©The Author(s) 2025.
World J Hepatol. Dec 27, 2025; 17(12): 111418
Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.111418
Published online Dec 27, 2025. doi: 10.4254/wjh.v17.i12.111418
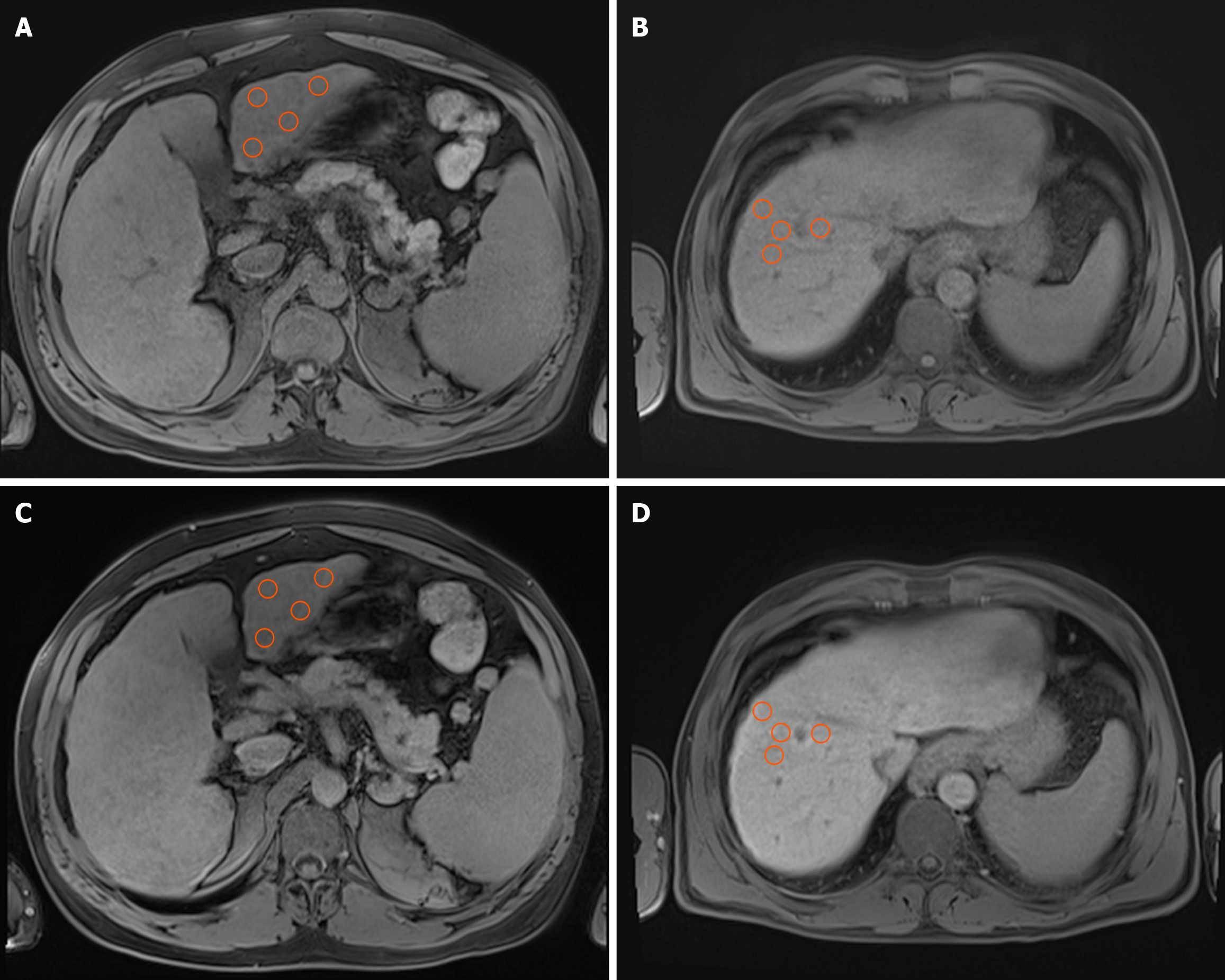
Figure 1 Illustration of signal intensity measurement methodology on axial T1-weighted volumetric interpolated breath-hold examination images with fat suppression, acquired during the native and hepatobiliary phases.
A and B: The native phases; C and D: The hepatobiliary phases. Images A and C depict signal intensity measurements in segment III, whereas images B and D demonstrate the same technique applied to segment VIII. Orange circles represent four manually positioned circular regions of interest, each spanning 1 cm2, situated within homogeneous liver parenchyma. The regions of interest were consistently replicated in identical locations across the two phases to ensure precise and comparable signal intensity evaluation.
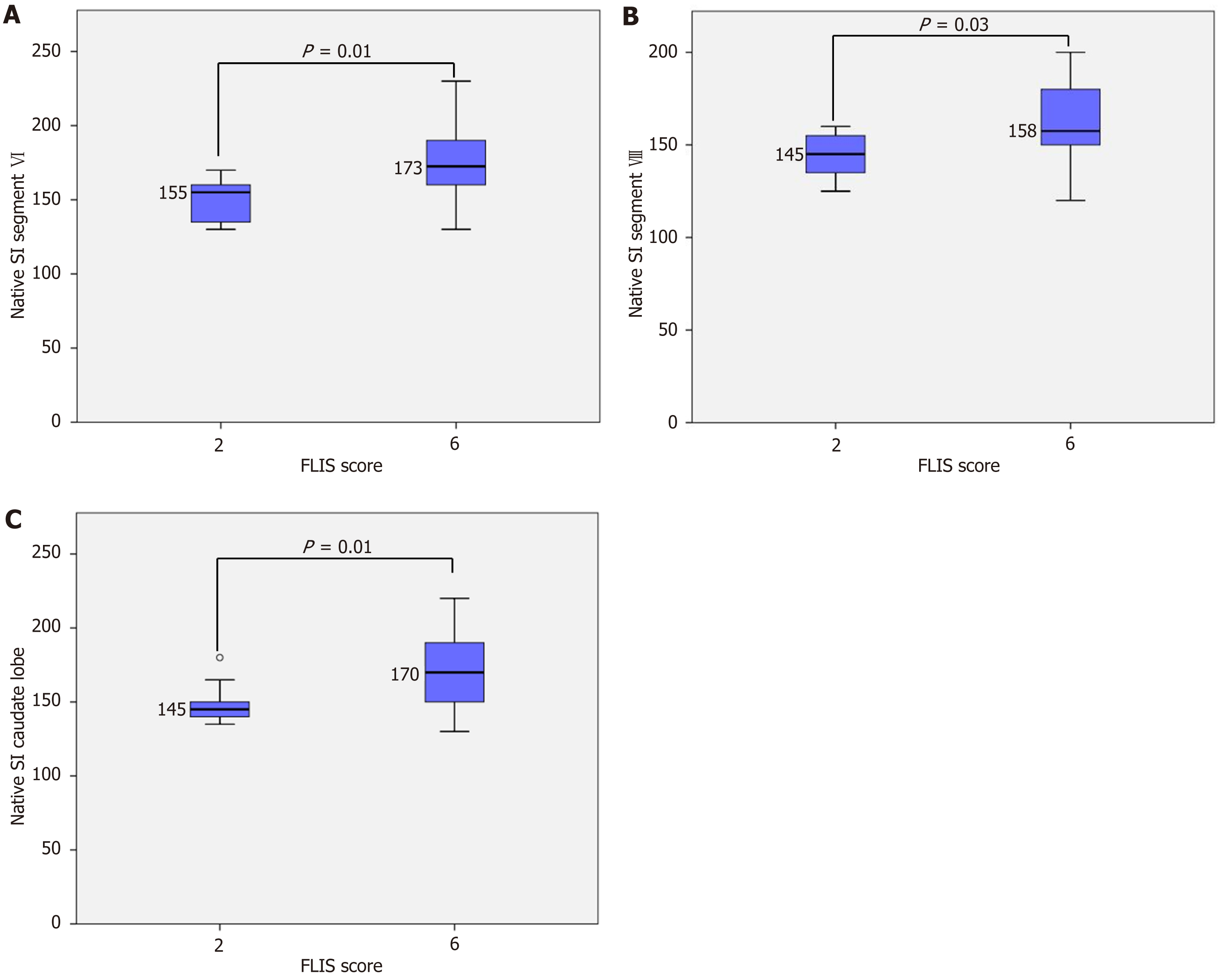
Figure 2 Segmental native signal intensity across different Functional Liver Imaging Score categories.
A: Native signal intensity (SI) segment VI; B: Native SI segment VIII; C: Native SI caudate lobe. Box plots illustrate native SI measured in liver segment VI (A), segment VII (B), and the caudate lobe (C). A significant increase in segmental SI was observed between Functional Liver Imaging Score (FLIS) 2 and FLIS 6 in all three regions (P = 0.01 for segments VI and caudate lobe, P = 0.03 for segment VII). Statistical analyses were performed using one-way analysis of variance with Tukey’s honest significant difference post-hoc test.
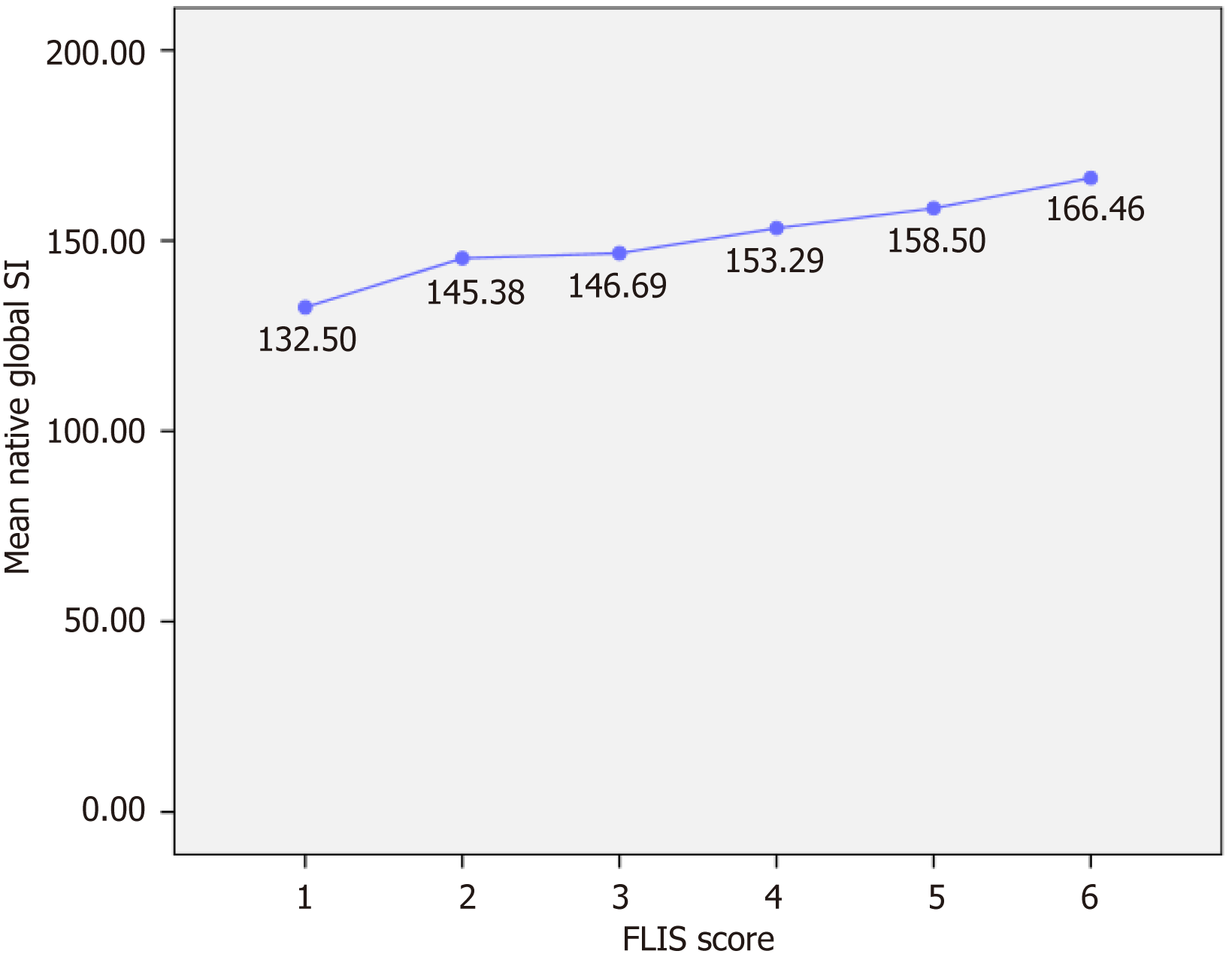
Figure 3 Variation in global native signal intensity based on Functional Liver Imaging Score categories.
The line chart illustrates a gradual yet modest increase in the average global native signal intensity (SI), corresponding to a higher Functional Liver Imaging Score (FLIS). No significant differences were observed between groups. Values are shown as mean SI with corresponding numeric labels. Statistical analyses did not find a significant difference in global SI across FLIS categories. These findings suggest that native SI is relatively independent of hepatocellular function.
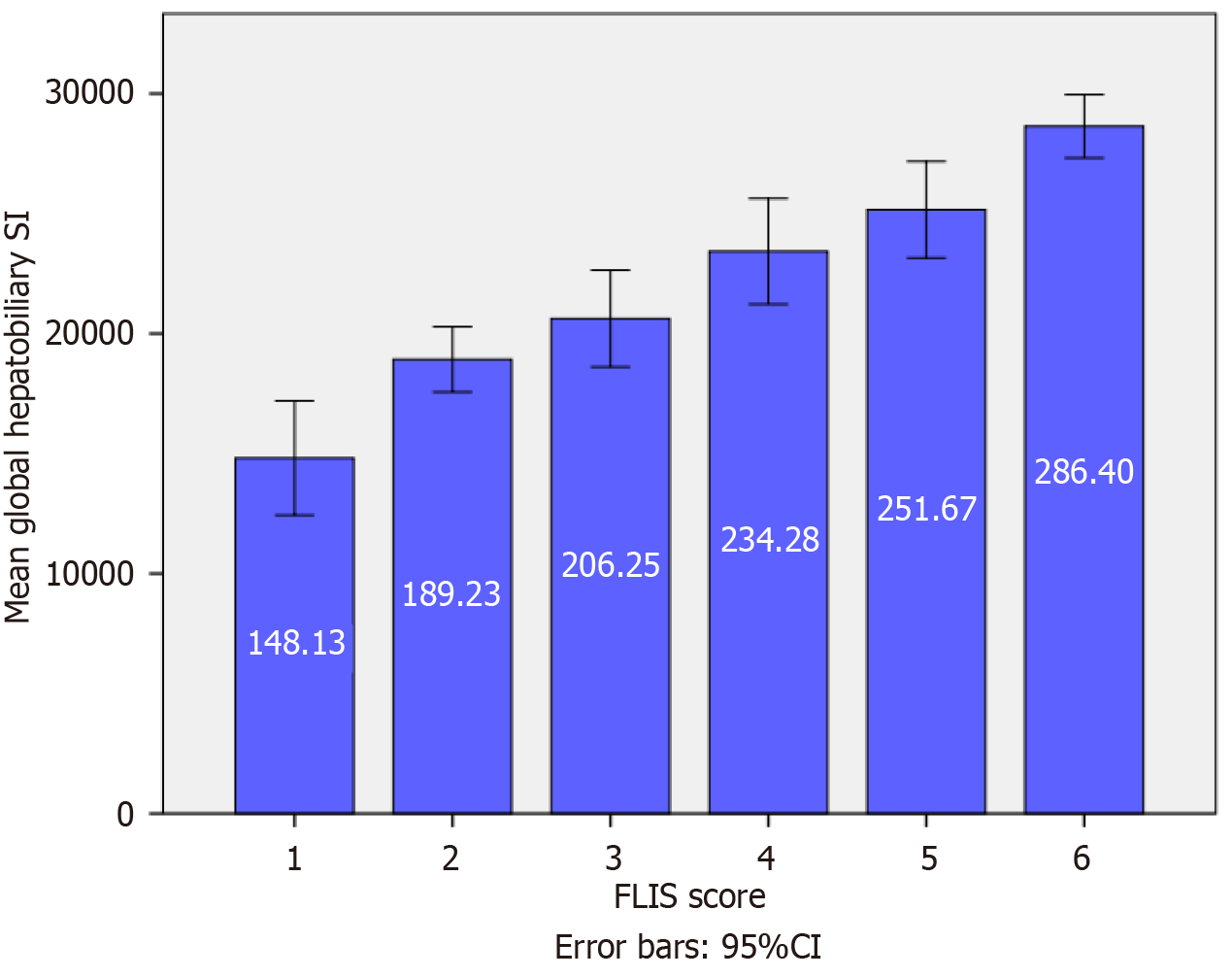
Figure 4 Mean global hepatobiliary-phase signal intensity across Functional Liver Imaging Score categories.
The bar chart illustrates a significant and progressive increase in mean global hepatobiliary signal intensity (SI) with higher Functional Liver Imaging Score (FLIS) (P < 0.001). The bar chart illustrates a substantial and progressive increase in mean global hepatobiliary SI, accompanied by higher FLIS scores (P < 0.001). Error bars represent the 95% confidence interval (95%CI). Post hoc Tukey’s honest significant difference analysis confirmed statistically significant differences between distant and adjacent FLIS categories, including FLIS 4 vs 6 and FLIS 5 vs 6. These results indicate that hepatobiliary-phase SI correlates closely with hepatocellular function as reflected by the FLIS score.
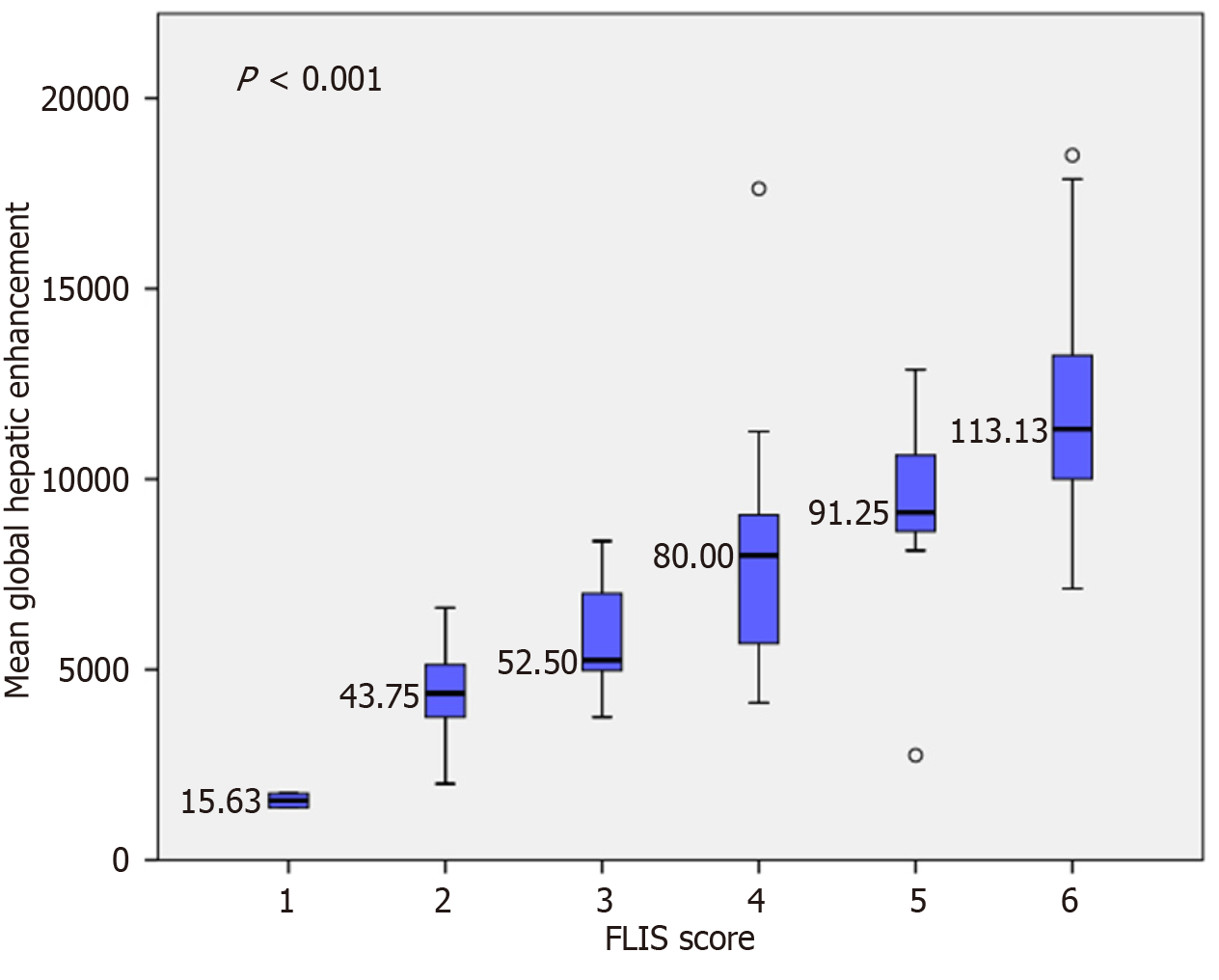
Figure 5 Variation in mean global hepatic enhancement according to the Functional Liver Imaging Score.
Box plots illustrate a progressive increase in global hepatic enhancement with a higher Functional Liver Imaging Score (FLIS) (F = 28.06, P < 0.001). Tukey’s honest significant difference post hoc analysis confirmed significant differences between lower and higher FLIS groups (e.g., FLIS 1-3 vs FLIS 4-6). However, no significant differences were observed between adjacent categories, such as FLIS 1 vs 2 and FLIS 2 vs 3.
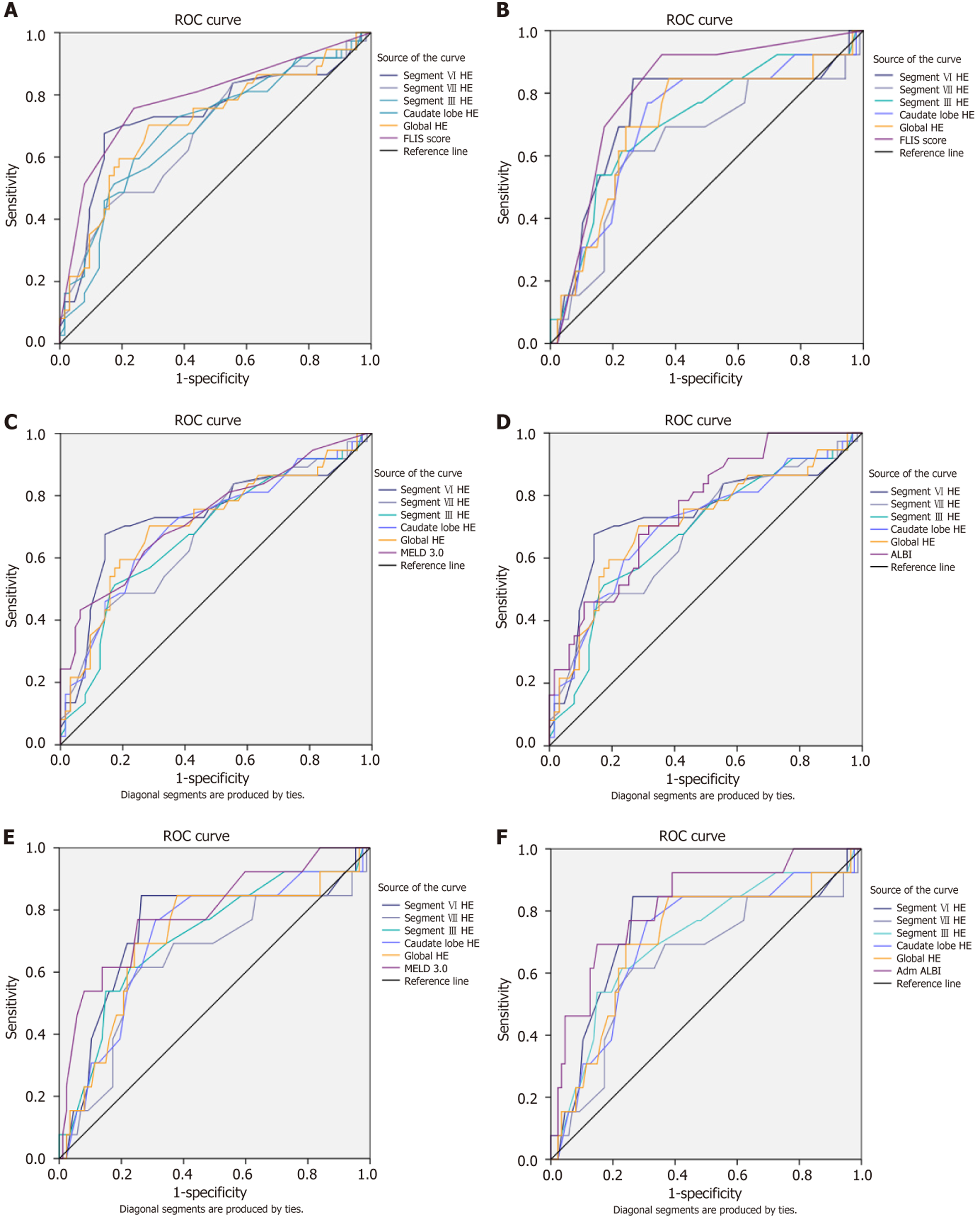
Figure 6 Receiver operating characteristic curves.
A and B: Receiver operating characteristic curves comparing the diagnostic performance of hepatic enhancement (HE) and Functional Liver Imaging Score (FLIS) for predicting outcomes during follow-up. A: Receiver operating characteristic (ROC) curves for decompensation. FLIS achieved the highest discriminatory accuracy, with an area under the receiver operating characteristic curve (AUC) of 0.79 (95% confidence interval [CI]: 0.69-0.88). Among HE measures, segment VI performed best, with an AUC of 0.74 (95%CI: 0.62-0.85). In direct comparisons, most HE measures were significantly less accurate than FLIS, although segment VI showed no significant difference (ΔAUC = -0.05, q = 0.123); B: ROC curves for mortality. FLIS achieved the highest discriminatory performance (AUC = 0.81, 95%CI: 0.69-0.93). Among HE measures, segment VI again performed best (AUC = 0.74, 95%CI: 0.57-0.91). In direct comparisons, segment VI HE did not differ significantly from either FLIS (ΔAUC = -0.07, q = 0.293), while segment VIII HE was significantly inferior to FLIS (ΔAUC = -0.18, q = 0.025); C-F: ROC curves comparing the diagnostic performance of HE, Model for End-Stage Liver Disease (MELD) 3.0, and albumin-bilirubin (ALBI) score for predicting outcomes during follow-up. C: ROC curves comparing HE and MELD 3.0 ability to discriminate for decompensation. MELD 3.0 (AUC = 0.73, 95%CI: 0.63-0.83) slightly outperformed most HE parameters, with ΔAUC values from -0.05 to 0.01. However, these differences were not statistically significant, and segment VI performed closest to both scores; D: ROC curves comparing HE and MELD 3.0 abilities to discriminate mortality. Among HE measures, segment VI performed best (AUC = 0.74, 95%CI: 0.57-0.91). MELD 3.0 performed similarly to HE (AUC = 0.79, 95%CI: 0.64-0.94), with ΔAUC values for segment VI vs MELD not being significant; E: ROC curves comparing HE and ALBI ability to discriminate for decompensation. ALBI (AUC = 0.77, 95%CI: 0.67-0.86) slightly outperformed most HE parameters, with ΔAUC values from -0.09 to -0.04. However, these differences were not statistically significant; F: ROC curves comparing HE and ALBI in their ability to discriminate mortality. Overall, ALBI proved to be the most accurate score for mortality (AUC = 0.82, 95%CI: 0.69-0.95). However, HE - particularly segment VI - maintained a strong discriminative ability and a very high negative predictive value (0.97).
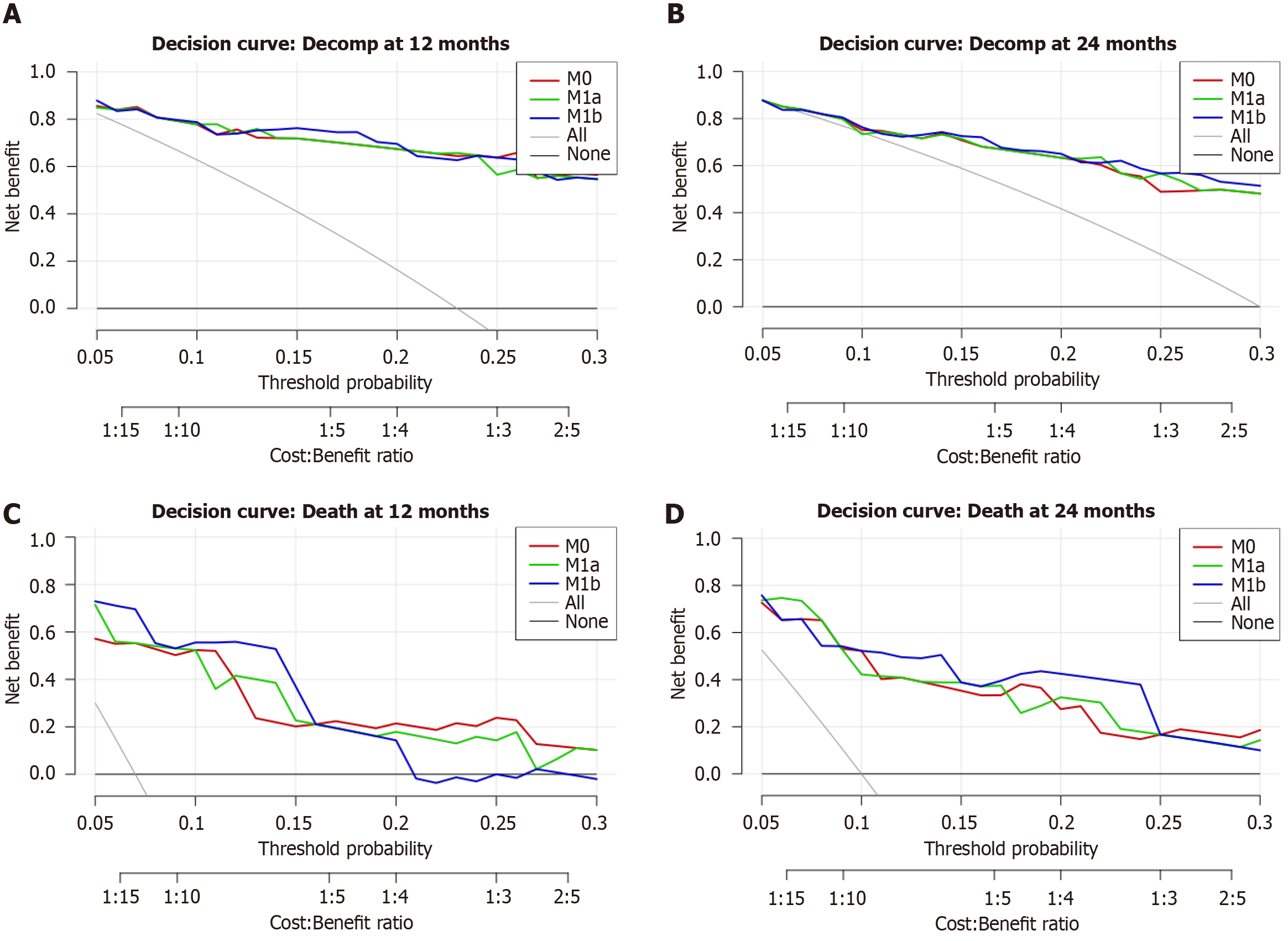
Figure 7 Decision-curve analysis.
A and B: Decision-curve analysis at 12 and 24-month decompensation. A: Decision-curve analysis for 12-month decompensation. Curves largely overlap across 5%-30% thresholds; no material net-benefit differences are apparent; B: Decision-curve analysis for 24-month decompensation. A minor advantage for hepatic enhancement (HE)-global is visible around 18%-24%; otherwise, the curves overlap; C and D: Decision-curve analysis at 12 and 24-month mortality; C: Decision-curve analysis for 12-month mortality. Net benefit is plotted against threshold probability. The global HE extension (M1b) modestly exceeds the reference model by 7%-16%; the HE-segment-VI extension (M1a) is comparable to M0, with a slight advantage of around 21%-27%; D: Decision-curve analysis for 24-month mortality. The HE-global extension (M1b) provides a small net-benefit gain over approximately 8%-23%; HE-segment-VI (M1a) overlaps the reference model.
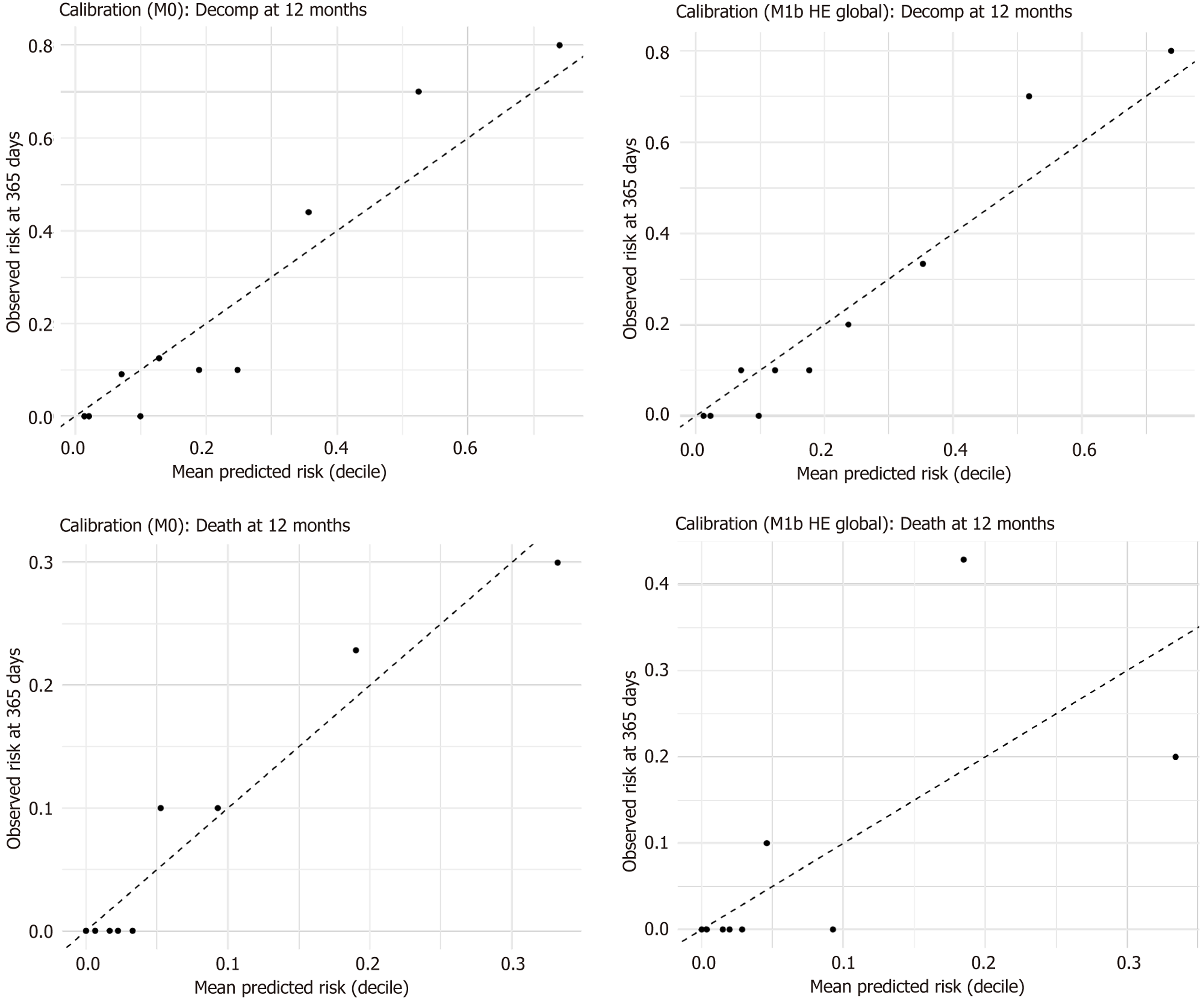
Figure 8 The observed risks across deciles closely aligned with the mean predicted risks, with no significant miscalibration resulting from the inclusion of hepatic enhancement.
HE: Hepatic enhancement.
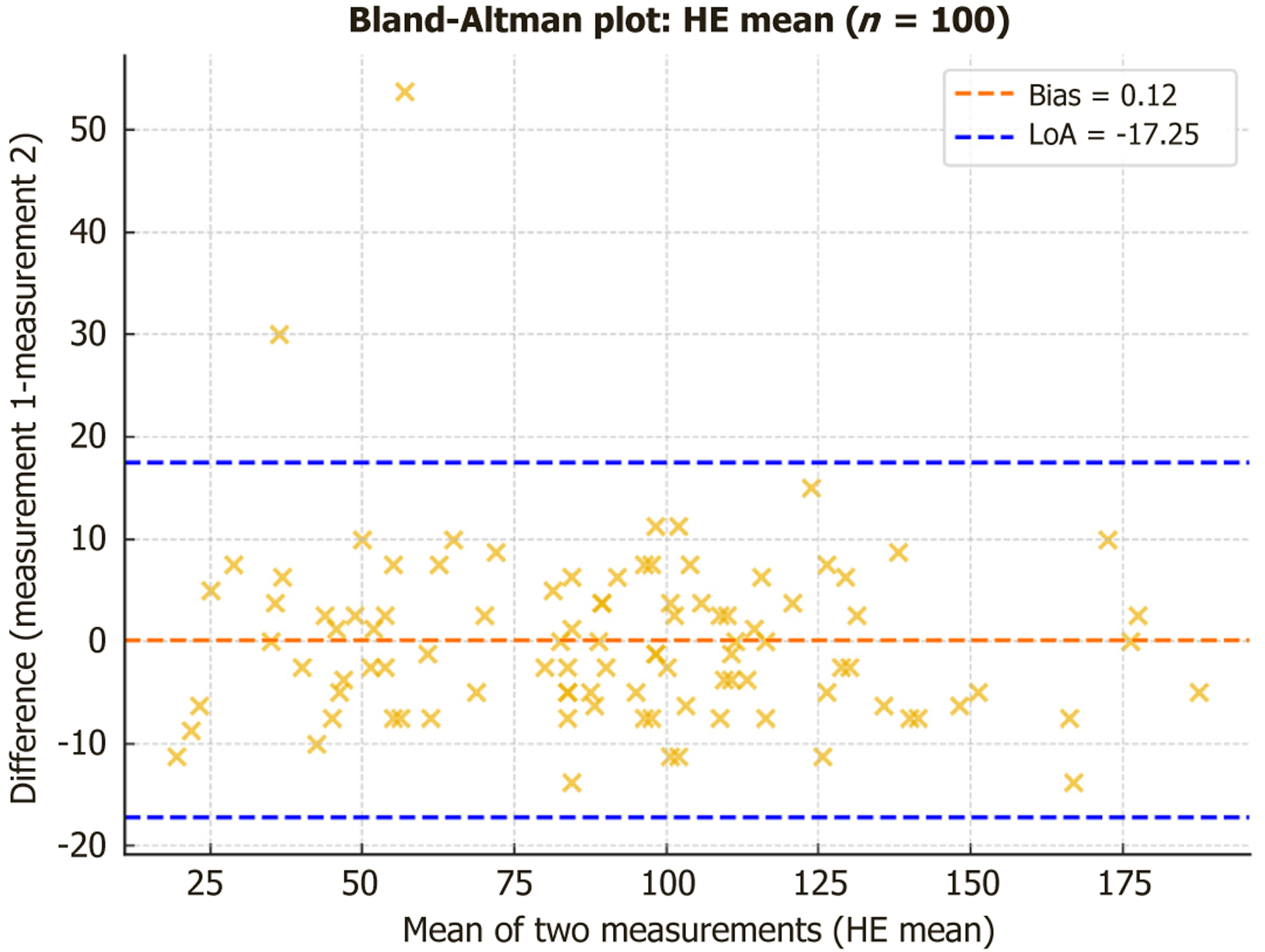
Figure 9 Bland-Altman analysis confirmed minimal systematic bias across parameters.
HE: Hepatic enhancement.
- Citation: Stanciu BI, Iojiban M, Morariu-Barb A, Caraiani C, Procopet B, Stefanescu H, Lupsor-Platon M. Hepatic enhancement and signal intensity analysis on magnetic resonance imaging as prognostic biomarkers in advanced chronic liver disease. World J Hepatol 2025; 17(12): 111418
- URL: https://www.wjgnet.com/1948-5182/full/v17/i12/111418.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i12.111418