©The Author(s) 2025.
World J Hepatol. Nov 27, 2025; 17(11): 110080
Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.110080
Published online Nov 27, 2025. doi: 10.4254/wjh.v17.i11.110080
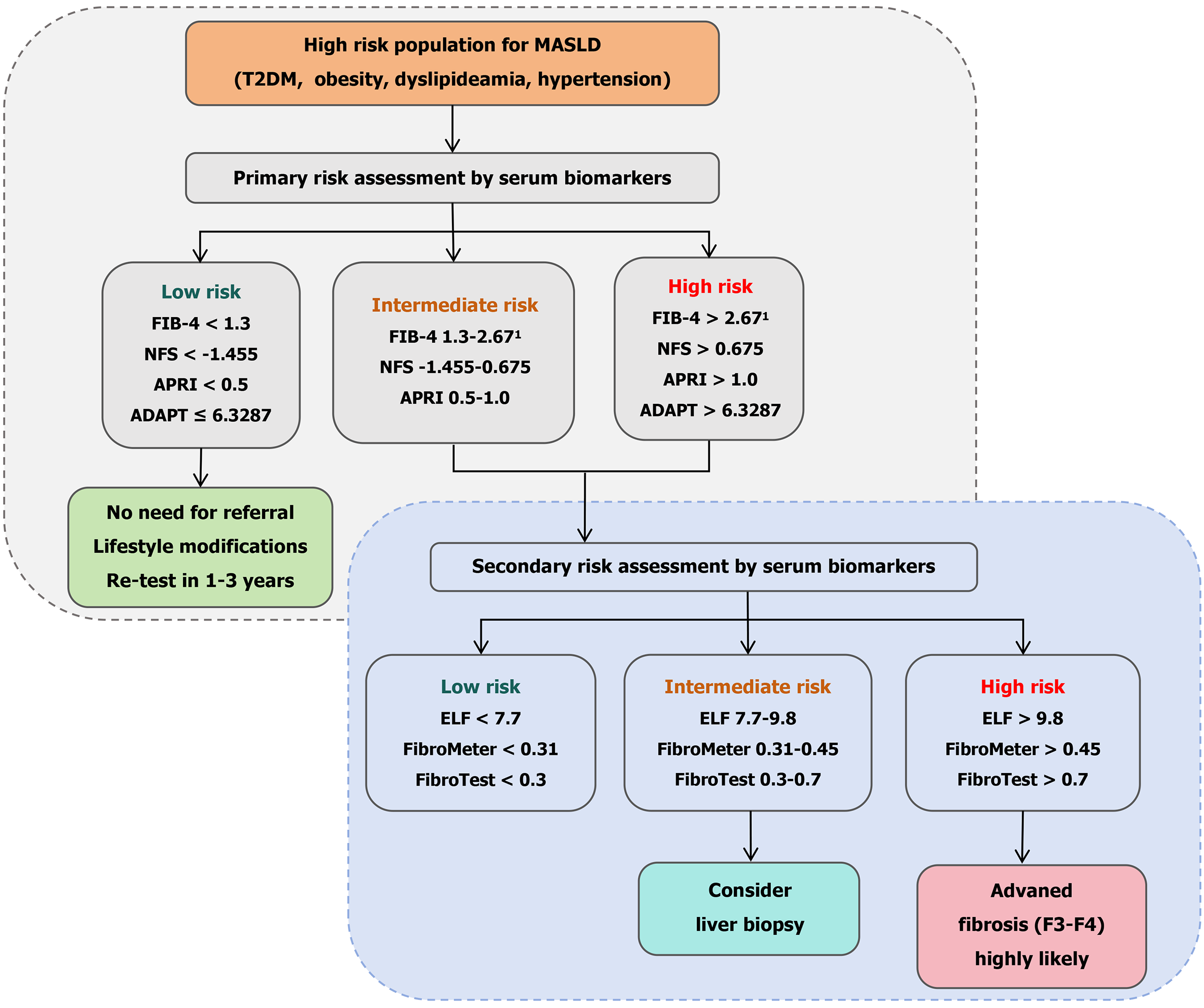
Figure 1 A suggested simplified algorithm for the use of non-invasive serum biomarkers for the assessment of patients with metabolic dysfunction-associated steatotic liver disease.
1The cutoff value of fibrosis-4 for individuals ≥ 65 years old is adjusted to 2.0. MASLD: Metabolic dysfunction-associated steatotic liver disease; T2DM: Type 2 diabetes; FIB-4: Fibrosis-4; NFS: Non-alcoholic fatty liver disease fibrosis score; ELF: Enhanced liver fibrosis; APRI: Aspartate aminotransferase-to-platelet ratio index; ADAPT: Age, diabetes status, propeptide of type 3 collagen, and thrombocyte.
- Citation: Zhao YH, Leng SS, Wang Y, Kui FZ, Gan W. Non-invasive blood biomarkers for assessment of liver fibrosis in metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(11): 110080
- URL: https://www.wjgnet.com/1948-5182/full/v17/i11/110080.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i11.110080