Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110026
Revised: June 28, 2025
Accepted: September 23, 2025
Published online: October 27, 2025
Processing time: 152 Days and 21.1 Hours
Liver metastases are a leading contributor to cancer-related illness and death, occurring far more frequently than primary liver tumors. Their management remains highly challenging due to the complexity of disease behavior and the need for an individualized, multidisciplinary approach. Effective care increasingly relies on integrating sophisticated diagnostic techniques, advanced systemic and locoregional therapies, and molecularly tailored treatment strategies. This review provides an in-depth analysis of the current clinicopathological perspectives on liver metastases. It explores their epidemiology, mechanisms of spread, histo
Core Tip: Liver metastases remain one of the most significant contributors to cancer-related deaths, occurring far more frequently than primary hepatic tumors. This review delivers a thorough overview of current clinicopathological under
- Citation: Ebrahim NAA, Farghaly TA, El-Sherif AA, Fahmy AM, Othman MO, Tahoun NS, Korany OM, Arafat A, Oreaba R, Soliman SMA. Clinicopathological insights and management of liver metastases: Current advances and future perspectives. World J Hepatol 2025; 17(10): 110026
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110026.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110026
Liver metastases are a major cause of cancer-related morbidity and mortality, vastly outnumbering primary liver cancers–by an estimated factor of 18-40. The liver’s unique vascular architecture, receiving dual blood supply from the portal vein and hepatic artery, makes it particularly vulnerable to metastatic spread from a variety of primary tumors. Colorectal cancer (CRC) is the most common source, accounting for roughly 40% of liver metastases, followed by gastric and pancreatic tumors (each approximately 20%), and lung and breast primaries (around 10% each). Other malignancies, such as neuroendocrine tumors (NETs), gastrointestinal stromal tumors, renal cell carcinomas, and melanomas, can also give rise to hepatic metastases, albeit less frequently[1-3].
The dissemination of tumor cells to the liver is influenced by both anatomical and biological factors, often described by the "seed and soil" hypothesis. Gastrointestinal primaries, such as colorectal and pancreatic cancers, typically spread via the portal venous system, while extra-abdominal tumors like lung and breast cancers tend to reach the liver through the hepatic artery. Once tumor cells arrive, the metastatic process involves several critical steps: (1) Adhesion to the sinusoidal endothelium; (2) Evasion of local immune defenses; (3) Induction of angiogenesis; and (4) Remodeling of the hepatic stroma to support tumor growth[4-6].
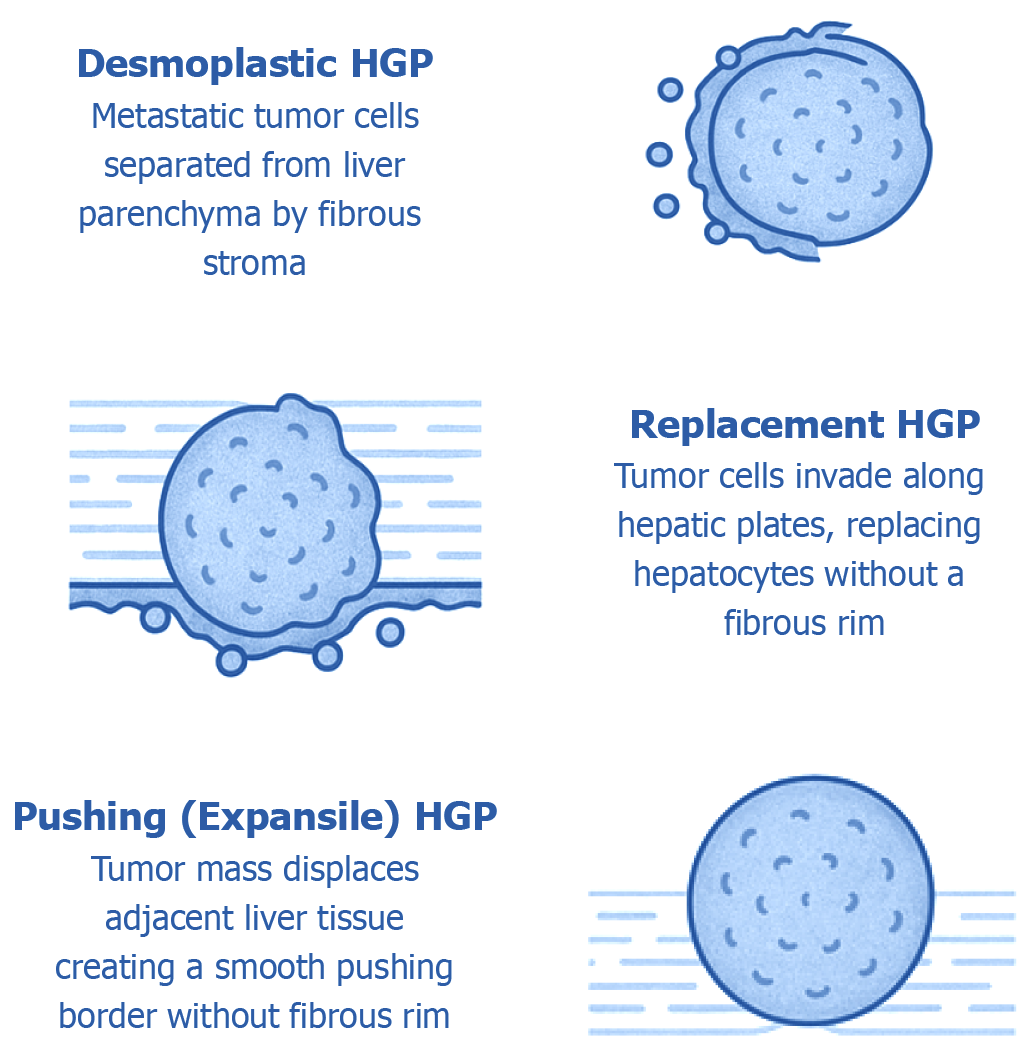
Histologically, liver metastases present in varied growth patterns at the tumor–liver interface, reflecting complex interactions between tumor cells and the hepatic microenvironment. Three primary patterns have been described: (1) Desmoplastic (characterized by a fibrotic rim separating tumor from liver tissue); (2) Replacement (where tumor cells infiltrate and replace normal hepatocytes); and (3) Pushing (where tumor expands by compressing adjacent liver parenchyma without significant invasion).
These patterns are not merely descriptive but carry significant prognostic implications. For instance, desmoplastic metastases–particularly in colorectal and breast cancers–are associated with better outcomes, whereas replacement–type lesions, which integrate into and co-opt hepatic structures, tend to predict poorer survival. Recent consensus guidelines have standardized the classification of these patterns across tumor types, underscoring their diagnostic and therapeutic relevance[7-9].
Grossly, liver metastases typically appear as multiple nodules scattered throughout an otherwise non-cirrhotic liver. However, their histological features can vary widely and sometimes mimic primary liver tumors, making accurate diagnosis essential. Immunohistochemical (IHC) stains and molecular assays are critical tools in this differentiation–for example, albumin in situ hybridization can help distinguish hepatocellular carcinoma (HCC) from metastatic tumors. The morphology[4], stromal response, and vascular patterns of metastases also provide valuable insights that inform prognosis and guide treatment strategies[5,10-13].
This review offers a comprehensive overview of the pathophysiology and pathology of liver metastases, incorporating recent advances in imaging-including magnetic resonance imaging (MRI) and computed tomography (CT) (with diffusion–weighted imaging and hepatocyte-specific contrast), positron emission tomography (PET)/CT, PET/MRI, and contrast-enhanced ultrasound (US)–as well as evolving molecular and circulating biomarkers. It also examines systemic therapies (chemotherapy, targeted agents, immunotherapy), surgical interventions (resection criteria and techniques), and locoregional treatments [such as ablation, transarterial chemoembolization (TACE), and radioembolization]. The importance of multidisciplinary care is highlighted, along with prognostic modeling, ongoing clinical trials, and future directions in the field, including liquid biopsies, artificial intelligence (AI) applications, organoid models, and precision oncology approaches. Together, these topics provide a thorough and up-to-date resource for clinicians and researchers navigating the complex landscape of liver metastases.
Liver metastases affect about 5.1% of cancer patients at initial diagnosis, according to Surveillance, Epidemiology, and End Results (SEER) data, with lung cancer emerging as the most common primary source of synchronous liver spread, followed by colorectal, pancreatic, gastric, and breast cancers. In contrast, CRC remains the leading source of liver metastases overall, largely because of its high rate of metachronous metastasis that develop after initial diagnosis-up to 25%–30% of CRC patients progress to liver involvement later. Thus, the SEER data reflect synchronous incidence, whereas the 5% numbers encompasses the total prevalence across the entire cancer population. The distribution of primary tumors also differs by age and sex: For instance, younger women are more likely to develop liver metastases from breast cancer, while younger men are more commonly affected by colorectal metastases. Older patients tend to present with a broader range of primaries, including esophageal and gastric cancers. Liver metastases are a major contributor to cancer–related mortality. In CRC specifically, around 17% of patients present with synchronous liver metastases at the time of diagnosis, translating to an incidence of approximately 6.9 per 100000 in men and 3.4 per 100000 in women. Although advances in cancer screening and systemic therapies have significantly reduced the incidence of metachronous colorectal liver metastases (CRLM)–from around 18.6% in the late 1970s to about 10% in the early 2010s–survival outcomes remain dismal. The 1-year overall survival rate for patients with synchronous liver metastases, regardless of primary tumor, is only about 15%. Specifically, patients with CRC have a 5-year survival rate of approximately 6% when liver metastases are synchronous and around 13% for metachronous metastases. Importantly, the prognosis for patients with liver metastases is closely tied to the primary tumor origin. Metastases from lung, pancreatic, or gastric cancers are associated with particularly poor outcomes, whereas liver metastases originating from colorectal or breast cancers tend to confer relatively better survival prospects[10,14].
These data highlight the substantial impact of liver metastases on patient outcomes. The presence of liver involvement often leads to a marked reduction in overall survival and is frequently accompanied by metastases to other organs. This underscores the need for vigilant surveillance, early detection, and aggressive management strategies to improve patient outcomes.
The liver’s distinctive anatomy and specialized microenvironment make it particularly susceptible to metastatic colonization by circulating tumor cells (CTCs). The well-known “seed and soil” hypothesis suggests that tumor cells ("seeds") preferentially establish in certain organs ("soil") that provide a favorable environment. In this context, the liver's extensive blood supply–receiving both nutrient–rich portal venous blood from the gastrointestinal tract and arterial blood from the systemic circulation–offers an ideal niche for metastatic tumor cells. This dual vascular inflow exposes the liver to tumor cells originating from various primary sites across the body. Notably, studies have shown that liver metastases develop in approximately 41% of patients with metastatic cancer, making the liver the second most common site of metastasis after lymph nodes[15].
Within the hepatic microenvironment, metastatic tumor cells interact with a diverse array of resident liver cells and extracellular matrix components. Hepatic sinusoidal endothelial cells, Kupffer macrophages, hepatic stellate cells, hepatocytes, and immune cells such as dendritic cells, natural killer cells, monocytes, and neutrophils collectively shape a permissive metastatic niche. These cells secrete cytokines and growth factors-such as hepatocyte growth factor and vascular endothelial growth factor (VEGF)–which promote tumor cell survival, facilitate neovascularization, and support metastatic progression. The liver’s abundant extracellular matrix, rich in collagen and fibronectin, also plays a critical role by sequestering and presenting growth factors to tumor cells, while hepatic stellate cells actively remodel the matrix, enabling invasion and expansion of metastatic deposits.
A key feature of the liver is its inherently tolerogenic immune landscape, which is evolutionarily designed to prevent excessive immune responses to harmless gut–derived antigens. However, this immunosuppressive environment can be co-opted by metastatic tumor cells, enabling them to evade immune surveillance and establish persistent growth. Crosstalk between resident hepatic immune cells further reinforces this immunosuppressive state, creating an environment that favors metastatic progression. Experimental models have shown that the liver facilitates multiple stages of the metastatic cascade, including tumor cell arrest in the sinusoidal microvasculature, extravasation into the parenchyma, dormancy, and eventual reactivation and proliferation into macroscopic lesions[16].
The metastatic process in the liver can be conceptually divided into distinct phases: (1) Microvascular phase: Tumor cells adhere to and exit through the sinusoidal endothelium into the liver tissue; (2) Pre-angiogenic phase: Tumor cells survive and adapt in the new environment before initiating vascularization; (3) Angiogenic phase: Neovascularization supports further growth of the metastasis; and (4) Macroscopic expansion: Tumor outgrowth becomes clinically evident and progresses into large metastatic nodules.
Both tumor-intrinsic factors and liver–specific host factors shape the metastatic potential. For instance, the expression of chemokine receptors (such as C-X-C chemokine receptor type 4) by tumor cells aligns with hepatic chemokine gradients, enhancing organ-specific colonization. Tumors with constitutive activation of survival pathways, such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) or Rat sarcoma (RAS)–rapidly accelerated fibrosarcoma (RAF)–mitogen-activated protein kinase (MEK)–extracellular-signal regulated kinase pathway; RAS/mitogen-activated protein kinase (MAPK) signaling axes, are better equipped to thrive in the liver’s unique environment. Furthermore, the condition of the liver itself can influence metastasis. For example, pre-existing liver disease, such as steatosis or chronic inflammation, may create a microenvironment that either facilitates or inhibits metastatic growth–some studies suggest fatty livers may even harbor an increased burden of metastases[17].
Altogether, liver metastasis is the result of a complex interplay between the biological characteristics of the tumor and the unique microenvironment of the liver.
Liver metastases typically retain the morphological features of their primary tumor. For instance, colorectal adenocarcinoma metastases often display glandular structures, while metastases from melanomas or NETs reflect the cellular architecture of their respective origins. However, emerging evidence highlights that beyond primary tumor histology, the pattern of tumor growth at the tumor–liver interface has significant prognostic and therapeutic relevance. In CRLM, three major histopathological growth patterns (HGPs) have been described (Figure 1).
This pattern is characterized by a distinct fibrous stromal rim that separates metastatic tumor cells from the surrounding liver parenchyma. It is typically associated with active tumor–driven angiogenesis and stromal remodeling. Notably, desmoplastic metastases often exhibit prominent immune cell infiltration within the stroma, suggesting a more immunologically "hot" microenvironment. In cases of CRLM, metastases displaying a purely desmoplastic histological growth pattern have been consistently linked to more favorable clinical outcomes. Evidence indicates that patients with desmoplastic–only lesions tend to achieve significantly longer overall survival compared to those exhibiting replacement or pushing growth patterns. These findings highlight the prognostic significance of the tumor–liver interface architecture in guiding risk stratification and therapeutic decision-making.
In this non-desmoplastic pattern, tumor cells infiltrate along hepatic plates, replacing hepatocytes without a fibrotic rim. Replacement growth typically involves vessel co-option-tumor cells hijack existing vasculature rather than inducing new blood vessels. This pattern tends to evade immune detection and is associated with poorer prognosis. In breast cancer liver metastases, a replacement growth pattern has been linked to worse progression–free and overall survival compared to desmoplastic metastases.
Here, the tumor mass expands by physically displacing adjacent liver tissue, creating a smooth, well-defined interface without a prominent fibrotic border. The pushing pattern is less common than desmoplastic or replacement types.
Figure 1 illustrates the three principal HGPs identified at the interface between metastatic tumors and liver parenchyma-namely, desmoplastic, replacement, and pushing patterns. Each pattern reflects a unique mode of tumor–liver interaction and has emerging relevance in prognostication and tailoring treatment strategies.
Two additional, less frequently described patterns include: (1) Sinusoidal HGP: Tumor cells infiltrate and fill sinusoidal spaces without disrupting the underlying liver architecture; and (2) Portal HGP: Tumor growth is localized within portal tracts.
The clinical significance of these rare patterns remains under investigation[2,18,19].
Interestingly, growth patterns tend to remain consistent across multiple liver metastases within a single patient, but can vary significantly between patients. For instance, in uveal melanoma liver metastases, two analogous patterns have been observed: (1) A “nodular” pattern, resembling the pushing HGP, where tumor nodules form in portal tracts; and (2) An “infiltrative” pattern, akin to the replacement HGP, where individual tumor cells intermingle with hepatocytes. Patients with infiltrative (replacement-like) patterns tend to have more aggressive disease and worse outcomes.
The classification of HGPs is increasingly recognized as an important biomarker in clinical practice. Beyond its prognostic implications, HGPs may also inform treatment strategies. For example, van Dam et al[18] demonstrated that desmoplastic metastases–relying on angiogenesis for growth–were more responsive to anti-angiogenic therapies like bevacizumab compared to non-desmoplastic (replacement) types, which utilize vessel co-option. Consequently, hist
Accurate imaging is essential for identifying liver metastases, guiding treatment decisions, and monitoring response to therapy. A variety of imaging techniques are employed in clinical practice, each offering distinct advantages and limitations.
Conventional B-mode US is often the initial tool for liver imaging due to its accessibility, low cost, and absence of ionizing radiation. While it has moderate sensitivity for larger lesions, its accuracy is limited for smaller or deep-seated metastases. Contrast-enhanced US (CEUS) using microbubble agents significantly enhance lesion detection and characterization. CEUS can reveal typical vascular patterns of metastases, such as rim-like enhancement during the arterial phase with subsequent washout. This technique is particularly helpful when CT or MRI findings are inconclusive. However, CEUS performance depends on operator skill and may be limited in obese patients or by lesion location.
Multiphase contrast-enhanced CT is a cornerstone of oncologic imaging, particularly for staging. Triple-phase CT (arterial, portal venous, delayed phases) provides good sensitivity for detecting liver metastases. CT is widely available, fast, and suitable for routine use, but its sensitivity decreases for lesions smaller than 1 cm or in fatty livers. Meta-analyses report that CT achieves sensitivity around 85% and specificity of approximately 94% in detecting CRLM. Advances in multidetector CT with thinner slice acquisition have improved detection, though MRI generally outperforms CT, especially for small or isodense lesions.
MRI offers superior soft–tissue contrast and multiple sequences (T1, T2, diffusion-weighted imaging) that improve detection and characterization of liver lesions. Gadolinium-enhanced MRI, particularly when using hepatocyte–specific contrast agents such as gadoxetic acid (Eovist), is considered the most sensitive modality for identifying focal liver lesions. Meta–analyses and expert guidelines consistently rank gadoxetic acid–enhanced MRI as the preferred technique for detecting liver metastases, with sensitivity often exceeding 90%. MRI is especially useful in evaluating indeterminate lesions on CT and in patients with hepatic steatosis. However, MRI is limited by longer scan times, higher cost, and contraindications in patients with certain implants or devices.
Fluorodeoxyglucose PET/CT (FDG-PET/CT) combines metabolic imaging with anatomical detail, offering high sensitivity for detecting metabolically active (FDG-avid) metastases, such as those from colorectal, lung, or melanoma primaries. However, some tumors (e.g., well-differentiated NETs) may not exhibit significant FDG uptake. PET/CT is particularly useful for identifying extrahepatic disease that may not be apparent on conventional imaging, thus influencing treatment plans. In CRC, meta-analyses report PET/CT sensitivity for liver metastases around 94% and specificity near 98%, surpassing CT. PET/CT is also valuable for evaluating treatment response based on metabolic activity. Novel tracers (e.g., prostate-specific membrane antigen, fibroblast activation protein inhibitor) and hybrid PET/MRI systems are under investigation for improved detection and characterization of liver lesions.
This technique combines real-time US with previously acquired CT or MRI datasets to enhance lesion detection and procedural accuracy, particularly in interventional settings such as biopsies or ablations. For instance, fusing MRI–defined lesions onto live US images can improve the targeting of small or iso-echoic metastases during procedures.
Investigational methods such as contrast-enhanced arterial spin labeling, magnetic resonance perfusion imaging, intravoxel incoherent motion, and radiomics–based approaches hold promise for further improving liver metastasis detection and characterization but are not yet standard in clinical practice[20-22].
Overall, PET/CT and MRI demonstrate the highest sensitivity for detecting liver metastases. Meta-analyses in CRC patients rank FDG-PET/CT as the most sensitive modality (approximately 94%), followed closely by MRI (approximately 93%), then CEUS (approximately 86%), and CT (approximately 85%). CEUS also shows high specificity (approximately 96%) but slightly lower sensitivity compared to PET/CT and MRI. In clinical workflows, CT remains the first-line modality for many patients due to its speed and availability. MRI, particularly with gadoxetic acid, is recommended when CT findings are inconclusive or for detailed preoperative planning. PET/CT is often reserved for staging or surveillance in specific tumor types or cases requiring whole-body assessment. Suspicious lesions typically undergo cross–modality confirmation to ensure diagnostic accuracy. Current guidelines emphasize a multimodal imaging strategy tailored to the individual patient and cancer type. For instance, Freitas et al[23] recommend CT and MRI as the primary tools for initial detection, follow-up, and response assessment, with MRI demonstrating superior sensitivity. Ichikawa et al[20] also affirm that gadoxetic acid–enhanced MRI is now the preferred choice for routine liver imaging due to its outstanding performance in metastasis detection[23-26].
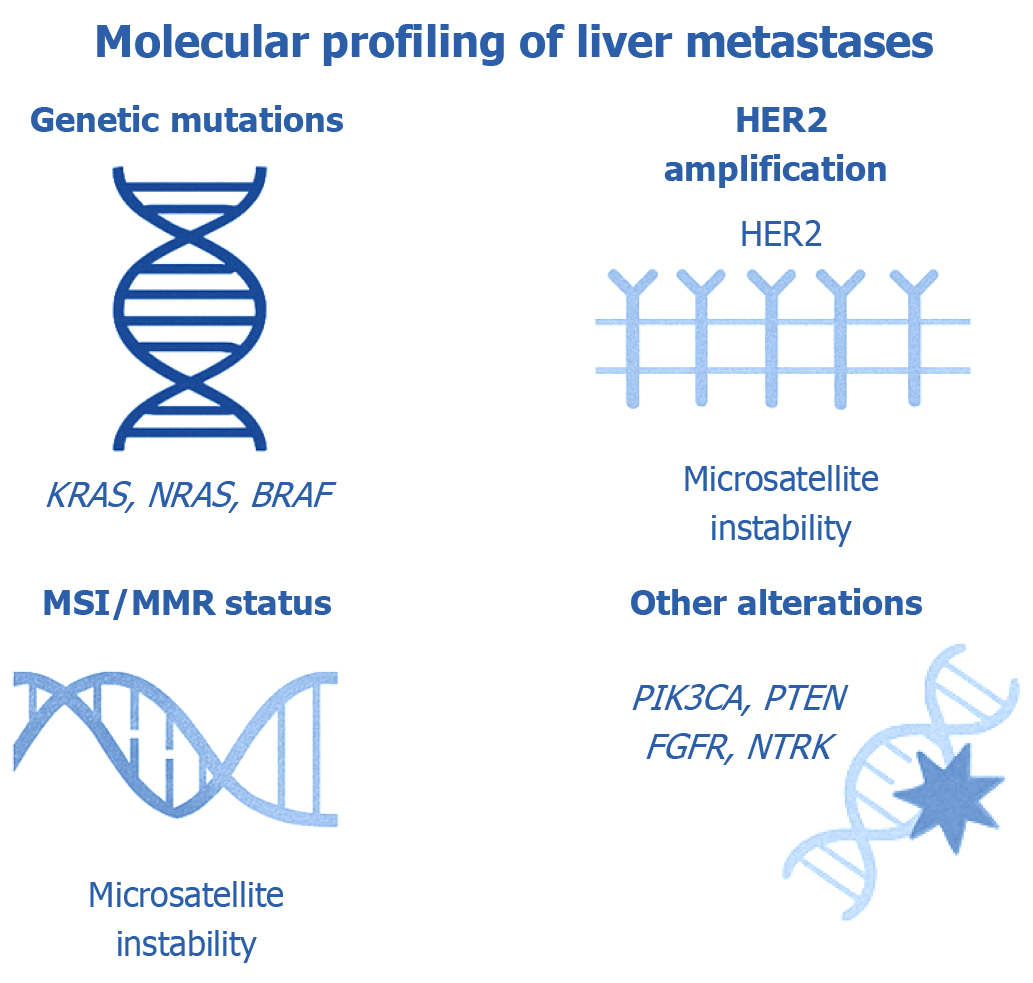
Comprehensive molecular profiling of liver metastases has become fundamental in determining both prognosis and individualized treatment strategies, particularly in CRC. By identifying specific genomic alterations within metastatic lesions, clinicians are better equipped to anticipate clinical outcomes and personalize systemic therapy accordingly. Notably, the liver is the predominant site of metastasis in CRC, and the molecular landscape of CRLM significantly influences survival rates and therapeutic decision-making[11,27].
Activating mutations in the RAS oncogene family, particularly KRAS and NRAS, occur in approximately 40%–50% of CRCs. In the context of CRLM, KRAS mutations are identified in around 30%–40% of cases. These mutations lead to constitutive activation of the MAPK signaling cascade, promoting tumor progression and metastatic spread. Clinically, RAS mutations are associated with poorer outcomes. In a large cohort of patients undergoing liver metastasectomy, KRAS mutations nearly doubled the hazard of mortality and were linked to higher recurrence rates. A meta–analysis further supported these findings, showing a twofold increase in death risk for KRAS–mutant cases post-surgery. NRAS mutations, though less frequent (approximately 5%), similarly correlate with inferior survival. Importantly, both KRAS and NRAS mutations are predictive of resistance to anti-epidermal growth factor receptor (EGFR) agents such as cetuximab and panitumumab, making RAS mutation testing mandatory before initiating EGFR–directed therapy. In contrast, RAS wild–type tumors are more responsive to these agents and generally carry a more favorable prognosis[11,27-29].
BRAF mutations, particularly the V600E variant, are observed in 5%–10% of CRC cases but are even rarer in patients selected for liver resection. Despite their low frequency, these mutations are associated with aggressive tumor biology, widespread dissemination, and poor prognosis. BRAF V600E mutations are linked to nearly a threefold increase in mortality compared to wild-type tumors. Post-metastasectomy survival in BRAF-mutant CRC is dismal, with median survival frequently under two years. Conversely, non-V600E BRAF variants tend to have a more indolent course. Due to the poor outcomes associated with V600E mutations, curative-intent surgery or liver transplantation is generally discouraged. Instead, these patients are managed with targeted systemic therapy, such as combined BRAF and EGFR inhibition[27,28].
Microsatellite instability-high (MSI-H) or deficient mismatch repair tumors represent a small subset (approximately 3%–5%) of metastatic CRC. These tumors, often associated with right-sided primaries and BRAF mutations, exhibit unique clinical and therapeutic behavior. Immunotherapy has revolutionized the treatment landscape for MSI-H CRC, as demonstrated in the KEYNOTE-177 trial, where pembrolizumab significantly improved progression-free survival compared to chemotherapy. MSI-H tumors are also less likely to develop widespread metastases and may paradoxically confer a better overall prognosis than microsatellite-stable (MSS) tumors. Consequently, MSI testing is essential in metastatic CRC, as it identifies candidates for programmed death-1 (PD-1) inhibitors (e.g., pembrolizumab or nivolumab), which can induce durable responses[27,29].
Although human EGFR-2 (HER2) amplification is well established in breast cancer, it also represents a therapeutically relevant target in CRC, particularly in patients with RAS/BRAF wild-type tumors. HER2 is amplified in 3%–5% of metastatic CRC cases and confers resistance to EGFR–targeted therapies. In these cases, HER2–directed treatments such as trastuzumab in combination with pertuzumab or lapatinib have shown efficacy. HER2–positive tumors that metastasize to the liver typically retain their oncogenic dependence on HER2, justifying the use of HER2 blockade. Given the potential for significant therapeutic benefit, HER2 testing is recommended in metastatic CRC, especially when standard RAS/BRAF mutations are absent[29,30].
Several less common but clinically actionable genetic alterations can influence the biology and treatment of CRLM. P110α catalytic subunit of class I PIK3CA mutations occur in approximately 10%–20% of CRCs and activate the PI3K/Akt signaling axis, often coexisting with other oncogenic drivers. Their presence may be associated with inferior survival following liver resection. Phosphatase and tensin homolog (PTEN) loss, another mechanism that enhances PI3K pathway activity, can confer resistance to targeted therapies, although direct inhibitors are currently unavailable. Fibroblast growth factor receptor-2 (FGFR2) fusions, while rare in CRC, are characteristic of intrahepatic cholangiocarcinoma (iCCA) and are targetable with FGFR inhibitors such as pemigatinib. Additionally, rare but highly actionable fusions involving neurotrophic tyrosine receptor kinase (NTRK) or anaplastic lymphoma kinase (ALK) genes can drive metastases across various tumor types. NTRK inhibitors (e.g., larotrectinib, entrectinib) and ALK inhibitors (e.g., crizotinib, alectinib) can elicit profound responses in tumors harboring these fusions. As such, comprehensive genomic profiling has become standard practice in assessing CRLM to uncover these rare but impactful targets[27,29,31].
Molecular analysis of liver metastases in CRC provides essential insights for prognosis and precision therapy. Mutations in KRAS, NRAS, and BRAF are routinely assessed to guide the use of EGFR inhibitors and to predict overall survival. MSI testing identifies patients who benefit from immune checkpoint inhibitors, while HER2 amplification opens the door to HER2–directed therapies. Additional alterations in PIK3CA, PTEN, FGFR, NTRK, and ALK, though less frequent, can significantly impact therapeutic planning when present. Integrating these biomarkers into clinical workflows enables a tailored approach that improves outcomes and refines eligibility for surgical and transplant-based interventions. In essence, molecular profiling has become a cornerstone of modern CRLM management, driving personalized oncology forward.
Histopathological markers: Immunohistochemistry remains essential for confirming tumor origin (e.g., cytokeratin 7 and cytokeratin 20 to distinguish gastrointestinal, pancreatic, or pulmonary primaries) and identifying therapeutic targets, such as hormone receptors (estrogen reseptor, progesterone reseptor, HER2 in breast cancer), androgen receptor in prostate cancer, or somatostatin receptors in NETs. Serum tumor markers like carcinoembryonic antigen (CEA), carbohydrate antigen 19-9, and alpha-fetoprotein can aid in tracking disease burden, although they lack specificity[4,27,32].
Liquid biopsy (circulating tumor DNA): One of the most significant recent advances is the ability to detect tumor-derived DNA fragments in the blood. In the setting of liver metastases, particularly CRC, the presence of circulating tumor DNA (ctDNA) reflects residual disease and is a strong predictor of recurrence. Studies have shown that detectable ctDNA after liver resection (hepatectomy) indicates a high risk of relapse. For example, in a prospective study of patients undergoing CRLM resection, the presence of ctDNA postoperatively-indicating minimal residual disease (MRD)–was an independent predictor of recurrence. Meta-analyses confirm these findings: Patients with detectable ctDNA before or after surgery have significantly worse recurrence–free and overall survival. One study strikingly reported that 63% of CRC patients with preoperative ctDNA experienced recurrence, compared to only 13% of those without ctDNA (odds ratio approximately 11.0). This demonstrates the power of ctDNA as a non-invasive tool for monitoring MRD and potentially guiding adjuvant therapy decisions. Similar approaches are being explored in other cancers[33-35].
CTCs and exosomes: Detection of CTCs in the bloodstream also holds prognostic value, as higher CTC counts are generally associated with poorer survival in metastatic cancers. Additionally, tumor-derived extracellular vesicles, such as exosomes containing RNA and DNA, are being investigated as potential biomarkers, although these are not yet standard in clinical practice[27,34].
In summary, the integration of molecular and histological biomarkers has become a cornerstone of liver metastasis management. Tsilimigras et al[16] emphasize that comprehensive genetic profiling provides critical insights for both prognosis and therapeutic decision-making. Advances in clinical sequencing panels and liquid biopsies now allow for broad molecular characterization without the need for repeated invasive procedures. For instance, a systematic review of colorectal metastases demonstrated that organoid cultures derived from multiple metastatic lesions can reveal intra–patient variability in drug responses, highlighting the importance of patient–specific assays. Looking ahead, combining genomics, transcriptomics, and liquid biopsy approaches will likely enable highly individualized treatment plans, moving toward a truly personalized oncology paradigm[16,36].
Figure 2 illustrates the key molecular biomarkers routinely analyzed in liver metastases, emphasizing their roles in diagnosis, prognosis, and therapeutic decision-making within the context of precision oncology.
Table 1 shows comprehensive understanding of the functions of multiple ligands and their associated receptors in the development and progression of HCC, iCCA, and metastatic liver tumors[37-55]. Differentiating liver metastases from primary hepatic neoplasms such as HCC and iCCA relies on a combination of histopathological assessment, IHC profiling, and radiologic features. The following Table 1 illustrates comparative summary highlights key diagnostic distinctions, aiding pathologists and radiologists in accurate diagnosis[37-55].
| Ligand | Receptor(s) | Functional role | Tumor association |
| HGF | c-MET (HGF receptor) | Facilitates cellular proliferation, migration, and invasive behavior | Highly expressed in HCC and iCCA; generally low in metastatic lesions |
| VEGF | VEGFR-1, VEGFR-2, VEGFR-3 | Mediates angiogenesis and increases vascular permeability | Elevated in poorly differentiated HCC, iCCA, and certain metastases such as colorectal carcinoma |
| TGF-β | TGF-β receptors 1 and 2 | Regulates EMT, fibrotic processes, and immune suppression | Upregulated in HCC and iCCA, contributing to fibrosis and EMT induction |
| PDGF | PDGFR-α, PDGFR-β | Activates stromal components and induces desmoplastic reactions | Predominantly expressed in iCCA and select metastatic adenocarcinomas (e.g., pancreatic, breast origin) |
| EGF | EGFR (human epidermal growth factor receptor 1) | Promotes cell growth, survival, and motility | EGFR signaling is active in iCCA, some HCC cases, and also in colorectal and pulmonary metastases |
| FGF | FGFR1 to FGFR4 | Involved in angiogenesis and cellular proliferation | FGFR2 gene fusions are characteristic of iCCA (especially small-duct subtype); less frequent in HCC |
| Wnt ligands (e.g., Wnt3a, Wnt5a) | Frizzled receptors and lipoprotein receptor-related protein 5/6 co-receptors | Regulates Wnt/β-catenin pathway affecting cell differentiation and proliferation | Aberrant Wnt/β-catenin activation is common in HCC; expression in metastases is variable |
| Notch ligands (Jagged1, DLL1) | Notch receptors 1 to 4 | Controls cellular differentiation and angiogenic processes | Notch signaling is upregulated in iCCA but less prominent in HCC or metastatic tumors |
| CXCL12 (stromal cell-derived factor 1) | CXCR4 | Directs chemotaxis, promotes metastasis and angiogenesis | High CXCR4/CXCL12 axis activity is observed in metastases from colorectal, breast, and pancreatic cancers |
| IL-6 | IL-6 receptor (via gp130/Janus kinase/signal transducer and activator of the transcription pathway) | Mediates inflammatory responses, proliferation, and survival | Elevated levels noted in HCC and metastatic colorectal carcinoma |
| Mucin-1 | Cell surface protein without classic receptor (functions as ligand) | Involved in tumor progression and immune evasion | Overexpressed in iCCA and certain metastatic adenocarcinomas, especially of breast and pancreatic origin |
This Table 1 outlines key ligand–receptor interactions involved in the molecular pathogenesis of primary liver cancers-such as HCC and iCCA-as well as liver metastases originating from extrahepatic primaries[37-55]. Each entry details the ligand, its cognate receptor(s), primary biological functions (e.g., cell proliferation, angiogenesis, epithelial–mesenchymal transition, immune evasion), and the specific tumor types in which these pathways are activated. These signaling axes not only contribute to tumor progression but also offer potential therapeutic targets, highlighting molecular differences between primary hepatic neoplasms and secondary metastatic deposits.
This Table 2 offers a side-by-side comparison of diagnostic criteria used to differentiate metastatic liver lesions from primary hepatic malignancies, specifically HCC and iCCA[32,56-59]. It integrates key morphologic characteristics-both gross and microscopic–with IHC marker profiles, reticulin framework alterations, vascular patterns, and radiologic enhancement behaviors. Each feature is contextualized based on its typical manifestation in metastatic vs primary tumors. Particular attention is given to architectural patterns, expression of hepatocytic and biliary markers, cytokeratin profiles, and contrast–enhancement dynamics on imaging, all of which play critical roles in reaching an accurate pathological and radiological diagnosis.
| Feature | Liver metastases | Primary liver tumors (HCC/iCCA) | Key insights |
| Lesion number and distribution | Frequently multiple, scattered nodules of varying sizes; often in a non-cirrhotic liver | HCC: Typically, a solitary mass, occasionally with satellite nodules; iCCA: Usually, a single mass, often subcapsular | Multiple lesions in a non-cirrhotic liver strongly suggest metastases; a solitary lesion in cirrhosis favors HCC |
| Liver background | Generally, arises in a normal liver without underlying chronic disease | HCC: Commonly associated with cirrhosis or chronic hepatitis; iCCA: May arise in chronic liver disease or normal liver | The presence of cirrhosis significantly increases the likelihood of HCC over metastases |
| Gross morphology | Well-defined, spherical nodules; cut surface varies by primary site (e.g., firm, mucinous, or necrotic) | HCC: Soft, tan-yellow mass, often with a pseudocapsule; iCCA: Firm, white mass with fibrotic stroma and capsular retraction | Capsular retraction suggests iCCA; a pseudocapsule is more typical of HCC; metastases lack a true capsule |
| Histologic pattern | Reflects the primary tumor morphology (e.g., glandular, mucinous, or neuroendocrine) | HCC: Thickened trabeculae of hepatocyte-like cells; iCCA: Irregular, malignant glandular structures with dense stroma | Tumor architecture in metastases mirrors the origin; HCC shows disrupted lobular architecture, while iCCA exhibits desmoplasia |
| Cytologic features | Variable: Mucin production, signet-ring cells, neuroendocrine differentiation, or squamous features, depending on primary | HCC: Polygonal cells with eosinophilic or clear cytoplasm, bile pigment may be present; iCCA: Columnar cells with intracellular mucin | Bile pigment is a hallmark of hepatocellular differentiation; mucin suggests cholangiocarcinoma or metastases |
| Reticulin staining | Reticulin framework usually preserved, outlining glandular or nested structures | HCC: Loss or fragmentation of reticulin due to thickened plates; iCCA: Often retains reticulin around malignant glands | Reticulin stain helps distinguish HCC (loss of framework) from metastases (preserved reticulin) |
| Vascular features | Lacks unpaired arteries; sinusoidal or vascular invasion typically at tumor-liver interface | HCC: Characteristically shows unpaired arteries and sinusoidal-like vasculature; iCCA: Tends to invade portal structures | Presence of unpaired arteries supports HCC; vascular invasion is common in both but more characteristic in HCC |
| Hepatocellular markers | Negative for hepatocytic markers such as HepPar1, arginase-1, and glypican-3 | HCC: Typically, positive for HepPar1, arginase-1 (most specific), and glypican-3; iCCA: Generally negative for these markers | Hepatocytic marker panel (HepPar1, arginase-1, glypican-3) is essential to confirm HCC |
| Cytokeratin profile | Varies by origin, e.g., colorectal (CK20+/CDX2+), breast (CK7+/GATA3+), lung (CK7+/ | HCC: Usually CK7−/CK19−; iCCA: CK7+/CK19+ in most cases | CK7/CK19 positivity favors cholangiocarcinoma or metastases; CDX2 positivity suggests colorectal origin |
| Additional immunohistochemical markers | Site-specific, e.g., CDX2 (colorectal), TTF-1 (lung), GATA3 (breast). Polyclonal CEA shows diffuse membranous pattern in adenocarcinomas | HCC: Polyclonal CEA shows canalicular (beaded) staining; alpha-fetoprotein may be positive (approximately 50% cases); iCCA: May express carbohydrate antigen 19-9 and luminal CEA | Canalicular CEA pattern is characteristic of HCC; diffuse membranous pattern suggests adenocarcinoma |
| Imaging characteristics | Typically, hypovascular on arterial phase with rim or peripheral enhancement (target sign); enhancement less than liver in portal phase | HCC: Arterial phase hyperenhancement with washout in portal/delayed phases; iCCA: Peripheral rim enhancement with gradual centripetal fill-in | Imaging enhancement pattern is key: Hypervascular with washout favors HCC; rim enhancement with delayed fill suggests iCCA/metastasis |
| Other imaging features | Often multiple, no pseudocapsule, calcifications possible (especially in mucinous tumors); no background liver disease | HCC: Solitary, in cirrhotic liver, with pseudocapsule; iCCA: Subcapsular location, capsular retraction, peripheral biliary dilatation | Capsular retraction and biliary dilation are typical of iCCA; multiple lesions in non-cirrhotic liver favor metastases |
The systemic management of liver metastases is tailored based on the primary tumor type, molecular profile, and patient-specific factors.
For most solid tumors with liver metastases, systemic chemotherapy forms the cornerstone of treatment. In CRC liver metastases, first-line regimens typically include combinations such as 5-fluorouracil/Leucovorin + oxaliplatin (FOLFOX), 5-fluorouracil/Leucovorin + irinotecan (FOLFIRI), or capecitabine + oxaliplatin. These are often combined with targeted agents according to KRAS and BRAF status to enhance efficacy. Such regimens can downsize tumors, potentially converting initially unresectable metastases into candidates for surgical resection[60].
In metastatic breast cancer, systemic options include anthracycline–based and taxane–based regimens, with additional agents like capecitabine or eribulin used in later lines. For NET metastases, options include streptozocin, temozolomide, or a combination of capecitabine plus temozolomide, although somatostatin analogs and targeted therapies (discussed below) also play an important role. For pancreatic cancer metastases, gemcitabine–based combinations, such as FOLFIRINOX, remain standard[61].
It is important to note that systemic chemotherapy for liver metastases is typically palliative. Historically, outcomes were poor. It was reported that chemotherapy for unresected CRC liver metastases rarely resulted in survival beyond three years. However, advances in combination regimens and sequencing strategies have significantly extended median survival in modern practice[62].
The advent of targeted therapies has dramatically improved outcomes for selects patient groups.
Anti-VEGF therapy: Bevacizumab, an anti-VEGF monoclonal antibody, inhibits angiogenesis and improves outcomes when added to chemotherapy in CRC metastases and other tumors. Studies like EORTC 40983 have shown that perioperative chemotherapy with bevacizumab can reduce progression events by approximately 25% in resectable CRC liver metastases. Tsilimigras et al[16] underscore that biologics have “revolutionized” the treatment landscape for metastatic liver disease.
Anti-EGFR therapy: In RAS wild-type CRC metastases, agents such as cetuximab and panitumumab (anti-EGFR; EGFR monoclonal antibodies) are effective. These therapies are guided by the molecular profile, as they lack efficacy in KRAS-mutant or NRAS-mutant tumors. In the resectable setting, anti-EGFR agents have been combined with chemotherapy, though caution is advised for right-sided CRC primaries due to data suggesting potential harm.
HER2-directed therapy: For HER2-amplified breast or (rarely) CRC metastases, therapies such as trastuzumab, pertu
For liver metastases from NETs, long-acting somatostatin analogs (octreotide, lanreotide) help control hormonal symptoms and slow tumor progression. Peptide receptor radionuclide therapy, such as 177 Lu-DOTATATE, delivers targeted radiation to somatostatin receptor-positive tumors and achieves high disease control rates.
Various tyrosine kinase inhibitors have roles across metastatic tumors. Sorafenib and lenvatinib are standards for HCC and are sometimes used off-label in other settings, such as thyroid or adrenal metastases. For BRAF–mutant melanoma or CRC metastases, BRAF/MEK inhibitors like dabrafenib plus trametinib are effective. Refractory CRC metastases may respond to multi-kinase inhibitors such as regorafenib. In cholangiocarcinoma metastases, IDH and FGFR inhibitors are options when the relevant alterations are present[63,64].
For hormone-driven tumors with liver metastases, endocrine therapy is central. In estrogen receptor (ER)–positive breast cancer, agents such as aromatase inhibitors, tamoxifen, or selective ER degraders like fulvestrant are frequently used, often in combination with CDK4/6 inhibitors (e.g., Ribociclib or palbociclib). For ERBB2–positive breast metastases, HER2–directed therapies remain essential[65]. In prostate cancer metastases to the liver, androgen deprivation therapy (using gonadotropin–releasing hormone analogs) is combined with novel anti-androgens (e.g., enzalutamide, abiraterone) or chemotherapy (docetaxel). Hormone-sensitive ovarian or endometrial metastases may respond to endocrine therapies when receptor–positive, also for castrate sensitive metastatic prostate cancer androgen-deprivation therapy play a major role.
Immune checkpoint inhibitors have transformed the management of certain metastatic cancers. Anti-PD-1 agents (pembrolizumab, nivolumab), alone or in combination with cytotoxic T-lymphocyte antigen-4 inhibitors (ipilimumab), can induce durable responses in select liver metastases, particularly MSI; MSI-H CRC (with response rates up to 40%–50%) and melanoma (response rates around 30%–50%). However, most other liver metastases (e.g., MSS CRC, breast, lung) have limited responses to immunotherapy. Tsilimigras et al[16] emphasize that these immunotherapeutic agents are “revolutionizing” the treatment landscape. Emerging approaches such as CAR-T cells, cancer vaccines, and bispecific antibodies are under investigation across tumor types[16,60,66].
Modern management of liver metastases often combines these therapies. For example, a patient with unresectable CRC liver metastases may receive FOLFIRINOX plus bevacizumab, potentially enabling subsequent surgical resection. A patient with breast cancer liver metastases will undergo HER2 and hormone receptor testing to guide targeted and endocrine therapies. Clinical trials are exploring novel combinations, such as immunotherapy with targeted agents, to improve outcomes. Ultimately, systemic therapy for liver metastases is rarely curative when used alone. Its primary roles are disease control, conversion to resect ability, and palliation of symptoms. Treatment selection is increasingly guided by the tumor’s molecular profile and prior therapeutic exposures, reflecting the growing emphasis on personalized oncology[67-69].
Clinical trial enrollment is critically important for patients with liver metastases, especially if they had exhausted the current effective therapeutic modalities, offering access to innovative therapies that can significantly improve outcomes. Furthermore, emerging immunotherapy approaches and loco-regional strategies in clinical trials continue to expand treatment possibilities. Together, these findings underscore that trial participation enables access to potent, cutting–edge therapies tailored to liver–involved metastatic disease and plays a vital role in advancing oncology care.
Surgical resection continues to represent the most definitive and potentially curative intervention for patients with liver–limited metastatic disease, particularly from CRC. Whereas early surgical protocols restricted eligibility to patients with a limited number of metastases confined to a single hepatic lobe, advances in operative techniques, high-resolution imaging, and perioperative planning have broadened respectability criteria. Contemporary surgical decision-making prioritizes the preservation of sufficient liver function, with respectability determined not by the number or location of lesions alone, but by the adequacy of the future liver remnant (FLR)[70,71]. To ensure safe postoperative recovery, surgical guidelines recommend that at least two contiguous liver segments be preserved with intact portal and arterial inflow, venous outflow, and biliary drainage. The minimal acceptable FLR volume is approximately 20% in healthy livers, increasing to 30% or more in patients with prior chemotherapy exposure or chronic liver disease. In cases where the FLR is insufficient, portal vein embolization (PVE) is routinely employed to induce hypertrophy of the contralateral liver lobe, enhancing functional capacity prior to resection. For patients requiring more rapid FLR expansion, the Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) procedure offers a two-step surgical strategy that achieves accelerated hypertrophy. Notably, the LIGRO randomized controlled trial demonstrated that ALPPS significantly increased the proportion of patients completing curative resection (92% vs 57%) compared to conventional two-stage hepatectomy, with similar rates of R0 resection and perioperative outcomes[70-72].
Modern hepatic resection strategies are highly tailored to tumor distribution and patient anatomy. Depending on the depth and anatomical location of metastatic lesions, surgeons may opt for anatomical resections, which follow the segmental vasculobiliary framework, or non-anatomical (wedge) resections, which preserve maximal parenchyma. The presence of multifocal or bilobar metastases–once viewed as a contraindication to surgery–is now frequently addressed through parenchymal–sparing techniques or staged resections, provided complete tumor clearance is feasible. In patients with synchronous primary colorectal tumors and hepatic metastases, a combined or staged resection strategy is selected based on tumor burden and patient physiology. The increasing use of minimally invasive liver surgery–including laparoscopic and robotic techniques–has further reduced operative morbidity and recovery time in appropriately selected cases[54,73,74].
For patients initially deemed unresectable, neoadjuvant systemic therapy offers a pathway to resect ability. Chemotherapy regimens such as FOLFOX or FOLFIRI, often combined with biological agents (e.g., bevacizumab or cetuximab), can achieve significant tumor downsizing. The EORTC 40983 trial demonstrated that perioperative FOLFOX chemotherapy, administered pre- and post-operatively, improved 3-year progression-free survival by approximately 25% compared to surgery alone, supporting its use in borderline resect able settings. In patients with extensive tumor burden, PVE and ALPPS remain crucial adjuncts in expanding surgical indications by facilitating sufficient hepatic hypertrophy[75,76].
Perioperative management strategies further optimize patient outcomes. In select high-risk cases, hepatic artery infusion (HAI) with floxuridine enables localized delivery of chemotherapeutic agents directly to the liver, reducing systemic toxicity and potentially improving disease-free survival. Additionally, adoption of Enhanced Recovery After Surgery protocols-featuring early ambulation, structured nutrition, and multimodal analgesia-has contributed to reduced postoperative complications and shorter hospitalization durations[73,77].
Collectively, these surgical and perioperative innovations have led to marked improvements in long-term survival for patients undergoing hepatic metastasectomy. Studies by Tsilimigras et al[16] and De Greef et al[78] report 5-year survival rates of 50%–70% following resection of CRLM, especially in experienced, high-volume centers. Meta-analyses confirm a wide survival range (16%-71%) post-resection, a stark contrast to the median survival of less than one year in patients managed without surgery. Although the EORTC 40983 trial did not show a clear overall survival benefit with perioperative chemotherapy, its demonstrated impact on progression-free survival, along with evidence from real-world settings, supports a multimodal, surgery-centered approach as the current standard for managing liver-limited metastatic CRC[16,78,79].
Historically viewed as experimental, liver transplantation for metastatic disease–particularly unresectable colorectal metastases–has shown promising results in carefully selected patients. The Secondary Cancer (SECA) trial series from Denmark reported 5-year survival rates of approximately 60% in a cohort of well-selected CRC patients (e.g., KRAS wild-type, stable disease). More recent studies have reported even higher survival rates, with Ros et al[33] describing 5-year survival of up to 83% in highly selected cases. These survival outcomes surpass those achieved with standard systemic therapies. Ongoing trials, including SECA-II and Translational Research on Neoadjuvant Strategy for METastatic (TRANSMET) CRC are refining patient selection criteria, incorporating biomarkers such as tumor genetics and ctDNA to identify patients with indolent disease who are most likely to benefit[33].
Transplantation for other liver metastases (e.g., NETs, cholangiocarcinoma) is much less common but is being investigated in select cases, typically when the liver is the dominant disease site, with strict exclusion of extrahepatic disease and strict tumor burden criteria[78].
Modern perioperative strategies are critical for optimizing outcomes. Patients undergoing liver surgery require thorough preoperative assessment, including nutritional optimization, liver function evaluation, and volumetric assessment of the liver. Postoperative care is increasingly standardized through enhanced recovery protocols–such as early feeding and mobilization–that reduce complications and accelerate recovery. Post-resection management is individualized based on pathology results, and systemic therapies are often integrated into care plans. Ultimately, the optimal management of liver metastases relies on multidisciplinary decision-making, balancing oncologic outcomes with surgical risk and patient-specific factors[33].
For patients who are not candidates for surgery–or as an adjunct to resection-locoregional therapies offer targeted options to control liver metastases directly.
Thermal ablation methods such as radiofrequency ablation (RFA) and microwave ablation use heat to destroy tumors, delivered either percutaneously or during surgery. RFA is most commonly applied to small (< 3 cm) CRLM. Compared to surgery, RFA is less invasive and carries a lower risk of complications. However, multiple meta-analyses have shown that RFA is associated with higher local and distant recurrence rates and reduced long-term survival compared to surgical resection. For instance, a study by van Amerongen et al[80] found that RFA led to significantly higher rates of local recurrence and lower 5-year disease-free and overall survival than surgery. Nevertheless, in patients with unresectable oligometastases or those who are poor surgical candidates, RFA and microwave ablation remain valuable options for achieving local tumor control with minimal invasiveness. These methods are often combined with systemic therapies. Cryoablation, which uses extreme cold to induce tumor necrosis, is an alternative technique but is less commonly employed due to limited evidence of efficacy[81].
TACE delivers chemotherapy (e.g., doxorubicin or cisplatin) directly into the hepatic artery, along with embolic particles that block blood flow, inducing ischemic tumor necrosis. While TACE is standard treatment for HCC, it is also used for liver metastases-especially from NETs.
Transarterial radioembolization (TARE), also known as selective internal radiation therapy, involves injecting Yttrium-90–loaded microspheres into the liver to deliver targeted radiation. TARE has demonstrated effectiveness in both colorectal and neuroendocrine liver metastases. A systematic review of neuroendocrine liver metastases found comparable outcomes for TACE and TARE in terms of overall survival, progression-free survival, and radiographic and symptom response.
In patients with CRLM, TACE and TARE are typically used as salvage therapy when systemic treatments have failed. Data from a large registry (n = 498) showed a median survival of approximately 15 months after Y-90 radioembolization for metastatic CRC[82].
Stereotactic body radiation therapy (SBRT) delivers precise, high-dose radiation in a few focused treatments. It is particularly useful for small tumors (usually ≤ 5 cm) that are unsuitable for surgery or ablation–especially those located near critical structures like blood vessels or bile ducts. SBRT achieves excellent local control rates, with meta-analyses reporting 1-year and 2-year local control rates of 70%–90%. SBRT is noninvasive, repeatable for new lesions, and offers a valuable option for selected patients. However, its limitations include the risk of radiation–induced liver toxicity, particularly when large volumes are treated, and the need for precise targeting to minimize harm to surrounding tissues[83].
HAI therapy uses an implanted pump to deliver high concentrations of chemotherapy (e.g., floxuridine, oxaliplatin) directly into the liver via the hepatic artery, achieving drug levels far higher than systemic routes. HAI has been employed as an adjuvant therapy after liver resection, where it has shown benefits in improving hepatic recurrence–free survival and as salvage therapy in patients with unresectable CRLM. Response rates of 40%–50% have been reported even in heavily pretreated, chemotherapy–refractory CRC cases. The main limitations of HAI are technical complications such as catheter dysfunction and risks of biliary toxicity[84].
Other investigational approaches include non-thermal ablation methods such as irreversible electroporation and electrochemotherapy, as well as high-intensity focused US. Some intraoperative methods–like intra-arterial chemotherapy delivered during open surgery and photodynamic therapy–are also being explored in select clinical settings, although their roles remain limited[70,85-88].
Locoregional therapies primarily aim to prolong disease control and alleviate symptoms in patients with liver-dominant disease. For example, Tsilimigras et al[16] have noted that TACE and TARE contribute to improved survival in patients with unresectable disease. The choice of therapy depends on multiple factors, including tumor type, size, distribution, and prior treatments. Importantly, optimal use of these therapies requires coordination within a multidisciplinary care team to ensure the best outcomes for each patient[16,80,81].
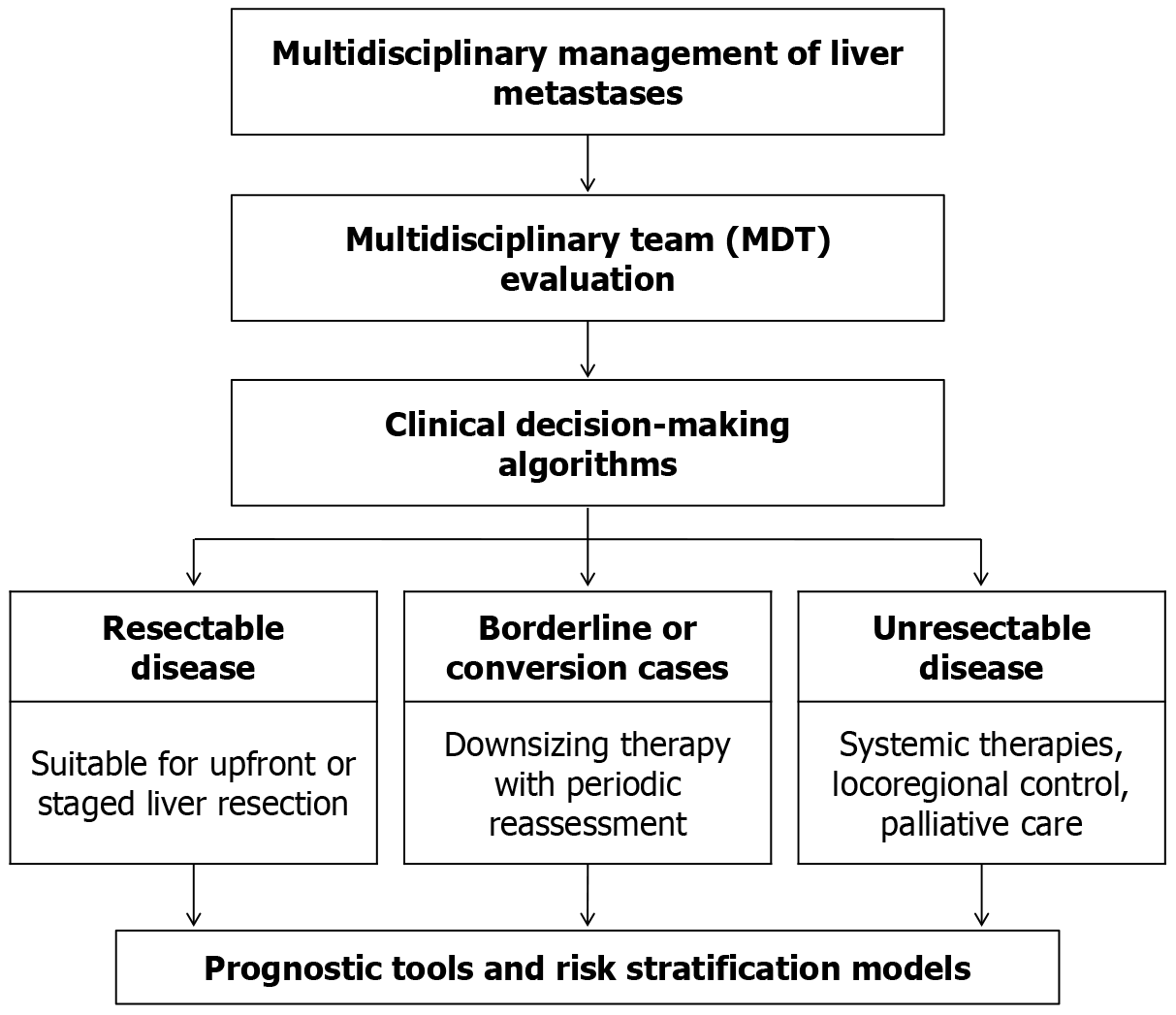
Given the complexity of managing liver metastases, a multidisciplinary team (MDT) approach is essential to ensure the best possible outcomes. Effective care requires close collaboration among hepatobiliary surgeons, medical oncologists, diagnostic and interventional radiologists, pathologists, radiation oncologists, and specialized nursing and support staff. MDT discussions involve a comprehensive review of each case, integrating imaging findings, pathology reports, and individual patient factors to tailor a personalized treatment strategy[89].
Patients are typically stratified into three broad categories within clinical decision-making algorithms: (1) Respectable disease: Patients suitable for upfront or staged liver resection, either directly or following a short course of neoadjuvant therapy; (2) Borderline or conversion cases: Patients who are not initially respectable but may become candidates for surgery after downsizing therapy (such as systemic chemotherapy or PVE). These cases require close monitoring and periodic reassessment to evaluate treatment response and surgical potential; and (3) Unrespectable disease: Patients with extensive or multifocal disease that cannot be safely removed with curative intent. For these individuals, the focus shifts toward systemic therapies, locoregional control, and palliative care to manage symptoms and prolong survival.
For instance, in CRC liver metastases, international guidelines such as European Society for Medical Oncology and by the National Comprehensive Cancer Network recommend that all patients undergo evaluation by an MDT. Patients with technically respectable, liver-limited disease should be offered surgery, often after a short course (2–3 months) of induction chemotherapy. In contrast, patients with initially unrespectable, liver–only metastases should receive aggressive systemic therapy aimed at converting them to respectable status, followed by re-evaluation for potential surgery or local ablation. For patients with widespread extrahepatic disease, curative approaches are typically not feasible; here, the emphasis is on extending survival with systemic therapy, supplemented by locoregional treatments as appropriate. Similar principles guide management for other malignancies with liver involvement, such as breast or lung cancer[78,89].
Prognostic tools and risk stratification models further guide treatment decisions. For CRC, the widely used Fong Clinical Risk Score incorporates factors such as the number of liver metastases, CEA level, and nodal status to predict the likelihood of recurrence after resection. Other tools, like the Nordlinger and Genetic and Morphological Evaluation (GAME) Score, provide additional risk assessments to help identify patients who might benefit most from aggressive surgical or multimodal interventions. The integration of molecular biomarkers (e.g., RAS/RAF status, MSI, ctDNA) further refines risk assessment and helps determine whether neoadjuvant therapy should precede surgery in high-risk cases[78,89].
Ultimately, a multidisciplinary, personalized approach (Figure 3) is a key to optimizing outcomes. As highlighted by De Greef et al[78] management decisions for liver metastases should be based on a combination of clinical findings, imaging, pathology, and molecular data. Collaborative, team-based decision–making ensures that patients receive the most appropriate treatment strategy–balancing the benefits of surgical intervention with the need for systemic and locoregional therapies. This strategy has led to more precise patient selection for surgery, better integration of emerging treatments, and ultimately, improved survival outcomes.
Accurate prognosis is fundamental to guiding treatment decisions in patients with liver metastases. Several prognostic tools and models have been developed to estimate outcomes and help tailor therapy to individual patients.
The Fong Clinical Risk Score (1999) remains a widely used tool for CRC liver metastases. It incorporates five factors–CEA > 200 ng/mL, disease-free interval < 12 months, node-positive primary, presence of multiple liver metastases, and largest lesion > 5 cm–to stratify patients into low-risk and high-risk categories. Patients with fewer risk factors tend to have significantly better long-term survival compared to those with multiple risk factors. Building on the Fong model, other scores such as the Nordlinger and GAME scores have integrated additional variables like KRAS mutation status, tumor histology, and other clinical parameters to improve predictive accuracy. Studies consistently demonstrate that these scores correlate with survival outcomes: For instance, low-risk patients may achieve 5-year survival rates of 60%–70%, while high-risk patients typically have survival rates below 30%.
More complex multivariate nomograms have been developed to generate individualized survival predictions. These models combine multiple factors–tumor size and number, CEA levels, response to chemotherapy, molecular markers, and more–to provide tailored risk estimates for each patient.
The application of AI and machine learning is an emerging frontier in prognostication. These models use large datasets–often incorporating imaging data (e.g., radiomics) and genomic profiles–to predict survival and treatment response. For example, AI algorithms analyzing CT imaging features have shown potential in stratifying CRC liver metastasis patients. However, such tools are still in development and not yet widely adopted in clinical practice.
Molecular markers increasingly inform prognostic assessment. For instance, CRC liver metastases harboring BRAFV600E mutations have an extremely poor outlook, with 5-year survival rates below 5%, even after resection. Similarly, ctDNA is a promising biomarker: The presence of detectable ctDNA after surgery is associated with a nearly threefold increased risk of recurrence. These molecular insights are progressively being integrated into clinical decision–making and predictive models[23,90].
In real-world practice, MDTs synthesize information from multiple sources–Clinical Risk Scores, imaging (extent and distribution of disease), molecular profiles, and treatment response–to estimate prognosis and guide therapy. Survival benchmarks are well-documented: Untreated CRC liver metastases have a median survival under 12 months, while resected cases achieve median survival of 40–60 months. Prognostic models help facilitate discussions with patients and families, guide the use of adjuvant therapies (e.g., offering more aggressive regimens to high-risk patients), and inform clinical trial design by enabling appropriate stratification[23,90].
While survival outcomes remain a primary goal in managing liver metastases, the impact of treatment on patients' quality of life (QoL) is equally critical. Interventions such as surgery, systemic chemotherapy, and locoregional therapies often carry significant side effects–including fatigue, pain, gastrointestinal disturbances, and neuropathy–that can affect daily functioning and long-term well-being. Although studies focusing specifically on patient-reported outcomes in individuals with liver metastases are relatively limited, the available data offer important insights. For instance, a study by Rees et al[91] assessed long-term survivors of metastatic CRC who had undergone liver resection, using validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ). Remarkably, these patients reported high overall QoL and functional status, with near-normal scores in emotional, social, and role functioning. Persistent severe symptoms, such as diarrhea or constipation, were uncommon–affecting no more than 10% of patients. A related study observed that although fatigue and pain were more pronounced in the immediate postoperative period, most symptoms returned to baseline within 12 months. These findings suggest that for many patients, the long-term impact of liver surgery and chemotherapy on QoL is minimal, and most survivors can expect a return to pre-treatment levels of functioning[91,92].
However, some studies highlight specific challenges. For example, sexual dysfunction has been reported in approximately one-third of patients following liver resection for CRC metastases. Chronic neurotoxicity–often related to oxaliplatin–and diarrhea from chemotherapy can persist in a smaller subset of patients, underscoring the need for tailored supportive care strategies. For patients with unrespectable disease, the literature on QoL is more limited, but management priorities shift toward symptom control–particularly addressing pain, fatigue, and psychosocial distress. In these cases, patient preferences must play a central role in guiding treatment intensity, as some individuals may choose less aggressive therapy to maintain QoL. In clinical practice, systematic monitoring of QoL–using validated tools like the EORTC QLQ–Core 30–should be integrated into the care of patients with liver metastases, both during and after treatment. Importantly, discussions around treatment choices should incorporate realistic expectations about QoL trajectories. Encouragingly, evidence suggests that for many patients, curative–intent liver metastasis surgery does not result in long-term QoL compromise. This supports the continued use of aggressive treatment approaches in app
The treatment of liver metastases represents a significant financial challenge due to the complexity of therapies and the often–prolonged nature of care. Direct healthcare expenditures encompass surgical procedures, hospital admissions, systemic chemotherapy, advanced imaging, interventional treatments, and the management of treatment–related complications. For CRC liver metastases, a United Kingdom–based cost-effectiveness analysis demonstrated that hepatic resection yields approximately 1.6 additional life-years at an incremental cost of £6742–equating to roughly £5236 per life-year gained–a number considered highly cost-effective by standard benchmarks. When longer survival projections are included, the cost per life-year gained drops further to about £1821. Even accounting for a subset of palliative resections (approximately 17%), the cost per life-year remains under £6000, reinforcing hepatic surgery as a highly economical option compared to non-surgical treatments[74,78,90].
Nevertheless, these cost estimates focus primarily on surgical intervention. The financial burden of systemic therapies, especially targeted agents and immunotherapies, is substantial. For example, combination biologic regimens for metastatic CRC or breast cancer may exceed annual costs of $100000. Patients frequently require multiple lines of che
Beyond direct medical expenses, indirect costs such as lost productivity, travel expenditures, and caregiver time contribute to the overall economic impact, though specific data on liver metastasis populations are scarce. Broader analyses indicate that metastatic CRC accounts for a large proportion of total colon cancer care costs, largely due to ongoing systemic treatment and supportive care requirements[74].
Healthcare resource utilization in this patient group is intensive, involving frequent imaging, laboratory monitoring, outpatient visits, and MDT consultations. Comparative data suggest that while patients undergoing resection face higher upfront costs due to surgery, they often experience prolonged survival and reduced long-term healthcare needs relative to those with unrespectable disease, who remain on continuous systemic therapy[93].
In summary, prioritizing effective, potentially curative interventions such as surgical resection may ultimately lower long-term healthcare expenditures by decreasing the need for subsequent therapies and improving survival. Cost-effectiveness analyses support investment in curative-intent multimodal care for appropriately selected patients. However, the financial toxicity experienced by patients–through out-of-pocket costs and lost income–must also be addressed, emphasizing the importance of cost-aware clinical decision–making and healthcare policies that promote equitable access to high-value treatments.
Liver metastases are relatively rare in pediatric oncology but present unique clinical challenges when they do occur. Childhood cancers known to spread to the liver include neuroblastoma, Wilms’ tumor, certain sarcomas such as rhabdomyosarcoma, and hepatoblastoma–a primary liver malignancy that can sometimes also metastasize to the lungs or liver. In neuroblastoma, one of the most common childhood tumors, liver involvement is frequently seen in infants under two years old, often as part of stage 4S or advanced disease. Treatment primarily relies on chemotherapy, with occasional use of liver–directed radiotherapy, since surgery is rarely curative in this setting. For metastatic Wilms’ tumor, lung metastases are more common and usually resected, but isolated liver metastases can also occur and may be surgically removed following chemotherapy[93,94].
Treatment strategies in pediatric patients largely parallel those used in adults, with chemotherapy as the initial approach for metastatic disease. Surgery and radiation are applied selectively, carefully weighing the benefits against potential long-term effects, such as damage to the developing liver. While children often tolerate intensive chemotherapy well, long-term survivors require ongoing monitoring for late treatment–related effects. Preserving QoL–including growth and organ function–is a critical concern in this population[93,95,96].
Among rare adult cancers, uveal (ocular) melanoma is notable for metastasizing almost exclusively to the liver, carrying a poor prognosis with median survival typically between six months and twelve months. Treatment options remain limited; localized interventions like surgical resection or ablation primarily offer symptom relief, while systemic immunotherapy shows modest response rates. Emerging therapies, including isolated hepatic perfusion and novel agents such as tebentafusp–a bispecific T-cell engager–are under active investigation in this difficult-to-treat disease[85,97].
Other uncommon tumors–such as adrenocortical carcinoma, Merkel cell carcinoma, and meningioma–may sporadically spread to the liver. Due to their rarity, these cases lack standardized management protocols, and treatment decisions are often guided by approaches used in more common metastatic cancers, including chemotherapy and tar
In summary, managing liver metastases from pediatric and rare tumors requires specialized expertise and often depends on multidisciplinary care teams experienced in these diseases. Enrollment in clinical trials is strongly encouraged to advance understanding and improve outcomes given the limited evidence base.
Advancements in AI and quantitative imaging analysis, known as radiomics, are set to transform the management of liver metastases. Radiomics involves extracting detailed quantitative features–such as texture, shape, and intensity–from CT or MRI scans that may reveal underlying tumor biology. AI and machine learning algorithms can process these complex data to assist in diagnosis, predict genetic mutations, or forecast patient outcomes. For example, recent research has demonstrated that CT–based radiomic patterns can correlate with KRAS mutation status or tumor microenvironment traits in CRLM, enabling noninvasive biomarker evaluation. Similarly, MRI radiomics has been explored for distinguishing desmoplastic vs replacement tumor growth patterns, which can influence treatment planning[21,98,99].
Deep learning methods also improve lesion detection and segmentation on imaging, potentially enhancing radiologists’ accuracy. Convolutional neural networks trained on extensive imaging datasets can identify subtle metastases that might otherwise be missed. AI can further standardize lesion size measurements and volumetric analysis, improving the consistency of treatment response assessments[98,100].
In the field of interventional radiology, fusion imaging techniques–which combine real-time US with pre-existing CT or MRI data–represent a sophisticated form of image guidance. For instance, electromagnetic-tracking fusion systems enhance precise lesion targeting during ablation or biopsy procedures. While not purely AI-driven, these hybrid technologies exemplify the growing role of advanced tools to assist image-guided interventions[98,100].
The emerging discipline of radiogenomics, which links imaging characteristics with genomic data, aims toward a “virtual biopsy” concept–where imaging could noninvasively predict molecular features like MSI and guide personalized therapies. Though still under investigation, radiomics and AI show great potential for early detection of recurrence, forecasting therapeutic response, and tailoring treatment strategies to individual patients[101,102].
Challenges remain, including the need for large, validated datasets, reproducibility of results, and seamless integration into clinical practice. Nonetheless, the future integration of AI–powered radiomic analysis with traditional imaging holds significant promise to enhance the precision and effectiveness of liver metastasis care.
Functional precision oncology is rapidly progressing, with increasing emphasis on patient-derived experimental systems such as patient–derived organoids (PDOs) and patient–derived xenografts (PDXs) that offer promising avenues for individualized cancer treatment. PDOs are three–dimensional cultures established from patient-derived tumor specimens–including liver metastases–that preserve key histopathological and genomic characteristics of the original malignancy. These models exhibit a robust establishment success rate of around 70%, and can typically be propagated within a few weeks post-biopsy, allowing for timely deployment in experimental workflows. Applications include high-throughput drug sensitivity screening, phenotypic characterization, and identification of resistance patterns[103,104]. For example, Bruun et al[105] developed PDOs from multiple CRC liver metastases and conducted comparative drug testing, uncovering substantial heterogeneity in therapeutic response even among metastases from a single patient–highlighting the complex biological diversity of metastatic CRC. Reinforcing this, recent findings reported in ESMO Open (2023) demonstrate that organoids derived from CRC liver lesions retain clinically relevant features and can accurately reflect resistance profiles observed in the clinical setting[105].
PDX models complement PDOs by offering an in vivo context through the implantation of human tumor tissue into immunocompromised murine hosts. These models enable the assessment of drug response, clonal dynamics, and biomarker modulation under physiologically relevant conditions. In one illustrative study, Jung et al[106] established PDXs from CRC liver metastases and treated them with various cytotoxic agents including doxorubicin, cisplatin, and docetaxel, with observed responses mirroring those of the patient’s tumor, thus validating their translational fidelity. Although PDX models require longer development timelines (ranging from 2 months to 6 months) and incur higher operational costs, they remain instrumental in preclinical pharmacodynamic evaluations and in vivo modeling of therapeutic regimens[106,107].
Despite their utility, both PDO and PDX systems face limitations that hinder routine clinical adoption. These include high infrastructure requirements, technical complexity, and a lack of standardized methodologies across institutions, which can impact reproducibility and scalability. Nonetheless, their integration into translational oncology pipelines holds great promise. Collectively, these models enable functional drug profiling, provide insights into resistance mechanisms, and support the design of patient-specific therapeutic strategies. In the future, it is conceivable that biopsies from metastatic lesions could be rapidly converted into PDOs or PDXs to test a suite of therapies and inform precision treatment decisions in real time. While currently confined to research-intensive environments, growing evidence supports their predictive accuracy, and with ongoing improvements in workflow efficiency and protocol harmonization, these platforms are expected to play an increasingly central role in clinical trial design and precision medicine initiatives[103,104,108].
There is a dynamic and expanding body of research focused on improving the management of liver metastases, with numerous clinical trials underway across all facets of care.
Many ongoing studies are investigating new systemic therapies for metastatic liver disease. In CRC metastases, trials are testing immunotherapy combinations in MSS tumors (e.g., adding PD-1 inhibitors to VEGF-targeting agents), novel KRAS inhibitors, and anti-HER2 therapies for HER2-positive cases. For breast cancer liver metastases, trials are evaluating CDK4/6 inhibitors, PI3K inhibitors, and emerging antibody-drug conjugates. There are also active trials for immunotherapy and targeted therapies in NETs and ocular melanoma liver metastases. Importantly, most of these studies include integrated biomarker analyses[12,33].
Randomized trials are assessing the best use of liver-directed therapies, such as comparing ablation, SBRT, and surgery. For instance, the SABR-COMET trial and other oligometastatic studies (though not specific to liver alone) have demonstrated improved survival with aggressive local treatments. A German study currently recruiting patients is comparing perioperative radioembolization plus chemotherapy vs chemotherapy alone for unrespectable CRC liver metastases. Other trials are investigating TARE in combination with systemic agents[12,33].
Studies such as TRANSMET and SECA-II are evaluating liver transplantation vs chemotherapy for patients with unrespectable CRC metastases, employing stringent selection criteria. Additionally, a ClinicalTrials.gov study (as of April 2023) is comparing surgical resection (hepatectomy) vs systemic therapy alone for breast cancer liver metastases[12,33].
Many clinical trials now incorporate translational components, including tissue and liquid biopsy analyses. As highlighted by Ros et al[33] ongoing transplant trials integrate molecular profiling and liquid biopsies to guide patient selection. This reflects a broader shift: Nearly all contemporary studies collect tumor and blood samples for next-generation sequencing, ctDNA analysis, and immunophenotyping to discover predictive biomarkers and improve therapy selection[12,33].
Early-phase studies are exploring cutting-edge strategies such as circulating tumor cell-targeting therapies, oncolytic viruses, cancer vaccines, and CAR-T cell therapies targeting antigens like CEA and glypican-3. Organoid-based drug screening is progressing toward “N-of-1” trials, where organoid responses directly inform individual treatment decisions. AI tools are also being piloted to predict recurrence risk and guide imaging surveillance[12,33].
In summary, the research landscape for liver metastases is both diverse and promising. As Ros et al[33] emphasize, current CRC liver metastasis trials aim to integrate clinical data with molecular insights to refine treatment strategies and improve outcomes. Similar translational research efforts are ongoing across various tumor types. Collectively, these studies are investigating every aspect of liver metastasis care–ranging from systemic and locoregional therapies to surgical interventions and biomarker discovery–working toward more personalized and effective treatment paradigms.
Looking ahead, the management of liver metastases is poised to become increasingly tailored through personalized medicine approaches. Several key areas are expected to shape future care.
The routine application of broad genomic and transcriptomic analyses–both from tumor tissue and liquid biopsies–will likely become standard at the time of metastatic diagnosis. This would allow the identification of actionable mutations, mutational burden, clonal dynamics, and potentially novel targets, extending beyond currently established biomarkers to include whole-exome sequencing and RNA profiling[16,33].
Serial ctDNA assessments are anticipated to play a critical role in tracking tumor evolution, detecting MRD, and identifying emerging resistance. This real-time feedback could enable early intervention, such as adjusting therapies before radiologic progression is evident[16,33].
The development of advanced imaging biomarkers–such as PET tracers specific to genetic alterations or tumor microenvironment characteristics–may improve surgical and ablative targeting. Personalized radiotherapy planning, informed by individual tumor biology, is also on the horizon[16,33].
Machine learning tools may soon assist in integrating complex datasets–including imaging, genomics, and laboratory results–to guide optimal treatment strategies. AI has already demonstrated utility in imaging and pathology workflows; its integration into multidisciplinary decision-making is a logical next step[16,33].
Innovations in interventional oncology, such as next–generation ablation devices, could enable treatment plans tailored to tumor–specific features. Combining ablation with immunomodulatory strategies (e.g., checkpoint inhibitors) holds potential for synergistic effects, particularly in converting immunologically “cold” tumors into “hot” and responsive lesions[16,33].
Future approaches may better stratify which patients or metastases are most likely to benefit from immunotherapy by characterizing the tumor immune microenvironment. Coupling liver–directed therapies (e.g., radiation, ablation) with immunotherapies could further enhance anti-tumor responses[16,33].
As discussed, the use of PDOs and xenograft models to test drug responses ex vivo is gaining traction. In the future, a patient’s liver metastasis could be both molecularly profiled and pharmacologically tested to identify the most effective therapy[16,33].
Research into novel therapeutic targets–including epigenetic regulators, metabolic pathways, and molecules that disrupt the metastatic niche (e.g., anti-angiopoietin, anti-platelet agents)–is expected to broaden systemic therapy options[16,33].
The combination of multi-layered data–genomic, transcriptomic, proteomic, and even microbiome information–with clinical factors may enable sophisticated disease modeling and simulation of therapeutic responses, allowing for truly personalized treatment plans[16,33].
In essence, the future of liver metastasis management is centered on precision: (1) Understanding the molecular underpinnings of each tumor; (2) Predicting therapeutic vulnerabilities; and (3) Applying tailored interventions. As Tsilimigras et al[16] emphasized, integrating molecular data and biomarkers will provide “more accurate information for decision-making and prognosis” (nature). Similarly, Ros et al[33] highlight the critical role of liquid biopsies and molecular profiling in ongoing and future clinical trials. Together, these innovations hold the promise of transforming outcomes for patients with liver metastases. The ultimate goal is for every patient to receive the treatment most likely to control their specific disease, extending survival and improving QoL in an era of personalized, precision oncology[16,33].
Liver metastases present a formidable clinical challenge across oncology. This review has synthesized current knowledge on their epidemiology, biology, diagnosis, and treatment. Key insights include the importance of comprehensive multimodal imaging, the prognostic value of HGPs, and the central role of multidisciplinary care integrating surgery, systemic and local therapies. Modern systemic treatments–from cytotoxic chemotherapy to targeted and immune therapies–have significantly extended survival, especially when combined with aggressive local treatment. Patient-derived models and biomarker-driven trials are paving the way to truly personalized strategies. Finally, QoL and economic factors must be weighed alongside clinical efficacy. As research advances, including AI and organoid technologies, we anticipate that liver metastases will become increasingly manageable, shifting the outlook toward long-term control and cure in suitable patients.
The authors extend their appreciation to UMM Al-Qura University, Saudi Arabia for funding this research work through grant number: 25UQU4350477GSSR05.
| 1. | Stremitzer S, Vermeulen P, Graver S, Kockx M, Dirix L, Yang D, Zhang W, Stift J, Wrba F, Gruenberger T, Lenz HJ, Scherer SJ. Immune phenotype and histopathological growth pattern in patients with colorectal liver metastases. Br J Cancer. 2020;122:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Barnhill R, van Dam PJ, Vermeulen P, Champenois G, Nicolas A, Rawson RV, Wilmott JS, Thompson JF, Long GV, Cassoux N, Roman-Roman S, Busam KJ, Scolyer RA, Lazar AJ, Lugassy C. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. J Pathol Clin Res. 2020;6:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Leduc S, De Schepper M, Richard F, Maetens M, Pabba A, Borremans K, Jaekers J, Latacz E, Zels G, Bohlok A, Van Baelen K, Nguyen HL, Geukens T, Dirix L, Larsimont D, Vankerckhove S, Santos E, Oliveira RC, Dede K, Kulka J, Borbala S, Salamon F, Madaras L, Marcell Szasz A, Lucidi V, Meyer Y, Topal B, Verhoef C, Engstrand J, Moro CF, Gerling M, Bachir I, Biganzoli E, Donckier V, Floris G, Vermeulen P, Desmedt C. Histopathological growth patterns and tumor-infiltrating lymphocytes in breast cancer liver metastases. NPJ Breast Cancer. 2023;9:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JF, Evans AJ. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 661] [Article Influence: 82.6] [Reference Citation Analysis (1)] |
| 6. | Wang C, Zhang Y, Zhang Y, Li B. A bibliometric analysis of gastric cancer liver metastases: advances in mechanisms of occurrence and treatment options. Int J Surg. 2024;110:2288-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, Ran X, Xiong L, Ran Y, Chen W, Wen Y. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. 2022;7:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 8. | Fernández Moro C, Bozóky B, Gerling M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol. 2018;5:e000217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Lincke T, Zech CJ. Liver metastases: Detection and staging. Eur J Radiol. 2017;97:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Horn SR, Stoltzfus KC, Lehrer EJ, Dawson LA, Tchelebi L, Gusani NJ, Sharma NK, Chen H, Trifiletti DM, Zaorsky NG. Epidemiology of liver metastases. Cancer Epidemiol. 2020;67:101760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 11. | Maino C, Vernuccio F, Cannella R, Cortese F, Franco PN, Gaetani C, Giannini V, Inchingolo R, Ippolito D, Defeudis A, Pilato G, Tore D, Faletti R, Gatti M. Liver metastases: The role of magnetic resonance imaging. World J Gastroenterol. 2023;29:5180-5197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (3)] |
| 12. | Selvaggi F, Catalano T, Lattanzio R, Cotellese R, Aceto GM. Wingless/It/β-catenin signaling in liver metastasis from colorectal cancer: A focus on biological mechanisms and therapeutic opportunities. World J Gastroenterol. 2023;29:2764-2783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ebrahim NAA, Othman MO, Fahmy AM, Tahoun NS, Salama RA, Mahmoud NM, Elfandy H. Diagnostic and clinical challenges of primary pulmonary solid adenocarcinoma with signet-ring cell features: a case report with unusual presentation and literature review. Int Cancer Conf J. 2025;14:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Wang ZG, He ZY, Chen YY, Gao H, Du XL. Incidence and survival outcomes of secondary liver cancer: a Surveillance Epidemiology and End Results database analysis. Transl Cancer Res. 2021;10:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Williamson T, Sultanpuram N, Sendi H. The role of liver microenvironment in hepatic metastasis. Clin Transl Med. 2019;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, D'Angelica MI, Endo I, Parks RW, Doyle M, de Santibañes E, Pawlik TM. Liver metastases. Nat Rev Dis Primers. 2021;7:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 369] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 17. | Sankar K, Pearson AN, Worlikar T, Perricone MD, Holcomb EA, Mendiratta-Lala M, Xu Z, Bhowmick N, Green MD. Impact of immune tolerance mechanisms on the efficacy of immunotherapy in primary and secondary liver cancers. Transl Gastroenterol Hepatol. 2023;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 18. | van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, Majeed AW, Eefsen RL, Coebergh van den Braak RRJ, Lazaris A, Fernandez MC, Galjart B, Laerum OD, Rayes R, Grünhagen DJ, Van de Paer M, Sucaet Y, Mudhar HS, Schvimer M, Nyström H, Kockx M, Bird NC, Vidal-Vanaclocha F, Metrakos P, Simoneau E, Verhoef C, Dirix LY, Van Laere S, Gao ZH, Brodt P, Reynolds AR, Vermeulen PB. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117:1427-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 19. | Bohlok A, Vermeulen P, Leduc S, Latacz E, Botzenhart L, Richard F, De Schepper M, Geukens T, Lucidi V, Ignatiadis M, Aftimos P, Sotiriou C, Piccart M, Hendlisz A, Van Laere S, Dirix L, Noël JC, Biganzoli E, Larsimont D, Desmedt C, Donckier V. Association between the histopathological growth patterns of liver metastases and survival after hepatic surgery in breast cancer patients. NPJ Breast Cancer. 2020;6:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Ichikawa S, Goshima S. Gadoxetic Acid-Enhanced Liver MRI: Everything You Need to Know. Invest Radiol. 2024;59:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Ebrahim NAA, Soliman SMA. Innovations in Functional Materials and Advanced Imaging Techniques for Targeting Extramural Venous Invasion (EMVI) in Colorectal Cancer: A Comprehensive Review. Biomed Mater Devices. 2025;4:261-272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Freitas PS, Janicas C, Veiga J, Matos AP, Herédia V, Ramalho M. Imaging evaluation of the liver in oncology patients: A comparison of techniques. World J Hepatol. 2021;13:1936-1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Singnurkar A, Poon R, Metser U. Head-to-Head Comparison of the Diagnostic Performance of FDG PET/CT and FDG PET/MRI in Patients With Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2024;223:e2431519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Musa Y, Ifeorah IM, Maiyaki AS, Almustapha RM, Maisuna YA, Saleh HT, Yakubu A. Liver cell cancer surveillance practice in Nigeria: Pitfalls and future prospects. World J Hepatol. 2024;16:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Veeramachaneni H, Moazzami B, Sharif N, Qayed E, Miller LS. Correlation of liver imaging and transient elastography among patients with hepatitis C at a safety net hospital. World J Hepatol. 2025;17:105065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Wang YY, Xin ZC, Wang K. Impact of Molecular Status on Metastasectomy of Colorectal Cancer Liver Metastases. Clin Colon Rectal Surg. 2023;36:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA Jr, Donehower RC, Hirose K, Ahuja N, Pawlik TM, Choti MA. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137-4144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Diener MK, Fichtner-Feigl S. Biomarkers in colorectal liver metastases: Rising complexity and unknown clinical significance? Ann Gastroenterol Surg. 2021;5:477-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Chang H, Hu T, Hu J, Ding T, Wang Q, Cheng J. Complete response in patient with liver metastasis of HER2-positive breast cancer following therapy with margetuximab: a case report. Anticancer Drugs. 2023;34:883-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Ebrahim NAA, Soliman SMA, Othman MO, Tahoun NS. Molecular mechanisms and clinical significance of perineural invasion in malignancies: the pivotal role of tumor-associated Schwann cells in cancer progression and metastasis. Med Oncol. 2025;42:171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 32. | Mocan LP, Rusu I, Melincovici CS, Boșca BA, Mocan T, Crăciun R, Spârchez Z, Iacobescu M, Mihu CM. The Role of Immunohistochemistry in the Differential Diagnosis between Intrahepatic Cholangiocarcinoma, Hepatocellular Carcinoma and Liver Metastasis, as Well as Its Prognostic Value. Diagnostics (Basel). 2023;13:1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Ros J, Salva F, Dopazo C, López D, Saoudi N, Baraibar I, Charco R, Tabernero J, Elez E. Liver transplantation in metastatic colorectal cancer: are we ready for it? Br J Cancer. 2023;128:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Wullaert L, van Rees JM, Martens JWM, Verheul HMW, Grünhagen DJ, Wilting SM, Verhoef C. Circulating Tumour DNA as Biomarker for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cells. 2023;12:2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Shi J, Zhu X, Yang JB. Advances and challenges in molecular understanding, early detection, and targeted treatment of liver cancer. World J Hepatol. 2025;17:102273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Bhimani N, Wong GYM, Molloy C, Pavlakis N, Diakos CI, Clarke SJ, Dieng M, Hugh TJ. Cost of treating metastatic colorectal cancer: a systematic review. Public Health. 2022;211:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 37. | Rothenberger NJ, Stabile LP. Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers (Basel). 2017;9:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Conway JW, Braden J, Lo SN, Scolyer RA, Carlino MS, Menzies AM, Long GV, Silva IPD. VEGF Inhibitors Improve Survival Outcomes in Patients with Liver Metastases across Cancer Types-A Meta-Analysis. Cancers (Basel). 2023;15:5012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | Masłowska K, Halik PK, Tymecka D, Misicka A, Gniazdowska E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers (Basel). 2021;13:1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Kato T. Biological roles of hepatocyte growth factor-Met signaling from genetically modified animals. Biomed Rep. 2017;7:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Gungor MZ, Uysal M, Senturk S. The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers (Basel). 2022;14:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Bastien AJ. VEGFR and PDGFR: Their Targeting in Liver Cancer. In: Nagaraju GP, eritor. Role of Tyrosine Kinases in Gastrointestinal Malignancies. Berlin: Springer, 2018. [DOI] [Full Text] |
| 43. | Zou X, Tang XY, Qu ZY, Sun ZW, Ji CF, Li YJ, Guo SD. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int J Biol Macromol. 2022;202:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 44. | He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (1)] |
| 45. | Zou G, Park JI. Wnt signaling in liver regeneration, disease, and cancer. Clin Mol Hepatol. 2023;29:33-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 46. | Berasain C, Ujue Latasa M, Urtasun R, Goñi S, Elizalde M, Garcia-Irigoyen O, Azcona M, Prieto J, Avila MA. Epidermal Growth Factor Receptor (EGFR) Crosstalks in Liver Cancer. Cancers (Basel). 2011;3:2444-2461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Wang H, Yang J, Zhang K, Liu J, Li Y, Su W, Song N. Advances of Fibroblast Growth Factor/Receptor Signaling Pathway in Hepatocellular Carcinoma and its Pharmacotherapeutic Targets. Front Pharmacol. 2021;12:650388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Ruan R, Li L, Li X, Huang C, Zhang Z, Zhong H, Zeng S, Shi Q, Xia Y, Zeng Q, Wen Q, Chen J, Dai X, Xiong J, Xiang X, Lei W, Deng J. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol Cancer. 2023;22:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 49. | Fu L, Gu X, Lou N, Li J, Xue C. Current research of the Notch pathway in hepatocellular carcinoma. Eur J Med Res. 2025;30:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Lujambio A, Maina F. Turning up our understanding of liver cancer by a notch. J Hepatol. 2021;74:502-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Anastasiadou DP, Quesnel A, Duran CL, Filippou PS, Karagiannis GS. An emerging paradigm of CXCL12 involvement in the metastatic cascade. Cytokine Growth Factor Rev. 2024;75:12-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (3)] |
| 52. | Shi Y, Riese DJ 2nd, Shen J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front Pharmacol. 2020;11:574667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 53. | Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front Oncol. 2021;11:760971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 54. | Calderon Novoa F, Ardiles V, de Santibañes E, Pekolj J, Goransky J, Mazza O, Sánchez Claria R, de Santibañes M. Pushing the Limits of Surgical Resection in Colorectal Liver Metastasis: How Far Can We Go? Cancers (Basel). 2023;15:2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 55. | Qing L, Li Q, Dong Z. MUC1: An emerging target in cancer treatment and diagnosis. Bull Cancer. 2022;109:1202-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 56. | Timek DT, Shi J, Liu H, Lin F. Arginase-1, HepPar-1, and Glypican-3 are the most effective panel of markers in distinguishing hepatocellular carcinoma from metastatic tumor on fine-needle aspiration specimens. Am J Clin Pathol. 2012;138:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Fernandez-Pineda I, Cabello-Laureano R. Differential diagnosis and management of liver tumors in infants. World J Hepatol. 2014;6:486-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000-4013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (2)] |
| 60. | Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol. 2017;9:907-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (4)] |
| 61. | Cidon EU. Systemic treatment of hepatocellular carcinoma: Past, present and future. World J Hepatol. 2017;9:797-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Jiang C, Zhang ZH, Li JX. Consideration on immunotherapy of liver metastases of malignant tumors. World J Gastrointest Surg. 2024;16:2374-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 63. | Lin ZP, Hu XL, Chen D, Huang DB, Zou XG, Zhong H, Xu SX, Chen Y, Li XQ, Zhang J. Efficacy and safety of targeted therapy plus immunotherapy combined with hepatic artery infusion chemotherapy (FOLFOX) for unresectable hepatocarcinoma. World J Gastroenterol. 2024;30:2321-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (3)] |
| 64. | Han Y, Zhi WH, Xu F, Zhang CB, Huang XQ, Luo JF. Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. World J Gastroenterol. 2021;27:2415-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 65. | Wang X, Zhao S, Xin Q, Zhang Y, Wang K, Li M. Recent progress of CDK4/6 inhibitors' current practice in breast cancer. Cancer Gene Ther. 2024;31:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 66. | Patauner S, Scotton G, Notte F, Frena A. Advanced hepatocellular carcinoma treatment strategies: Are transarterial approaches leading the way? World J Gastrointest Oncol. 2025;17:99834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Liang J, Bai Y, Ha FS, Luo Y, Deng HT, Gao YT. Combining local regional therapy and systemic therapy: Expected changes in the treatment landscape of recurrent hepatocellular carcinoma. World J Gastrointest Oncol. 2023;15:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Gao YX, Ning QQ, Yang PX, Guan YY, Liu PX, Liu ML, Qiao LX, Guo XH, Yang TW, Chen DX. Recent advances in recurrent hepatocellular carcinoma therapy. World J Hepatol. 2023;15:460-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 69. | Ebrahim NAA, Hussein MA, Sobeih ME, Amin NH. From biomarker to targeted therapy: investigating trophoblast cell-surface antigen 2 expression in triple-negative breast cancer- insights from the national cancer institute. BMC Cancer. 2025;25:1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Kovács A, Iezzi R, Cellini F, Lancellotta V, Bischoff P, Carchesio F, Tagliaferri L, Kovács G, Gambacorta MA. Critical review of multidisciplinary non-surgical local interventional ablation techniques in primary or secondary liver malignancies. J Contemp Brachytherapy. 2019;11:589-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 72. | Kabir T, Syn N, Goh BKP. Current status of laparoscopic liver resection for the management of colorectal liver metastases. J Gastrointest Oncol. 2020;11:526-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Liang C, He Z, Tao Q, Tang X, Jiang L, Tu X, Liu Z, Chen H, Xie F, Zheng Y. From Conversion to Resection for Unresectable Hepatocellular Carcinoma: A Review of the Latest Strategies. J Clin Med. 2023;12:7665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Beard SM, Holmes M, Price C, Majeed AW. Hepatic resection for colorectal liver metastases: A cost-effectiveness analysis. Ann Surg. 2000;232:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M, Björnsson B. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 76. | Wong P, Vien P, Kessler J, Lafaro K, Wei A, Melstrom LG. Augmenting the Future Liver Remnant Prior to Major Hepatectomy: A Review of Options on the Menu. Ann Surg Oncol. 2025;32:5694-5709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP, Fonseca GM, Jeismann VB, Cecconello I, Herman P. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 78. | De Greef K, Rolfo C, Russo A, Chapelle T, Bronte G, Passiglia F, Coelho A, Papadimitriou K, Peeters M. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J Gastroenterol. 2016;22:7215-7225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | Patel RK, Rahman S, Schwantes IR, Bartlett A, Eil R, Farsad K, Fowler K, Goodyear SM, Hansen L, Kardosh A, Nabavizadeh N, Rocha FG, Tsikitis VL, Wong MH, Mayo SC. Updated Management of Colorectal Cancer Liver Metastases: Scientific Advances Driving Modern Therapeutic Innovations. Cell Mol Gastroenterol Hepatol. 2023;16:881-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 80. | van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Fütterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford). 2017;19:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 81. | Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK, Duckworth A, Heister P, Praseedom R, Jah A, Balakrishnan A, Harper S, Liau S, Kosmoliaptsis V, Huguet E. Colorectal liver metastases: Current management and future perspectives. World J Clin Oncol. 2020;11:761-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (10)] |
| 82. | Mheid S, Allen S, Ng SSW, Hall WA, Sanford NN, Aguilera TA, Elamir AM, Bahij R, Intven MPW, Radhakrishna G, Mohamad I, De Leon J, Tan H, Lewis S, Gani C, Stanecu T, Dell'Acqua V, Hosni A. Local Control Following Stereotactic Body Radiation Therapy for Liver Oligometastases: Lessons from a Quarter Century. Curr Oncol. 2023;30:9230-9243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 83. | Emmons EC, Bishay S, Du L, Krebs H, Gandhi RT, Collins ZS, O'Hara R, Akhter NM, Wang EA, Grilli C, Brower JS, Peck SR, Petroziello M, Abdel Aal AK, Golzarian J, Kennedy AS, Matsuoka L, Sze DY, Brown DB. Survival and Toxicities after (90)Y Transarterial Radioembolization of Metastatic Colorectal Cancer in the RESIN Registry. Radiology. 2022;305:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Victory Srinivasan N, Venugopal S. A Comparison of the Outcomes of Transarterial Chemoembolization and Transarterial Radioembolization in the Management of Neuroendocrine Liver Metastases in Adults: A Systematic Review. Cureus. 2023;15:e40592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Obiorah IE, Ozdemirli M. Incidental Metastatic Meningioma Presenting as a Large Liver Mass. Case Reports Hepatol. 2018;2018:1089394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | de Senneville BD, Moonen C, Ries M. MRI-Guided HIFU Methods for the Ablation of Liver and Renal Cancers. Adv Exp Med Biol. 2016;880:43-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 87. | Robinson TP, Pebror T, Krosin ME, Koniaris LG. Ablative Therapy in Non-HCC Liver Malignancy. Cancers (Basel). 2023;15:1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 88. | Adnane F, Soliman SMA, ElZayat E, Abdelsalam EM, Fahmy HM. Evaluation of chlorophyll-loaded mesoporous silica nanoparticles for photodynamic therapy on cancer cell lines. Lasers Med Sci. 2024;39:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Li H, Gu GL, Li SY, Yan Y, Hu SD, Fu Z, Du XH. Multidisciplinary discussion and management of synchronous colorectal liver metastases: A single center study in China. World J Gastrointest Oncol. 2023;15:1616-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 90. | Montalti R, Pepe F, Cassese G, Russo G, Malapelle U, Carlomagno C, Giglio MC, Falco G, Alagia M, De Simone G, Geboes K, Troncone G, Troisi RI, Rompianesi G. Prognostic role of postoperative persistence of ctDNA molecular signature after liver resection for colorectal liver metastases: Preliminary results from a prospective study. Eur J Surg Oncol. 2025;51:109706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Rees JR, Blazeby JM, Brookes ST, John T, Welsh FK, Rees M. Patient-reported outcomes in long-term survivors of metastatic colorectal cancer needing liver resection. Br J Surg. 2014;101:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Ebrahim NAA, Soliman SMA, Othman MO, Salama RA, Tahoun NS. Nanotechnology in Neuro-Oncology: Evaluating the Potential of Graphene and Boron Nitride Nanostructures. Biomed Mater Devices. 2025;3:691-701. [DOI] [Full Text] |
| 93. | Kaštelan S, Mrazovac Zimak D, Ivanković M, Marković I, Gverović Antunica A. Liver metastasis in uveal melanoma - treatment options and clinical outcome. Front Biosci (Landmark Ed). 2022;27:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Ebrahim NAA, Soliman SMA. Advances in Stem Cell Integration and Calcium Hydroxyapatite Utilization in 3D-Printed Scaffolds for Pediatric Bone Repair Following Conservative Surgery. Biomed Mater Devices. 2025;. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 95. | Ehrlich PF, Ferrer FA, Ritchey ML, Anderson JR, Green DM, Grundy PE, Dome JS, Kalapurakal JA, Perlman EJ, Shamberger RC. Hepatic metastasis at diagnosis in patients with Wilms tumor is not an independent adverse prognostic factor for stage IV Wilms tumor: a report from the Children's Oncology Group/National Wilms Tumor Study Group. Ann Surg. 2009;250:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Ebrahim NAA, Othman MO, Salama RA, Abdelfatah D, Tahoun NS. Diagnostic insights into solid pseudopapillary neoplasms of the pancreas: a decade of experience with pediatric representation. Diagn Pathol. 2025;20:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 97. | McDonald HG, Sarvestani AL, Owen JW, Rueckert J, Wierman ME, Kiseljak-Vassiliades K, Pandalai PK, Ellis CS, Patel RA, Del Rivero J, Hernandez JM, Cavnar MJ. Response of adrenocortical carcinoma liver metastases to hepatic artery infusion floxuridine. HPB (Oxford). 2023;25:1446-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Bodalal Z, Trebeschi S, Nguyen-Kim TDL, Schats W, Beets-Tan R. Radiogenomics: bridging imaging and genomics. Abdom Radiol (NY). 2019;44:1960-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 99. | Ebrahim NAA, Soliman SMA. Advanced Biomaterials and Biomedical Devices for Studying Tumor-Associated Fibroblasts: Current Trends, Innovations, and Future Prospects. Biomed Mater Devices. 2025;4:287-301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 100. | Zhou J, Wang W, Lei B, Ge W, Huang Y, Zhang L, Yan Y, Zhou D, Ding Y, Wu J, Wang W. Automatic Detection and Classification of Focal Liver Lesions Based on Deep Convolutional Neural Networks: A Preliminary Study. Front Oncol. 2020;10:581210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 101. | Ying H, Liu X, Zhang M, Ren Y, Zhen S, Wang X, Liu B, Hu P, Duan L, Cai M, Jiang M, Cheng X, Gong X, Jiang H, Jiang J, Zheng J, Zhu K, Zhou W, Lu B, Zhou H, Shen Y, Du J, Ying M, Hong Q, Mo J, Li J, Ye G, Zhang S, Hu H, Sun J, Liu H, Li Y, Xu X, Bai H, Wang S, Cheng X, Xu X, Jiao L, Yu R, Lau WY, Yu Y, Cai X. A multicenter clinical AI system study for detection and diagnosis of focal liver lesions. Nat Commun. 2024;15:1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 102. | Li WH, Wang S, Liu Y, Wang XF, Wang YF, Chai RM. Differentiation of histopathological growth patterns of colorectal liver metastases by MRI features. Quant Imaging Med Surg. 2022;12:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Liu L, Yu L, Li Z, Li W, Huang W. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J Transl Med. 2021;19:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 104. | Wang E, Xiang K, Zhang Y, Wang XF. Patient-derived organoids (PDOs) and PDO-derived xenografts (PDOXs): New opportunities in establishing faithful pre-clinical cancer models. J Natl Cancer Cent. 2022;2:263-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 105. | Bruun J, Kryeziu K, Eide PW, Moosavi SH, Eilertsen IA, Langerud J, Røsok B, Totland MZ, Brunsell TH, Pellinen T, Saarela J, Bergsland CH, Palmer HG, Brudvik KW, Guren T, Dienstmann R, Guren MG, Nesbakken A, Bjørnbeth BA, Sveen A, Lothe RA. Patient-Derived Organoids from Multiple Colorectal Cancer Liver Metastases Reveal Moderate Intra-patient Pharmacotranscriptomic Heterogeneity. Clin Cancer Res. 2020;26:4107-4119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 106. | Jung J, Kim J, Lim HK, Kim KM, Lee YS, Park JS, Yoon DS. Establishing a colorectal cancer liver metastasis patient-derived tumor xenograft model for the evaluation of personalized chemotherapy. Ann Surg Treat Res. 2017;93:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Gao J, Lan J, Liao H, Yang F, Qiu P, Jin F, Wang S, Shen L, Chao T, Zhang C, Zhu Y. Promising preclinical patient-derived organoid (PDO) and xenograft (PDX) models in upper gastrointestinal cancers: progress and challenges. BMC Cancer. 2023;23:1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 108. | Hedayat S, Valeri N. Patient-Derived Organoids: Promises, Hurdles and Potential Clinical Applications. Clin Oncol (R Coll Radiol). 2020;32:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/