Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.108695
Revised: May 29, 2025
Accepted: July 3, 2025
Published online: August 26, 2025
Processing time: 122 Days and 16.8 Hours
Young women’s physical and mental health is seriously impacted by recurrent spontaneous abortion (RSA), a prevalent obstetric complication that is becoming more commonplace worldwide. Therefore, a thorough investigation into the pathophysiology of RSA and the development of novel therapeutic strategies are imperative. Recent developments suggest that mesenchymal stem cell (MSC)-based therapies may be viable for addressing RSA. Through a variety of mech
Core Tip: The reproductive health of women of childbearing age is significantly impacted by recurrent spontaneous abortion. Although there are several therapeutic options available today, the curative efficacy is unsatisfactory. By controlling immunological factors, mesenchymal stem cell therapy is one possible treatment option that can enhance pregnancy outcomes. Mesenchymal stem cell treatment and its associated mechanisms are briefly explained in this article.
- Citation: Xiao Y, Zeng FY, Chen ZY, Zhao F, Sun JL. Advances in mesenchymal stem cell-based therapies for recurrent spontaneous abortion. World J Stem Cells 2025; 17(8): 108695
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/108695.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.108695
The American College of Obstetrics and Gynecology defines recurrent spontaneous abortion (RSA) as the failure of three or more successive pregnancies before 20 weeks of conception with the same sexual partner[1]. Due to varying definitions, the mean occurrence of RSA is between 1% and 4%[2]. In addition to affecting women’s health[3], RSA also causes psychological distress and places economic and emotional strain on the entire family[4-6]. There are numerous causes of RSA, including immunological disorders, chromosomal abnormalities, endometrial dysfunction, and uterine malformations[6,7]. Mesenchymal stem cell (MSC) therapy, hydrogels, or synthetic biosimilars containing MSCs have been shown by Rodríguez-Eguren et al[8] to stimulate tissue healing, vascular growth, and endometrial proliferation. These actions may aid in the restoration of endometrial function and, eventually, fertility[8]. Accurate personalized treatment is still challenging, as the exact cause of RSA is unknown and requires more research[9].
Because of their ability to proliferate, self-renew, and differentiate either unidirectionally or multidirectionally, stem cells may be used for wound repair, tissue regeneration, restoration of organ and tissue function and maintenance of the normal state of the body[10,11]. Stem cell therapy is one type of immunotherapy that may be used for the treatment of RSA[6]. Depending upon the source they originate from, stem cells can be classified as either adult or embryonic. Due to their rich sources, ease of separation and culture, and minimal immunogenicity, stem cells are regarded as an effective therapeutic approach. At present, an increasing number of studies have demonstrated that MSC therapy can enhance pregnancy outcome in RSA and preserve the immunological tolerance of the maternal-fetal interface[12-14].
To provide specific treatment for RSA, it is necessary to determine the pertinent mechanisms of MSCs from various origins in the management of RSA. This will have a definite effect on unexplained RSA. Understanding the mechanism of RSA aids in the advancement of MSC research and can provide a basis for the clinical use of MSCs in RSA treatment.
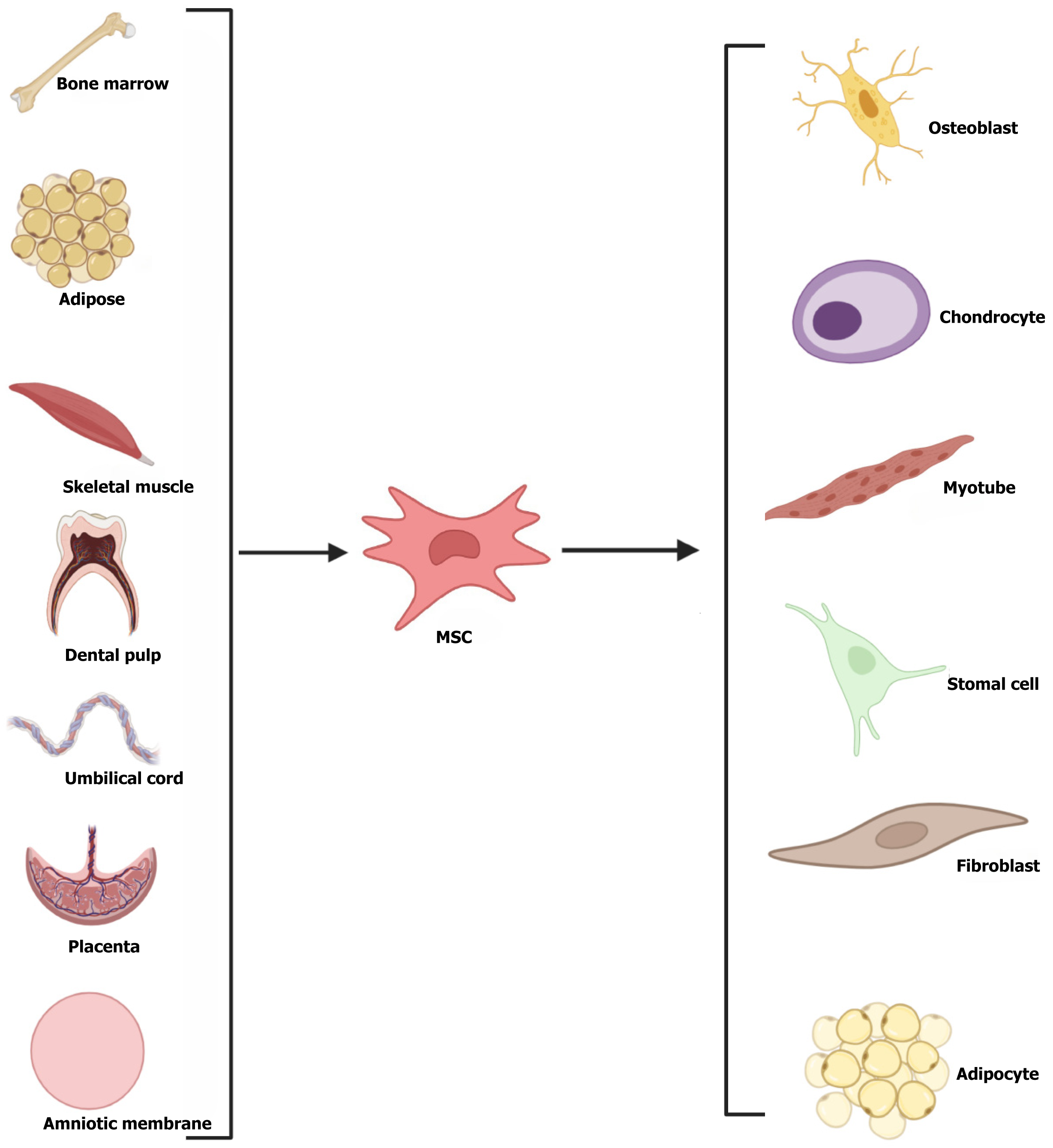
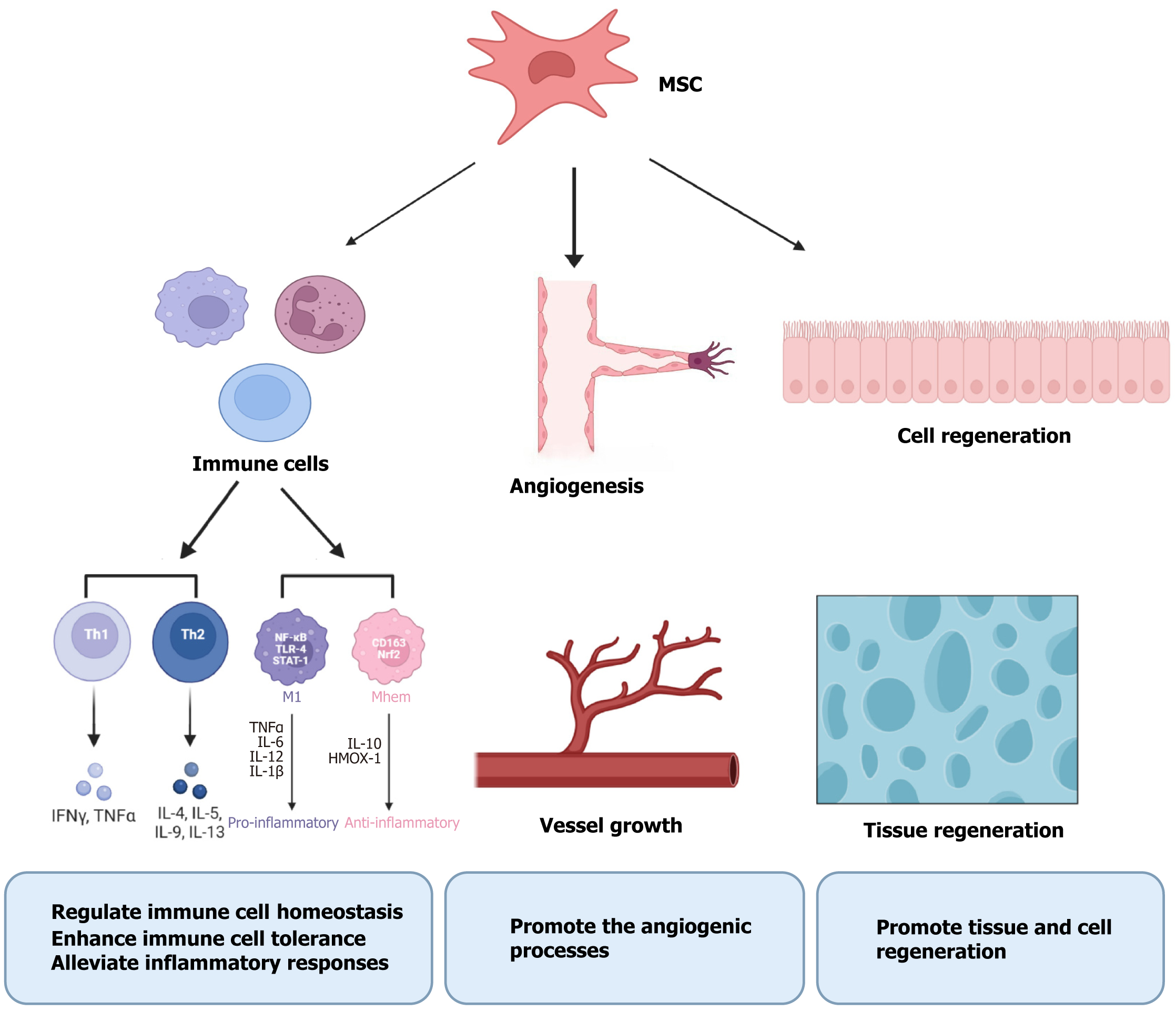
MSCs are multifunctional adult stem cells that can be generated from a variety of organs, including bone marrow, fat, umbilical cord, umbilical cord blood, skeletal muscle, dental pulp, placenta, amniotic fluid, and amniotic membrane[15-17] (Figure 1). MSCs could enhance the results of pregnancies by modifying the immunological milieu at the maternal-fetal interaction through various mechanisms, including regulating immune cell homeostasis, enhancing immune tolerance, alleviating inflammatory responses, promoting angiogenic processes, and promoting tissue regeneration (Figure 2). We performed a systematic search of full-text articles available in PubMed to identify original peer-reviewed studies published in English. The search terms included: “Stem cell”, “mesenchymal stem cell”, “abortion”, “miscarriage”, and “recurrent spontaneous abortion”. Preclinical and clinical studies were included. According to the literature review, there are currently five origins of MSCs that satisfy the requirements: Bone marrow, adipose, umbilical cord, amniotic, and decidual.
The adult stem cells most frequently utilized in therapeutic research to date are bone marrow derived MSCs (BMSCs), which have minimal immunogenicity. Multiple disorders affecting the reproductive system have been treated with these cells[18,19]. Numerous animal studies have been conducted to investigate the mechanism by which BMSCs could treat RSA. Although BMSCs do not affect CD4+ T cells and macrophages in the entire body, they can decrease the rate of embryo absorption in mice and act locally on CD4+ T cells and macrophages at the interaction site between mother and fetus[20]. At the site of BMSC injection, the secretion of interleukin (IL)-4 and IL-10 increased while tumor necrosis factor (TNF)-α and interferon (IFN)-γ secretion was inhibited. This indicated that BMSCs improve the outcome of pregnancy by suppressing the T helper type 1 (Th1) immunological response, encouraging the Th2 immunological response, and making the Th2 immune response a key player in the interaction between the mother and the fetus[21]. Following BMSC injection, M1-type macrophages transformed into M2-type macrophages, which increased IL-10 release and decreased IL-12 secretion, shifting the macrophage balance to an anti-inflammatory state[22,23]. Additionally, by interacting with M1-type macrophages, BMSCs can alleviate abortion by immunosuppressing TNF-stimulating gene-6 and promoting its paracrine function[24-26]. In one study, exosomes were extracted from BMSC culture medium and injected into the uterine horn. These exosomes induced macrophages to polarize to M2-type in addition to transporting miR-101 to T cells to boost the Th2 immune response. At the interaction site between mother and fetus, exosomes regulate T cell and macrophage activity, which decreases the rate of embryo absorption[27,28].
Adipose-derived MSCs (ADMSCs) are less invasive than BMSCs, have high proliferative capacity, and release a range of growth factors[29]. The primary way that ADMSCs modulate the immune system is by controlling the equilibrium between Th1 and Th2 inflammatory agents both locally and systemically. ADMSC therapy has an immunomodulatory effect by downregulating IFN-γ and TNF-α gene expression and upregulating the production of transforming growth factor-β and IL-10 in the decidua, thereby reducing the rate of embryo absorption and protecting the fetus[30]. Salek Farrokhi et al[31] discovered that the identical alterations took place in the spleen of mice and that ADMSCs might reduce the immunological rejection of paternal antigens by the spleen. Rezaei Kahmini et al[32] discovered that ADMSCs improve the tolerance environment of the maternal-fetal interaction by reducing CD49b+ natural killer (NK) cell infiltration into the interface and modifying the NK cell cytokine spectrum from an inflammatory to a tolerance spectrum. ADMSCs prevented excessive complement C3 activation and deposition during pregnancy and enhanced angiogenic equilibrium at the interaction site between mother and fetus, which benefited the fetus and enhanced intrauterine pregnancy[33]. By examining the data from relevant studies on the use of ADMSCs to treat abortion, Zhao et al[34] validated the aforementioned perspective, which was crucial in supporting the ensuing clinical data translation.
Human umbilical cord-derived MSCs, known as Wharton’s jelly-derived MSCs (WJMSCs), are capable of tissue healing and immunoregulation[35,36]. They are regarded as the perfect stem cell type in regenerative medicine due to their lack of intrusion[37]. Chen et al[38] discovered that injection of WJMSCs improved early abortion by increasing IL-10 expression in the mouse placenta and decreasing IFN-γ and IL-17 expression. WJMSCs decreased the rate of embryo absorption by increasing the production of Th2 immune indicators through the Janus kinase/signal transducer and activator of transcription pathway, as confirmed by Ding et al[39]. The connection between decidual tissues in the abortion model, particularly that between stromal cells and NK cells, was found to be suppressed using single cell sequencing. Treatment with WJMSCs may improve RSA at the single cell scale by partially repairing barriers between decidual tissues[40]. The precise mechanism of WJMSCs requires further investigation, as there has been little preclinical research on their use in treating RSA.
Amniotic membrane is simple to obtain, is noninvasive, has almost no ethical limitations, and has therapeutic potential in the field of regenerative medicine. After repeated passage and growth, amniotic MSCs (AMSCs), which have a high initial order of magnitude and minimal immunogenicity, can maintain good proliferation and differentiation potential[41-43]. By suppressing proinflammatory cytokines and stimulating anti-inflammatory molecule release, AMSCs may decrease intrauterine adhesion and promote early miscarriage. By controlling the population of regulatory T cells, AMSCs may additionally improve the number of glands, decrease areas of ovarian fibrosis, and encourage endometrial regeneration[44]. Research has verified that human AMSCs enhanced angiogenesis, facilitated macrophages to polarize into M2-type macrophages, reduced the rate of embryo absorption and inflammatory cell infiltration, and markedly increased IL-10 and transforming growth factor-β expression while decreasing that of IL-1β and IL-6[45]. In order to cure disorders of the reproductive system, AMSCs can stimulate follicular creation, endometrial regeneration, gland development, and the restoration of hormone levels (such as anti-Müllerian hormone and estradiol)[46]. Even though these studies offer a theoretical framework for treating abortion, more investigation is required to validate these findings and determine how to use them in a clinical setting.
With the patient’s consent, decidual tissue is extracted from samples of induced abortions between 6 and 8 weeks of pregnancy. This tissue is a promising candidate for treatment in regenerative medicine and has good angiogenesis, anti-inflammatory, and antiapoptotic properties[47,48]. Although there are currently limited investigations on decidual MSCs (DMSCs), related research has shown that like MSCs from other origins, DMSCs can play a role in immunomodulation. DMSCs have been shown to boost the proportion of regulatory T cells and suppress apoptosis and proliferation, both of which are beneficial for preserving immunological resistance at the junction between the mother and the fetus. Nevertheless, the study also discovered that spontaneous abortion had a better immunoregulatory impact than RSA following DMSC administration[49]. To ensure successful pregnancy in the future, a customized diagnosis and treatment plan should be developed, as DMSCs have distinct immunomodulatory effects on spontaneous abortion and RSA.
Immunology is essential for preserving the immunological tolerance of the maternal-fetal interface and preventing pathogen invasion, which can preserve the equilibrium of immune cells, cytokines, and autoantibodies. The majority of RSAs are caused by bodily imbalances. Thus, controlling the immunological response to RSA may be a successful therapeutic approach. At present, immune regulation methods primarily include intravenous immunoglobulin, paternal lymphocyte immunization, granulocyte colony stimulating factor and MSC therapy[6]. MSC therapy is an effective immune regulation method.
MSCs’ low immunogenicity, repeatability, and immunomodulatory action make them promising for use in regenerative therapy. In addition to improving pregnancy outcome in RSA, MSCs can be utilized as regenerative materials in other medical specialties, including neurology, orthopedics, and cardiovascular medicine[50]. Although MSCs in tissue engineering have advanced due to the use of biocompatible scaffolds, 3D culture systems, and organ-like architectures, there are still technical obstacles to overcome[51].
Although there is some evidence that MSCs are a viable and successful treatment for RSA, clinical implementation of this treatment is still in its infancy. The precise techniques and procedures of MSC treatment are still being investigated. Through uterine horn or intraperitoneal injection, MSCs are primarily extracted from the bone marrow, fat, umbilical cord, amniotic membrane, and decidua. They are involved in immune cell balance regulation, immune tolerance enhancement, inflammation inhibition, angiogenesis promotion, and tissue regeneration[12] (Table 1). Abortion safety with stem cell therapy remains a prominent concern. After stem cell transplantation, there remains a risk of immunological rejection, carcinogenicity, and other negative outcomes[52,53]. The study of MSC exosomes has recently attracted a lot of interest. According to research by Xiang et al[27], BMSC exosomes can carry miR-101, decreasing the rate of embryo absorption and preserving immunological tolerance. Researchers also need to investigate the unclear mechanism of additional MSC exosomes in the treatment of RSA. Hormone levels may be impacted by MSC treatment, for instance, by preventing iron death and preserving apoptosis, MSCs and their exosome vesicles can alleviate polycystic ovarian syndrome and premature ovarian failure. Therefore, more research is required to determine whether MSCs can alter hormone levels and whether MSCs have an impact on hormone homeostasis[54,55]. More preclinical research is required before clinical trials are initiated on the assumption of safety and efficacy. The research of MSCs in RSA therapy is still at the animal experimental stage. Although MSC treatment now has certain restrictions and challenges, its regeneration potential and therapeutic impact cannot be disregarded. In order to advance treatment to the clinic as soon as possible, a number of animal studies and preclinical research are still required to determine how MSC therapy can safely and efficiently improve RSA outcomes.
| Origin | Mechanism | Ref. |
| Bone marrow | Act locally on CD4+ T cells and macrophages at the maternal-fetal interface | Meng et al[20] |
| Inhibit the Th1 immune response and promote the Th2 immune response | Aggarwal and Pittenger[21] | |
| Promote macrophage polarization to M2-type | Maggini et al[23] | |
| Immunosuppress TNF-stimulating gene-6 | Vallés et al[25] | |
| Promote paracrine function | Li et al[26] | |
| Exosomes regulate T cells and macrophages activity | Xiang et al[27] | |
| Adipose | Control the balance of Th1 and Th2 cytokines | Sadighi-Moghaddam et al[30] |
| Decrease immunological rejection of paternal antigens by the spleen | Salek Farrokhi et al[31] | |
| Reduce the infiltration of CD49b+ NK cells into the interface | Rezaei Kahmini et al[32] | |
| Enhance the balance of angiogenesis | Shahgaldi et al[33] | |
| Wharton’s jelly | Increase expression of Th2 immune factors | Chen et al[38], Ding et al[39] |
| Suppress stromal cells and NK cells | Jin et al[40] | |
| Amniotic membrane | Suppress proinflammatory cytokines and control the population of Treg cells | Gan et al[44] |
| Enhance angiogenesis and macrophage polarization | Xiao et al[45] | |
| Stimulate endometrial regeneration and restore the hormone levels | Naeem et al[46] | |
| Decidual | Stimulate angiogenesis | Chen et al[47] |
| Increase the proportion of Treg cells and suppress apoptosis and proliferation | Büyükbayrak et al[49] |
| 1. | Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 376] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 2. | Rasmark Roepke E, Matthiesen L, Rylance R, Christiansen OB. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet Gynecol Scand. 2017;96:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Gudnadottir U, Du J, Hugerth LW, Engstrand L, Schuppe-Koistinen I, Wiberg Itzel E, Fransson E, Brusselaers N. Pre-pregnancy complications - associated factors and wellbeing in early pregnancy: a Swedish cohort study. BMC Pregnancy Childbirth. 2023;23:153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Iordăchescu DA, Paica CI, Boca AE, Gică C, Panaitescu AM, Peltecu G, Veduță A, Gică N. Anxiety, Difficulties, and Coping of Infertile Women. Healthcare (Basel). 2021;9:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Holt-Kentwell A, Ghosh J, Devall A, Coomarasamy A, Dhillon-Smith RK. Evaluating interventions and adjuncts to optimize pregnancy outcomes in subfertile women: an overview review. Hum Reprod Update. 2022;28:583-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Guan D, Sun W, Gao M, Chen Z, Ma X. Immunologic insights in recurrent spontaneous abortion: Molecular mechanisms and therapeutic interventions. Biomed Pharmacother. 2024;177:117082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 7. | Dimitriadis E, Menkhorst E, Saito S, Kutteh WH, Brosens JJ. Recurrent pregnancy loss. Nat Rev Dis Primers. 2020;6:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 8. | Rodríguez-Eguren A, Bueno-Fernandez C, Gómez-Álvarez M, Francés-Herrero E, Pellicer A, Bellver J, Seli E, Cervelló I. Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review. Hum Reprod Update. 2024;30:584-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Lai H, Yang Y, Zhang J. Advances in post-translational modifications and recurrent spontaneous abortion. Gene. 2024;927:148700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023;199:3-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 11. | Blau HM, Daley GQ. Stem Cells in the Treatment of Disease. N Engl J Med. 2019;380:1748-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Pourakbari R, Ahmadi H, Yousefi M, Aghebati-Maleki L. Cell therapy in female infertility-related diseases: Emphasis on recurrent miscarriage and repeated implantation failure. Life Sci. 2020;258:118181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Rezayat F, Esmaeil N, Rezaei A. Potential Therapeutic Effects of Human Amniotic Epithelial Cells on Gynecological Disorders Leading to Infertility or Abortion. Stem Cell Rev Rep. 2023;19:368-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Song YT, Liu PC, Tan J, Zou CY, Li QJ, Li-Ling J, Xie HQ. Stem cell-based therapy for ameliorating intrauterine adhesion and endometrium injury. Stem Cell Res Ther. 2021;12:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Lisini D, Nava S, Pogliani S, Avanzini MA, Lenta E, Bedini G, Mantelli M, Pecciarini L, Croce S, Boncoraglio G, Maccario R, Parati EA, Frigerio S. Adipose tissue-derived mesenchymal stromal cells for clinical application: An efficient isolation approach. Curr Res Transl Med. 2019;67:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Angelopoulos I, Brizuela C, Khoury M. Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential. Cell Transplant. 2018;27:967-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Gasiūnienė M, Petkus G, Matuzevičius D, Navakauskas D, Navakauskienė R. Angiotensin II and TGF-β1 Induce Alterations in Human Amniotic Fluid-Derived Mesenchymal Stem Cells Leading to Cardiomyogenic Differentiation Initiation. Int J Stem Cells. 2019;12:251-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010;19:1449-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | He Y, Chen D, Yang L, Hou Q, Ma H, Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Meng YH, Zhu XH, Yan LY, Zhang Y, Jin HY, Xia X, Li R, Qiao J. Bone mesenchymal stem cells improve pregnancy outcome by inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in mouse. Placenta. 2016;47:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3303] [Article Influence: 150.1] [Reference Citation Analysis (1)] |
| 22. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1847] [Article Influence: 108.6] [Reference Citation Analysis (1)] |
| 23. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 24. | Song HB, Park SY, Ko JH, Park JW, Yoon CH, Kim DH, Kim JH, Kim MK, Lee RH, Prockop DJ, Oh JY. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol Ther. 2018;26:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Vallés G, Bensiamar F, Crespo L, Arruebo M, Vilaboa N, Saldaña L. Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials. 2015;37:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D, Du M. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Xiang YJ, Hou YY, Yan HL, Liu H, Ge YX, Chen N, Xiang JF, Hao CF. Mesenchymal stem cells-derived exosomes improve pregnancy outcome through inducing maternal tolerance to the allogeneic fetus in abortion-prone mating mouse. Kaohsiung J Med Sci. 2020;36:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Qin W, Tang Y, Yang N, Wei X, Wu J. Potential role of circulating microRNAs as a biomarker for unexplained recurrent spontaneous abortion. Fertil Steril. 2016;105:1247-1254.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Tal R, Shaikh S, Pallavi P, Tal A, López-Giráldez F, Lyu F, Fang YY, Chinchanikar S, Liu Y, Kliman HJ, Alderman M 3rd, Pluchino N, Kayani J, Mamillapalli R, Krause DS, Taylor HS. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17:e3000421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Sadighi-Moghaddam B, Salek Farrokhi A, Namdar Ahmadabad H, Barati M, Moazzeni SM. Mesenchymal Stem Cell Therapy Prevents Abortion in CBA/J × DBA/2 Mating. Reprod Sci. 2018;25:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Salek Farrokhi A, Zarnani AH, Moazzeni SM. Mesenchymal stem cells therapy protects fetuses from resorption and induces Th2 type cytokines profile in abortion prone mouse model. Transpl Immunol. 2018;47:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Rezaei Kahmini F, Shahgaldi S, Moazzeni SM. Mesenchymal stem cells alter the frequency and cytokine profile of natural killer cells in abortion-prone mice. J Cell Physiol. 2020;235:7214-7223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Shahgaldi S, Rezaei Kahmini F, Moazzeni SM. Mesenchymal stem cell therapy attenuates complement C3 deposition and improves the delicate equilibrium between angiogenic and anti-angiogenic factors in abortion-prone mice. Mol Immunol. 2022;141:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Zhao X, Hu Y, Xiao W, Ma Y, Shen D, Jiang Y, Shen Y, Wang S, Ma J. Efficacy of mesenchymal stromal cells in the treatment of unexplained recurrent spontaneous abortion in mice: An analytical and systematic review of meta-analyses. PLoS One. 2023;18:e0294855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Shin YH, Choi SJ, Kim JK. Mechanisms of Wharton's Jelly-derived MSCs in enhancing peripheral nerve regeneration. Sci Rep. 2023;13:21214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Chen P, Tang S, Li M, Wang D, Chen C, Qiu Y, Fang Z, Zhang H, Gao H, Weng H, Hu K, Lin J, Lin Q, Tan Y, Li S, Chen J, Chen L, Chen X. Single-Cell and Spatial Transcriptomics Decodes Wharton's Jelly-Derived Mesenchymal Stem Cells Heterogeneity and a Subpopulation with Wound Repair Signatures. Adv Sci (Weinh). 2023;10:e2204786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 37. | Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J Cell Physiol. 2020;235:9230-9240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Chen X, Yang X, Wu R, Chen W, Xie H, Qian X, Zhang Y. Therapeutic effects of Wharton jelly-derived mesenchymal stem cells on rat abortion models. J Obstet Gynaecol Res. 2016;42:972-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Ding X, Wu R, Jin B, Zhu C, Zhang Y, Yang X. Human Wharton's jelly-derived mesenchymal stem cells prevent pregnancy loss in a rat by JAK/STAT-mediated immunomodulation. J Obstet Gynaecol Res. 2023;49:2417-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Jin B, Ding X, Dai J, Peng C, Zhu C, Wei Q, Chen X, Qiang R, Ding X, Du H, Deng W, Yang X. Deciphering decidual deficiencies in recurrent spontaneous abortion and the therapeutic potential of mesenchymal stem cells at single-cell resolution. Stem Cell Res Ther. 2024;15:228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 41. | Gasiūnienė M, Valatkaitė E, Navakauskienė R. Long-term cultivation of human amniotic fluid stem cells: The impact on proliferative capacity and differentiation potential. J Cell Biochem. 2020;121:3491-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Topoluk N, Hawkins R, Tokish J, Mercuri J. Amniotic Mesenchymal Stromal Cells Exhibit Preferential Osteogenic and Chondrogenic Differentiation and Enhanced Matrix Production Compared With Adipose Mesenchymal Stromal Cells. Am J Sports Med. 2017;45:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Yang L, Zhu S, Li Y, Zhuang J, Chen J, Huang H, Chen Y, Wen Y, Wen Y, Guo H, Fan X, Yuan W, Jiang Z, Wang Y, Wu X, Zhu P. Overexpression of Pygo2 Increases Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Cardiomyocyte-like Cells. Curr Mol Med. 2020;20:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Gan L, Duan H, Xu Q, Tang YQ, Li JJ, Sun FQ, Wang S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Xiao Y, Zeng F, Sun J. The improvement of inflammatory infiltration and pregnancy outcome in mice with recurrent spontaneous abortion by human amniotic mesenchymal stem cells. Biol Reprod. 2024;111:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Naeem A, Gupta N, Naeem U, Elrayess MA, Albanese C. Amniotic stem cells as a source of regenerative medicine to treat female infertility. Hum Cell. 2023;36:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 47. | Chen K, Bai L, Lu J, Chen W, Liu C, Guo E, Qin X, Jiao X, Huang M, Tian H. Human Decidual Mesenchymal Stem Cells Obtained From Early Pregnancy Improve Cardiac Revascularization Postinfarction by Activating Ornithine Metabolism. Front Cardiovasc Med. 2022;9:837780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Ning G, Guo X, Zhu K, Xu Z, Cai P, Dang Y, Lu C, Xu F, Shen R, Kang N, Zhang R, Chen K. Human decidual mesenchymal stem cells obtained from early pregnancy attenuate bleomycin-induced lung fibrosis by inhibiting inflammation and apoptosis. Int Immunopharmacol. 2024;142:113224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Büyükbayrak EE, Gündoğdu NEÖ, Gürkan N, Kahraman FR, Akalın M, Akkoç T. Immunological effects of human decidual mesenchymal stem cells in spontaneous and recurrent abortions. J Reprod Immunol. 2024;162:104193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Zhidu S, Ying T, Rui J, Chao Z. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res Ther. 2024;15:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 51. | Hoseini SM, Montazeri F. Cell origin and microenvironment: The players of differentiation capacity in human mesenchymal stem cells. Tissue Cell. 2025;93:102709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Volarevic V, Bojic S, Nurkovic J, Volarevic A, Ljujic B, Arsenijevic N, Lako M, Stojkovic M. Stem cells as new agents for the treatment of infertility: current and future perspectives and challenges. Biomed Res Int. 2014;2014:507234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Rahimi Darehbagh R, Seyedoshohadaei SA, Ramezani R, Rezaei N. Stem cell therapies for neurological disorders: current progress, challenges, and future perspectives. Eur J Med Res. 2024;29:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 54. | Dai W, Xu B, Ding L, Zhang Z, Yang H, He T, Liu L, Pei X, Fu X. Human umbilical cord mesenchymal stem cells alleviate chemotherapy-induced premature ovarian insufficiency mouse model by suppressing ferritinophagy-mediated ferroptosis in granulosa cells. Free Radic Biol Med. 2024;220:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Fu Y, Zhang M, Sui B, Yuan F, Zhang W, Weng Y, Xiang L, Li C, Shao L, You Y, Mao X, Zeng H, Chen D, Zhang M, Shi S, Hu X. Mesenchymal stem cell-derived apoptotic vesicles ameliorate impaired ovarian folliculogenesis in polycystic ovary syndrome and ovarian aging by targeting WNT signaling. Theranostics. 2024;14:3385-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/