominantly expressed in high-metabolic-activity tissues such as the liver, heart, and skeletal muscle, and regulates critical physiological processes, including fatty acid β-oxidation, inflammatory responses, and immune modulation by controlling the transcription of downstream target genes[62]. Within the hepatic lobular microenvironment, PPARα acts as a central metabolic regulator of fatty acid oxidative catabolism. Upon binding the fatty acids absorbed by hepatocytes, PPARα initiates transcriptional programs for key enzymes such as acyl-CoA oxidase and CPT1A, forming a precise lipid catabolic cascade that maintains the dynamic equilibrium of lipid uptake, synthesis, oxidation, and export in the hepatobiliary system[63]. By promoting fatty acid oxidation, PPARα alleviates pathological lipid accumulation in hepatocytes, thereby exerting protective effects against MASLD. Abnormalities in the expression of the acyl-coenzyme A oxidase 1 gene, which is regulated by PPARα, are closely linked to fatty acid metabolic disorders and TG deposition[64,65]. While mitochondrial β-oxidation primarily processes medium-to-long-chain fatty acids, peroxisomal β-oxidation targets very-long-chain fatty acids. Acyl-coenzyme A oxidase, the rate-limiting enzyme initiating peroxisomal β-oxidation, specifically catalyzes the dehydrogenation of long/very-long-chain fatty acids to form α,β-unsaturated enoyl-CoA derivatives[66]. In contrast to PPARα, which plays a pro-oxidative role, SREBP-1c promotes DNL by upregulating ACC to enhance malonyl-CoA synthesis, which inhibits fatty acid β-oxidation and exacerbates the lipogenesis-fatty acid oxidation imbalance.
TG storage itself is not inherently lipotoxic, and may act as a lipid buffer by reducing the accumulation of toxic lipids (e.g., FFAs and diacylglycerol) in the early stages of MASLD. However, the inhibition of DGAT2, while alleviating hepatic steatosis in obese mice, exacerbates liver injury and fibrosis by increasing hepatic FFAs, lipid peroxidation, and oxidative stress[67]. Impaired TG synthesis prevents effective FFA esterification, leading to their accumulation and the subsequent induction of apoptosis[68]. Prolonged TG storage, however, also promotes toxic intermediate accumulation[69-71], which exacerbates hepatocyte damage via mitochondrial respiratory chain dysfunction, oxidative stress, and inflammatory responses[72,73]. Excessive TG deposition drives pathological FFA and ceramide accumulation, a key mechanism of liver injury[74]. Excess FFAs—particularly saturated fatty acids like palmitate—enter the mitochondria and undergo β-oxidation, overproducing reducing equivalents (reduced nicotinamide adenine dinucleotide/reduced flavin adenine dinucleotide), which overload the electron transport chain and increase electron leakage, generating excessive ROS. The sustained overproduction of ROS damages organelles through multiple pathways: (1) Disrupting ER redox balance (reduced glutathione/oxidized glutathione), causing misfolded protein accumulation and ER stress; (2) Peroxidizing mitochondrial cardiolipin and impairing electron transport chain complexes, thereby suppressing ATP synthesis and causing energy failure; and (3) Oxidizing polyunsaturated fatty acids in membranes, producing lipid peroxidation products (e.g., malondialdehyde) that crosslink ER membrane proteins and increase ER permeability. Additionally, FFAs activate serine palmitoyltransferase-mediated de novo ceramide synthesis, elevating cytotoxic ceramide levels. Both FFAs and ceramides inhibit sarcoplasmic/ER Ca2+-ATPase pump activity, disrupting ER calcium homeostasis[75-77]. Saturated FFAs directly impair ER protein-folding capacity and alter membrane fluidity in the ER and lysosomes, exacerbating ER stress and lysosomal membrane permeabilization. Furthermore, excessive TGs may overload the ER—the initial site of lipid droplet formation—leading to structural and functional alterations in the ER membrane.
During the early phase of MASLD, hepatic ATGL may be transiently upregulated to counteract lipid overload[78]. As the disease progresses, hyperinsulinemia suppresses ATGL expression by inhibiting transcription factors like FoxO1[79,80]. Pro-inflammatory cytokines such as TNF-α and IL-6 further inhibit ATGL transcription via the NF-κB pathway. FFAs and ceramides may also directly impair ATGL activity. Reduced ATGL activity exacerbates hepatocellular TG accumulation, while excessive ATGL/HSL-mediated lipolysis-induced FFA overflow aggravates hepatocyte injury. In mouse models, ATGL inhibition reduces FFA release from TG lipolysis; although ATGL deficiency leads to progressive hepatic steatosis, it paradoxically alleviates histological inflammation, fibrosis, and ER stress[81-83]. Thus, ATGL plays a dual role in MASLD pathogenesis: Its initial upregulation serves as a protective mechanism against lipid overload, whereas its subsequent downregulation or dysfunction contributes to both lipid accumulation and the paradoxical mitigation of lipotoxicity-driven inflammation and fibrosis. PPARα agonists (e.g., fibrates) upregulate ATGL expression and improve hepatic steatosis, but their efficacy in steatohepatitis may be limited by their potential cytotoxicity[84].
In MASLD, mitochondrial fatty acid β-oxidation and oxidative phosphorylation are impaired[54,85], leading to the accumulation of toxic metabolic intermediates that exacerbate hepatocellular damage. The core mechanism of hepatic lipotoxicity involves the multi-level dysregulation of TG catabolism. In MASLD, hepatocyte autophagy activity is reduced, particularly lipophagy (selective degradation of lipid droplets via autophagosomes). Lipophagy, a protective mechanism dependent on double-membrane vesicles, mitigates hepatic lipid accumulation by hydrolyzing TGs. Impaired lipophagy directly diminishes TG hydrolysis, leading to abnormal lipid droplet accumulation, and is closely linked to lysosomal dysfunction[86]. Excess FFAs (e.g., palmitate) exacerbate lipotoxicity by activating ER stress, mitochondrial dysfunction, and oxidative stress cascades, mediated by the mTORC1-G9a-H3K9me2 axis-induced epigenetic suppression of autophagy-related genes[87,88]. Lysosomes, regulated by transcription factor EB-driven autophagy-lysosomal gene networks, compensate by selectively degrading lipid droplets to release FFAs for oxidation[89,90]. Early compensatory signals, such as regulator of calcineurin 2 deficiency, activate transcription factor EB to enhance lipophagy and sustain fatty acid oxidation[91]. However, persistent lipotoxicity disrupts lysosomal acidification and membrane stability, triggering protease leakage, inflammation, and a vicious cycle of autophagy blockade and lipid accumulation[92]. Peroxisomes, via the PPARα-dependent fatty acid oxidation of very-long-chain fatty acids and sirtuin 6-mediated lipophagy enhancement, may aggravate oxidative damage due to excessive ROS[93]. Collectively, disrupted TG catabolism drives lipotoxicity through multi-level interactions involving fatty acid oxidation, lipophagy, lysosomal homeostasis, and peroxisomal function.
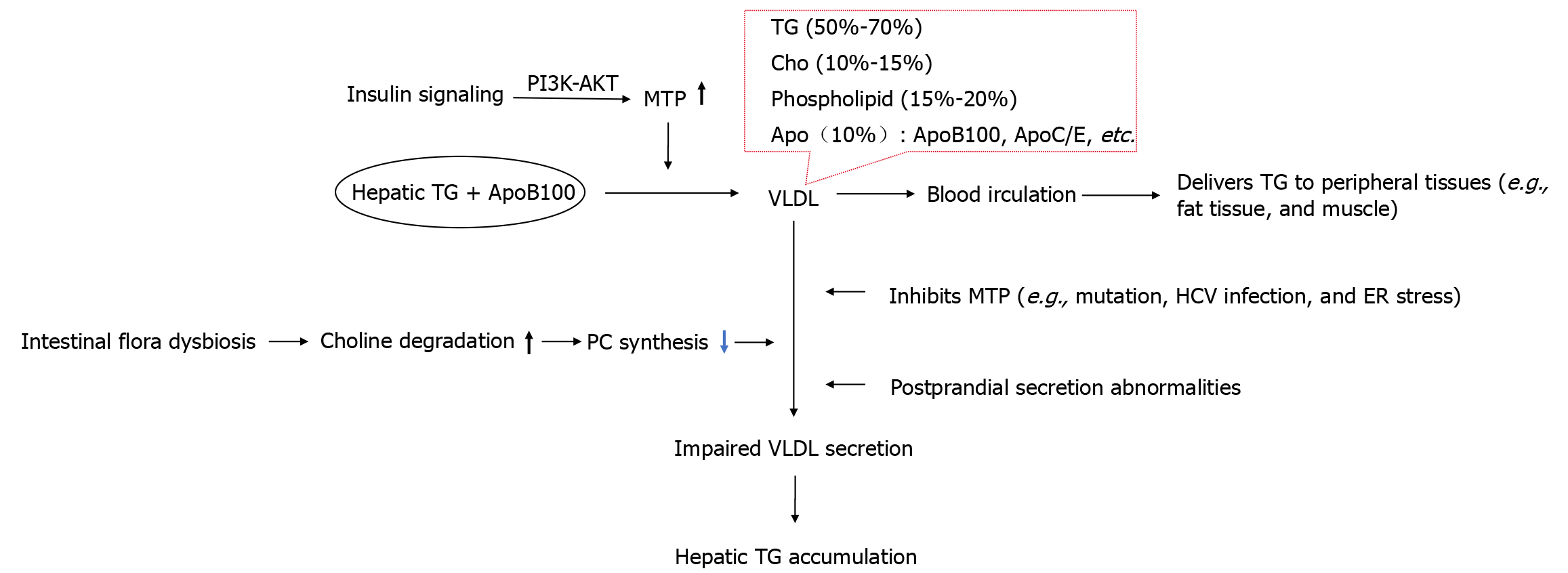
The secretion of TGs by hepatocytes via VLDL is the primary pathway for hepatic TG efflux, which is closely related to the pathogenesis of MASLD (Figure 4). This process involves multi-level molecular regulation. Newly synthesized TGs in the ER lumen are loaded onto ApoB100 via microsomal TG transfer protein (MTP)-mediated lipid transfer, forming a nascent VLDL precursor. Subsequently, the lipid composition is optimized in the Golgi apparatus, and the mature VLDL particles are finally released into the circulation via exocytosis. This mechanism delivers liver-synthesized TGs to peripheral tissues (e.g., adipose tissue, muscle) to support energy metabolism or TG storage in adipocytes[94,95]. Mature VLDL particles primarily consist of TGs (50%-70%), cholesterol (10%-15%), phospholipids (15%-20%), Apo (about 10%), and FFAs. Newly secreted VLDL particles from hepatocytes initially contain only ApoB100 on their surface but acquire ApoC and ApoE from HDL during circulation[96].
ApoB100 serves as the molecular scaffold for VLDL biogenesis, and its reduced synthesis efficiency or structural instability exacerbates hepatic TG accumulation. MTP drives VLDL assembly and secretion through a dual mechanism: Acting as a lipid chaperone to catalyze the loading of neutral lipids like TGs and cholesteryl esters onto ApoB100, forming precursor VLDL particles, and maintaining the conformational stability of ApoB100 to prevent its ubiquitination degradation triggered by ER-associated degradation. This biochemical cascade constitutes the rate-limiting step in VLDL biosynthesis. The regulatory function of MTP directly impacts hepatic lipid metabolic homeostasis[97]. Dysregulated MTP expression is closely linked to MASLD development[98,99]. ApoB100 mutations impair lipid loading and VLDL secretion, causing familial hypobetalipoproteinemia[100], while MTP mutations lead to abetalipoproteinemia (e.g., Anderson disease), characterized by disrupted hepatic lipid transfer, abnormal lipid droplet accumulation, and plummeting plasma Apo levels[101]. Liver-specific Mtp knockout or MTP inhibition induces marked hepatic steatosis due to blocked VLDL assembly/secretion and subsequent lipid retention. Conversely, MTP overexpression enhances ApoB-mediated VLDL production, promoting TG efflux. These findings underscore the pivotal role of MTP in hepatic lipid homeostasis. FoxO1 mediates the regulation of MTP production by insulin. In the fed state, insulin suppresses FoxO1, thereby promoting VLDL assembly. However, in MASLD-associated hyperinsulinemia, hepatic IR may impair this regulation, leading to sustained FoxO1 activity and MTP suppression—a potential contributor to VLDL secretion defects[102]. Hepatitis C virus inhibits MTP activity, blocking TG-enriched VLDL assembly/secretion and causing hepatic lipid deposition, a core mechanism underlying hepatitis C virus-associated hepatic steatosis[103].
Postprandial VLDL-TG secretion is a critical mechanism for maintaining hepatic lipid metabolic homeostasis. In obesity or MASLD, impaired postprandial VLDL secretion exacerbates intrahepatic lipid deposition[104,105]. IR acts as a central driver through dual mechanisms: (1) Inducing postprandial plasma fatty acid profile abnormalities (elevated saturated fatty acids and reduced polyunsaturated fatty acids), which promote intrahepatic lipid accumulation and oxidative stress[106]; and (2) Disrupting insulin-regulated glucose-lipid metabolic balance via mTORC1 signaling dysregulation, forming a self-reinforcing metabolic vicious cycle[107]. Additionally, gut-liver axis dysregulation accelerates this pathology: Gut microbiota modulate hepatic lipid metabolism through bile acid metabolic reprogramming and short-chain fatty acid production[108,109]. Dysbiosis-induced postprandial endotoxemia activates hepatic inflammatory responses via the Toll-like receptor 4/NF-κB pathway and upregulates lipogenic enzymes (e.g., ACC and FASN)[110]. Furthermore, gut bacteria promote host choline deficiency by accelerating choline degradation, impairing hepatic VLDL assembly and indirectly driving TG accumulation[111-113]. Animal studies confirm that microbiota depletion significantly alters hepatic lipidomic profiles (e.g., sphingolipid dysregulation)[114], highlighting the pivotal role of mic
Obese and lean MASLD exhibit significant differences in metabolic mechanisms (Table 1). The primary initiating factors in obese MASLD are systemic IR and nutrient excess, while lean MASLD is predominantly driven by genetic variations, abnormal visceral fat distribution, adaptive metabolic responses, and gut-liver axis dysfunction[115,116]. Genetic susceptibility is particularly prominent in lean MASLD patients. The rs738409 polymorphism in the patatin-like phospholipase domain-containing protein 3 gene can inhibit hepatocyte TG hydrolysis[117]. Additionally, a variant of the trans
| Characteristic | Obesity-related MASLD | Lean MASLD |
| Primary initiating factors | Systemic IR, overnutrition | Genetic variants, dysfunctional visceral adipose tissue, and gut-liver axis dysregulation |
| FFA source | Peripheral lipolysis | Visceral adipose tissue, gut microbiota-derived metabolites |
| DNL | Enhanced | Enhanced |
| Fatty acid oxidation | Impaired | May be markedly reduced |
| VLDL | Early compensatory increase (relative insufficiency), later absolute insufficiency | Genetic secretion defects (e.g., TM6SF2 variants) or normal |
| Inflammatory triggers | Early adipose tissue macrophage activation, later accompanied by hepatic innate immune activation | Gut-derived LPS translocation, hepatic innate immune activation |
| Genetic predisposition | Polygenic susceptibility with cumulative minor effects | Monogenic strong effects (e.g., PNPLA3) |
| Prognosis | Higher incidence of cardiovascular complications | Higher incidence of liver disease and all-cause mortality |
| Clinical management focus | Weight reduction, improving IR, managing metabolic syndrome | Fructose restriction, correcting malnutrition, targeted genetic interventions |
Currently, two experimental models are commonly used to simulate this pathological process: In vivo hepatic steatosis models established via high-fat diet, high-fat high-sucrose diet, or high-fat high-sucrose high-cholesterol diet, and in vitro hepatocyte steatosis models induced by FFAs. Both models replicate the core pathological features of MASLD by increasing hepatocyte lipid uptake and elevating lipid burden. This modeling strategy not only highlights the universality of extrahepatic lipid metabolic dysregulation in driving hepatic TG deposition via promoting hepatocyte lipid uptake, but also reveals the central role of this dysregulated metabolic pathway in the development and progression of MASLD through reproducible experimental systems. During MASLD progression, circulating FFA levels are significantly higher in patients than in healthy individuals, and their concentration positively correlates with the stage of liver fibrosis. IR and hyperinsulinemia likely upregulate the expression of fatty acid transporter protein and CD36 on hepatocyte membranes, markedly enhancing hepatic FFA uptake and exacerbating hepatic steatosis. As the disease advances, the liver’s capacity for lipid uptake continues to increase. However, concurrent SREBP-1c-mediated DNL hyperactivity, combined with impaired PPARα-mediated fatty acid oxidation and VLDL-mediated TG export, worsens TG metabolic imbalance, driving progression to more severe stages[48,64,65].
The HNF-1 family, comprising two members (HNF-1α and HNF-1β), is enriched in the liver and also distributed in the kidneys, small intestine, and pancreatic β-cells[119]. As a pivotal transcription factor, HNF-1 plays a critical role in liver development and the maintenance of liver-specific functions by regulating multiple genes associated with hepatic activities[120]. HNF-1β is expressed during the early stages of liver development, where it regulates hepatocyte differentiation, biliary system development, and metabolic gene expression, ensuring normal hepatic maturation and functional integrity. In contrast, HNF-1α is expressed in the late differentiation stages, primarily in highly differentiated hepatocytes, and is responsible for sustaining their differentiated phenotype[121]. Studies indicate that HNF-1 plays essential roles in various aspects of hepatocellular lipid metabolism by activating the transcription of lipid metabolism-related genes[122,123]. Reduced expression or functional abnormalities of HNF-1 may lead to disruptions in lipid metabolism, thereby contributing to the onset of related metabolic disorders[124,125].
HNF-1α suppresses SREBP-1c-, ChREBP-, and PPARγ-mediated lipogenic gene expression, thereby reducing FFA and TG synthesis, while enhancing fatty acid oxidation by activating the PPARα-mediated transcriptional regulation of mitochondrial fatty acid β-oxidation genes (Table 2)[126]. HNF-1α may inhibit lipid synthesis by competitively binding or regulating the promoter region of the SREBP-1c target gene; when IR occurs, this inhibitory effect weakens, and SREBP-1c-driven lipid synthesis dominates[127]. The transcription of SREBP-1c is regulated by epigenetic mechanisms such as histone modification and DNA methylation[128,129]. However, whether HNF-1α directly regulates the SREBP-1c promoter or participates in its epigenetic modifications, and the dynamic interaction mechanisms between the two in IR need to be further explored in the future.
| HNF | Model | Target | Mechanism | Main results | Ref. |
| HNF-1α | C57BL/6J mice | L-FABP↑ | Promotes the transport of long-chain fatty acids from the intracellular space to specific organelles | Promotes lipid synthesis | [133] |
| C57BL/6J mice | PPAR-γ↓ | Reduces synthesis of TGs and Cho | Inhibits lipid synthesis | [126] | |
| C57BL/6J mice | SREBP-1c↓ | Inhibits the expression of its target lipogenesis genes | Inhibits TG synthesis | [130] | |
| HepG2 cells and Huh7 cells | Sigma receptor 1↓ | Decreases intracellular lipid droplet formation rate and lipid storage capacity | Inhibits lipid synthesis | [136] | |
| C57BL/6J mice | PCSK9↑ | Mediates degradation of LDL receptors and increases plasma LDL-C levels | Reduces Cho intake | [142] | |
| - | CYP7A1↑, BSEP↑, NTCP↑ | Promotes the elimination of BAs | Reduces Cho accumulation | [143] | |
| HepG2 cells | MiR-122↓ | Enhances miR-122-inhibited SCAP expression and interferes with SREBP-2 maturation | Reduces lipid synthesis and absorption | [130] | |
| HNF-1β | - | Angiopoietin-like protein 8↑ | Inhibits LPL activity | Reduces TG hydrolysis | [147,148] |
| 3T3-L1 preadipocytes | PPAR-γ↓ | Enhances mitochondrial oxidative phosphorylation | Accelerates TG decomposition | [149] | |
| C57BL/6J mice | GPX1↑ | Reduces ROS levels, which in turn reduces the expression of SREBP-1, ACC, and FASN | Indirectly inhibits TG synthesis | [151] | |
| AML-12 cells | SREBP-1c↓ | Inhibits the expression of its target lipogenesis genes | Inhibits lipid synthesis | [146] | |
| FOXA1 | HepG2 cells | GPAT1↓, DGAT2↓, MTP↓, ApoB↓ | Inhibits the expression of its target genes related to TG synthesis and secretion | Inhibits TG synthesis and secretion | [157] |
| HepG2 cells | FABP1↑ | Activates its transcription | Promotes intracellular transport of fatty acids | [158] | |
| HepG2 cells | UCP1↑ | Enhances its expression and reduces mitochondrial membrane potential | Reduces lipid storage | [157] | |
| HepG2 cells | HMGCS2↑ | Enhances ketone production | Promotes TG catabolism | [157] | |
| FOXA2 | HepG2 cells | hFABP1↑ | Activates its transcription | Involved in the transport of long-chain fatty acids | [160] |
| - | PGC-1β↑ | Activates mitochondrial fatty acid oxidation | Enhances FA metabolism | [166] | |
| FOXA3 | C57BL/6J mice | ApoA-1↑ | Mediates reverse Cho transport by macrophages | Promotes liver cell steatosis | [173] |
| HNF-4α | HFD mice | ApoB↓ | Reduces secretion of VLDL | Promotes lipid accumulation | [187] |
| C57BL/6J mice | ULK1↑ | Activates lipophagy | Reduces lipid storage | [199] | |
| HFD mice | CDKL3↑ | Induces phosphorylation of FoxO1 | Reduces lipid accumulation | [207] | |
| HNF-6 | BDL mice | CYP7A1↓ | Reduces Cho-to-BA conversion | Severely impairs Cho clearance | [208] |
By inhibiting the expression of the ApoC3 gene, HNF-1α alleviates the ApoC3-mediated suppression of LPL activity, thereby indirectly upregulating LPL function to accelerate TG breakdown in the circulation. Consequently, HNF-1α deficiency or reduced activity may upregulate ApoC3 expression, increasing ApoC3 protein levels and impairing LPL-mediated TG hydrolysis. Studies demonstrate that small interfering RNA-mediated HNF-1α downregulation in HepG2 cells induces excessive lipid droplet accumulation and promotes the intracellular deposition of TGs and cholesterol[130,131]. Overexpression of HNF-1α suppresses PPARγ expression, which reduces TG and cholesterol synthesis, and ameliorates hepatocellular lipid metabolic disorders. Additionally, HNF-1α activates transcription of the liver fatty acid-binding protein (L-FABP) gene. L-FABP binds to long-chain fatty acids and facilitates their transport from the cytoplasm to organelles such as mitochondria and peroxisomes for β-oxidation, thereby reducing lipid accumulation in hepatocytes[132,133]. L-FABP may further modulate oxidative metabolism by interacting with PPARδ. HNF-1α functions as an autophagy cargo receptor, directly binding to microtubule-associated protein 1A/1B light chain 3 through its light chain 3-interacting region domain to facilitate autophagosome formation. This interaction specifically induces K33-linked ubiquitin chain modification on TANK-binding kinase 1, promoting its autophagic degradation and ultimately alleviating fatty acid accumulation in vivo[134,135]. Furthermore, HNF-1α inhibits the expression of sigma receptor 1 on the ER membrane, significantly reducing lipid droplet formation and storage capacity, which alleviates hepatic steatosis[136]. Dysfunction of HNF-1α may impair autophagy, leading to TG accumulation, and induce lipotoxicity, both of which are closely associated with the pathogenesis of MASLD.
In cases of hepatic steatosis, HNF-1α expression in hepatocytes tends to decline[122], leading to the activation of SREBP-1c, which increases the synthesis of saturated fatty acids, including palmitic acid, thereby inducing mitochondrial oxidative stress and ER stress[73,137]. Under these conditions, the buffering capacity of lipid droplets is compromised, and the disruption of lipophagy prevents lipid droplets from effectively sequestering excessive fatty acids. Consequently, FFAs are released into the cytoplasm, activating lipotoxicity[68,138].
HNF-1α is a key regulator of hepatic glucose metabolic homeostasis. The reduced expression or activity of HNF-1α downregulates fructose-1,6-bisphosphatase, phosphoenolpyruvate carboxykinase, and glucose-6-phosphate transporter, thereby suppressing gluconeogenesis and reducing hepatic glucose output into the bloodstream[139,140]. Impaired gluconeogenesis leads to the accumulation of gluconeogenic precursors (lactate, glycerol, and amino acids), which are diverted toward TG synthesis. Concurrently, reduced hepatic glucose production lowers fasting blood glucose, triggering enhanced TG breakdown in adipose tissue and increasing FFA influx into the liver. HNF-1α inactivation also downregulates insulin-like growth factor 1, contributing to IR. Furthermore, HNF-1α deficiency reduces the transcription of glucose transporter 2 (encoded by SLC2A2), reducing the expression of its encoded protein, glucose transporter 2. This impairs postprandial hepatic glucose uptake (leading to postprandial hyperglycemia) and fasting glucose release (causing fasting hypoglycemia)[141]. The alternation between fasting hypoglycemia and postprandial hyperglycemia, lipid accumulation, IR, and chronic inflammation collectively drive the progression of metabolic syndrome. HNF-1α directly upregulates the transcription of the microRNA (miRNA) miR-122, thereby enhancing the miR-122-mediated suppression of SCAP expression and interfering with the maturation of SREBP-2, ultimately leading to reduced lipid biosynthesis and decreased lipid uptake in HepG2 hepatocytes[130]. Hepatic HNF-1α transcriptionally upregulates proprotein convertase subtilisin/kexin type 9, which reduces hepatic LDL endocytosis and cholesterol uptake by mediating LDL receptor degradation and increasing plasma LDL-cholesterol levels[142]. Cholesterol 7α-hydroxylase (CYP7A1), a liver-specific enzyme, catalyzes the rate-limiting step in cholesterol degradation to bile acids. HNF-1α binds to the proximal promoter region of the CYP7A1 gene and contributes to its basal transcriptional activity[143]. Downregulation of HNF-1α thus decreases CYP7A1 expression, which reduces the expressions of bile salt export pump and sodium taurocholate cotransporting polypeptide, thereby impairing bile acid excretion and ultimately leading to hepatic cholesterol accumulation. HNF-1α downregulation also enhances acyl-CoA: Cholesterol acyltransferase activity, promoting cholesterol esterification in hepatocytes. HNF-1α further binds to the promoters of apolipoprotein genes (ApoA1, ApoA2, ApoC3, and ApoE). Genome-wide association studies indicate that HNF1A mutations are risk factors for elevated LDL, high TG, and low HDL levels[144]. Another study corroborates the link between HNF1A polymorphisms and serum lipid profiles[145].
HNF-1β plays a critical role in the regulation of lipid metabolism. Studies show that its overexpression suppresses the expression of SREBP-1c and lipogenic enzymes (e.g., L-FABP, ACC, and FAS), thereby reducing lipid synthesis and absorption[146]. Additionally, HNF-1β upregulates angiopoietin-like protein 8, which inhibits LPL activity in C57BL/6J mice, slowing the hydrolysis of TGs into FFAs and disrupting TG metabolic balance[147,148]. Concurrently, HNF-1β enhances mitochondrial oxidative phosphorylation and accelerates the catabolism of TGs and FFAs by downregulating PPARγ and its downstream target genes, while significantly improving cholesterol conversion to bile acids[149,150]. Overexpression of HNF-1β also upregulates glutathione peroxidase 1, lowering ROS levels and subsequently reducing SREBP1, ACC, and FASN expression to ameliorate lipid metabolic disorders[151]. Dipeptidyl peptidase 4 (DPP4) degrades glucagon-like peptide-1, which stimulates insulin secretion from pancreatic β-cells. DPP4 inhibitors have been introduced for the treatment of type 2 diabetes[152]. The promoter region of the DPP4 gene contains functional HNF1 binding sites[153]. Conversely, knockdown of the Hnf1b gene in mice exacerbates high-fat diet-induced hepatic steatosis through two mechanisms: (1) Upregulation of DPP4, which aggravates IR; and (2) Enhanced nicotinamide adenine dinucleotide phosphate oxidase 1 expression, which promotes oxidative stress[154]. Whether HNF-1β directly regulates the expression of DPP4 and subsequently affects lipogenesis needs further investigation. Thus, aberrant expression or functional impairment of HNF-1β may impair mitochondrial oxidative capacity, hinder lipid catabolism, and disrupt lipid homeostasis, ultimately contributing to the development of lipid metabolism-related diseases.
The FOXA family members (FOXA1, FOXA2, and FOXA3, corresponding to HNF-3α, HNF-3β, and HNF-3γ, respectively) maintain TG metabolic homeostasis by regulating hepatic lipid synthesis, oxidative catabolism, and VLDL-TG secretion-related gene expression. Impairment of FOXA1/2 function or hyperactivation of FOXA3 disrupts TG metabolic homeostasis, leading to pathological TG deposition, which is closely associated with the development of MASLD[155,156].
As a pivotal anti-steatosis regulator, FOXA1 suppresses hepatic TG synthesis, accumulation, and secretion by downregulating the expression of key genes, including glycerol-3-phosphate acyltransferase, DGAT2, MTP, and APOB. Concurrently, FOXA1 enhances intracellular fatty acid transport and oxidation efficiency through the transcriptional activation of L-FABP1. Furthermore, FOXA1 synergistically activates the PPARα signaling pathway to promote fatty acid oxidation. Notably, the hepatic overexpression of FOXA1 significantly upregulates uncoupling protein 1 expression, which dissipates cellular energy as heat and reduces mitochondrial membrane potential, thereby diminishing lipid storage[157,158]. Mechanistically, FOXA1 augments ketogenic metabolism in hepatocytes by elevating the expression of 3-hydroxy-3-methylglutaryl-CoA synthase within the mitochondrial matrix, thereby accelerating TG catabolism[157]. In MASLD, impaired SUMOylation of FOXA1 Leads to decreased protein expression in the liver. Experiments using hepatocyte-specific Foxa1 knockout mouse models reveal suppressed fatty acid oxidation and prominent intracellular lipid droplet accumulation, which can be ameliorated by overexpression of the deacetylase sirtuin 6[159].
FOXA2 promotes fatty acid oxidation through activation of PPARα and fibroblast growth factor 21. Under IR conditions, the phosphorylation-induced inactivation of FOXA2 suppresses the transcription of genes related to fatty acid oxidation, leading to lipid accumulation. Human liver fatty acid-binding protein 1 gene is highly transcribed in hepatocyte-derived cells. FOXA2 regulates human liver fatty acid-binding protein 1 expression, thereby participating in cellular long-chain fatty acid transport and lipid metabolism modulation[160]. Hepatic FOXA2 mRNA levels are significantly reduced in both MASH patients and murine models[161,162]. As a key transcriptional regulator of augmenter of liver regeneration, FOXA2 mitigates ER stress by inhibiting JNK activation and pro-apoptotic C/EBP homologous protein, while upregulating proteins involved in lipid detoxification (FABP1, CPT1A) and downregulating the toxic lipid transporter elongation of long-chain fatty acids family member 6, thereby reducing lipotoxicity and exerting anti-apoptotic effects. Its downregulation exacerbates MASLD progression through enhanced lipid deposition and impaired mitochondrial fatty acid oxidation[161]. Contrastingly, hepatic Foxa2 mRNA expression is markedly upregulated in diet-induced insulin-resistant mice and Zucker diabetic fatty rats, and this can be normalized by treatment with the insulin sensitizer pioglitazone[163]. These divergent findings suggest context-dependent FOXA2 regulation across steatotic liver disease models, though most evidence supports its downregulation in hepatosteatosis[162]. This discrepancy may reflect differences in pathological stages: MASH models represent advanced disease, whereas insulin-resistant models may exhibit compensatory transient Foxa2 upregulation to counteract lipid overload. Mechanistically, FOXA2 governs ketogenesis and fatty acid oxidation by transcriptionally activating CPT1A and 3-hydroxy-3-methylglutaryl-CoA synthase 2 promoters[164]. The LPL promoter contains FOXA2-binding sites, and FOXA2 dose-dependently enhances its transcriptional activity[165]. Furthermore, FOXA2 cooperates with PPARγ coactivator 1β to co-activate mitochondrial fatty acid oxidation genes and induce MTP expression, thereby promoting TG/VLDL secretion and maintaining lipid homeostasis[166]. Hepatocyte-specific Foxa2 knockout mice exhibit significantly impaired ketogenesis and fatty acid oxidation capacity, particularly under high-fat diet conditions, confirming the essential role of FOXA2 in hepatic lipid catabolism[167]. Notably, Foxa2 ablation via the Cre-loxP system reduces bile acid transporter gene transcription at hepatocyte membranes, inducing intrahepatic cholestasis. Consistently, FOXA2 expression is markedly decreased in human cholestatic liver specimens, suggesting its deficiency aggravates liver injury[168]. Pharmacologically, the antidepressant imipramine alleviates hepatic steatosis through FOXA2/CPT2 pathway activation[169].
Genetic studies reveal that the FOXA3 single nucleotide polymorphism rs28666870 is significantly associated with human metabolic traits, including elevated body mass index and increased lean body mass, particularly appendicular lean mass[170]. Hepatic FOXA3 expression is upregulated in both MASLD patients and high-fat diet-fed mice[171]. In contrast to the lipid-homeostatic roles of FOXA1/2, FOXA3 functions as a pro-lipogenic regulator by activating the period circadian regulator 1/SREBP-1c transcriptional axis, thereby upregulating lipogenic gene expression and enhancing lipid synthesis[171]. Notably, conflicting evidence shows FOXA3 downregulation in the livers of MASLD patients and high-fat diet-fed murine models, and hepatic FOXA3 overexpression enhances fatty acid oxidation and inhibits lipid synthesis via the activation of Takeda G protein-coupled receptor 5[172,173]. FOXA3 overexpression elevates ApoA1 expression and serum HDL-cholesterol levels, whereas its knockdown exerts opposing effects. Mechanistically, FOXA3 has been demonstrated to increase the expression of ApoA1 by directly binding to the promoter region of the ApoA1 gene and activating its transcription[173]. ApoA1 is the main structural protein of HDL and is responsible for the retrograde transport of cholesterol from peripheral tissues to the liver for metabolism, a process known as reverse cholesterol transport[174,175]. While hepatocyte FOXA3-mediated ApoA1 induction may protect against atherosclerosis by enhancing macrophage reverse cholesterol transport[172], this mechanism concurrently increases the amount of cholesterol delivered by HDL to the liver; if hepatic processing capacity is insufficient, this may lead to cholesterol accumulation, and promote hepatic steatosis. In summary, FOXA1 and FOXA2 inhibit TG accumulation in hepatic steatosis, but their expression tends to be downregulated; in contrast, FOXA3 promotes TG production, but its expression tends to be upregulated.
HNF-4α (nuclear receptor subfamily 2 group A member 1), a nuclear hormone receptor highly enriched in the liver, serves as a central transcriptional regulator by binding to HNF-4α response elements in the promoters of approximately 40% of actively transcribed hepatic genes, thereby maintaining metabolic homeostasis[176,177]. Its regulatory network spans glucose-lipid metabolism, amino acid metabolism, and transport systems. Upregulation of HNF-4α enhances the transcriptional activity of multiple functional genes in embryonic stem cells, including those encoding fatty acid oxidation rate-limiting enzymes (e.g., CPT1A), Apo (ApoA1, ApoB, and ApoC3), gluconeogenic enzymes (phosphoenolpyruvate carboxykinase, fructose-1,6-bisphosphatase), glycogen metabolism enzymes (glycogen synthase, glucose-6-phosphatase), amino acid metabolic enzymes (phenylalanine hydroxylase), and transport proteins (transferrin, retinol-binding protein)[178,179]. HNF-4α deficiency induces multifaceted abnormalities in embryonic murine hepatocytes: It suppresses liver-specific proteins (albumin, α-fetoprotein), lipid metabolism regulators (Apo, L-FABP), and iron-related proteins (transferrin), and disrupts epithelial integrity via E-cadherin loss; metabolically, it reduces hepatic glycogen reserves, promotes lipid accumulation with paradoxical decreases in cholesterol and TGs, and elevates bile acid levels[180,181]. Structurally, it disrupts hepatic cord-sinusoid architecture, leading to profound liver disorganization. These findings establish HNF-4α as a master regulator that connects metabolic regulation with hepatic development and pathology.
Experimental results have demonstrated that in MASLD model rats, HNF-4α activity exhibits a progressive decline, and the magnitude of its reduction shows a positive correlation with disease progression and a significant negative correlation with TG content. This phenomenon has been widely validated in both clinical specimens and animal models: Significant downregulation of HNF-4α expression was observed in the liver tissues of MASLD patients and MASH mouse models[182], which is consistent with findings in human chronic liver diseases (including MASLD/MASH) where gradual loss of HNF-4α expression/activity characterizes disease progression[183], suggesting that HNF-4α functional impairment may critically drive lipid metabolic dysregulation and pathological advancement in MASLD. Genome-wide association studies have identified single nucleotide polymorphisms at the HNF-4α locus that are significantly associated with MASLD susceptibility. Mechanistically, HNF-4α is a highly conserved, constitutively active nuclear receptor that binds DNA exclusively as a homodimer. The nucleocytoplasmic shuttling of HNF-4α is triggered by the phosphorylation of a conserved serine residue (Ser78) within its DNA-binding domain, a process mediated by PKC. This phosphorylation exposes a nuclear export signal and enables binding to the nuclear export receptor chromosomal region maintenance 1 (exportin 1). Treatment with PKC activators increases the cytoplasmic localization of HNF-4α and reduces endogenous HNF-4α protein levels[184]. This process is triggered by oxidative stress resulting from microenvironmental factors associated with steatosis such as lipotoxicity (high FFAs), IR (hyperinsulinemia), and inflammation. Conversely, PKA and AMP-activated protein kinase (AMPK) phosphorylate HNF-4α at Ser134 in the N-terminal half of the DNA-binding domain and at Ser304 in the C-terminal half of the ligand-binding domain, respectively, impairing its dimerization and DNA-binding activity while promoting its nuclear retention[185,186]. Oxidative stress, ER stress, and dysfunction of molecular chaperones cause decompensation of this protective effect, exacerbating the imbalance and ultimately promoting fat accumulation in the liver. Future research should further elucidate the functions of specific phosph
The liver-specific knockdown of HNF-4α induces marked reductions in plasma TGs, total cholesterol, and HDL-cholesterol levels, accompanied by impaired VLDL secretion and diminished hepatic DNL, ultimately triggering hepatic steatosis[192]. Conversely, the hepatocyte-specific overexpression of HNF-4α reduces intrahepatic TG accumulation by enhancing TG lipolysis, fatty acid oxidation, and VLDL-associated TG secretion[193,194]. Mechanistically, activating transcription factor 3 exerts preventive effects against the progression from metabolic dysfunction-associated steatotic liver to MASH by modulating fatty acid oxidation through HNF-4α-dependent pathways[195]. Therapeutically, the lipid nanoparticle-mediated delivery of Hnf4a mRNA significantly attenuates fibrogenesis during hepatic steatosis progression, as evidenced by a 60% reduction in α-smooth muscle actin expression[196]. At the epigenetic level, sirtuin 1-mediated deacetylation enhances HNF-4α transcriptional activity while suppressing phosphoenolpyruvate carboxykinase gene expression, thereby coordinating glucose and lipid metabolism[197]. Furthermore, HNF-4α activates mitochondrial fatty acid oxidation genes (e.g., CPT1A) through a PPARγ coactivator-1α-dependent mechanism, highlighting its central role in metabolic reprogramming.
Beyond direct transcriptional regulation of metabolic genes, HNF-4α also modulates hepatic lipid homeostasis through autophagy-dependent pathways. Patients with MASLD exhibit reduced autophagy activity, a self-protective mechanism involving the selective degradation of lipid droplets via double-membrane vesicles (autophagosomes), which plays a critical role in alleviating hepatic lipid accumulation[198]. Unc-51-like autophagy activating kinase 1 (ULK1), a core regulator of autophagy, is essential for maintaining lipid homeostasis; the liver-specific depletion of ULK1 directly induces hepatocyte steatosis, while HNF-4α overexpression enhances autophagy activity by upregulating ULK1, thereby mitigating hepatic lipid deposition[199]. HNF-4α reduces hepatic lipid storage by activating lipophagy, a selective autophagy process targeting lipid droplets, but this protective mechanism is suppressed in MASLD. Notably, HNF-4α agonists can restore lipophagy activity[200]. Recently, miRNAs, such as miR-34a, miR-449a, miR-24, and miR-124, have emerged as regulators of many diverse functions of HNF-4α, modulating the expression of metabolic enzymes[201], the cell cycle[202], and hepatocellular oncogenesis[203]. Studies reveal an inverse correlation between hepatic miR-34a and HNF-4α expression, where miR-34a promotes TG accumulation by inhibiting HNF-4α, suggesting that the miR-34a-HNF-4α axis is a potential therapeutic target in MASLD[182,204]. HNF-4α transcriptionally regulates hepatic miR-338-3p expression, which targets protein phosphatase 4 regulator subunit 1 to enhance protein phosphatase 4 activity, thereby ameliorating TNF-α-induced IR and restoring hepatic IR[205]. HNF-4α regulates miR-122 to mediate hepatic gluconeogenesis and lipid metabolism abnormalities, and its effect can be blocked by miR-122 inhibitors[206]. Additionally, cyclin-dependent kinase-like 3, a direct transcriptional target of HNF-4α, has emerged as a key regulator in MASLD progression. Suppression of HNF-4α during MASLD reduces cyclin-dependent kinase-like 3 expression for the ubiquitination-dependent degradation of FoxO1, exacerbating hepatic inflammation, fibrosis, and glucose metabolism disorders[207]. These findings collectively highlight the central role of autophagy-related pathways in MASLD pathogenesis and thera
HNF-6, a member of the ONECUT transcription factor family, generates two functional isoforms—HNF-6α and HNF-6β—through alternative splicing of exons. Both isoforms contain a conserved DNA-binding domain and an N-terminal STP-box domain, with the STP-box mediating nuclear localization and facilitating transcriptional activation by recruiting coactivators to target genes. HNF-6 dynamically regulates hepatic physiological functions (e.g., lipid metabolism) and pathological processes (e.g., liver fibrosis and cancer metastasis) by modulating hepatocyte proliferation, differentiation, and organ development[208].
HNF-6 forms a liver-specific regulatory network with other nuclear factors to critically modulate hepatic lipid metabolism. HNF-6 deficiency in the liver upregulates multiple lipogenic genes, leading to hepatic steatosis[209]. For instance, HNF-6 directly enhances the transcription of CYP7A1, thereby promoting cholesterol conversion into bile acids, and reducing hepatic cholesterol levels[210]. In bile duct ligation animal models, both HNF-6 and CYP7A1 expression are significantly downregulated, resulting in the severe impairment of cholesterol catabolism[208].
Furthermore, HNF-6 collaborates with the circadian nuclear receptor Rev-erbα to form a functional regulatory network in hepatic lipid metabolism. Studies reveal that the liver-specific knockout of HNF-6 in adult mice induces hepatic steatosis and alters the expression of multiple lipid metabolism-related genes[209]. Furthermore, this study also demonstrates the synergistic gene regulatory role of HNF-6 and Rev-erbα: HNF-6 suppresses lipid synthesis gene expression by recruiting Rev-erbα and its co-repressor complex. Besides, HNF-6 and Rev-erbα maintain hepatic metabolic homeostasis through independent regulatory mechanisms on distinct gene sets[209]. This finding not only identifies a novel function of HNF-6 in adult hepatic lipid metabolism but also provides mechanistic insights into the complex transcriptional regulatory network of liver metabolism, offering potential therapeutic targets for metabolic disorders such as fatty liver. Notably, given that Rev-erbα serves as a core circadian rhythm regulator, HNF-6 may integrate circadian signaling into the control of lipid metabolism.
Hierarchical regulation and synergistic interactions of HNFs: Members of the HNF family form a dynamic regulatory network through complex transcriptional cascades and synergistic interactions. As the central hub of hepatic lipid metabolism, HNF-4α orchestrates key metabolic processes by: (1) Promoting fatty acid β-oxidation through the transcriptional activation of PPARα and CPT1A; (2) Driving VLDL assembly and secretion via the upregulation of ApoB and MTP expression; and (3) Enhancing lipolysis and autophagy. As a top-level regulator, HNF-4 establishes transcriptional hierarchy by activating the HNF-1 promoter, which is critical for maintaining the differentiated phenotype of hepatocytes[211]. HNF-4α enhances HNF-1α transcriptional activity in a DNA-binding-independent manner, while HNF-1α reciprocally downregulates HNF-4α-mediated transcriptional activation, forming a bidirectional regulatory loop[212]. Transcriptomic interaction studies reveal that HNF-1α and HNF-4α co-regulate shared targets[213]. HNF-1α exerts multifaceted control over hepatic lipid homeostasis by transcriptionally repressing the key lipogenic regulators SREBP-1c, ChREBP, and PPARγ, thereby suppressing TG and cholesterol synthesis. Complementarily, miR-122 targets the SCAP-SREBP-2 complex to inhibit cholesterol uptake and biosynthesis. Furthermore, HNF-1α activates PPARα-mediated fatty acid β-oxidation while transcriptionally repressing ApoC3, which relieves inhibition of LPL activity to accelerate circulating TG hydrolysis. Concurrently, it upregulates L-FABP, facilitating fatty acid transport into mitochondria for oxidation, thereby promoting systemic lipid clearance[126]. Additionally, HNF-6, acting as a co-activator, enhances FOXA2 transcription, whereas the FOXA2 protein inhibits the DNA-binding activity of HNF-6, thereby blocking the transcriptional activation of its target genes[214]. Inactivation of HNF-4α in adult liver tissue leads to the reduced expression of other HNFs, indicating that network stability correlates with its complexity[215]. During the progression of MASLD, the cytoplasmic localization of HNF-4α is triggered by the phosphorylation of a conserved Ser78 within its DNA-binding domain, a process mediated by PKC[184,216]. Oxidative stress induced by a lipotoxic microenvironment (high FFAs/inflammation/IR) activates the PKC-mediated phosphorylation of HNF-4α at Ser78, leading to its cytoplasmic retention, the downregulation of hepatic ApoB expression and decreased ApoB protein levels in the serum, and reduced VLDL secretion[187]. This subsequently suppresses HNF-1α expression; the loss of HNF-1α, on the one hand, lifts the transcriptional repression on SREBP-1c/ChREBP/PPARγ (increasing lipogenesis), and on the other hand, weakens the activation of PPARα/L-FABP and the suppression of ApoC3 (reducing fatty acid oxidation and increasing VLDL secretion, while impairing blood TG hydrolysis)[126]. Concurrently, it suppresses the expression of the rate-limiting enzyme in bile acid synthesis CYP7A1 and its transporters, indirectly impairing HNF-6-dependent bile acid homeostasis. PKA phosphorylates HNF-4α at Ser134, located in the N-terminal half of the DNA-binding domain, impairing its DNA-binding ability but counteracting the above process by helping retain HNF-4α in the nucleus[185]. However, oxidative stress and ER stress form a positive feedback loop that persistently amplifies the aberrant phosphorylation of HNF-4α, ultimately overwhelming this protective mechanism. This results in a self-reinforcing vicious cycle: HNF-4α → cytoplasmic retention → collapse of the downstream transcriptional regulatory network (including HNF-1α) → comprehensive imbalance in hepatic lipid synthesis, oxidation, and secretion metabolism → further exacerbation of lipotoxicity, hepatic lipid deposition, inflammatory response, and IR, driving the progression of MASLD. Therefore, targeted intervention in this regulatory network is a core strategy to interrupt MASLD progression, for instance, using AMPK agonists to stabilize the nuclear localization of HNF-4α. However, single-target intervention may be insufficient to fully reverse established stress feedback loops. Combining AMPK agonists with HNF-1α activators could synergistically correct the triad of crucial metabolic disturbances: Excessive lipid synthesis, impaired fatty acid oxidation, and abnormal VLDL secretion: Providing a core direction for therapy.
HNFs regulate hepatocyte differentiation and lineage specification: HNF-6 and HNF-1β are key regulators of hepatobiliary lineage differentiation. During embryonic development, HNF-6 directs the differentiation of hepatoblasts into hepatocytes or cholangiocytes by regulating HNF-1β expression[217,218]. The small interfering RNA-mediated suppression of HNF-1β leads to the comprehensive downregulation of HNF-4α, HNF-1α, HNF-3 (including HNF-3α, β, and γ), and HNF-6, positioning HNF-1β as a central node in the regulatory network[219]. HNF-1β and FOXA3 collaboratively reprogram mouse embryonic fibroblasts into induced hepatic stem cells capable of differentiating into hepatocytes or cholangiocytes, highlighting their lineage-determining capacity[220].
The core pathological manifestation of MASLD in the early stages is hepatocellular TG overload. Disease progression is characterized by the sequential compensatory activation of SREBP-1c-mediated DNL, impaired TG catabolism with the activation of lipotoxic cascades, and defective TG export. The synergistic interplay of these multiple metabolic dysregulations drives disease progression. This complex pathophysiology highlights the need for MASLD treatment to transcend the “hepatocentric paradigm” and adopt an integrated strategy combining “peripheral metabolic regulation with hepatoprotective detoxification synergy”, while incorporating personalized regimens. Potential therapeutic strategies are listed below: (1) Source intervention: Suppression of abnormal lipid influx into the liver. For example, glucagon-like peptide-1 receptor agonists regulate blood glucose and inhibit adipocyte HSL phosphorylation via cAMP/PKA to reduce adipose tissue lipolysis, thereby lowering hepatic lipid input[221]. Modulation of the gut microbiota (e.g., probiotics) reduces endotoxin-induced CD36 upregulation and dietary fatty acid absorption; (2) Metabolic diversion: Enhancement of peripheral lipid processing. Exercise promotes muscle FFA uptake and oxidation. Cold exposure induces non-shivering thermogenesis, activating brown adipose tissue to enhance systemic lipid clearance; (3) Detoxification and hepatoprotection: Restoration of lipid homeostasis by optimizing mitochondrial function and regulating lipid droplet dynamics to improve hepatic lipid oxidation/export; (4) Metabolic reprogramming: Implementing precision-controlled caloric intake, optimizing carbohydrate-lipid metabolic balance[222,223], and adopting evidence-based nutritional and weight management[224]; (5) Systemic management of glucolipid metabolic disorders and comorbidities; and (6) Targeted genetic interventions for inherited variants.
HNFs, through their specific functions in maintaining hepatic physiological homeostasis, serve as central regulatory nodes governing TG metabolic balance in MASLD. Current research is shifting from single-molecule mechanistic studies to multi-dimensional regulatory network integration. Future efforts urgently require the convergence of basic research and clinical translation to develop HNF pathway-targeted therapeutic strategies, while further exploring the potential of HNF networks in early disease detection, metabolic reprogramming, and fibrosis reversal. Leveraging technological innovations such as multi-omics integration, spatiotemporal dynamic monitoring, and organoid models, alongside breakthroughs in interdisciplinary research paradigms, may overcome current barriers in treatment due to molecular complexity and enable precise and individualized management for MASLD patients.
| 1. | Fan JG, Xu XY, Yang RX, Nan YM, Wei L, Jia JD, Zhuang H, Shi JP, Li XY, Sun C, Li J, Wong VW, Duan ZP; Chinese Society of Hepatology, Chinese Medical Association. Guideline for the Prevention and Treatment of Metabolic Dysfunction-associated Fatty Liver Disease (Version 2024). J Clin Transl Hepatol. 2024;12:955-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Polyzos SA, Mantzoros CS. Metabolic dysfunction-associated steatotic liver disease: Recent turning points for its diagnosis and management. Metabolism. 2024;157:155936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 3. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 4. | Kawaguchi T, Tsutsumi T, Nakano D, Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res. 2022;52:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 5. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 3163] [Article Influence: 527.2] [Reference Citation Analysis (2)] |
| 6. | Allen AM, Pose E, Reddy KR, Russo MW, Kamath PS. Nonalcoholic Fatty Liver Disease Gets Renamed as Metabolic Dysfunction-Associated Steatotic Liver Disease: Progress But With Challenges. Gastroenterology. 2024;166:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Lou TW, Yang RX, Fan JG. The global burden of fatty liver disease: the major impact of China. Hepatobiliary Surg Nutr. 2024;13:119-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 8. | Lekakis V, Papatheodoridis GV. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 9. | Mak LY, Liu K, Chirapongsathorn S, Yew KC, Tamaki N, Rajaram RB, Panlilio MT, Lui R, Lee HW, Lai JC, Kulkarni AV, Premkumar M, Lesmana CRA, Hsu YC, Huang DQ. Liver diseases and hepatocellular carcinoma in the Asia-Pacific region: burden, trends, challenges and future directions. Nat Rev Gastroenterol Hepatol. 2024;21:834-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 98] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Sandireddy R, Sakthivel S, Gupta P, Behari J, Tripathi M, Singh BK. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front Cell Dev Biol. 2024;12:1433857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 11. | Prasoppokakorn T, Pitisuttithum P, Treeprasertsuk S. Pharmacological Therapeutics: Current Trends for Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). J Clin Transl Hepatol. 2021;9:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Çubuk H, Yalçın Çapan Ö. A Review of Functional Characterization of Single Amino Acid Change Mutations in HNF Transcription Factors in MODY Pathogenesis. Protein J. 2021;40:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Badmus OO, Hillhouse SA, Anderson CD, Hinds TD, Stec DE. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci (Lond). 2022;136:1347-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 231] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 14. | Vos DY, Wijers M, Smit M, Huijkman N, Kloosterhuis NJ, Wolters JC, Tissink JJ, Pronk ACM, Kooijman S, Rensen PCN, Kuivenhoven JA, van de Sluis B. Cargo-Specific Role for Retriever Subunit VPS26C in Hepatocyte Lipoprotein Receptor Recycling to Control Postprandial Triglyceride-Rich Lipoproteins. Arterioscler Thromb Vasc Biol. 2023;43:e29-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2672] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 16. | Wu SA, Kersten S, Qi L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol Metab. 2021;32:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 17. | Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537-2564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1300] [Cited by in RCA: 1404] [Article Influence: 280.8] [Reference Citation Analysis (36)] |
| 18. | Kersten S. ANGPTL4/8 promotes plasmin-mediated cleavage of LPL inhibitors. J Lipid Res. 2023;64:100438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Alves-Bezerra M, Cohen DE. Triglyceride Metabolism in the Liver. Compr Physiol. 2017;8:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 450] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 20. | Lampidonis AD, Rogdakis E, Voutsinas GE, Stravopodis DJ. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene. 2011;477:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1610] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 22. | Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806-4815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 483] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Nguyen HP, Xue P, Xie Y, Yi D, Lin F, Dinh J, Viscarra JA, Ibe NU, Duncan RE, Sul HS. ApoL6 associates with lipid droplets and disrupts Perilipin1-HSL interaction to inhibit lipolysis. Nat Commun. 2024;15:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Lan D, Li S, Tang W, Zhao Z, Luo M, Yuan S, Xu J, Wang Y. Glycerol is Released from a New Path in MGL Lipase Catalytic Process. J Chem Inf Model. 2022;62:2248-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Shen S, Huang H, Wang J, Tang Z, Shen C, Xu C. Positive Association Between the Chinese Visceral Adiposity Index and Nonalcoholic Fatty Liver Disease in Lean Adults. Dig Dis Sci. 2023;68:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Li R, Liu J, Han P, Shi R, Zhao L, Li J. Associations between abdominal obesity indices and pathological features of non-alcoholic fatty liver disease: Chinese visceral adiposity index. J Gastroenterol Hepatol. 2023;38:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Tchernof A, Bélanger C, Morisset AS, Richard C, Mailloux J, Laberge P, Dupont P. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 475] [Article Influence: 22.6] [Reference Citation Analysis (8)] |
| 29. | Bensussen A, Torres-Magallanes JA, Roces de Álvarez-Buylla E. Molecular tracking of insulin resistance and inflammation development on visceral adipose tissue. Front Immunol. 2023;14:1014778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 30. | Shi T, Yu L, Zhuang R, Xi J, He R, Shao Y, Huang J, Liu S, Yang X. Regulation of Mitochondrial Function by Natural Products for the Treatment of Metabolic Associated Fatty Liver Disease. Can J Gastroenterol Hepatol. 2021;2021:5527315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol. 2016;22:1664-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc. 2016;91:452-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 33. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 956] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 34. | Anwar SD, Foster C, Ashraf A. Lipid Disorders and Metabolic-Associated Fatty Liver Disease. Endocrinol Metab Clin North Am. 2023;52:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Liao Y, Chen Q, Liu L, Huang H, Sun J, Bai X, Jin C, Li H, Sun F, Xiao X, Zhang Y, Li J, Han W, Fu S. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. 2024;36:2437-2448.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 36. | Xin X, Chen C, Xu X, Lv S, Sun Q, An Z, Chen Y, Xiong Z, Hu Y, Feng Q. Caffeine ameliorates metabolic-associated steatohepatitis by rescuing hepatic Dusp9. Redox Biol. 2025;80:103499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 37. | Longo M, Paolini E, Di Benedetto P, Tomassini E, Meroni M, Dongiovanni P. DGAT1 and DGAT2 Inhibitors for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management: Benefits for Their Single or Combined Application. Int J Mol Sci. 2024;25:9074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552-8557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Ido-Kitamura Y, Sasaki T, Kobayashi M, Kim HJ, Lee YS, Kikuchi O, Yokota-Hashimoto H, Iizuka K, Accili D, Kitamura T. Hepatic FoxO1 integrates glucose utilization and lipid synthesis through regulation of Chrebp O-glycosylation. PLoS One. 2012;7:e47231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Zhang C, Chen X, Zhu RM, Zhang Y, Yu T, Wang H, Zhao H, Zhao M, Ji YL, Chen YH, Meng XH, Wei W, Xu DX. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol Lett. 2012;212:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Yellaturu CR, Deng X, Park EA, Raghow R, Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP).SREBP-1c complex. J Biol Chem. 2009;284:31726-31734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Choi WI, Jeon BN, Park H, Yoo JY, Kim YS, Koh DI, Kim MH, Kim YR, Lee CE, Kim KS, Osborne TF, Hur MW. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN). J Biol Chem. 2008;283:29341-29354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB. Insulin activates the rat sterol-regulatory-element-binding protein 1c (SREBP-1c) promoter through the combinatorial actions of SREBP, LXR, Sp-1 and NF-Y cis-acting elements. Biochem J. 2005;385:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245-11250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 435] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 45. | Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100:3155-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 240] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Hegarty BD, Bobard A, Hainault I, Ferré P, Bossard P, Foufelle F. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc Natl Acad Sci U S A. 2005;102:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 47. | Casali C, Malvicini R, Erjavec L, Parra L, Artuch A, Fernández Tome MC. X-box binding protein 1 (XBP1): A key protein for renal osmotic adaptation. Its role in lipogenic program regulation. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Jiang SY, Yang X, Yang Z, Li JW, Xu MQ, Qu YX, Tang JJ, Li YF, Wang L, Shao YW, Meng XY, Hu H, Song BL, Rao Y, Qi W. Discovery of an insulin-induced gene binding compound that ameliorates nonalcoholic steatohepatitis by inhibiting sterol regulatory element-binding protein-mediated lipogenesis. Hepatology. 2022;76:1466-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Chen X, Zhang P, Ma W, Pan H, Hong W, Chen G, Ding H, Tang W, Lin G, Zhang Z. Protective effects of methyl protodioscin against lipid disorders and liver injury in hyperlipidemic gerbils. Heliyon. 2023;9:e22785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Kawamura S, Matsushita Y, Kurosaki S, Tange M, Fujiwara N, Hayata Y, Hayakawa Y, Suzuki N, Hata M, Tsuboi M, Kishikawa T, Kinoshita H, Nakatsuka T, Sato M, Kudo Y, Hoshida Y, Umemura A, Eguchi A, Ikenoue T, Hirata Y, Uesugi M, Tateishi R, Tateishi K, Fujishiro M, Koike K, Nakagawa H. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis. J Clin Invest. 2022;132:e151895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 51. | Chen M, Li H, Zheng S, Shen J, Chen Y, Li Y, Yuan M, Wu J, Sun Q. Nobiletin targets SREBP1/ACLY to induce autophagy-dependent cell death of gastric cancer cells through PI3K/Akt/mTOR signaling pathway. Phytomedicine. 2024;128:155360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 52. | Zhan X, Qiu R, He Y, Zhao Z, Huang M, Liu Q, Zhi F, Long W. The Aurora Kinase Inhibitor TAK901 Inhibits Glioblastoma Growth by Blocking SREBP1-Mediated Lipid Metabolism. Cancers (Basel). 2022;14:5805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 53. | Ma DB, Liu XY, Jia H, Zhang Y, Jiang Q, Sun H, Li X, Sun F, Chai Y, Feng F, Liu L. A Novel Small-Molecule Inhibitor of SREBP-1 Based on Natural Product Monomers Upregulates the Sensitivity of Lung Squamous Cell Carcinoma Cells to Antitumor Drugs. Front Pharmacol. 2022;13:895744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | He Y, Jiang J, Ou L, Chen Y, Abudukeremu A, Chen G, Zhong W, Jiang Z, Nuermaimaiti N, Guan Y. Impaired RelA signaling and lipid metabolism dysregulation in hepatocytes: driving forces in the progression of metabolic dysfunction-associated steatotic liver disease. Cell Death Discov. 2025;11:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 55. | Cai J, Ye Z, Hu Y, Ye L, Gao L, Wang Y, Sun Q, Tong S, Zhang S, Wu L, Yang J, Chen Q. Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. Cell Death Dis. 2023;14:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 56. | Ma X, Zhao T, Yan H, Guo K, Liu Z, Wei L, Lu W, Qiu C, Jiang J. Fatostatin reverses progesterone resistance by inhibiting the SREBP1-NF-κB pathway in endometrial carcinoma. Cell Death Dis. 2021;12:544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 58. | Gao X, Li K, Hui X, Kong X, Sweeney G, Wang Y, Xu A, Teng M, Liu P, Wu D. Carnitine palmitoyltransferase 1A prevents fatty acid-induced adipocyte dysfunction through suppression of c-Jun N-terminal kinase. Biochem J. 2011;435:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 60. | Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab. 2008;294:E969-E977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 61. | Orellana-Gavaldà JM, Herrero L, Malandrino MI, Pañeda A, Sol Rodríguez-Peña M, Petry H, Asins G, Van Deventer S, Hegardt FG, Serra D. Molecular therapy for obesity and diabetes based on a long-term increase in hepatic fatty-acid oxidation. Hepatology. 2011;53:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 62. | Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci. 2020;21:2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 63. | Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852-G858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 593] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 64. | Yang W, Ling X, He S, Cui H, Yang Z, An H, Wang L, Zou P, Chen Q, Liu J, Ao L, Cao J. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: An integrated approach. Environ Int. 2023;178:108138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 118] [Reference Citation Analysis (0)] |
| 65. | Xu Y, Denning KL, Lu Y. PPARα agonist WY-14,643 induces the PLA2/COX-2/ACOX1 pathway to enhance peroxisomal lipid metabolism and ameliorate alcoholic fatty liver in mice. Biochem Biophys Res Commun. 2022;613:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Shang Z, Gao Y, Xue Y, Zhang C, Qiu J, Qian Y, Fang M, Zhang X, Sun X, Kong X, Gao Y. Shenge Formula attenuates high-fat diet-induced obesity and fatty liver via inhibiting ACOX1. Phytomedicine. 2024;123:155183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 67. | Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 798] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 68. | Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1590] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 69. | Musso G, Cassader M, Paschetta E, Gambino R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:282-302.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 70. | Frades I, Andreasson E, Mato JM, Alexandersson E, Matthiesen R, Martínez-Chantar ML. Integrative genomic signatures of hepatocellular carcinoma derived from nonalcoholic Fatty liver disease. PLoS One. 2015;10:e0124544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 411] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 72. | Sommer J, Mahli A, Freese K, Schiergens TS, Kuecuekoktay FS, Teufel A, Thasler WE, Müller M, Bosserhoff AK, Hellerbrand C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget. 2017;8:13059-13072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Kim YR, Lee EJ, Shin KO, Kim MH, Pewzner-Jung Y, Lee YM, Park JW, Futerman AH, Park WJ. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp Mol Med. 2019;51:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Liu J, Han L, Zhu L, Yu Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis. 2016;15:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 75. | Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:479348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Liu Z, Xia Y, Li B, Xu H, Wang C, Liu Y, Li Y, Li C, Gao N, Li L. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca(2+) homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 2014;4:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 77. | Ogino N, Miyagawa K, Kusanaga M, Hayashi T, Minami S, Oe S, Honma Y, Harada M. Involvement of sarco/endoplasmic reticulum calcium ATPase-mediated calcium flux in the protective effect of oleic acid against lipotoxicity in hepatocytes. Exp Cell Res. 2019;385:111651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087-13099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284:13296-13300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Wang W, Liu K, Xu H, Zhang C, Zhang Y, Ding M, Xing C, Huang X, Wen Q, Lu C, Song L. Sleep deprivation induced fat accumulation in the visceral white adipose tissue by suppressing SIRT1/FOXO1/ATGL pathway activation. J Physiol Biochem. 2024;80:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 81. | Fuchs CD, Claudel T, Kumari P, Haemmerle G, Pollheimer MJ, Stojakovic T, Scharnagl H, Halilbasic E, Gumhold J, Silbert D, Koefeler H, Trauner M. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 82. | Fuchs CD, Radun R, Dixon ED, Mlitz V, Timelthaler G, Halilbasic E, Herac M, Jonker JW, Ronda OAHO, Tardelli M, Haemmerle G, Zimmermann R, Scharnagl H, Stojakovic T, Verkade HJ, Trauner M. Hepatocyte-specific deletion of adipose triglyceride lipase (adipose triglyceride lipase/patatin-like phospholipase domain containing 2) ameliorates dietary induced steatohepatitis in mice. Hepatology. 2022;75:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 84. | Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rülicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 85. | Hughey CC, Puchalska P, Crawford PA. Integrating the contributions of mitochondrial oxidative metabolism to lipotoxicity and inflammation in NAFLD pathogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 86. | Miyagawa K, Oe S, Honma Y, Izumi H, Baba R, Harada M. Lipid-Induced Endoplasmic Reticulum Stress Impairs Selective Autophagy at the Step of Autophagosome-Lysosome Fusion in Hepatocytes. Am J Pathol. 2016;186:1861-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 87. | Guo R, Li Y, Song Q, Huang R, Ge X, Nieto N, Jiang Y, Song Z. Increasing cellular NAD(+) protects hepatocytes against palmitate-induced lipotoxicity by preventing PARP-1 inhibition and the mTORC1-p300 pathway activation. Am J Physiol Cell Physiol. 2025;328:C776-C790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 88. | Mushtaq A, Ashraf NU, Altaf M. The mTORC1-G9a-H3K9me2 axis negatively regulates autophagy in fatty acid-induced hepatocellular lipotoxicity. J Biol Chem. 2023;299:102937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 89. | Li L, Lin J, Huang C, Liu J, Yuan Y, Liu Z, Li Y, Li W, Diao A. The TFEB activator clomiphene citrate ameliorates lipid metabolic syndrome pathology by activating lipophagy and lipolysis. Biochem Pharmacol. 2025;232:116694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 90. | Lin J, Huang C, Zhao J, Li L, Wu Z, Zhang T, Li Y, Li W, Guo B, Liu Z, Diao A. The novel TFEB agonist desloratadine ameliorates hepatic steatosis by activating the autophagy-lysosome pathway. Front Pharmacol. 2024;15:1449178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 91. | Shan L, Guo P, Wen M, Sun Y, Gao F, Zhang K, Zhang N, Yang B. Knockdown of regulator of Calcineurin 2 promotes transcription factor EB-mediated lipophagy to prevent non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 2025;495:117210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Zhu L, Guo L, Xu J, Xiang Q, Tan Y, Tian F, Du X, Zhang S, Wen T, Liu L. Postprandial Triglyceride-Rich Lipoproteins-Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Inflammation in White Adipocytes. J Nutr. 2024;154:1619-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Naiman S, Huynh FK, Gil R, Glick Y, Shahar Y, Touitou N, Nahum L, Avivi MY, Roichman A, Kanfi Y, Gertler AA, Doniger T, Ilkayeva OR, Abramovich I, Yaron O, Lerrer B, Gottlieb E, Harris RA, Gerber D, Hirschey MD, Cohen HY. SIRT6 Promotes Hepatic Beta-Oxidation via Activation of PPARα. Cell Rep. 2019;29:4127-4143.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 94. | Pirahanchi Y, Anoruo M, Sharma S. Biochemistry, Lipoprotein Lipase. 2023 Jul 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 95. | Jayaraman S, Pérez A, Miñambres I, Sánchez-Quesada JL, Gursky O. Heparin binding triggers human VLDL remodeling by circulating lipoprotein lipase: Relevance to VLDL functionality in health and disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Venugopal SK, Anoruo M, Jialal I. Biochemistry, Low Density Lipoprotein. 2023 Apr 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 97. | Iqbal J, Jahangir Z, Al-Qarni AA. Microsomal Triglyceride Transfer Protein: From Lipid Metabolism to Metabolic Diseases. Adv Exp Med Biol. 2020;1276:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Chen Z, Newberry EP, Norris JY, Xie Y, Luo J, Kennedy SM, Davidson NO. ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. J Lipid Res. 2008;49:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Zhang J, Jazii FR, Haghighi MM, Alvares D, Liu L, Khosraviani N, Adeli K. miR-130b is a potent stimulator of hepatic very-low-density lipoprotein assembly and secretion via marked induction of microsomal triglyceride transfer protein. Am J Physiol Endocrinol Metab. 2020;318:E262-E275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 101. | Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 102. | Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347-2364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 103. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, Bréchot C. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 429] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 104. | Grandt J, Jensen AH, Werge MP, Rashu EB, Møller A, Junker AE, Hobolth L, Mortensen C, Johansen CD, Vyberg M, Serizawa RR, Møller S, Gluud LL, Wewer Albrechtsen NJ. Postprandial dysfunction in fatty liver disease. Physiol Rep. 2023;11:e15653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 105. | Kim SQ, Mohallem R, Franco J, Buhman KK, Kim KH, Aryal UK. Global landscape of protein complexes in postprandial-state livers from diet-induced obese and lean mice. Biochem Biophys Res Commun. 2022;629:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 106. | Gijbels A, Erdős B, Trouwborst I, Jardon KM, Adriaens ME, Goossens GH, Blaak EE, Feskens EJM, Afman LA. Hepatic insulin resistance and muscle insulin resistance are characterized by distinct postprandial plasma metabolite profiles: a cross-sectional study. Cardiovasc Diabetol. 2024;23:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 107. | Uehara K, Lee WD, Stefkovich M, Biswas D, Santoleri D, Garcia Whitlock A, Quinn W 3rd, Coopersmith T, Creasy KT, Rader DJ, Sakamoto K, Rabinowitz JD, Titchenell PM. mTORC1 controls murine postprandial hepatic glycogen synthesis via Ppp1r3b. J Clin Invest. 2024;134:e173782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, Greiner TU, Perkins R, Bäckhed F. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66:429-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 109. | Deng M, Qu F, Chen L, Liu C, Zhang M, Ren F, Guo H, Zhang H, Ge S, Wu C, Zhao L. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 2020;245:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 110. | Sun W, Jia J, Liu G, Liang S, Huang Y, Xin M, Chang Z, Liu X, Ma C, Song X, He F, Song Y, Wu M. Polysaccharides Extracted from Old Stalks of Asparagus officinalis L. Improve Nonalcoholic Fatty Liver by Increasing the Gut Butyric Acid Content and Improving Gut Barrier Function. J Agric Food Chem. 2025;73:6632-6645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 111. | Du X, Wu Z, Xu Y, Liu Y, Liu W, Wang T, Li C, Zhang C, Yi F, Gao L, Liang X, Ma C. Increased Tim-3 expression alleviates liver injury by regulating macrophage activation in MCD-induced NASH mice. Cell Mol Immunol. 2019;16:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 112. | Piras IS, Raju A, Don J, Schork NJ, Gerhard GS, DiStefano JK. Hepatic PEMT Expression Decreases with Increasing NAFLD Severity. Int J Mol Sci. 2022;23:9296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Romano KA, Martinez-Del Campo A, Kasahara K, Chittim CL, Vivas EI, Amador-Noguez D, Balskus EP, Rey FE. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe. 2017;22:279-290.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 114. | Han H, Wang M, Zhong R, Yi B, Schroyen M, Zhang H. Depletion of Gut Microbiota Inhibits Hepatic Lipid Accumulation in High-Fat Diet-Fed Mice. Int J Mol Sci. 2022;23:9350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 115. | Sato-Espinoza K, Chotiprasidhi P, Huaman MR, Díaz-Ferrer J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J Hepatol. 2024;16:452-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 116. | Rotaru A, Stafie R, Stratina E, Zenovia S, Nastasa R, Minea H, Huiban L, Cuciureanu T, Muzica C, Chiriac S, Girleanu I, Singeap AM, Sfarti C, Stanciu C, Trifan A. Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut-Liver Axis. Life (Basel). 2025;15:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 117. | Wang Y, Hong S, Hudson H, Kory N, Kinch LN, Kozlitina J, Cohen JC, Hobbs HH. PNPLA3(148M) is a gain-of-function mutation that promotes hepatic steatosis by inhibiting ATGL-mediated triglyceride hydrolysis. J Hepatol. 2025;82:871-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 118. | Zhang X, Lau HC, Ha S, Liu C, Liang C, Lee HW, Ng QW, Zhao Y, Ji F, Zhou Y, Pan Y, Song Y, Zhang Y, Lo JCY, Cheung AHK, Wu J, Li X, Xu H, Wong CC, Wong VW, Yu J. Author Correction: Intestinal TM6SF2 protects against metabolic dysfunction-associated steatohepatitis through the gut-liver axis. Nat Metab. 2025;7:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 119. | Begum S. Hepatic Nuclear Factor 1 Alpha (HNF-1α) In Human Physiology and Molecular Medicine. Curr Mol Pharmacol. 2020;13:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Chandra S, Srinivasan S, Batra J. Hepatocyte nuclear factor 1 beta: A perspective in cancer. Cancer Med. 2021;10:1791-1804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Browne JA, Yang R, Eggener SE, Leir SH, Harris A. HNF1 regulates critical processes in the human epididymis epithelium. Mol Cell Endocrinol. 2016;425:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 122. | Tan J, Xu J, Wei G, Zhang L, Sun L, Wang G, Li F, Jiang F. HNF1α Controls Liver Lipid Metabolism and Insulin Resistance via Negatively Regulating the SOCS-3-STAT3 Signaling Pathway. J Diabetes Res. 2019;2019:5483946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 123. | Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, Park SW, Liu J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res. 2010;51:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 124. | Huang T, Wang T, Heianza Y, Sun D, Ivey K, Durst R, Schwarzfuchs D, Stampfer MJ, Bray GA, Sacks FM, Shai I, Qi L. HNF1A variant, energy-reduced diets and insulin resistance improvement during weight loss: The POUNDS Lost trial and DIRECT. Diabetes Obes Metab. 2018;20:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Liu F, Zhu X, Jiang X, Li S, Lv Y. Transcriptional control by HNF-1: Emerging evidence showing its role in lipid metabolism and lipid metabolism disorders. Genes Dis. 2022;9:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 126. | Patitucci C, Couchy G, Bagattin A, Cañeque T, de Reyniès A, Scoazec JY, Rodriguez R, Pontoglio M, Zucman-Rossi J, Pende M, Panasyuk G. Hepatocyte nuclear factor 1α suppresses steatosis-associated liver cancer by inhibiting PPARγ transcription. J Clin Invest. 2017;127:1873-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 127. | Zuvairiya U, Jayaraman S, Sankaran K, Veeraraghavan VP, R G. Studies on the Effect of Piperine on Hepatocyte Nuclear Factor 1 Alpha (HNF-1α) and Sterol Regulatory Element-Binding Protein 1c (SREBP-1c) Levels in High-Fat-Diet and Sucrose-Induced Type 2 Diabetes Mellitus Rats. Cureus. 2024;16:e54061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Speckmann B, Schulz S, Hiller F, Hesse D, Schumacher F, Kleuser B, Geisel J, Obeid R, Grune T, Kipp AP. Selenium increases hepatic DNA methylation and modulates one-carbon metabolism in the liver of mice. J Nutr Biochem. 2017;48:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Viscarra JA, Wang Y, Nguyen HP, Choi YG, Sul HS. Histone demethylase JMJD1C is phosphorylated by mTOR to activate de novo lipogenesis. Nat Commun. 2020;11:796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 130. | Hu M, Huang X, Han X, Ji L. Loss of HNF1α Function Contributes to Hepatocyte Proliferation and Abnormal Cholesterol Metabolism via Downregulating miR-122: A Novel Mechanism of MODY3. Diabetes Metab Syndr Obes. 2020;13:627-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 131. | Wang X, Hassan W, Zhao J, Bakht S, Nie Y, Wang Y, Pang Q, Huang Z. The impact of hepatocyte nuclear factor-1α on liver malignancies and cell stemness with metabolic consequences. Stem Cell Res Ther. 2019;10:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 132. | Lopez AP, Foscaldi SA, Perez MS, Rodriguez M, Traversa M, Puchulu FM, Bergada I, Frechtel GD. HNF1 alpha gene coding regions mutations screening, in a Caucasian population clinically characterized as MODY from Argentina. Diabetes Res Clin Pract. 2011;91:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 133. | Graham RP, Terracciano LM, Meves A, Vanderboom PM, Dasari S, Yeh MM, Torbenson MS, Cruise MW. Hepatic adenomas with synchronous or metachronous fibrolamellar carcinomas: both are characterized by LFABP loss. Mod Pathol. 2016;29:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Qin Y, Qiu D, Zhang Q. HNF1A regulates the crosstalk between innate immune responses and MAFLD by mediating autophagic degradation of TBK1. Autophagy. 2023;19:1026-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 135. | He J, Du C, Peng X, Hong W, Qiu D, Qiu X, Zhang X, Qin Y, Zhang Q. Hepatocyte nuclear factor 1A suppresses innate immune response by inducing degradation of TBK1 to inhibit steatohepatitis. Genes Dis. 2023;10:1596-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 136. | Villemain L, Prigent S, Abou-Lovergne A, Pelletier L, Chiral M, Pontoglio M, Foufelle F, Caruso S, Pineau R, Rebouissou S, Chevet E, Zucman-Rossi J, Combettes L. Sigma 1 Receptor is Overexpressed in Hepatocellular Adenoma: Involvement of ERα and HNF1α. Cancers (Basel). 2020;12:2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Lair B, Laurens C, Van Den Bosch B, Moro C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int J Mol Sci. 2020;21:6358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 138. | Zhang Z, Yu Z, Liang D, Song K, Kong X, He M, Liao X, Huang Z, Kang A, Bai R, Ren Y. Roles of lipid droplets and related proteins in metabolic diseases. Lipids Health Dis. 2024;23:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 139. | Ozaki K, Harada K, Terayama N, Matsui O, Saitoh S, Tomimaru Y, Fujii T, Gabata T. Hepatocyte nuclear factor 1α-inactivated hepatocellular adenomas exhibit high (18)F-fludeoxyglucose uptake associated with glucose-6-phosphate transporter inactivation. Br J Radiol. 2016;89:20160265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 140. | Hiraiwa H, Pan CJ, Lin B, Akiyama TE, Gonzalez FJ, Chou JY. A molecular link between the common phenotypes of type 1 glycogen storage disease and HNF1alpha-null mice. J Biol Chem. 2001;276:7963-7967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | David-Silva A, Freitas HS, Okamoto MM, Sabino-Silva R, Schaan BD, Machado UF. Hepatocyte nuclear factors 1α/4α and forkhead box A2 regulate the solute carrier 2A2 (Slc2a2) gene expression in the liver and kidney of diabetic rats. Life Sci. 2013;93:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 142. | Shende VR, Wu M, Singh AB, Dong B, Kan CF, Liu J. Reduction of circulating PCSK9 and LDL-C levels by liver-specific knockdown of HNF1α in normolipidemic mice. J Lipid Res. 2015;56:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 143. | Chen J, Cooper AD, Levy-Wilson B. Hepatocyte nuclear factor 1 binds to and transactivates the human but not the rat CYP7A1 promoter. Biochem Biophys Res Commun. 1999;260:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF, Bonnycastle LL, Jackson AU, Crawford G, Surti A, Guiducci C, Burtt NP, Parish S, Clarke R, Zelenika D, Kubalanza KA, Morken MA, Scott LJ, Stringham HM, Galan P, Swift AJ, Kuusisto J, Bergman RN, Sundvall J, Laakso M, Ferrucci L, Scheet P, Sanna S, Uda M, Yang Q, Lunetta KL, Dupuis J, de Bakker PI, O'Donnell CJ, Chambers JC, Kooner JS, Hercberg S, Meneton P, Lakatta EG, Scuteri A, Schlessinger D, Tuomilehto J, Collins FS, Groop L, Altshuler D, Collins R, Lathrop GM, Melander O, Salomaa V, Peltonen L, Orho-Melander M, Ordovas JM, Boehnke M, Abecasis GR, Mohlke KL, Cupples LA. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1146] [Cited by in RCA: 1122] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 145. | Zhou YJ, Yin RX, Hong SC, Yang Q, Cao XL, Chen WX. Association of the HNF1A polymorphisms and serum lipid traits, the risk of coronary artery disease and ischemic stroke. J Gene Med. 2017;19:e2941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 146. | Mattijssen F, Kersten S. Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 147. | Watanabe T, Ozawa A, Masuda S, Yoshino S, Ishida E, Kondo Y, Matsumoto S, Katano-Toki A, Horiguchi K, Nakajima Y, Yamada E, Tomaru T, Saito T, Ishii S, Shibusawa N, Okada S, Satoh T, Yamada M. Transcriptional Regulation of the Angptl8 Gene by Hepatocyte Nuclear Factor-1 in the Murine Liver. Sci Rep. 2020;10:9999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 148. | Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci U S A. 2013;110:16109-16114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 149. | Wang X, Wu H, Yu W, Liu J, Peng J, Liao N, Zhang J, Zhang X, Hai C. Hepatocyte nuclear factor 1b is a novel negative regulator of white adipocyte differentiation. Cell Death Differ. 2017;24:1588-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Wang W, Zhao M, Zhao Y, Shen W, Yin S. PDGFRα/β-PI3K-Akt pathway response to the interplay of mitochondrial dysfunction and DNA damage in Aroclor 1254-exposed porcine granulosa cells. Environ Pollut. 2020;263:114534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 151. | Wu H, Yu W, Meng F, Mi J, Peng J, Liu J, Zhang X, Hai C, Wang X. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-κB signaling via downregulation of HNF1b. Redox Biol. 2017;12:300-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 152. | Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 153. | Wu YJ, Guo X, Li CJ, Li DQ, Zhang J, Yang Y, Kong Y, Guo H, Liu DM, Chen LM. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism. 2015;64:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Long Z, Cao M, Su S, Wu G, Meng F, Wu H, Liu J, Yu W, Atabai K, Wang X. Inhibition of hepatocyte nuclear factor 1b induces hepatic steatosis through DPP4/NOX1-mediated regulation of superoxide. Free Radic Biol Med. 2017;113:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 155. | Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142-146. [PubMed] |
| 156. | Yu C, Li X, Zhao Y, Hu Y. The role of FOXA family transcription factors in glucolipid metabolism and NAFLD. Front Endocrinol (Lausanne). 2023;14:1081500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 157. | Moya M, Benet M, Guzmán C, Tolosa L, García-Monzón C, Pareja E, Castell JV, Jover R. Foxa1 reduces lipid accumulation in human hepatocytes and is down-regulated in nonalcoholic fatty liver. PLoS One. 2012;7:e30014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Guzmán C, Benet M, Pisonero-Vaquero S, Moya M, García-Mediavilla MV, Martínez-Chantar ML, González-Gallego J, Castell JV, Sánchez-Campos S, Jover R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARα; and repressed by C/EBPα: Implications in FABP1 down-regulation in nonalcoholic fatty liver disease. Biochim Biophys Acta. 2013;1831:803-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 159. | Zou D, Liao J, Xiao M, Liu L, Dai D, Xu M. Impaired SUMOylation of FoxA1 promotes nonalcoholic fatty liver disease through down-regulation of Sirt6. Cell Death Dis. 2024;15:674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 160. | Wu YL, Peng XE, Wang D, Chen WN, Lin X. Human liver fatty acid binding protein (hFABP1) gene is regulated by liver-enriched transcription factors HNF3β and C/EBPα. Biochimie. 2012;94:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 161. | Weiss TS, Lupke M, Ibrahim S, Buechler C, Lorenz J, Ruemmele P, Hofmann U, Melter M, Dayoub R. Attenuated lipotoxicity and apoptosis is linked to exogenous and endogenous augmenter of liver regeneration by different pathways. PLoS One. 2017;12:e0184282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Yang L, Ma Q, Chen J, Kong X, Yu X, Wang W. Foxa2 attenuates steatosis and inhibits the NF-κB/IKK signaling pathway in nonalcoholic fatty liver disease. PeerJ. 2023;11:e16466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Kurtz CL, Peck BC, Fannin EE, Beysen C, Miao J, Landstreet SR, Ding S, Turaga V, Lund PK, Turner S, Biddinger SB, Vickers KC, Sethupathy P. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes. 2014;63:3141-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 164. | Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282:9768-9776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 165. | Kanaki M, Kardassis D. Regulation of the human lipoprotein lipase gene by the forkhead box transcription factor FOXA2/HNF-3β in hepatic cells. Biochim Biophys Acta Gene Regul Mech. 2017;1860:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 166. | Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 167. | Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 168. | Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 169. | Liu X, Hou S, Xiang R, Hu C, Chen Z, Li N, Yan H, Yu X, Li X, Chi Y, Yang J. Imipramine activates FAM3A-FOXA2-CPT2 pathway to ameliorate hepatic steatosis. Metabolism. 2022;136:155292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 170. | Adler-Wailes DC, Alberobello AT, Ma X, Hugendubler L, Stern EA, Mou Z, Han JC, Kim PW, Sumner AE, Yanovski JA, Mueller E. Analysis of variants and mutations in the human winged helix FOXA3 gene and associations with metabolic traits. Int J Obes (Lond). 2015;39:888-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 171. | Liu C, Zhou B, Meng M, Zhao W, Wang D, Yuan Y, Zheng Y, Qiu J, Li Y, Li G, Xiong X, Bian H, Zhang H, Wang H, Ma X, Hu C, Xu L, Lu Y. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J Hepatol. 2021;75:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 172. | Gopoju R, Wang J, Pan X, Hu S, Lin L, Clark A, Xu Y, Yin L, Wang X, Zhang Y. Hepatic FOXA3 overexpression prevents Western diet-induced obesity and MASH through TGR5. J Lipid Res. 2024;65:100527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 173. | Li Y, Xu Y, Jadhav K, Zhu Y, Yin L, Zhang Y. Hepatic Forkhead Box Protein A3 Regulates ApoA-I (Apolipoprotein A-I) Expression, Cholesterol Efflux, and Atherogenesis. Arterioscler Thromb Vasc Biol. 2019;39:1574-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 174. | Nakamura T, Fox-Robichaud A, Kikkawa R, Kashiwagi A, Kojima H, Fujimiya M, Wong NC. Transcription factors and age-related decline in apolipoprotein A-I expression. J Lipid Res. 1999;40:1709-1718. [PubMed] |
| 175. | Bhale AS, Venkataraman K. Leveraging knowledge of HDLs major protein ApoA1: Structure, function, mutations, and potential therapeutics. Biomed Pharmacother. 2022;154:113634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 176. | Pan X, Zhang Y. Hepatocyte nuclear factor 4α in the pathogenesis of non-alcoholic fatty liver disease. Chin Med J (Engl). 2022;135:1172-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 177. | Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F. Multidomain integration in the structure of the HNF-4α nuclear receptor complex. Nature. 2013;495:394-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 178. | Chen L, Vasoya RP, Toke NH, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder EM, Su X, Verzi MP. HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice. Gastroenterology. 2020;158:985-999.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 179. | Beinsteiner B, Billas IML, Moras D. Structural insights into the HNF4 biology. Front Endocrinol (Lausanne). 2023;14:1197063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 180. | Tsai TY, Wang WT, Li HK, Chen WJ, Tsai YH, Chao CH, Wu Lee YH. RNA helicase DDX3 maintains lipid homeostasis through upregulation of the microsomal triglyceride transfer protein by interacting with HNF4 and SHP. Sci Rep. 2017;7:41452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 181. | Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 182. | Xu Y, Zalzala M, Xu J, Li Y, Yin L, Zhang Y. A metabolic stress-inducible miR-34a-HNF4α pathway regulates lipid and lipoprotein metabolism. Nat Commun. 2015;6:7466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 183. | Gunewardena S, Huck I, Walesky C, Robarts D, Weinman S, Apte U. Progressive loss of hepatocyte nuclear factor 4 alpha activity in chronic liver diseases in humans. Hepatology. 2022;76:372-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 184. | Sun K, Montana V, Chellappa K, Brelivet Y, Moras D, Maeda Y, Parpura V, Paschal BM, Sladek FM. Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol. 2007;21:1297-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 185. | Viollet B, Kahn A, Raymondjean M. Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol Cell Biol. 1997;17:4208-4219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 146] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 186. | Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495-27501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 187. | Yu D, Chen G, Pan M, Zhang J, He W, Liu Y, Nian X, Sheng L, Xu B. High fat diet-induced oxidative stress blocks hepatocyte nuclear factor 4α and leads to hepatic steatosis in mice. J Cell Physiol. 2018;233:4770-4782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 188. | Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal. 2001;3:535-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 189. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (5)] |
| 190. | Tian J, Brown LA, Jones DP, Levin MS, Wang L, Rubin DC, Ziegler TR. Intestinal redox status of major intracellular thiols in a rat model of chronic alcohol consumption. JPEN J Parenter Enteral Nutr. 2009;33:662-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 191. | Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G643-G651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 192. | Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4α is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 193. | Xu Y, Zhu Y, Hu S, Xu Y, Stroup D, Pan X, Bawa FC, Chen S, Gopoju R, Yin L, Zhang Y. Hepatocyte Nuclear Factor 4α Prevents the Steatosis-to-NASH Progression by Regulating p53 and Bile Acid Signaling (in mice). Hepatology. 2021;73:2251-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 194. | Thymiakou E, Tzardi M, Kardassis D. Impaired hepatic glucose metabolism and liver-α-cell axis in mice with liver-specific ablation of the Hepatocyte Nuclear Factor 4α (Hnf4a) gene. Metabolism. 2023;139:155371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 195. | Xu Y, Hu S, Jadhav K, Zhu Y, Pan X, Bawa FC, Yin L, Zhang Y. Hepatocytic Activating Transcription Factor 3 Protects Against Steatohepatitis via Hepatocyte Nuclear Factor 4α. Diabetes. 2021;70:2506-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 196. | Yang T, Poenisch M, Khanal R, Hu Q, Dai Z, Li R, Song G, Yuan Q, Yao Q, Shen X, Taubert R, Engel B, Jaeckel E, Vogel A, Falk CS, Schambach A, Gerovska D, Araúzo-Bravo MJ, Vondran FWR, Cantz T, Horscroft N, Balakrishnan A, Chevessier F, Ott M, Sharma AD. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol. 2021;75:1420-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 197. | Yang J, Kong X, Martins-Santos ME, Aleman G, Chaco E, Liu GE, Wu SY, Samols D, Hakimi P, Chiang CM, Hanson RW. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4alpha. J Biol Chem. 2009;284:27042-27053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 198. | Ren Q, Sun Q, Fu J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy. 2024;20:221-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 79] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 199. | Lee DH, Park SH, Ahn J, Hong SP, Lee E, Jang YJ, Ha TY, Huh YH, Ha SY, Jeon TI, Jung CH. Mir214-3p and Hnf4a/Hnf4α reciprocally regulate Ulk1 expression and autophagy in nonalcoholic hepatic steatosis. Autophagy. 2021;17:2415-2431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 200. | Lee SH, Veeriah V, Levine F. Liver fat storage is controlled by HNF4α through induction of lipophagy and is reversed by a potent HNF4α agonist. Cell Death Dis. 2021;12:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 201. | Ramamoorthy A, Li L, Gaedigk A, Bradford LD, Benson EA, Flockhart DA, Skaar TC. In silico and in vitro identification of microRNAs that regulate hepatic nuclear factor 4α expression. Drug Metab Dispos. 2012;40:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 202. | Takagi S, Nakajima M, Kida K, Yamaura Y, Fukami T, Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4alpha, modulating the expression of metabolic enzymes and cell cycle. J Biol Chem. 2010;285:4415-4422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 203. | Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, Karin M, Struhl K, Hadzopoulou-Cladaras M, Iliopoulos D. An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147:1233-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 393] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 204. | Lamba V, Ghodke Y, Guan W, Tracy TS. microRNA-34a is associated with expression of key hepatic transcription factors and cytochromes P450. Biochem Biophys Res Commun. 2014;445:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 205. | Dou L, Wang S, Sun L, Huang X, Zhang Y, Shen T, Guo J, Man Y, Tang W, Li J. Mir-338-3p Mediates Tnf-A-Induced Hepatic Insulin Resistance by Targeting PP4r1 to Regulate PP4 Expression. Cell Physiol Biochem. 2017;41:2419-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 206. | Wei S, Zhang M, Yu Y, Xue H, Lan X, Liu S, Hatch G, Chen L. HNF-4α regulated miR-122 contributes to development of gluconeogenesis and lipid metabolism disorders in Type 2 diabetic mice and in palmitate-treated HepG2 cells. Eur J Pharmacol. 2016;791:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 207. | Pang Z, Zhang H, Zheng S, Yang X, Liu C, Han Q, Chen Y, Li Z, Zhang X, Cao L, Wang Q, Cao Y, Sun X, Zhao P, Li X, Zheng Q, Sheng R. HNF4α-CDKL3 axis restricts MASLD progression by targeting FoxO1 via noncanonical phosphorylation. Hepatology. 2025;82:702-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 208. | Wang M, Chen M, Zheng G, Dillard B, Tallarico M, Ortiz Z, Holterman AX. Transcriptional activation by growth hormone of HNF-6-regulated hepatic genes, a potential mechanism for improved liver repair during biliary injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G357-G366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 209. | Zhang Y, Fang B, Damle M, Guan D, Li Z, Kim YH, Gannon M, Lazar MA. HNF6 and Rev-erbα integrate hepatic lipid metabolism by overlapping and distinct transcriptional mechanisms. Genes Dev. 2016;30:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 210. | Wang D, Hartmann K, Seweryn M, Sadee W. Interactions Between Regulatory Variants in CYP7A1 (Cholesterol 7α-Hydroxylase) Promoter and Enhancer Regions Regulate CYP7A1 Expression. Circ Genom Precis Med. 2018;11:e002082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 211. | McNair A, Cereghini S, Brand H, Smith T, Breillat C, Gannon F. Synergistic activation of the Atlantic salmon hepatocyte nuclear factor (HNF) 1 promoter by the orphan nuclear receptors HNF4 and chicken ovalbumin upstream promoter transcription factor I (COUP-TFI). Biochem J. 2000;352 Pt 2:557-564. [PubMed] |
| 212. | Eeckhoute J, Formstecher P, Laine B. Hepatocyte nuclear factor 4alpha enhances the hepatocyte nuclear factor 1alpha-mediated activation of transcription. Nucleic Acids Res. 2004;32:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 213. | Boj SF, Petrov D, Ferrer J. Epistasis of transcriptomes reveals synergism between transcriptional activators Hnf1alpha and Hnf4alpha. PLoS Genet. 2010;6:e1000970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 214. | Rausa FM, Tan Y, Costa RH. Association between hepatocyte nuclear factor 6 (HNF-6) and FoxA2 DNA binding domains stimulates FoxA2 transcriptional activity but inhibits HNF-6 DNA binding. Mol Cell Biol. 2003;23:437-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 215. | Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev. 2006;20:2293-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 216. | Lee SH, Na SI, Heo JS, Kim MH, Kim YH, Lee MY, Kim SH, Lee YJ, Han HJ. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: involvement of Ca2+/PKC and MAPKs-induced EGFR transactivation. J Cell Biochem. 2009;106:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 217. | Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 218. | Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 219. | Tanaka T, Tomaru Y, Nomura Y, Miura H, Suzuki M, Hayashizaki Y. Comprehensive search for HNF-1beta-regulated genes in mouse hepatoma cells perturbed by transcription regulatory factor-targeted RNAi. Nucleic Acids Res. 2004;32:2740-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 220. | Yu B, He ZY, You P, Han QW, Xiang D, Chen F, Wang MJ, Liu CC, Lin XW, Borjigin U, Zi XY, Li JX, Zhu HY, Li WL, Han CS, Wangensteen KJ, Shi Y, Hui LJ, Wang X, Hu YP. Reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Cell Stem Cell. 2013;13:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 221. | Monfeuga T, Norlin J, Bugge A, Gaalsgaard ED, Prada-Medina CA, Latta M, Veidal SS, Petersen PS, Feigh M, Holst D. Evaluation of long acting GLP1R/GCGR agonist in a DIO and biopsy-confirmed mouse model of NASH suggest a beneficial role of GLP-1/glucagon agonism in NASH patients. Mol Metab. 2024;79:101850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 222. | Kawaguchi T, Charlton M, Kawaguchi A, Yamamura S, Nakano D, Tsutsumi T, Zafer M, Torimura T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin Liver Dis. 2021;41:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 223. | Yki-Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18:770-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 224. | Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Dhalwani NN, Kendrick S, Celis-Morales C, Waterworth DM, Alazawi W, Sattar N. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/