Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110029
Revised: July 29, 2025
Accepted: September 29, 2025
Published online: October 27, 2025
Processing time: 149 Days and 1.5 Hours
Synchronous double primary malignancies of the gallbladder and liver are exceedingly rare clinically and prone to misdiagnosis as metastatic lesions. Due to anatomic contiguity and overlapping imaging characteristics, distinguishing primary carcinomas from metastatic disease is challenging, often delaying curative-intent treatment. Current lack of consensus on management underscores the imperative to investigate their pathologic features and individualized stra
This study presents a rare case of synchronous double primary malignancies in
The critical insights obtained from this case, integrated with a review of current literature, identify the key diagnostic challenges in differentiating primary vs metastatic lesions and propose a multidisciplinary management framework.
Core Tip: The synchronous double primary malignancies of the gallbladder and liver represent an exceedingly rare clinical entity with no established management consensus. We present a distinctive case of a 70-year-old patient who achieved no evidence of disease status following multidisciplinary intervention. This case highlights the diagnostic challenges in distinguishing synchronous primaries from metastatic disease, underscores the critical role of histopathological confirmation, and demonstrates the potential for favorable outcomes through aggressive surgical intervention. Our experience provides valuable insights for managing such complex cases.
- Citation: Zhang K, Liu HL. Unusual presentation of synchronous double primary gallbladder and hepatic malignancies: A case report. World J Hepatol 2025; 17(10): 110029
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110029.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110029
Synchronous multiple primary cancers (MPCs) refer to the occurrence of two or more independently originating malignant tumors diagnosed in the same patient within a 6-month interval, posing significant challenges in clinical diagnosis and management. Synchronous double primary carcinomas of the gallbladder and liver are exceptionally rare and are frequently misdiagnosed as metastatic disease, leading to inappropriate therapeutic strategies. Studies indicate that synchronous MPCs account for approximately 2%-8% of all cancer cases, with a notable predominance in the digestive system; however, reports of synchronous gallbladder and hepatocellular carcinoma (HCC) remain exceedingly scarce[1]. This article presents a case of a 70-year-old female with moderately differentiated gallbladder adenocarcinoma coexisting with HCC, highlighting the diagnostic pitfalls and therapeutic approaches through multidisciplinary collaboration, radiological and pathological evaluation, aiming to provide valuable insights for clinical practice.
A 70-year-old female patient was admitted on July 1, 2024, with a 20-day history of abdominal distension and incidentally discovered gallbladder space-occupying lesions identified 9 days previously.
Initial symptoms included intermittent epigastric and dorsal pain without fever, chills, nausea, or vomiting. Previous ultrasound at an external hospital revealed gallbladder occupation, hepatic space-occupying lesions, cholelithiasis, and liver cirrhosis. Notable clinical features included reduced appetite, mild fatigue, a 5 kg weight loss, and normal bowel/bladder function.
The patient’s medical history included a 40-year chronic hepatitis B virus (HBV) infection without regular monitoring or antiviral therapy, 15 years of untreated cholelithiasis, and a remote hysterectomy for uterine fibroids.
The patient denies any history of tobacco use or alcohol consumption. There is no family history of similar conditions.
The patient has an Eastern Cooperative Oncology Group performance status of 1 and a numerical rating scale score of 2, with mild icterus noted in the skin and sclera. The abdomen is flat and symmetrical without abdominal wall varicosities, visible peristalsis, or intestinal patterns. On palpation, the abdomen is soft and non-tender with no rebound tenderness or palpable masses. The liver and spleen are non-palpable below the costal margin with negative Murphy’s sign. Percussion reveals the upper liver border at the 5th intercostal space on the right midclavicular line without hepatorenal tenderness or shifting dullness. Bowel sounds are normal at 4/minute with no vascular bruits auscultated.
Liver function tests showed no abnormalities: Albumin (36 g/L), total bilirubin (12.55 μmol/L), alanine aminotransferase (25 U/L), aspartate aminotransferase (33 U/L), alanine aminotransferase/aspartate aminotransferase (0.76), prothrombin time (12.0 seconds), international normalized ratio (1.11), and Child-Pugh grade A. Results of tests for tumor markers were as follows: Cancer antigen-199 227.3 U/mL (elevated), alpha-fetoprotein 5.8 ng/mL (normal), and protein induced by vitamin K absence-II 42.39 mAU/mL (elevated). Serology showed positive hepatitis B surface antigen and HBV DNA of 7.513 × 105 IU/mL.
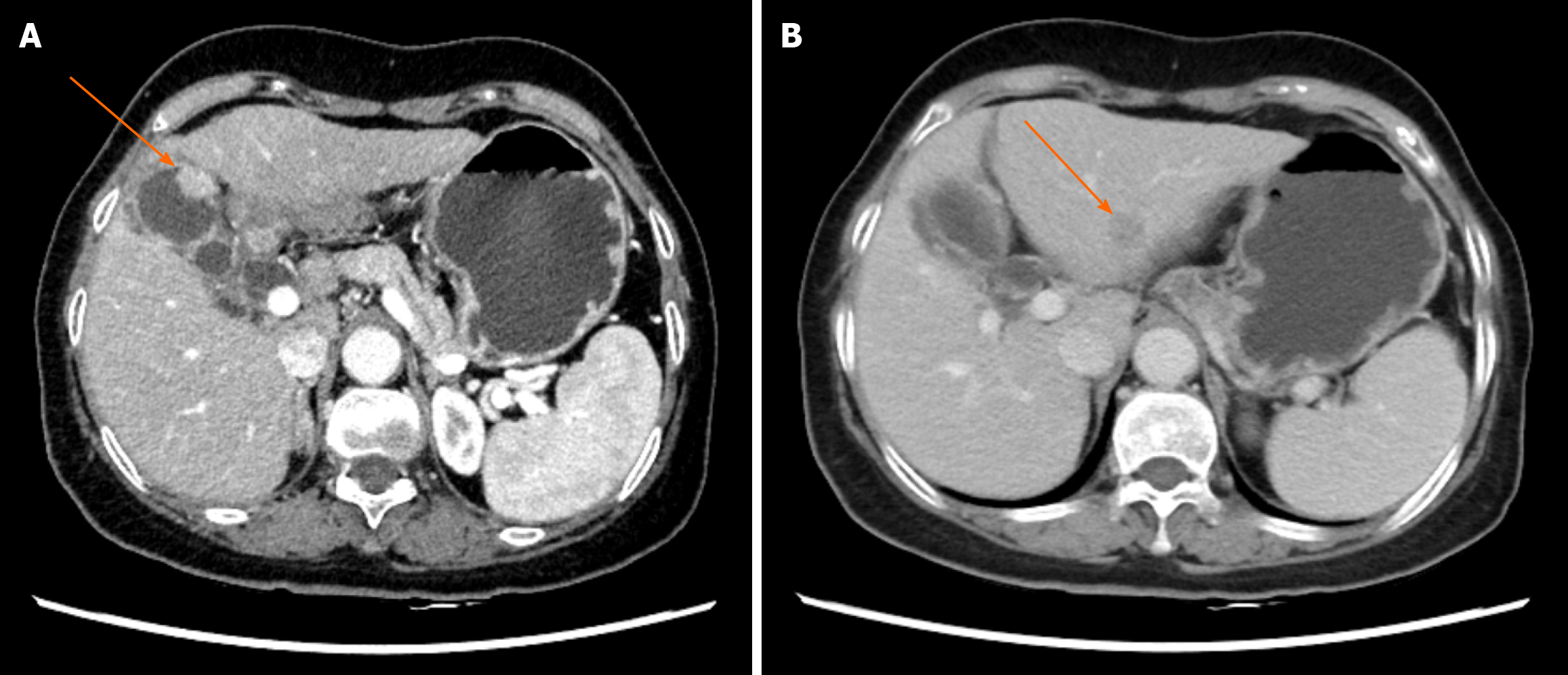
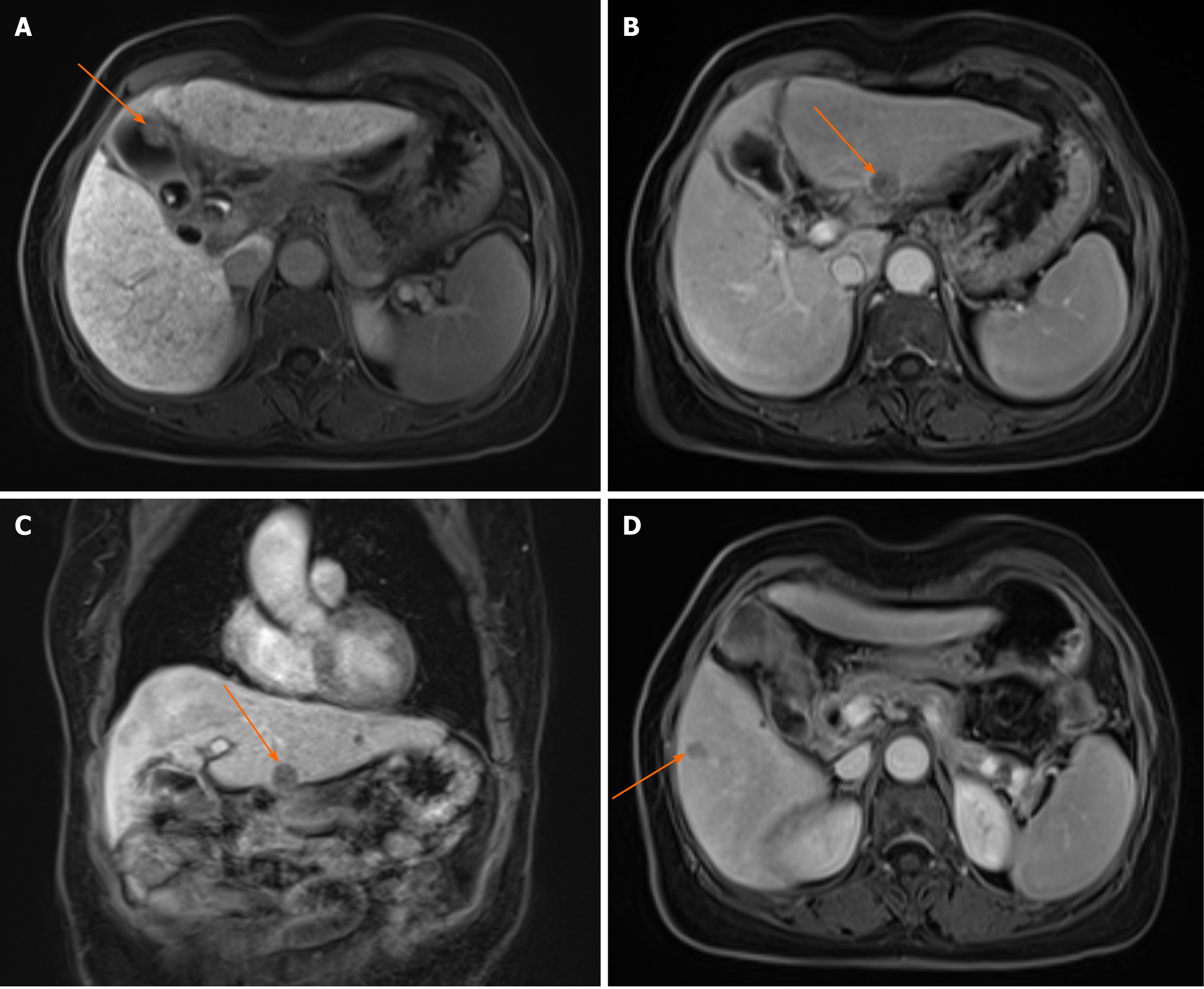
Dynamic contrast-enhanced abdominal computed tomography (CT) demonstrated irregular gallbladder wall thickening, enlarged porta hepatis lymph nodes (suspected metastasis), and multiple hypodense hepatic lesions (Figure 1). Magnetic resonance imaging with contrast revealed T1-short/T2-long nodular lesions with diffusion restriction and enhancement, strongly suggestive of gallbladder carcinoma (Figure 2A). Additional hepatic lesions showed variable enhancement patterns (Figure 2B-D), warranting differentiation between HCC and metastasis. Positron emission tomography (PET)-CT confirmed metabolically active gallbladder lesions [maximum standardized uptake value (SUVmax): 8.0] and suspicious lymph nodes (SUVmax: 5.3). A left hepatic lobe nodule (SUVmax: 3.5) remained indeterminate.
Two ultrasound-guided liver biopsies (July 5 and 9) revealed chronic active hepatitis with cirrhotic changes but no definitive malignancy. Multidisciplinary consensus recommended laparoscopic excision for histopathological con
Gallbladder adenocarcinoma and HCC.
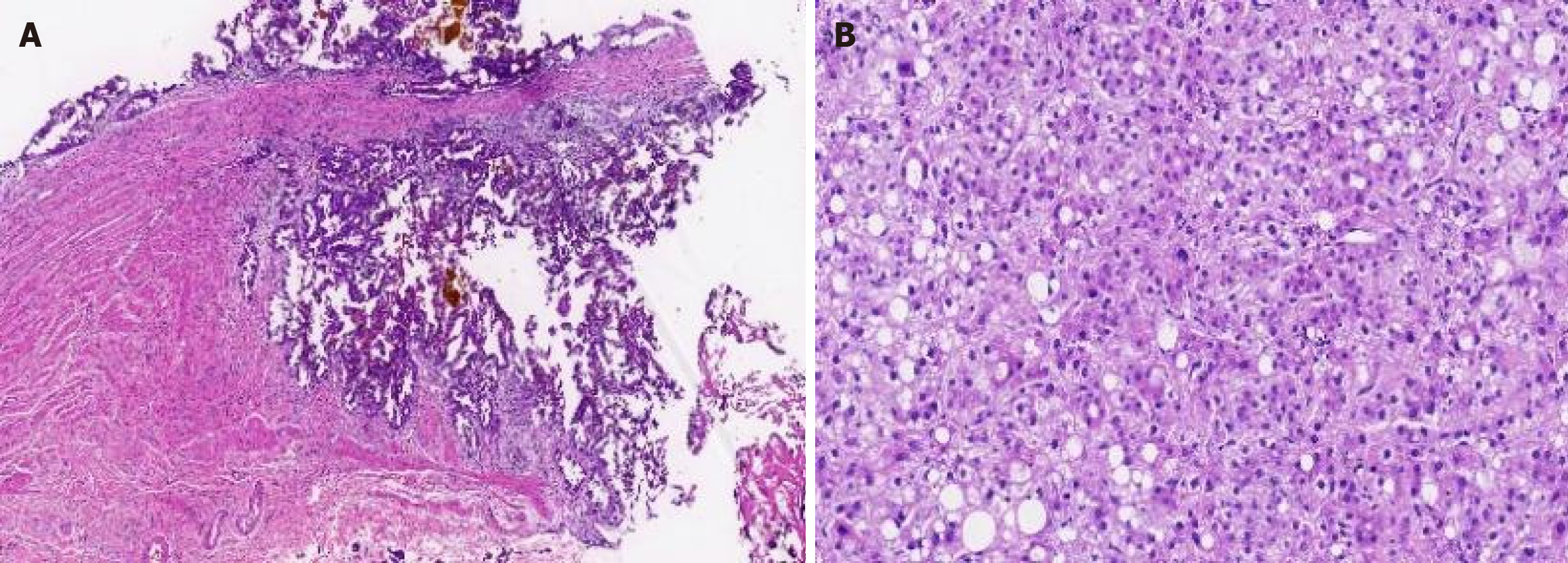
Cholecystectomy and lesion resections were performed, and the pathology of intraoperative frozen sections showed the following: Moderately differentiated adenocarcinoma in the gallbladder (T1b, Figure 3A); HCC in the left liver mass (grade II, Figure 3B); and a dysplastic nodule in the segment VI nodule. Radical resection involving pericholecystic hepatic parenchyma (2 cm margin) and lymphadenectomy (stations 8, 9, 12, 13) showed no metastasis.
The patient had an uneventful recovery and was discharged on postoperative day 5. The current surveillance protocol (imaging and tumor marker monitoring every 3 months in the first year, and 6 months thereafter) showed no evidence of disease status and no recurrence or metastasis. The patient also received regular postoperative oral antiviral therapy with entecavir (0.5 mg orally once daily).
According to the diagnostic criteria established by Warren and Gates[2], this case represents a rare instance of MPCs. MPCs refer to the occurrence of two or more independent primary malignancies in the same individual, either synchronously or metachronously. Based on the diagnostic interval between tumors (< 6 months), this case qualifies as synchronous MPCs. The incidence of MPCs among cancer patients ranges from 2% to 8%, with gastrointestinal tumors being particularly common[1]. MPC cases primarily involve the digestive system, with initial tumors predominantly occurring in the stomach and colorectum, while synchronous dual primary cancers in the liver and gallbladder are exceptionally rare[3].
The diagnostic challenge in this case was differentiating MPCs from “gallbladder cancer with hepatic metastasis”. Despite the patient’s history of chronic HBV infection and liver cirrhosis suggesting a potential secondary primary malignancy, the absence of elevated alpha-fetoprotein levels, lack of typical HCC “rapid wash-in and wash-out” enhancement patterns on CT, and the inherent rarity of dual primary cancers complicated preoperative diagnosis. Previous studies have demonstrated statistically significant differences in SUVmax values (18 fluorine-fluorodeoxyglucose PET/CT) between primary and metastatic liver cancers, with primary HCC typically showing SUVmax ≈ 8.657 ± 1.169[4]. However, in the present study, the patient’s SUVmax of 3.5 deviated from these findings, highlighting both the limitations of PET/CT in MPC diagnosis and the irreplaceable “gold standard” status of pathological confirmation.
No standardized treatment protocol exists for MPC patients, with limited literature guidance. Current approaches follow general oncological principles, prioritizing management of tumors with poorer prognosis, immediate life-threatening potential, or symptomatic burden[5]. In this case, both primary cancers demonstrated early-stage features on intraoperative frozen section pathology, enabling successful R0 resection. Lyu et al[3] demonstrated through clinical analysis that radical treatment for all cancer foci significantly improves median survival (168 months vs 68 months in incomplete treatment groups). Kourie et al[6] emphasized surgical intervention and chemotherapy for synchronous MPCs, advocating R0 resection when feasible and dual-effective chemotherapeutic regimens for inoperable cases. When clinically permissible, aggressive radical treatments (including surgically manageable procedures and tolerable systemic therapies) should be implemented to optimize outcomes. Determining optimal intervention timing, treatment sequencing, and therapeutic combinations requires case-specific analysis and ongoing clinical exploration. With regard to the prognosis of MPCs, numerous studies suggest comparable survival outcomes between MPCs and single primary malignancies[7-9]. Li et al[10] reported comparable long-term survival in 14 cases of hepatogastric MPCs following radical resection to single-cancer counterparts. Notably, literature data indicate that MPC patient survival depends on the most aggressive tumor focus rather than tumor multiplicity[11]. Numerous studies have confirmed improved outcomes with radical MPC treatment[12-15].
This study has several limitations. First, the single-case design employed in this research indicates that our findings may not be representative of a wider population. While single-case designs facilitate in-depth analysis of individual cases, the results may be influenced by the specific characteristics of the patient, thereby limiting the generalizability of the findings. Therefore, we recommend that future studies adopt a multicenter design with larger sample sizes to enhance the representativeness and external validity of the results. Second, our study only included a short-term follow-up, which may restrict our understanding of the long-term treatment effects and potential delayed complications. To more comprehensively evaluate therapeutic outcomes, future research should incorporate long-term follow-up to monitor patients’ prolonged prognosis and quality of life.
In the present case, the patient underwent surgical resection of the tumor, resulting in the resolution of abdominal discomfort and significant improvement in nutritional status. At the six-month postoperative follow-up, the patient’s weight had increased by 5 kg. Based on our follow-up observations, the patient currently exhibits no evidence of disease and has experienced substantial improvement in quality of life. Since its establishment, our center has collected postoperative patients’ subjective perceptions of treatment efficacy through telephone interviews and questionnaires, including symptom relief, daily activity capacity, and psychosocial support. Preliminary assessments indicate that, despite some challenges during treatment, most patients experienced an overall improvement in quality of life post-treatment, particularly in physical function and psychological well-being. However, these findings require further validation in future studies with larger sample sizes and extended follow-up periods.
Key clinical insights from this case include the following: (1) Maintain diagnostic suspicion: Clinicians must maintain awareness of MPCs when evaluating multi-organ malignancies, rigorously clarifying tumor relationships rather than defaulting to “Occam’s razor” assumptions of metastasis that may delay appropriate treatment; (2) Standardized management: Implement comprehensive protocols encompassing preoperative multidisciplinary team discussions, intraoperative pathology-guided decision-making, and guideline-compliant postoperative surveillance; and (3) MPC ≠ advanced disease: As demonstrated by this case and a literature review, favorable prognoses remain achievable in MPC patients, warranting active therapeutic approaches. The exceptional rarity of synchronous hepatobiliary MPCs (both being aggressive gastrointestinal malignancies) underscores the need for multicenter studies to elucidate risk factors and prognostic patterns. The authors have compiled relevant data from national and international studies on such dual-primary cases (Table 1) for reference.
| Patient number | Age (years) | Gallbladder lesion | Liver lesion | Treatment modalities | Prognosis of patients (not lost) (OS) |
| 1 | 65 | 3 cm × 3 cm, highly differentiated adenocarcinoma | 6.2 cm × 4.5 cm, hepatocellular carcinoma complicated with cholangiocarcinoma | Surgical treatment | 8 months, dead |
| 2 | 7 | 3.5 cm × 3 cm, moderately differentiated adenocarcinoma | 2.8 cm × 2.4 cm, hepatocellular carcinoma complicated with cholangiocarcinoma | N/A | 10 months, dead |
| 3 | 56 | 1.9 cm × 1.5 cm, moderately differentiated adenocarcinoma | 5 cm × 3 cm, hepatocellular carcinoma | N/A | 28 months, dead |
| 4 | 81 | The cavity was full of moderately differentiated adenocarcinoma | 7.8 cm × 6.5 cm, hepatocellular carcinoma | N/A | 24 months, alive |
| 5 | 54 | Invasion of the whole layer, moderately differentiated adenocarcinoma | 11 cm × 10 cm, hepatocellular carcinoma | N/A | 2 months, dead |
| 6 | 57 | Invasion of the whole layer, moderately differentiated adenocarcinoma | 5 cm × 5 cm, hepatocellular carcinoma | N/A | 3 months, dead |
| 7 | 63 | 5 cm × 3 cm, moderately differentiated adenocarcinoma | 2.2 cm × 1.5 cm, hepatocellular carcinoma | Surgical treatment | 17 months, alive |
| 8 | 70 | 0.7 cm × 0.7 cm, highly differentiated adenocarcinoma | 4.5 cm × 5.5 cm, hepatocellular carcinoma | Conservative treatment | 4 months, dead |
| 9 | 70 | 2 cm × 1 cm, moderately differentiated adenocarcinoma | 1.6 cm × 1.5 cm, hepatocellular carcinoma | Surgical treatment | 13 months, alive |
| 1. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 2. | Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Lyu JM, Xiong HC, Wu B, Zhou XQ, Hu J. [Clinical analysis of 138 multiple primary cancers diagnosed of digestive system malignant tumor initially]. Zhonghua Zhong Liu Za Zhi. 2018;40:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Wang X, Yu ZP, Wang H, Dai PF. [The value of 18F-FDG PET/CT dynamic imaging in the diagnosis of primary hepatocellular carcinoma with liver metastases]. Anhui Yike Daxue Xuebao. 2024;59:869-873. [DOI] [Full Text] |
| 5. | Corey L, Ruterbusch J, Shore R, Ayoola-Adeola M, Baracy M, Vezina A, Winer I. Incidence and Survival of Multiple Primary Cancers in US Women With a Gynecologic Cancer. Front Oncol. 2022;12:842441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Kourie HR, Markoutsaki N, Roussel H, Rahmi G, Van der Stiegel M, Palazzo L, Fabre M, Cuenod CA, Dubreuil O, Landi B, Rougier P, Taieb J. Double pancreatic and gastric adenocarcinomas: a rare association. Clin Res Hepatol Gastroenterol. 2013;37:e137-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003;65:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, Lee KU. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | He S, Liu Y, Liu X, Dou L, Zhang Y, Ni X, Lai S, Yu X, Zhang L, Wang G. [Clinical characteristics of multiple primary cancer associated with esophageal squamous carcinoma]. Zhonghua Yi Xue Za Zhi. 2015;95:2868-2870. [PubMed] |
| 10. | Li Z, Liu K, Duan JC, Huan Y, Yang JH, Shen F, Wu MC. [Analysis of surgery for multiple primary cancers in liver and gastrium: a report of 14 cases]. Zhonghua Gandan Waike Zazhi. 2010;16:570-572. [DOI] [Full Text] |
| 11. | Zhu LF, Xue P, Wang LW. [A clinical retrospective study of 65 cases of multiple primary cancers]. Fudan Xuebao (Yixueban). 2010;37:591-593. [DOI] [Full Text] |
| 12. | Pan Y, Wang J, Liang H. [116 multiple primary cancers in the digestive system]. Zhonghua Zhong Liu Za Zhi. 2002;24:191-193. [PubMed] |
| 13. | Li F, Zhong WZ, Niu FY, Zhao N, Yang JJ, Yan HH, Wu YL. Multiple primary malignancies involving lung cancer. BMC Cancer. 2015;15:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Zhao J, Tan Y, Wu Y, Zhao W, Wu J, Ji M, Shi L, Jiang J, Wu C. A rare case of eight multiple primary malignant neoplasms in a female patient: A case report and review of the literature. Oncol Lett. 2015;9:587-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wang Y. [Clinical analysis and research on 89 cases of multiple primary cancers in the digestive system]. Zhongguo Yiyao Zhinan. 2013;9:595. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/