Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.107717
Revised: April 30, 2025
Accepted: July 4, 2025
Published online: August 26, 2025
Processing time: 145 Days and 15.1 Hours
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, are characterized by the progressive loss of neuronal function and structure, leading to severe morbidity and mortality. Current therapeutic approaches are ineffective at stopping or reversing disease pro
Core Tip: This review emphasizes the incidence and social burden of neurodegenerative diseases, focusing on disease pathogenesis and available therapeutic approaches. We have thoroughly investigated the potential of mesenchymal stem cells in treating neurodegenerative diseases and explored their therapeutic effects on different diseases. This review aims to elucidate how mesenchymal stem cells can alleviate neurodegenerative disease progression by modulating molecular pathways related to abnormal protein accumulation, neuroinflammation, oxidative stress, and neurodegeneration.
- Citation: Cui CX, Shao XN, Li YY, Qiao L, Lin JT, Guan LH. Therapeutic potential of mesenchymal stem cells in neurodegenerative diseases. World J Stem Cells 2025; 17(8): 107717
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/107717.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.107717
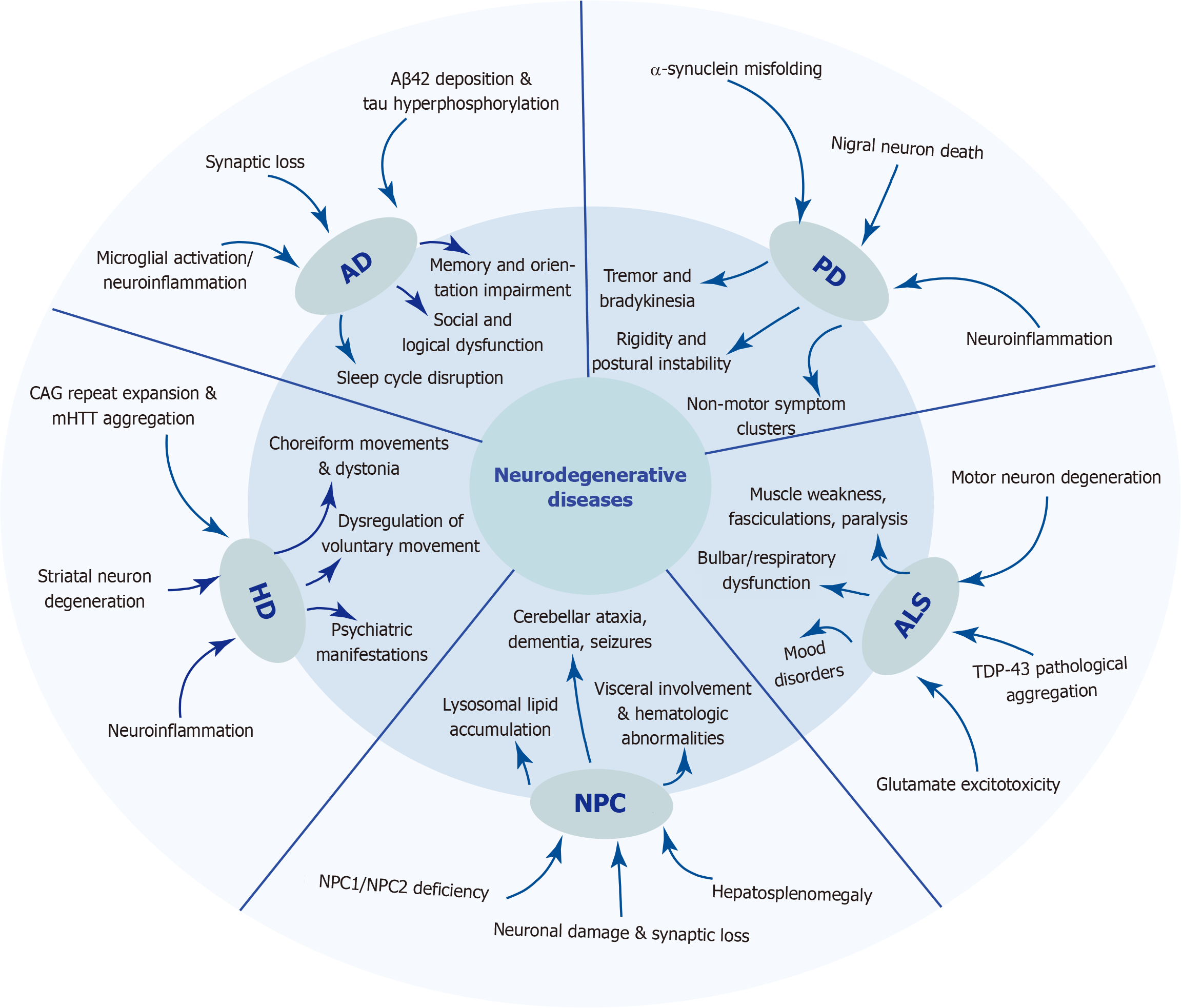
Neurodegenerative diseases are disorders characterized by the progressive loss of neuronal function and structure in the central nervous system, and they are often accompanied by myelin damage and synaptic dysfunction[1].These conditions tend to gradually worsen over time, resulting in severe neurological deficits. These diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS) and Niemann-Pick disease type C (NPC). Neurodegenerative diseases are associated with aging; as age increases, misfolded protein accumulation in the brain eventually leads to progressive loss of cognitive and/or motor functions in patients[2]. Figure 1 summarizes the main pathogenic mechanisms and clinical manifestations of five neurodegenerative diseases. With the increasing aging of the global population, the incidence and prevalence of neurodegenerative diseases have significantly increased[3]. Neuronal degeneration not only severely affects patients’ quality of life, but has a series of secondary impacts, imposing a heavy burden on society[4]. The etiology of neurodegenerative diseases is multifaceted, with certain conditions such as AD, HD, and ALS being precipitated by genetic mutations. Moreover, a lack of exercise and unhealthy diet can also increase the risk of disease onset. Currently, interventions for neurodegenerative diseases such as AD, PD, HD, ALS, and NPC primarily focus on symptomatic treatment and non-pharmacological therapies, such as lifestyle modifications and peer and caregiver support[5].
The therapeutic management of AD primarily relies on pharmacological interventions, with cholinesterase inhibitors often used in combination with memantine. Notably, phase III trials of anti-amyloid-β (anti-Aβ) monoclonal antibodies, such as lecanemab and donanemab, have validated the clinical viability of disease-modifying therapies that target pathological proteins[6,7]. In PD, therapeutic strategies primarily focus on modulating α-synuclein (αSN) pathology, complemented by a well-established dopaminergic treatment system including levodopa, dopamine receptor agonists, and monoamine oxidase B inhibitors. The drug development pipeline for PD remains robust, with a wide variety of approaches being developed and evaluated in phase I and II. However, only a limited number of disease-modifying therapies have advanced to phase III[8]. Therapeutic options for ALS are currently relatively limited, and only three disease-modifying drugs are currently approved by the United States Food and Drug Administration (FDA). These agents target various pathological mechanisms, including excitotoxicity, oxidative stress, mitochondrial dysfunction, protein homeostasis, nucleocytoplasmic transport, and neuroinflammation. However, the therapeutic efficacy of these agents against this devastating disease remains modest[9]. This has stimulated a rapidly expanding pipeline of investigational drugs, and superoxide dismutase 1 (SOD1)-targeting antisense oligonucleotides have established a precedent for gene-specific therapies[10]. Tetrabenazine and deutetrabenazine are currently approved for treating HD-associated chorea, whereas several other investigational drugs are in clinical trials[11]. Therapeutic options for NPC remain limited, with only two United States FDA-approved disease-modifying drug currently available (Miplyffa and Aqneursa). Nevertheless, several promising investigational therapies, including intravenous cyclodextrin and acetyl-L-leucine, have demonstrated encouraging preliminary results in late-stage clinical trials[12]. However, no approaches have halted or reversed disease progression[4].
Stem cells are endowed with self-renewal capacity and the potential to differentiate into multiple lineages. Stem cell transplantation represents a promising and rapidly advancing therapeutic approach for several neurological disorders[13]. Stem cell-derived extracellular vesicles (EVs) are considered natural therapeutic agents and innate drug delivery systems for the treatment of brain diseases because of their low immunogenicity, high biosafety, ability to effectively penetrate the blood-brain barrier (BBB), and anti-inflammatory and antioxidant effects[14]. Furthermore, engineered EVs obtained by modifying natural EVs via gene editing, chemical modification or bioengineering techniques can achieve precise targeting of neurodegenerative disease lesions by decoration of their surfaces with targeting peptides, nanobodies, or other molecules[15,16]. Mesenchymal stem cells (MSCs) are adult stem cells that originate from the mesoderm and can self-renew and undergo multipotent differentiation into various lineages[17]. MSCs are easy to collect, isolate and culture, they exhibit low immunogenicity, and perform immunomodulatory functions; furthermore, they have the potential to differentiate into neurons and glial cells[18]. Therefore, MSCs and their derivatives hold great promise as therapeutic approaches for treating neurodegenerative diseases.
Although neurodegenerative diseases exhibit significant differences in clinical manifestations and pathological features, they share several common biological mechanisms. Protein misfolding and aggregation represent one of the core pathological hallmarks of neurodegenerative diseases[2]. Together with mechanisms such as mitochondrial dysfunction, oxidative stress, neuroinflammation, and synaptic dysfunction, protein misfolding and aggregation jointly drive neuronal degeneration and death[4]. Here, we provide an overview of the characteristics and traditional treatment methods for AD, PD, HD, ALS, and NPC.
AD is a highly prevalent neurodegenerative disease characterized as progressive and irreversible[19]. It leads to varying degrees of mental and cognitive impairments, including impairments in attention, social cognition, processing speed, working memory, verbal learning, and visual learning, and poses a serious threat to human health[20,21]. In the United States, approximately 1 in 9 people aged 65 years and above currently have AD[22]. It is projected that the number of individuals worldwide with AD will exceed 100 million by 2050[23]. People 65 years and older can survive an average of 4-8 years after being diagnosed with AD[19].
The etiology of AD is complex and is influenced by a combination of factors, including age, genetics, and the environment[24]. Currently, the mainstream pathophysiology of AD is believed to involve the progressive accumulation of amyloid plaques (also known as senile plaques) and neurofibrillary tangles, which lead to the loss of neurons related to cognition and memory in the brain[25-27]. This results in brain atrophy, depletion of cholinergic neurons and ace
The approved drugs for AD can be categorized into three main classes: Acetylcholinesterase inhibitors, N-methyl-D-aspartate receptor modulators (memantine), and anti-Aβ monoclonal antibodies[32-34]. However, these available drugs can only provide symptomatic relief for AD patients and are not capable of effectively curing the disease. In the past few decades, most clinical drugs have been discontinued because of their limited efficacy or adverse reactions[35]. Although the anti-Aβ monoclonal antibodies abucanumab, which was approved by the United States FDA in June 2021, and lecanemab, which was approved in July 2023, have shown good therapeutic effects, their long-term effectiveness and safety require further validation[21]. The multifactorial nature of AD onset, compounded by the intricate interplay among various factors, presents formidable challenges for drug development and substantially raises the bar for the next generation of AD therapeutics[21].
PD is the second most prevalent neurodegenerative disorder in the world and affects more than 6 million people globally, posing a significant threat to human health[36-39]. It is characterized by the progressive degeneration of dopaminergic neurons and the formation of αSN protein aggregates in the substantia nigra, which are known as Lewy bodies and Lewy neurites[40-42]. These pathological changes lead to motor symptoms, such as bradykinesia, muscle stiffness, resting tremor, and postural instability, as well as nonmotor symptoms such as hyposmia, depression, sleep disturbance, and cognitive deficits[36,43,44]. The exact mechanisms that lead to neuronal death in PD are not fully understood, but they are likely multifactorial and involve neuroinflammation, mitochondrial dysfunction, oxidative stress, genetic factors, glial dysfunction, excitotoxicity and other factors[45,46].
To date, there are no effective treatments to reverse the pathological progression of dopaminergic neurodegeneration[47]. Traditional treatment methods primarily include pharmacological therapy, surgical therapy, and physical therapy[48]. Levodopa, dopamine agonists, monoamine oxidase B inhibitors, and catechol-O-methyltransferase inhibitors are common drug treatments for PD, but long-term administration may reduce their efficacy and lead to side effects, including involuntary motor movements and movement disorders, affecting patients’ quality of life[49]. Deep brain stimulation (DBS), including subthalamic nucleus DBS and medial globus pallidus DBS, is used to treat motor fluctuations and dyskinesia. This treatment can reduce the dependence of patients on drugs such as levodopa, reduce side effects caused by drugs and improve patient quality of life. However, the destruction of neural nuclei caused by DBS surgery may lead to irreversible nerve injury[50,51]. Physical therapy plays an important role in the treatment of PD, helping to increase muscle strength and flexibility of patients[52]. Nevertheless, the effect of physical therapy is limited and requires continuous investment. In general, the treatment of PD faces many challenges, and new therapeutic methods and strategies to improve patient quality of life are needed.
HD is a fatal, incurable autosomal dominant inherited neurodegenerative disorder caused by an autosomal dominant mutation in the Huntingtin (HTT) gene[53]. The molecular pathology of HD is complex and involves aggregate formation, transcriptional dysregulation, synaptic plasticity alterations, and glial dysfunction[54]. Clinically, HD patients exhibit uncontrolled movements, mood disorders, and progressive cognitive dysfunction[54,55]. As the disease progresses, it may develop into dystonia, rigidity, and ataxia[56].
Treatment for HD primarily focuses on the symptomatic management of chorea, as well as psychiatric and cognitive symptoms. Tetrabenazine and deuterated tetrabenazine, which are United States FDA-approved medications, are effective in reducing HD-associated chorea[57,58]. HD-associated dementia symptoms can be treated with clozapine or acetylcholinesterase inhibitors, albeit with limited success[11]. For patients who exhibit suboptimal responses to pharmacological treatments or who experience severe adverse drug reactions, DBS may present an effective therapeutic option. However, current therapies are primarily aimed at alleviating symptoms rather than reversing or halting neuronal damage. Consequently, more clinical trials and research studies are needed to further explore treatment options for HD[59].
ALS is a severe neurodegenerative disease characterized by progressive muscle paralysis due to motor neuron degeneration in the motor cortex of the brainstem and spinal cord[60]. The primary clinical symptoms include progressive muscle weakness, muscle atrophy, dysphagia, and dysarthria, and in rare cases, episodes of respiratory failure[61,62]. As a heterogeneous syndrome, ALS pathogenesis is very complex and involves numerous different cellular mechanisms such as oxidative stress, mitochondrial dysfunction, glutamate excitotoxicity, axonal transport, impaired protein degradation, hypermetabolism, and RNA defects[63]. The global prevalence and incidence rates of ALS are estimated at 4.42 cases per 100000 people and 1.59 cases per 100000 person-years, respectively[64]. Most patients die within 2-5 years after initial symptom onset[65]. The United States FDA has approved three drugs for the treatment of ALS, namely riluzole, edaravone, and tofersen injection[9]. However, the contributions of these drugs to extending patient survival time and improving quality of life are limited, and they also have side effects on other organs[66-68].
NPC is a neurodegenerative lysosomal cholesterol storage disorder caused by mutations in the NPC1 or NPC2 genes. NPC is characterized by the accumulation of cholesterol and glycolipids within the endosomal/Lysosomal system, leading to hepatosplenomegaly and progressive neurodegeneration[12]. The disease is classified into the following categories according to the age of symptom onset: Early infantile form (onset before 2 years), late infantile form (onset between 2 and 6 years), juvenile form (onset between 6 and 15 years), and adult form (onset after 15 years)[69].
The first approved therapeutic agent with the potential to modify NPC progression was miglustat, an inhibitor of glycosphingolipid synthesis[70]. Miglustat has several limitations in the treatment of NPC, as it cannot completely prevent disease progression and does not easily restore damaged tissues and organs to a normal state. Furthermore, its applicability is limited to a subset of patients and is accompanied by numerous adverse effects. Recently, Miplyffa (arimoclomol) and Aqneursa (levacetylleucine) have been approved by the United States FDA for the treatment of NPC[71,72]. When used in combination with miglustat, Miplyffa can improve the neurological symptoms associated with NPC in children aged 2 years and older and in adults[73]. However, the drug may cause allergic reactions such as urticaria and angioedema, as well as side effects including respiratory tract infections, diarrhea, and weight loss. Early diagnosis and treatment are crucial for improving patient outcomes. Future research is necessary to gain a better understanding of the pathomechanism of these diseases and to explore new therapeutic options[12].
MSCs are a class of adult stem cells with multidirectional differentiation potential, self-renewal capacity, and immunomodulatory properties, that are widely present in a variety of tissues[17]. Many preclinical studies and clinical trials have demonstrated that MSCs have great potential in the treatment of neurodegenerative diseases.
MSCs can be extracted from a variety of tissues, such as the bone marrow, umbilical cord, placenta, adipose tissue, and dental pulp (Table 1). MSCs from different sources have tissue-specific differentiation potential. To describe MSCs more specifically, the abbreviation of the source tissue is typically added before their name[74]. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has established the minimal criteria for defining human MSCs. First, MSCs must be plastic-adherent under standard culture conditions. Second, MSCs must express CD105, CD73, and CD90, but lack expression of the CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules. Third, MSCs must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro[75].
| Type | Advantage | Limitations | Ref. |
| BM-MSCs | First to be discovered, abundant sources, possesses good differentiation capacity and immune regulatory functions | Invasive collection process, age-related decline in proliferation capacity and differentiation potential | [76-79] |
| ADSCs | Abundant sources, relatively easy collection process | Cell quality influenced by the age, weight, and disease status of donor | [80,81] |
| UC-MSCs | Convenient collection, no ethical issues, strong proliferative capacity, high differentiation potential, and low immunogenicity | Requires establishment of cord blood banks for preservation and management | [82,83] |
| PMSCs | Easily accessible, convenient collection, limited ethical concerns and low immunogenicity | Placental tissue has complex origin and requires strict screening and processing, needs to be collected from a healthy mother giving birth to a healthy baby | [84] |
| DPSCs | No ethical concerns, low immunogenicity, and high regenerative potential | Limited opportunities for collection | [85,86] |
| MenSCs | No ethical concerns, low immunogenicity, and strong cell proliferation capacity | Proliferation capacity influenced by donor age, passage number, and storage time | [87,88] |
Bone marrow-derived MSCs (BM-MSCs) were the first adult MSCs to be discovered and have become one of the most widely used sources of MSCs[76]. BM-MSCs exhibit well-defined differentiation potential, robust immunomodulatory capacity and broad clinical applicability. BM-MSCs are typically isolated from bone marrow aspirates obtained via iliac crest or sternal puncture[77]. Despite the invasive harvesting procedure, limited initial cell yield, and age-dependent decline in donor cells, BM-MSCs possess remarkable expansion capacity and have tremendous potential in bone repair, cartilage regeneration, and immunomodulation[78,79]. The primary advantages of BM-MSCs over other types of MSCs include the abundance of tissue sources for isolation, the ease of tissue collection and cell isolation, and their therapeutic potential.
Adipose-derived MSCs (ADSCs) are predominantly derived from adipose tissue within the human body. ADSCs have the advantages of abundant sources, minimally invasive harvesting procedures, high cell yields, and rapid proliferation rates. ADSCs also possess superior adipogenic differentiation ability and are more suitable for autologous trans
Human umbilical cord-derived MSCs (hUC-MSCs) and placenta-derived MSCs (PMSCs) are isolated from the umbilical cord and placenta, respectively. Both types of MSCs are characterized by noninvasive harvesting techniques, low immunogenicity, robust proliferative capacity, and fewer ethical issues. These features make them suitable for allogeneic transplantation and endow them with unique advantages in clinical applications[82-84]. However, the placenta is a complex tissue, and it is important to obtain it from healthy mothers who have given birth to healthy babies. Dental pulp-derived MSCs (DPSCs) are isolated from the pulp of exfoliated deciduous teeth or wisdom teeth. Although the opportunities for acquisition are limited, DPSCs raise no ethical concerns and possess low immunogenicity and high regenerative potential[85,86]. Additionally, MSCs can also be isolated from synovial tissue and menstrual blood, expanding the range of accessible sources for regenerative therapies[87]. Menstrual blood-derived endometrial stem cells (MenSCs) have increased proliferation rates and low immunogenicity with few ethical issues, and they can be obtained noninvasively; therefore, MenSCs have been extensively studied in recent years[88].
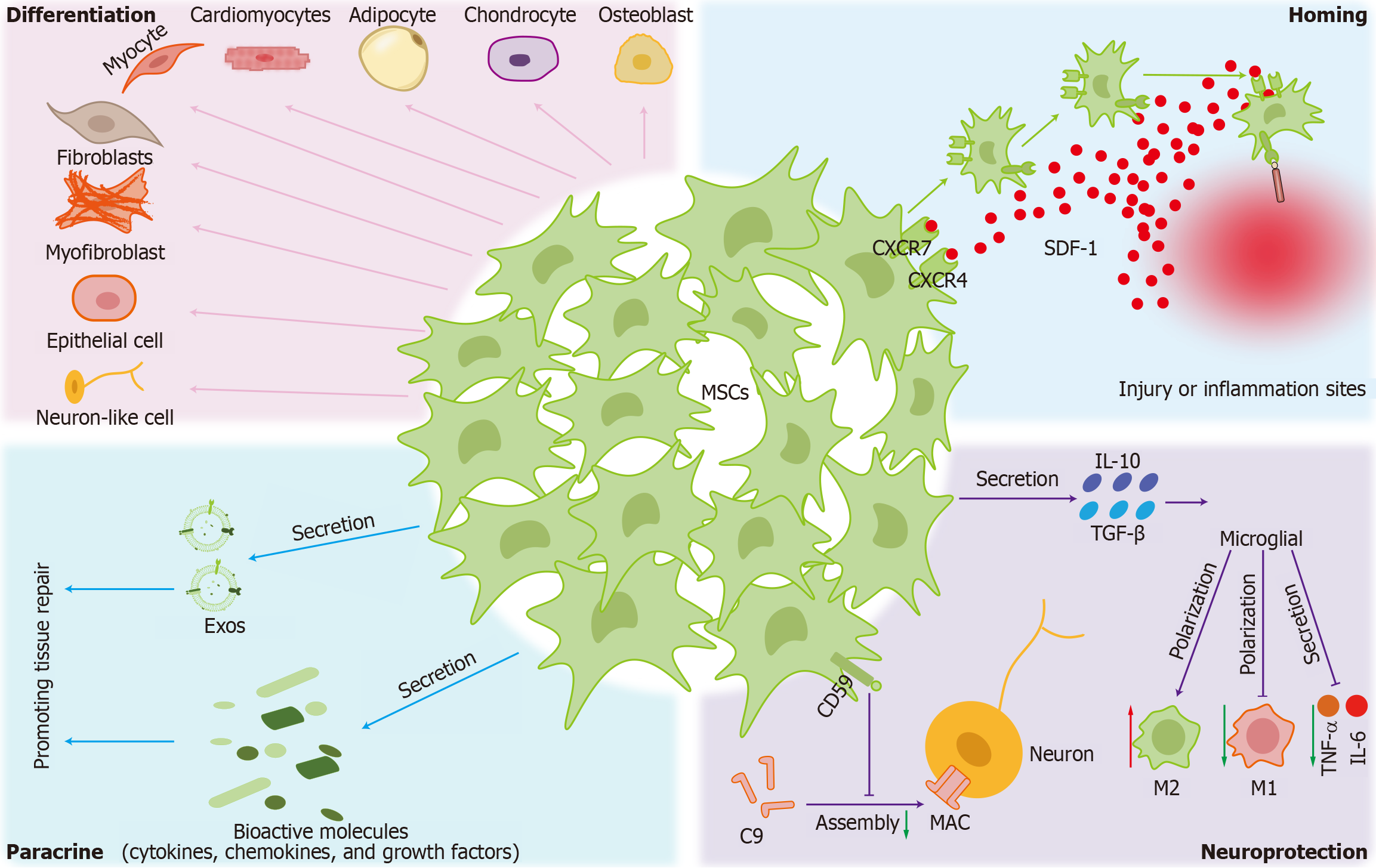
The therapeutic mechanisms of MSCs involve several complex and interrelated biological processes, including homing, differentiation capacity, paracrine effects, and neuroprotective actions (Figure 2).
Homing: The homing mechanism of MSCs involves multiple steps and the interaction of various molecules[89]. First, when MSCs enter the bloodstream, CD44 on their surface binds transiently and reversibly to selectins, such as E-selectin, P-selectin, and L-selectin on endothelial cell surfaces, resulting in a rolling phenomenon. During this rolling process, MSCs encounter chemokines, such as stromal cell-derived factor 1 produced by local tissues. These chemokines bind to G-protein-coupled chemokine receptors on the surface of MSCs, activating intracellular signaling pathways. MSCs express various chemokine receptors, such as C-X-C chemokine receptor type 4 (CXCR4) and CXCR7. After binding to their receptors, chemokines activate signaling pathways within MSCs, leading to conformational changes that activate integrins, enhancing MSC adhesion to endothelial cells[90]. After activation, the integrins on MSCs bind to their corresponding ligands on endothelial cell surfaces, forming a strong adhesion that allows MSCs to stably attach to endothelial cells. MSCs secrete various proteases, such as matrix metalloproteinases, which can degrade the extracellular matrix and the connections between endothelial cells. Through these proteases, MSCs can break through the endothelial layer and enter the tissue interstitium. Subsequently, MSCs migrate along the chemokine concentration gradient and eventually reach the site of injury or disease. These MSCs then exert their therapeutic effects, promote tissue regeneration, and regulate the immune response[91].
Differentiation: MSCs possess multipotent differentiation capacity, enabling their commitment to both mesodermal and non-mesodermal lineages. In addition to their well-documented osteogenic, adipogenic, and chondrogenic potential, MSCs can differentiate into myocytes, cardiomyocytes, fibroblasts, myofibroblasts, epithelial cells, and neuron-like cells, highlighting their remarkable plasticity[92]. MSCs isolated from different tissues exhibit strong phenotypic similarity in their differentiation potential, while their lineage commitment is significantly influenced by in vitro culture conditions, particularly growth factor stimulation[93]. Culturing conditions, such as passage number, cell density, and oxygen concentration, can affect MSC differentiation efficiency. MSC multipotency has broad prospects for regenerative medicine. By optimizing induction conditions and the microenvironment, the differentiation efficiency of MSCs can be further increased, providing new strategies for clinical treatment[94].
Paracrine signaling: MSCs modulate the microenvironment and promote tissue repair by secreting exosomes (Exos) and bioactive molecules, including cytokines, chemokines, growth factors and EVs. MSCs produce extensive secretemany factors, such as interleukin (IL)-10, stromal cell-derived factor 1, IL-6, transforming growth factor-β (TGF-β) and some growth factors and chemokine ligands[95]. Although initial studies suggested that MSCs may play a pivotal role in tissue repair, the paracrine efficacy of MSCs can be affected by in vitro factors, including number of passages, culture conditions and donor source, as well as in vivo administration methods. Accumulating evidence has revealed low survival rate and engraftment potential in damaged tissue regions, significantly limiting their therapeutic efficacy in regenerative applications[96-98]. Further research indicates that the therapeutic benefits of MSCs in tissue repair are primarily due to their paracrine signaling, which involves EV secretion[99]. Emerging studies have demonstrated that Exos can function as substitutes for whole MSC-based therapies in various injury and disease models, offering a cell-free alternative with comparable therapeutic effects[100].
Neuroprotection: In neurodegenerative diseases and neural injuries, MSCs perform significant anti-inflammatory functions. MSCs exert neuroprotective effects by secreting TGF-β and IL-10 to suppress the microglial release of proinflammatory factors (e.g., tumor necrosis factor-α and IL-6) and promote microglial polarization toward the anti-inflammatory M2 phenotype, thereby mitigating neuronal damage[101]. MSCs also express CD59, which inhibits the formation of the membrane attack complex, thereby modulating the complement system to protect neurons[102]. Through paracrine secretion of neurotrophic factors and direct differentiation, MSCs promote axonal regeneration and synaptic remodeling[103,104]. In addition, MSCs can regulate neuronal cell survival and function through secreted Exos and microRNAs[105], and MSCs can further improve neuronal function through electrophysiological modulation and metabolic regulation[106].
The bioactive substances released by MSCs through paracrine action, collectively known as MSC derivatives, possess immunoregulatory and regenerative repair potential similar to that of MSCs[107-109]. Owing to their low immunogenicity, low risk of tumor formation, and favorable safety profile, MSC derivatives may be superior to MSC trans
In recent years, substantial advances have been achieved in the application of MSCs and their derivatives for the treatment of AD. A multitude of preclinical studies utilizing diverse animal models have consistently demonstrated the neuroprotective potential of MSCs and their derivatives. These effects include reducing Aβ deposition, modulating neuroinflammation, and promoting neuroregeneration, ultimately leading to improvements in AD-associated cognitive impairment. Table 2 provides a comprehensive summary of the current major studies on MSCs and their derivatives in the context of AD treatment.
| Animal model | Source | Type | Administration method | Main outcomes | Ref. |
| C57BL/6 mice with Aβ25-35 | Mice | BM-MSCs | IV | Upregulate BDNF expression, downregulate GSK-3β activity, improve cognitive dysfunction | [116] |
| C57BL/6 mice with Aβ25-35 | Mice | BM-MSCs | IV | Inhibit microglial activation, improve behavioral deficits, reduce neuroinflammatory cytokines | [115] |
| APP/PS1 mice | Human | hUC-MSCs, SHED, ADSCs | IV | Reduce amyloid plaques, improve behavioral deficits, and increase neuronal and Nissl body density in brain regions | [117] |
| APPswe/PS1dE9 mice | Human | OM-MSCs | SI | Alleviate AD symptoms and promote Aβ clearance | [118] |
| C57BL/6 mice with Aβ1-42 | Human | BM-MSC-EVs | SI | Stimulate neurogenesis in the subventricular zone, alleviate cognitive impairment | [121] |
| C57BL/6 mice with STZ | Human | iPSC-MSC-sEVs | SI | Alleviate neuroinflammation, reduce amyloid deposition and neuronal apoptosis, and mitigate cognitive dysfunction | [120] |
| C57BL/6 mice with AlCl3 | Mice | BM-MSC-EVs | IP | Regulate autophagy through the PI3K/AKT/mTOR pathway, promote Aβ degradation, modulate immunity, and improve memory and neurological dysfunction | [122] |
| C57BL/6 mice with AlCl3 | Mice | ADSC | IV | Reduce amyloid deposition, mitigate cognitive dysfunction | [119] |
| Zebrafish with LPS | Human | ADSC-EVs | IV | Reduce LPS-induced inflammatory cytokines | [123] |
| C57BL/6 mice with Aβ1-42 | Mice | BM-MSCs | IV | Induce mitophagy in neuronal cells, alleviating mitochondrial damage-mediated apoptosis and NLRP3 inflammasome activation | [125] |
| C57BL/6 mice with STZ | Mice | BM-MSC-Exos | SI | Modulate hippocampal glial cell activation, alleviate neuroinflammation, mitigate cognitive dysfunction | [127] |
| APP/PS1 mice | Human | hUC-MSCs | IV | Inhibit glial cell activation and oxidative stress | [128] |
| APP/PS1 mice | Mice | BM-MSCs | HIPP injection | Reduce number of Aβ plaques and increase M2 microglial polarization | [101] |
| C57BL/6 mice | Mice | BM-MSCs | SI | Stimulate endogenous neurogenesis | [130] |
| C57BL/6 mice with Aβ1-42 | Human | OE-MSCs | IN | Upregulate BDNF and NMDAR; reduce neuronal loss | [132] |
| C57BL/6 mice with STZ | Human | hUC-MSC-Exos | IV | Increase adiponectin levels and protect neurons | [133] |
| C57BL/6 mice with AlCl3 | Mice | ADSCs | IV | Reduce amyloid deposition, mitigate cognitive dysfunction | [119] |
| SH-SY5Y cell with Aβ1-40 | Human | Exosomes derived from the serum of AD patients | Coculture | Reduce apoptosis through the PI3K/AKT signaling pathway | [134] |
| APP/PS1 mice | Human | hUC-MSCs | IV | Enhance targeting ability of hUC-MSCs and promote production of neuroprotective factors; improve cognitive function | [136] |
Remove abnormally accumulated proteins: Aβ accumulation is a general feature of AD[113,114]. Intravenous injection of BM-MSCs during pregnancy can reduce the Aβ burden induced by Aβ25-35 Levels in female mice, thereby improving cognitive dysfunction[115,116]. The administration of MSCs derived from the umbilical cord, dental pulp, and adipose tissue via the tail vein can significantly reduce Aβ levels, behavioral deficits, and phosphorylated tau in the hippocampus and frontal cortex of APP/PS1 model mice, while increasing neuronal count and Nissl body density in brain regions[117]. Olfactory mucosa MSC (OM-MSC) transplantation through brain stereotaxic injection can alleviate AD symptoms and promote Aβ clearance[118]. Intravenous injection of ADSCs may inhibit amyloid production induced by intraperitoneal aluminum chloride injection in AD rats by activating of the sirtuin-1 signaling pathway, thereby alleviating cognitive dysfunction[119]. Therefore, MSCs can improve AD-associated cognitive impairment by reducing Aβ deposition.
MSC derivatives can also reduce Aβ accumulation in AD. For example, the intracisternal administration of BM-MSC-derived Exos or EVs can mitigate the cognitive dysfunction induced by Aβ1-42 or streptozotocin (STZ)[120,121]. In addition, intraperitoneal injection of BM-MSC-derived EVs(BM-MSC-EVs) can regulate autophagy through the phos
Neuroinflammation reduction: Persistent reactive oxygen species (ROS) and neuroinflammation are key factors that contribute to AD[123]. Intravenous injection of ADSC-derived EVs into the zebrafish larval brain significantly decreased the lipopolysaccharide-induced levels of proinflammatory cytokines and oxidative stress[123]. The NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is a multimeric protein complex that participates in the innate immune system and plays a vital role in inflammatory reactions[124]. The intracisternal administration of BM-MSC-EVs inhibits neuroinflammation and mitigates cognitive dysfunction in AD mice by suppressing the NLRP3/gasdermin D pathway mediated by miR-223-3p[120]. Nanoscale EVs prepared from BM-MSCs with high tyrosine phosphatase-2 expression can significantly induce mitophagy in neuronal cells, thereby alleviating mitochondrial damage-mediated apoptosis and NLRP3 inflammasome activation[125]. Therefore, MSC-derived EVs inhibit neuroinflammation primarily through the NLRP3-mediated signaling pathway and restore the damaged neuronal microenvironment[126].
Activated microglia are among the key sources of neuroinflammation. BM-MSCs not only reduce the number and size of activated microglia, but also markedly increase dendrite length and reverse proinflammatory cytokine elevation in offspring[116]. Furthermore, lateral ventricle administration of BM-MSC-derived Exos can regulate hippocampal glial cell activation and related neuroinflammation in an STZ-induced sporadic AD mouse model, thus improving the cognitive ability of AD mice[127]. Intravenous injection of hUC-MSCs inhibits glial cell activation and oxidative stress by activating nuclear factor erythroid 2-related factor signaling[128]. Stereotaxic injection of OM-MSCs or BM-MSCs into the brain can downregulate the inflammatory response by increasing the ratio of M2/M1 microglia[101,118]. Therefore, MSCs and their derivatives can reduce neuroinflammation by inhibiting proinflammatory cytokines, ameliorating oxidative stress, and reducing glial cell activation.
Promotion of neurogenesis: Progressive neuronal loss in the brain tissue of AD patients leads to cognitive deficits[121,129]. Stereotaxic injection of BM-MSCs into the subventricular zone of mice can stimulate endogenous neurogenesis and exert a neuroprotective effect on the hippocampus of AD rats[130,131]. Intranasal administration of olfactory ecto-MSCs (OE-MSCs) significantly reduced neuronal loss by increasing the levels of brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate receptor in an AD rat model[132]. Intravenous injection of hUC-MSC-derived Exos into an STZ-induced AD mouse model increased adiponectin levels in the peripheral central nervous system and promoted neuroprotective function by improving adult neurogenesis[133]. Additionally, this treatment reduced the apoptotic response in AD through the PI3K/AKT/mTOR signaling pathway[134]. hUC-MSC-derived EVs can treat the pathological characteristics of the APP/PS1 mouse model by regulating the synaptic vesicle cycle signaling pathway[135]. When coated with Fe3O4@PDA, hUC-MSC targeting efficiency can be increased and the memory and cognitive ability of AD mice can be improved via the excessive generation of neuroprotective factors[136]. Therefore, the transplantation of MSCs and their derivatives can induce the sustained production of neurotrophic factors such as BDNF and nerve growth factor, making them excellent therapeutic agents for neuronal repair and regeneration[137,138].
There has been a recent surge of interest in exploring the therapeutic potential of MSCs and their derivatives for the treatment of PD. A growing body of evidence suggests that MSCs exert neuroprotective effects through multiple mechanisms, such as promotion of dopaminergic neuron survival, suppression of neuroinflammation, reduction of αSN aggregation, and increased secretion of neurotrophic factors. Moreover, MSCs derived from different sources have varying degrees of therapeutic efficacy in animal models of PD. Table 3 provides a systematic summary of the key studies investigating MSC-based therapies for PD.
| Animal model | Source | Type | Administration method | Main outcomes | Ref. |
| C57BL/6 mice with MPTP | Human | hUC-MSCs | IN | Inhibit activated glial cells, repair dopamine neuron degeneration, improve motor behavior in mice | [158] |
| C57BL/6 mice with 6-OHDA | Human | hUC-MSCs | IN | Upregulate SATB1, activate Wnt/β-catenin pathway, improve motor behavior, reduce neuronal damage | [159] |
| C57BL/6 mice with 6-OHDA | Human | hUC-MSCs | ST | Increase dopamine levels and improve motor dysfunction | [160] |
| C57BL/6 mice with 6-OHDA | Human | hUC-MSCs | ST | Improve rotational behavior, provide neuroprotection, exhibit anti-neuroinflammatory effects | [161] |
| C57BL/6 mice with 6-OHDA | Human | hUC-MSCs-BDNF | IV | Increase neuronal survival rate | [162] |
| C57BL/6 mice with 6-OHDA | Human | DPSCs | SI | Promote recovery of dopaminergic neurons, reduce dopaminergic neuron loss, improve motor behavior | [163] |
| C57BL/6 mice with 6-OHDA | Human | DPSCs | IV | Improve rotational and forelimb asymmetry behaviors, enhance anti-apoptotic Bcl-2/Bax axis | [164] |
| Zebrafish with 6-OHDA | Human | DPSCs | Yolk sac injection | Improve motor dysfunction | [166] |
| Zebrafish with rotenone | Mice | BM-MSCs | IV | Improve motor and behavioral performance | [167] |
| C57BL/6 mice with MPTP | Mice | BM-MSCs | IN | Restore dopaminergic neurons in the substantia nigra and nerve terminals in the striatum, improve motor deficits | [168] |
| C57BL/6 mice with 6-OHDA | Human | OE-MSCs | IN | Improve motor dysfunction | [169] |
| C57BL/6 mice with MnCl2 | Human | hnmMSC-sEVs | IN | Restore motor dysfunction and enhance neurogenesis | [170] |
| C57BL/6 mice with MPTP | Human | hUC-MSC-Exos | IN | Increase number of dopaminergic neurons in the SNPC region, rescue death of substantia nigra dopaminergic neurons, alleviate inflammatory responses, and improve local microenvironment | [149] |
| C57BL/6 mice with 6-OHDA | Human | hUC-MSC-Exos | IV and LV | Repair damage to the nigrostriatal dopamine system, inhibit microglial activation | [150] |
| C57BL/6 mice with MPTP | Human | hUC-MSCs | IV | Alleviate dopaminergic neuron degeneration, exhibit anti-inflammatory effects | [151] |
| PD patients | Human | OM-MSCs | IT | Promote recovery of neural function, modulate neuroinflammation | [152] |
| C57BL/6 mice and cells with MPTP | Human | T-MSC-Exos | IV | Protect DA neurons through the Nox4-ROS-Nrf2 axis, maintain function of the nigrostriatal system, improve motor deficits, and reduce oxidative stress | [153] |
| C57BL/6 mice with MPTP | Human | BM-MSCs | IV | Reduce neuronal loss, damage, and inflammatory responses, inhibit cell apoptosis | [154] |
| C57BL/6 mice with MPTP | Human | ADSCs | SI | Secrete neuroprotective factors to prevent neuronal damage, reduce dopaminergic neuron loss and alleviate neuroinflammation; protect dopaminergic neurons | [155] |
| SH-SY5Y cells with rotenone | Human | NI-hADSC-CM | Coculture | Provide neuroprotection, alleviate αSN aggregation | [143] |
| Moderate PD patients | Human | BM-MSCs | IV | Safe, well tolerated, and not immunogenic | [142] |
| C57BL/6 mice with AAV 1/2A 53 T-a-syn | Human | BM-MSCs | SI | Reduce αSN levels; protect dopaminergic neurons, modulate microglial cells | [145] |
| C57BL/6 mice with rotenone | Mice | BM-MSCs | SI, IV | Improve motor function, protection of the nigrostriatal system, and improve striatal dopamine release | [146] |
Reduction in αSN aggregate accumulation: One of the main causes of PD is the accumulation of αSN protein aggregates[139]. αSN aggregates can be transmitted to neighboring cells in the form of Lewy bodies and Lewy neurites, thus spreading to a large area of the brain with severe damage[140,141]. Therefore, inhibiting aggregation or directing self-assembly to less toxic aggregates could be therapeutic. In fact, numerous experiments have shown that the secretome of MSCs can trigger neuronal differentiation and protect dopaminergic neurons in vitro and in vivo, reversing the PD phenotype in 6-hydroxydopamine (6-OHDA)-induced PD models[142,143]. The hybrid of Exos from hUC-MSCs and nanoliposomes containing baicalein and oleuropein decreased αSN pathogenicity and increased drug internalization into cells, surpassing the efficacy of nanoliposomes alone; additionally, this formulation crossed the BBB in an in vitro cellular model[144].
Injection of BM-MSCs into the substantia nigra pars compacta (SNPC) and striatum significantly reduced αSN levels in the SNPC in AAV 1/2A 53 T-a-syn-induced neurodegeneration[145]. In addition, intracerebral and tail vein injection of BM-MSCs ameliorated the motor function of PD model rats induced by subcutaneous injection of rotenone and normalized tyrosine hydroxylase, 3,4-dihydroxyphenylalanine decarboxylase, and αSN expression to levels comparable to controls in the nigral and striatal tissues of male rats[146].
Curcumin (Cur) can effectively reduce αSN aggregates in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice[147,148]. The intranasal administration of a microfluidic electroporation chip prepared with MSC-derived neuron-like cell membrane-coated Cur nanoparticles can significantly increase the levels of several neurotransmitters and ameliorate movement disorders in PD mice[106]. A self-oriented nanocarrier termed PR-EXO/PP@Cur, which combines therapeutic MSC-derived Exos with Cur, can improve the motor and coordination abilities of mice with MPTP-induced PD and promote neuronal function recovery following intranasal administration[147].
Alleviation of inflammatory responses: Intranasal administration of hUC-MSC-derived Exos reverses dopaminergic neuron death in mice with MPTP-induced PD and reduces the inflammatory response[149]. hUC-MSC-derived Exos can also be absorbed by dopaminergic neurons and microglia in the substantia nigra, repairing nigral-striatal dopamine system damage and inhibiting microglial activation[150]. Tail vein injection of hUC-MSCs attenuate MPTP-induced microglial activation in the striatum and NLRP3 inflammasome levels[151]. In addition, trophoblast stage-derived MSCs, BM-MSC-derived Exos, ADSCs, and MenSC conditioned medium can also inhibit MPP+-induced cytotoxicity and ameliorate neuroinflammation through different reaction mechanisms[152-156].
Restoration of dopaminergic neurons: hUC-MSCs can significantly alleviate motion defects in PD. Dopaminergic neurons in the SNPC play central roles in motor control. The loss of dopaminergic neurons and the presence of cytoplasmic protein aggregates known as Lewy bodies in the remaining dopaminergic neurons contribute to PD[157]. The intranasal administration of hUC-MSCs significantly alleviates locomotor deficits and rescues dopaminergic neurons by inhibiting neuroinflammation in a MPTP-induced PD mouse model[158]. In addition, intranasal administration of hUC-MSC-derived EVs mitigates motor deficits in a 6-OHDA-induced PD model by upregulating special AT-rich sequence-binding protein-1 and activating the Wnt/β-catenin signaling pathway[159]. Moreover, hUC-MSCs can be efficiently differentiated into dopaminergic neurons by a one-step differentiation system using a YFBP cocktail (Y27632, forskolin, SB431542, and SP600125), which increases dopamine levels in the striatum and significantly ameliorates motor impairments in PD model mice by transiently activating Wnt/β-catenin signaling[160]. hUC-MSCs modified with BDNF and transplanted into the right striatum can improve the rotational behavior of PD model rats induced by 6-OHDA, exerting neuroprotective and anti-neuroinflammatory effects[161]. Similarly, tail vein injection of BDNF-loaded Exos derived from hUC-MSCs can significantly increase neuronal survival in PD models[162].
DPSCs can effectively promote the recovery of dopaminergic neurons. Transplanting DPSCs into the SNPC of PD model mice via stereotaxic brain injection of 6-OHDA can reduce the loss of dopaminergic neurons in the SNPC and improve dopaminergic function[163]. Intravenous injection of human gingival-derived MSCs improves rotational behavior and forelimb misalignment in PD model rats[164]. This finding is consistent with the results of Fujii et al[165]. Chen et al[166] injected stem cells from human exfoliated deciduous teeth (SHED)-conditioned medium at different doses into the yolk sac of zebrafish with 6-OHDA-induced PD. They reported that SHED-conditioned medium repaired motor deficits in a zebrafish PD model similar to the drug nomifensin, which has potential in treating PD. In addition, BM-MSCs and OE-MSCs transplanted into different PD models can restore dopaminergic neurons in the substantia nigra and nerve terminals in the striatum, improve PD-like motor deficits, and enhance neurogenesis[167-170].
HD is a progressive neurodegenerative disorder characterized primarily by the loss of striatal neurons and the development of motor and cognitive dysfunction. Emerging evidence suggests that MSCs may help alleviate the pathological features of HD through multiple mechanisms, including reducing mutant HTT protein aggregation, modulating synaptic plasticity, suppressing neuroinflammation, and providing neurotrophic support. Table 4 summarizes the major research advances in MSC-based therapies for HD, providing a valuable reference for the development of stem cell treatment strategies.
| Animal model | Source | Type | Administration method | Main outcomes | Ref. |
| R62 mice | Human | hUC-MSCs | IP | Regulate microglia; improve neurological dysfunction | [171] |
| R62 mice | Mice | BM-MSCs | IN | Improve neuroinflammation and dopaminergic signaling, increase survival rate | [172] |
| C57BL/6 mice with 3-NP | Human | hUC-MSCs | SI | Decrease gliosis, ameliorate motor coordination and muscle activity, along with an increase in striatal volume and dendritic length of the striatum | [173] |
| C57BL/6 mice with 3-NP | Mice | BM-MSCs | IV | Inhibit 3-NP-induced neurological insults via modulation of the Ca2+/CaN/NFATc4 and Wnt/β-catenin signaling pathways | [174] |
| C57BL/6 mice with 3-NP | Human | DPSCs | IV | Neuroprotection | [175] |
Amelioration of neuroinflammation: Mutation of the HTT protein triggers neuroinflammation, activating microglia and other cells to release inflammatory factors, thereby significantly influencing HD progression. Human full-term placental amniotic MSC-conditioned medium can ameliorate neurological dysfunction in R6/2 mice by regulating microglia[171]. Intranasal administration of BM-MSCs can improve neuroinflammation and dopaminergic signaling in R6/2 mice and increase their survival rates[172].
Neuroprotective effects: The striatum contains various types of neurons and plays a pivotal role in motor control. MSCs can participate in neuroprotection through mechanisms such as the regulation of neurotransmitters. hUC-MSC and hUC-MSC-conditioned medium transplantation into the bilateral striatum can ameliorate motor dysfunction and striatal atrophy in rats with 3-nitropropionic acid (3-NP)-induced PD[173]. The intravenous injection of BM-MSCs in combination with lercanidipine can inhibit 3-NP-induced neurotoxicity by modulating the Ca2+/CaN/NFATc4 and Wnt/β-catenin signaling pathways. This treatment also increases BDNF, forkhead box protein 3, Wnt, and β-catenin expression in the striatum. These findings indicate that the combination of lercanidipine and BM-MSCs has neuroprotective effects and may represent a feasible approach to increasing the beneficial effects of stem cell therapy for treating HD[174]. Furthermore, intravenous administration of DPSCs can exert neuroprotective effects by restoring BDNF expression in the cortex and striatum of 3-NP-induced rats[175].
MSCs and their derivatives have shown significant potential for neuroprotection and anti-inflammatory effects in the treatment of ALS. The pathophysiology of ALS is characterized by motor neuron degeneration, neuroinflammation, and glutamate excitotoxicity. Current research suggests that MSCs may slow disease progression through multiple mecha
| Animal model | Source | Type | Administration method | Main outcomes | Ref. |
| In vitro SIM-A9 hSOD1 (G93A) microglial cells | Mice | ADSCs | Coculture | Reduce metabolic activity of microglia, decrease iNOS+ cells, and increase CD206+ cells | [176] |
| BV-2 cells with SOD 1-G93 A | Human | hUC-MSC-CM | IV | Extend lifespan and reduce expression of pro-inflammatory cytokines and iNOS | [178] |
| SOD 1G 93 A mice | Human | BM-MSCs | IV | Mediate anti-inflammatory responses through the CX3CL1/CX3CR1 axis | [179] |
| SOD 1G 93 A mice | Mice | BM-MSCs | IV | Upregulate expression of Nrf2 and NQO1, promote antioxidant responses, and reduce accumulation of reactive oxygen species | [180] |
| ALS patients | Human | BM-MSCs | IT | Feasible and safe | [181] |
| ALS patients | Human | BM-MSCs | IT, IV | Feasible and safe | [182] |
| ALS patients | Human | BM-MSCs | IT | Feasible and safe | [183] |
| hSOD 1 G93 A mice | Human | hUC-MSCs | IM | Extend lifespan; enhance motor function | [184] |
| ALS cell model expressing TDP-43 mutant M337V | Human | hUC-MSCs | Coculture | Activate the Nrf-2/HO-1 axis to exert antioxidant and neuroprotective effects | [185] |
| SOD 1G 93 A-NSC 34 cell model | Human | hUC-MSCs | Coculture | Inhibit the NF-κB/Bcl-2 signaling pathway; reduce cell apoptosis | [186] |
| ALS patients | Human | hUC-MSCs | IT | Feasible and safe | [187] |
| ALS patients | Human | ADSCs | LP | Feasible and safe | [188] |
| ALS patients | Human | hUC-MSCs | IN | Feasible and safe, lifespan extended by 2-fold | [189] |
| SOD 1G 93 A rat | Human | BM-MSCs | IT, IM | Increase survival of motor neurons | [190] |
| SOD 1G 93 A mice | Mice | BM-MSCs | IV | Reduce astrocyte activation and expression of neuroinflammatory factors | [180] |
| SOD 1G 93 A rat | Not mentioned | BM-MSCs | IV | Prolong survival period and protect the motor function | [191] |
| SOD 1G 93 A mice | Human | ADSCs | IV | Delay disease progression, prolong survival rate, and enhance neuron survival | [192] |
| SOD 1G 93 A mice | Human | iPSC-sEVs | IN | Improve motor performance and survival time | [193] |
Reduction in neuroinflammation: Recently, the role of neuroinflammation in ALS pathogenesis has been emphasized. In particular, numerous studies have shown that microglia are highly activated in ALS mouse models and are closely associated with the severity of motor neuron degeneration in ALS[176,177]. ADSCs were cocultured with SIM-A9 microglia expressing the G93A mutant human Cu/Zn SOD1 protein for 24 hours. This coculture significantly reduced the metabolic activity of microglia by decreasing ROS levels, polarizing the microglia toward an anti-inflammatory phenotype[176]. The intravenous administration of hUC-MSC-conditioned medium can prolong the lifespan of hSOD1-G93A mice. In an ALS cell model of BV-2 cells overexpressing hSOD1-G93A, hUC-MSC-conditioned medium suppressed the lipopolysaccharide-induced inflammatory response, including a reduction in proinflammatory cytokine and inducible nitric oxide synthase expression[178]. When cocultured with primary motor neurons from female hSOD1-G93A mice, BM-MSCs and their conditioned medium mediated anti-inflammatory responses through the C-X3-C motif chemokine ligand 1/C-X3-C motif chemokine receptor 1 axis[179]. Single intravenous injection of BM-MSCs in hSOD1-G93A mice can promote an antioxidant response by increasing the expression of nuclear factor erythroid 2-related factor and NAD(P)H quinone dehydrogenase 1, thereby reducing ROS accumulation[180].
Moreover, the safety of BM-MSC therapy has been validated in ALS patients. Repeated intrathecal injections of autologous BM-MSCs in ALS patients have significantly improved cerebrospinal biomarkers of neuroinflammation, neurodegeneration, and neurotrophic factors[181]. Current research indicates that the simultaneous intrathecal and intravenous administration of autologous BM-MSCs to ALS patients is feasible and safe. During the follow-up period, mild headache was the most common treatment-related adverse reaction[182]. A potential mechanism is that BM-MSCs mediate the conversion of proinflammatory cells to anti-inflammatory cells. Additionally, transplanting autologous BM-MSCs into the lumbar spinal cord region is feasible[182,183].
Extension of lifespan and promotion of neuronal survival: As potential therapeutic agents for ALS, hUC-MSCs prolong lifespan and increase motor function in hSOD1-G93A mice[184]. Treatment of an ALS cell model expressing the transactive response DNA-binding protein 43 mutant M337V with hUC-MSC-conditioned medium for 24 hours had antioxidant effects by activating the nuclear factor erythroid 2-related factor/heme oxygenase-1 axis, confirming that hUC-MSC-conditioned medium has potential neuroprotective effects[185]. Ginsenoside-Rg1 combined with hUC-MSC-conditioned medium effectively reduces cell apoptosis in a hSOD1-G93A-NSC34 cell model after 24 hours of treatment by inhibiting the nuclear factor-kappa B/B-cell lymphoma 2 signaling pathway[186]. ALS patients have shown good tolerance to intrathecal injection of hUC-MSCs and ADSCs[187,188].The survival period was extended in all treatment groups, and no serious adverse reactions were observed[189].
The combined administration of BM-MSCs via lumbar puncture and intramuscular (quadriceps) injection can increase motor neuron survival in the hSOD1-G93A mouse model[190]. In vitro BM-MSC-derived EV exposure reverses the neurotoxicity of ALS astrocytes from both humans and mice toward motor neurons[180]. Moreover, repeated administration of MSCs (four times weekly) increases survival times, protects motor functions, and reduces the deterioration of locomotor activity[191].
Furthermore, intravenous injection of ADSCs via the dorsal tail vein delays disease progression in hSOD1-G93A mice, significantly increasing survival by 20 days, increasing neuronal survival, and promoting a less degenerative neuronal microenvironment[192]. The intranasal delivery of EVs derived from human induced pluripotent stem cells (iPSCs) significantly improves motor performance and survival time and alleviates motor neuron-related pathological changes in the hSOD1-G93A mouse model[193].
NPC is a rare lysosomal storage disorder caused by mutations in the NPC1 or NPC2 genes, resulting in impaired cholesterol metabolism that triggers neuronal apoptosis and cerebellar degeneration. Emerging experimental studies suggest that MSCs may help attenuate disease progression through four principal mechanisms: Restoring cholesterol homeostasis, reducing oxidative stress, providing neurotrophic support, and promoting neuronal repair. Table 6 compiles representative studies investigating MSC-derived therapies for NPC, offering critical insights for translational research.
| Animal model | Source | Type | Administration method | Main outcomes | Ref. |
| NP-C GsbsGFP mice | Mice | BM-MSCs | IV | Improve degenerative loss of Purkinje neurons | [194] |
| BALB/c npcnih (NPC) mice | Mice | BM-MSCs | Cerebellum injection | Promote neuronal networks with functional synaptic transmission | [195] |
| BALB/c npcnih (NPC) mice | Mice | BM-MSCs | Cerebellum injection | Upregulate fusion ability of Purkinje neurons and donor-derived BM-MSCs | [196] |
| BALB/c npcnih (NPC) mice | Mice | BM-MSCs | Cerebrum injection | Modulate endogenous NPC NSCs, stimulate NSC proliferation and neuronal differentiation | [199] |
| BALB/c npcnih (NPC) mice | Mice | BM-MSCs | Cerebellum injection | Inhibit activation of astrocytes and microglia, reduce inflammation | [197] |
| BALB/c npcnih (NPC) mice | Mice | BM-MSCs | Cerebellum injection | Release bioactive neurotrophic factors, modulate sphingolipid metabolism of endogenous NPC Purkinje neurons | [198] |
| BALB/c npcnih (NPC) mice | Mice | ADSCs | Cerebellum injection | Rescue Purkinje neurons, restore motor coordination, and alleviate inflammatory responses | [201] |
| BALB/c npcnih (NPC) mice | Human | hUC-MSCs | HIPP injection | Stimulate endogenous neurogenesis, diminish intracellular cholesterol accumulation, and safeguard motor functionality | [200] |
| BALB/c npcnih (NPC) mice | Human | hUC-MSCs | IN | Reduce cholesterol levels, decrease loss of Purkinje cells in the cerebellum, delay motor dysfunction | [202] |
| Npc1KO N2a cells | Human | MenSCs-CM | Coculture | Increase survival rate, significantly alleviate inflammatory responses and apoptosis | [203] |
Purkinje cell loss is a prominent feature of NPC. Tail vein injection of BM-MSCs ameliorates the degenerative loss of Purkinje cells in the cerebellum of NPC mice[194]. In addition, cerebellar transplantation of BM-MSCs promotes the formation of neuronal networks with functional synaptic transmission in NPC mice[195,196]. Moreover, BM-MSCs can reduce inflammatory responses by inhibiting astrocyte and microglia activation[197,198]. Similarly, transplanting BM-MSCs into the brain can promote the neural stem cell proliferation and neuronal differentiation in the subventricular zone[199]. The transplantation of ADSCs and hUC-MSCs can also rescue damaged Purkinje cells in NPC mice and alleviate inflammatory responses[200,201]. Additionally, hUC-MSCs can reduce intracellular cholesterol accumulation, activate cholesterol transporters to improve cholesterol metabolism, and delay motor dysfunction in NPC mice[202]. Our previous study revealed that conditioned medium of MenSCs can also ameliorate cholesterol accumulation and inhibit neuroinflammation and apoptosis in Npc1 knockout neuro-2a cells[203].
Currently, human embryonic stem cells and human iPSCs are commonly employed in the clinical translation research of stem cell therapy for neurodegenerative diseases[204]. An open-label Phase I clinical trial (NCT04802733) transplanted bemdaneprocel, a dopaminergic neuron progenitor cell product derived from human embryonic stem cells, bilaterally into the putamen of patients with PD[205]. 18Fluoro-DOPA positron emission tomography (PET) scans indicated graft survival, and patients receiving high-dose therapy showed improvements in motor dysfunction. In a phase I/II clinical trial (jRCT2090220384), iPSC-derived dopaminergic progenitors were bilaterally transplanted into the putamen of patients with PD, leading to varying degrees of motor improvement without evidence of tumor formation[206]. Although MSCs have numerous advantages in preclinical studies of neurodegenerative diseases, significant challenges remain regarding their source and quality control; delivery methods; long-term survival, differentiation and functional integration in vivo; and safety and ethical concerns in clinical translational research.
MSCs derived from different tissue sources all exhibit multipotent differentiation capacity, have low immunogenicity, and perform immunomodulatory functions, but have heterogeneous biological characteristics that may influence their therapeutic effects. Compared to those isolated from adult tissues, fetal MSCs exhibit superior proliferative capacity in vitro[207,208]. For example, compared to BM-MSCs and ADSCs, hUC-MSCs demonstrate superior migration[209,210]. However, the proliferative potential of ADSCs is greater than that of BM-MSCs[211]. In addition, studies have revealed that SHEDs exhibit greater proliferative capacity than hUC-MSCs[212]. MSCs from different tissue sources exhibit distinct immunogenic and immunomodulatory properties. Specifically, compared to BM-MSCs, hUC-MSCs demonstrate lower immunogenicity and enhanced immunomodulatory capacity[115,212]. Compared with BM-MSCs, AD-MSCs exert superior immunomodulatory effects[211]. Moreover, the secretory profiles of MSCs vary significantly across tissue sources. Compared with BM-MSCs, hUC-MSCs demonstrate substantially higher cytokine levels (granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, hepatocyte growth factor, IL-6, IL-8, and IL-11) secretion. In contrast, BM-MSCs exhibit greater vascular endothelial growth factor production than hUC-MSCs, suggesting their greater potential for promoting angiogenesis[213]. Consequently, the therapeutic efficacy of MSCs varies significantly depending on their tissue origin, requiring further preclinical and clinical studies for comprehensive validation.
Quality control is critical during MSC manufacturing. However, non-standardized isolation and culture techniques pose challenges to the production and quality management of MSC-based products[214]. Variations in donor age and culture passage number contribute to batch-to-batch differences in cellular senescence levels among MSCs, ultimately leading to inconsistent immunomodulatory potency[215]. Wang et al[216] developed a microcarrier-based stirred bioreactor system for hUC-MSC expansion, which enhanced the proliferative capacity and delayed the process of cell aging while preserving the essential stem cell properties of hUC-MSCs compared with conventional planar culture. Therefore, establishing standardized isolation and culture protocols for MSCs from different tissue sources will increase their therapeutic efficacy.
The in vivo fate and therapeutic efficacy of MSCs are critically influenced by the delivery method, and distinct pharmacokinetic and pharmacodynamic outcomes are associated with each administration route. Systemic administration delivers MSCs via the circulatory system, offering whole-body distribution with clinical practicality. Although intravenous injection is the clinically preferred administration route, the average diameter of MSCs exceeds that of pulmonary capillaries, leading to pulmonary retention and limited dose delivery to target organs[217]. Arterial injection of MSCs bypasses pulmonary circulation but carries risks of cellular accumulation in cerebral arteries[218]. Intraperitoneal administration of MSCs prevents their entrapment in capillary networks, although its therapeutic application is anatomically restricted to the treatment of peritoneal and pelvic cavity disorders due to localized biodistribution[219]. Localized administration through direct MSC injection into target tissues or lesion sites significantly increases homing efficiency and therapeutic efficacy. This approach is suitable for treating localized injuries or pathologies, such as osteoarthritis, and offers targeted therapy with minimal systemic exposure[220]. Intrathecal, intracerebral, and endocardial injection methods constitute invasive surgical interventions that carry inherent risks of iatrogenic tissue damage and procedure-related complications. Intranasal administration represents a noninvasive delivery route whereby transplanted cells can migrate to the brain via the olfactory epithelium, although its bioavailability may be constrained by multiple physiological factors, particularly the absorptive capacity of the nasal mucosa. Therefore, selecting the appropriate administration route should be based on the specific disease type, therapeutic objectives, and individual patient characteristics. Moreover, combining multiple delivery methods may further enhance therapeutic efficacy.
The in vivo survival of MSCs is influenced by multiple factors, including donor source, delivery route, and host immune status. Typically, MSC viability progressively decreases within days to weeks post-transplantation[221,222]. MSCs derived from different tissue sources exhibit variations in immunogenicity and viability. For example, compared with BM-MSCs, hUC-MSCs may exhibit stronger immune-privileged properties and longer survival times[223]. Through genetic modification, MSCs can produce or overexpress functional genes, enabling these cells to withstand hostile microenvironments and resist apoptosis, increasing their migration and homing capacity and potentiating paracrine effects. Zhu et al[224] proposed a novel in situ empowerment of autologous MSC strategies in which bone morphogenetic protein 2-loaded bioactive materials are implanted to establish bone-like tissue in vivo to induce the immunomodulatory functions of MSCs with the help of the inflammatory microenvironment in early developmental stages. The autologous MSCs obtained through this method have reduced immunogenicity, which promotes their long-term survival in the host while maintaining their immunomodulatory capacity longer. The in vivo persistence of MSCs depends on the administration route. MSCs disappear within a few days after intravenous injection, while MSCs are detected within 3 to 4 weeks after intraperitoneal or subcutaneous injection[222]. However, after intramuscular injection, the hUC-MSCs and BM-MSCs survived in situ for more than 5 months[222]. MSCs transplanted into the host may encounter hostile microenvironment conditions including hypoxia, oxidative stress, and chronic inflammation, ultimately leading to cellular apoptosis[225]. Because MSCs can recognize stimuli in the microenvironment and remember them, preconditioning MSCs can achieve the desired effects and reverse their inactivation. Pretreatment with hypoxia, inflammatory factors, and bioactive compounds can enhance MSC proliferation, survival, and paracrine function[226,227].
MSC differentiation in vivo is profoundly influenced by the microenvironment, making it challenging to precisely direct their differentiation into specific cell lineages. For example, an inflammatory environment may induce MSCs to differentiate into unexpected cell types, thus altering the therapeutic effect. Furthermore, even in an ideal microenvironment, the differentiation efficiency of MSCs in vivo may be relatively low, which can limit their role in tissue repair and regeneration. Even when MSCs successfully differentiate into the desired cell types, their functionality often remains incomplete, resulting in failure to fully recapitulate native tissue performance. For example, in neural regeneration, MSC-derived neurons frequently exhibit inferior electrophysiological properties and synaptic transmission capacity than endogenous neural cells.
The most common adverse reaction following MSC therapy is fever. Although MSCs exhibit low immunogenicity, the febrile response may be associated with acute inflammatory reactions triggered by allogeneic MSC infusion. Notably, patients with MSC-induced pyrexia typically recover spontaneously without requiring specific treatment. Multiple studies have demonstrated that MSCs may acquire chromosomal abnormalities during in vitro expansion; however, these genetic alterations do not lead to tumor growth. For example, studies have revealed that even when MSCs develop chromosomal abnormalities and undergo senescence due to prolonged culture, they still fail to form tumors upon transplantation into mouse models[228]. MSCs that are derived from different sources present distinct ethical considerations. For example, the collection of hUC-MSCs and MenSCs is a noninvasive, painless, and safe procedure, posing no significant ethical concerns[82]. In contrast, BM-MSCs require invasive harvesting procedures, which may involve more ethical considerations. Consequently, stem cell secretory products, including the secretome, Exos, and EVs, offer superior safety and ethical profiles compared with whole-cell therapies[229].
MSCs demonstrate great potential in neurodegenerative diseases due to their self-renewal capacity, multipotent differentiation potential, low immunogenicity, immune regulatory functions, and minimal ethical concerns. Moreover, derivatives of MSCs, such as the secretome, Exos, and EVs, show even greater advantages in terms of safety and ethics. Preclinical studies have shown that MSCs and their derivatives can ameliorate neuroinflammation in AD, PD, HD, ALS, and NPC. MSCs and their derivatives can reduce Aβ accumulation and promote neurogenesis, thereby improving cognitive deficits in AD. They can also decrease αSN aggregation and restore dopaminergic neuron function, thus alleviating motor impairments in PD. In HD, MSCs and their derivatives exert neuroprotective effects, thereby mitigating motor dysfunction. In ALS, they increase neuronal survival, thereby prolonging survival and protecting motor function. MSCs and their derivatives can reduce cholesterol accumulation and increase Purkinje cell numbers, thereby delaying motor dysfunction in NPC. Although there are still challenges related to the sources, delivery methods, safety and ethics considerations of MSCs, an increasing number of studies are addressing these limitations. For example, given that the paracrine effects of MSCs are widely recognized as playing a crucial role in disease therapy, the study of secretome, Exos, and EVs will likely be a key focus for future research in neurodegenerative diseases. In conclusion, MSCs have remarkable promise for treating neurodegenerative diseases. As research advances and technologies evolve, MSC-based therapies are expected to emerge as clinically viable treatment options.
| 1. | Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017;9:a028035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 1103] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 2. | Khanam H, Ali A, Asif M, Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur J Med Chem. 2016;124:1121-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3259] [Cited by in RCA: 2793] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 4. | Jiang Q, Liu J, Huang S, Wang XY, Chen X, Liu GH, Ye K, Song W, Masters CL, Wang J, Wang YJ. Antiageing strategy for neurodegenerative diseases: from mechanisms to clinical advances. Signal Transduct Target Ther. 2025;10:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 5. | Cheng YJ, Lin CH, Lane HY. From Menopause to Neurodegeneration-Molecular Basis and Potential Therapy. Int J Mol Sci. 2021;22:8654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023;388:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2962] [Cited by in RCA: 3318] [Article Influence: 1106.0] [Reference Citation Analysis (0)] |
| 7. | Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM; TRAILBLAZER-ALZ 2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330:512-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 1682] [Article Influence: 560.7] [Reference Citation Analysis (0)] |
| 8. | McFarthing K, Buff S, Rafaloff G, Pitzer K, Fiske B, Navangul A, Beissert K, Pilcicka A, Fuest R, Wyse RK, Stott SRW. Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2024 Update. J Parkinsons Dis. 2024;14:899-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Ilieva H, Vullaganti M, Kwan J. Advances in molecular pathology, diagnosis, and treatment of amyotrophic lateral sclerosis. BMJ. 2023;383:e075037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 10. | Johnson SA, Fang T, De Marchi F, Neel D, Van Weehaeghe D, Berry JD, Paganoni S. Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents. Drugs. 2022;82:1367-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Stahl CM, Feigin A. Medical, Surgical, and Genetic Treatment of Huntington Disease. Neurol Clin. 2020;38:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Tirelli C, Rondinone O, Italia M, Mira S, Belmonte LA, De Grassi M, Guido G, Maggioni S, Mondoni M, Miozzo MR, Centanni S. The Genetic Basis, Lung Involvement, and Therapeutic Options in Niemann-Pick Disease: A Comprehensive Review. Biomolecules. 2024;14:211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Sakowski SA, Chen KS. Stem cell therapy for central nervous system disorders: Metabolic interactions between transplanted cells and local microenvironments. Neurobiol Dis. 2022;173:105842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Bang OY, Kim JE. Stem cell-derived extracellular vesicle therapy for acute brain insults and neurodegenerative diseases. BMB Rep. 2022;55:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Shao W, Yuan H, Xiao R, Zhang Y, Wei C, Ni X, He N, Chen G, Gui S, Cheng Z, Wang Q. Engineered Extracellular Vesicle-Based Nanoformulations That Coordinate Neuroinflammation and Immune Homeostasis, Enhancing Parkinson's Disease Therapy. ACS Nano. 2024;18:23014-23031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Lin J, Xiang K, Shi T, Guo B. Omnidirectional improvement of mitochondrial health in Alzheimer's disease by multi-targeting engineered activated neutrophil exosomes. J Control Release. 2024;376:470-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Rohban R, Pieber TR. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017;2017:5173732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Kuang Q, Xu J, Lin Q, Chi H, Yu D. MSC-Based Cell Therapy in Neurological Diseases: A Concise Review of the Literature in Pre-Clinical and Clinical Research. Biomolecules. 2024;14:538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 19. | 2024 Alzheimer's disease facts and figures. Alzheimers Dement. 2024;20:3708-3821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 1348] [Article Influence: 674.0] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Xiao X, Yang Y, Yao R, Yang Q, Zhu Y, Yang X, Zhang S, Shen L, Jiao B. The risk of Alzheimer's disease and cognitive impairment characteristics in eight mental disorders: A UK Biobank observational study and Mendelian randomization analysis. Alzheimers Dement. 2024;20:4841-4853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Recent advances in Alzheimer's disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024;9:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 488] [Reference Citation Analysis (0)] |
| 22. | GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23:344-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 975] [Article Influence: 487.5] [Reference Citation Analysis (0)] |
| 23. | Athar T, Al Balushi K, Khan SA. Recent advances on drug development and emerging therapeutic agents for Alzheimer's disease. Mol Biol Rep. 2021;48:5629-5645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (17)] |
| 24. | Singh MK, Shin Y, Ju S, Han S, Kim SS, Kang I. Comprehensive Overview of Alzheimer's Disease: Etiological Insights and Degradation Strategies. Int J Mol Sci. 2024;25:6901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 25. | Anitha K, Singh MK, Kohat K, Sri Varshini T, Chenchula S, Padmavathi R, Amerneni LS, Vishnu Vardhan K, Mythili Bai K, Chavan MR, Bhatt S. Recent Insights into the Neurobiology of Alzheimer's Disease and Advanced Treatment Strategies. Mol Neurobiol. 2025;62:2314-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Yin Q, Ma Y, Hong Y, Hou X, Chen J, Shen C, Sun M, Shang Y, Dong S, Zeng Z, Pei JJ, Liu X. Lycopene attenuates insulin signaling deficits, oxidative stress, neuroinflammation, and cognitive impairment in fructose-drinking insulin resistant rats. Neuropharmacology. 2014;86:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease. Neurotherapeutics. 2018;15:1036-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1845] [Article Influence: 230.6] [Reference Citation Analysis (0)] |
| 29. | Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Kovacs GG. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int J Mol Sci. 2016;17:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 31. | Desai AK, Grossberg GT. Diagnosis and treatment of Alzheimer's disease. Neurology. 2005;64:S34-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Kim AY, Al Jerdi S, MacDonald R, Triggle CR. Alzheimer's disease and its treatment-yesterday, today, and tomorrow. Front Pharmacol. 2024;15:1399121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 33. | Shukla D, Suryavanshi A, Bharti SK, Asati V, Mahapatra DK. Recent Advances in the Treatment and Management of Alzheimer's Disease: A Precision Medicine Perspective. Curr Top Med Chem. 2024;24:1699-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Saeedi M, Mehranfar F. Challenges and Approaches of Drugs Such as Memantine, Donepezil, Rivastigmine, and Aducanumab in the Treatment, Control and Management of Alzheimer's Disease. Recent Pat Biotechnol. 2022;16:102-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Dave BP, Shah YB, Maheshwari KG, Mansuri KA, Prajapati BS, Postwala HI, Chorawala MR. Pathophysiological Aspects and Therapeutic Armamentarium of Alzheimer's Disease: Recent Trends and Future Development. Cell Mol Neurobiol. 2023;43:3847-3884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3143] [Cited by in RCA: 4413] [Article Influence: 401.2] [Reference Citation Analysis (40)] |
| 37. | Kumar S, Goyal L, Singh S. Tremor and Rigidity in Patients with Parkinson's Disease: Emphasis on Epidemiology, Pathophysiology and Contributing Factors. CNS Neurol Disord Drug Targets. 2022;21:596-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Kulcsarova K, Skorvanek M, Postuma RB, Berg D. Defining Parkinson's Disease: Past and Future. J Parkinsons Dis. 2024;14:S257-S271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Deliz JR, Tanner CM, Gonzalez-Latapi P. Epidemiology of Parkinson's Disease: An Update. Curr Neurol Neurosci Rep. 2024;24:163-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 40. | Cramb KML, Beccano-Kelly D, Cragg SJ, Wade-Martins R. Impaired dopamine release in Parkinson's disease. Brain. 2023;146:3117-3132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 41. | Galper J, Dean NJ, Pickford R, Lewis SJG, Halliday GM, Kim WS, Dzamko N. Lipid pathway dysfunction is prevalent in patients with Parkinson's disease. Brain. 2022;145:3472-3487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 42. | Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2340] [Cited by in RCA: 2626] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 43. | Lima MM, Martins EF, Delattre AM, Proenca MB, Mori MA, Carabelli B, Ferraz AC. Motor and non-motor features of Parkinson's disease - a review of clinical and experimental studies. CNS Neurol Disord Drug Targets. 2012;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Simon DK, Tanner CM, Brundin P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin Geriatr Med. 2020;36:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 683] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 45. | Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1500] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 46. | Machado V, Zöller T, Attaai A, Spittau B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson's Disease-Lessons from Transgenic Mice. Int J Mol Sci. 2016;17:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2018;33:1248-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 678] [Article Influence: 84.8] [Reference Citation Analysis (1)] |
| 48. | Kouli A, Torsney KM, Kuan WL. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018. [PubMed] |
| 49. | Chen X, Feng Y, Quinn RJ, Pountney DL, Richardson DR, Mellick GD, Ma L. Potassium Channels in Parkinson's Disease: Potential Roles in Its Pathogenesis and Innovative Molecular Targets for Treatment. Pharmacol Rev. 2023;75:758-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Bhattacharyya KB. The Story of Levodopa: A Long and Arduous Journey. Ann Indian Acad Neurol. 2022;25:124-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 51. | Przytuła F, Dulski J, Sobstyl M, Sławek J. Battery for deep brain stimulation depletion in Parkinson's Disease and dystonia patients - a systematic review. Neurol Neurochir Pol. 2021;55:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 52. | Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. 2017;13:689-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 53. | A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6006] [Cited by in RCA: 6236] [Article Influence: 189.0] [Reference Citation Analysis (0)] |
| 54. | Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. Huntington's Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb Perspect Med. 2017;7:a024240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 55. | Liu L, Prime ME, Lee MR, Khetarpal V, Brown CJ, Johnson PD, Miranda-Azpiazu P, Chen X, Clark-Frew D, Coe S, Davis R, Dickie A, Ebneth A, Esposito S, Gadouleau E, Gai X, Galan S, Green S, Greenaway C, Giles P, Halldin C, Hayes S, Herbst T, Herrmann F, Heßmann M, Jia Z, Kiselyov A, Kotey A, Krulle T, Mangette JE, Marston RW, Menta S, Mills MR, Monteagudo E, Nag S, Nibbio M, Orsatti L, Schaertl S, Scheich C, Sproston J, Stepanov V, Svedberg M, Takano A, Taylor M, Thomas W, Toth M, Vaidya D, Vanräs K, Weddell D, Wigginton I, Wityak J, Mrzljak L, Munoz-Sanjuan I, Bard JA, Dominguez C. Imaging Mutant Huntingtin Aggregates: Development of a Potential PET Ligand. J Med Chem. 2020;63:8608-8633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Berlet R, Galang Cabantan DA, Gonzales-Portillo D, Borlongan CV. Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson's Disease, and Huntington's Disease. Front Cell Dev Biol. 2022;10:798826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 57. | Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 58. | Huntington Study Group; Frank S, Testa CM, Stamler D, Kayson E, Davis C, Edmondson MC, Kinel S, Leavitt B, Oakes D, O'Neill C, Vaughan C, Goldstein J, Herzog M, Snively V, Whaley J, Wong C, Suter G, Jankovic J, Jimenez-Shahed J, Hunter C, Claassen DO, Roman OC, Sung V, Smith J, Janicki S, Clouse R, Saint-Hilaire M, Hohler A, Turpin D, James RC, Rodriguez R, Rizer K, Anderson KE, Heller H, Carlson A, Criswell S, Racette BA, Revilla FJ, Nucifora F Jr, Margolis RL, Ong M, Mendis T, Mendis N, Singer C, Quesada M, Paulsen JS, Brashers-Krug T, Miller A, Kerr J, Dubinsky RM, Gray C, Factor SA, Sperin E, Molho E, Eglow M, Evans S, Kumar R, Reeves C, Samii A, Chouinard S, Beland M, Scott BL, Hickey PT, Esmail S, Fung WL, Gibbons C, Qi L, Colcher A, Hackmyer C, McGarry A, Klos K, Gudesblatt M, Fafard L, Graffitti L, Schneider DP, Dhall R, Wojcieszek JM, LaFaver K, Duker A, Neefus E, Wilson-Perez H, Shprecher D, Wall P, Blindauer KA, Wheeler L, Boyd JT, Houston E, Farbman ES, Agarwal P, Eberly SW, Watts A, Tariot PN, Feigin A, Evans S, Beck C, Orme C, Edicola J, Christopher E. Effect of Deutetrabenazine on Chorea Among Patients With Huntington Disease: A Randomized Clinical Trial. JAMA. 2016;316:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 59. | Shah S, Mansour HM, Lucke-Wold B. Advances in Stem Cell Therapy for Huntington's Disease: A Comprehensive Literature Review. Cells. 2025;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Younger DS, Brown RH Jr. Amyotrophic lateral sclerosis. Handb Clin Neurol. 2023;196:203-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 61. | Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1471] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 62. | Kano O, Iwamoto K, Ito H, Kawase Y, Cridebring D, Ikeda K, Iwasaki Y. Limb-onset amyotrophic lateral sclerosis patients visiting orthopedist show a longer time-to-diagnosis since symptom onset. BMC Neurol. 2013;13:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Chiò A, Calvo A, Moglia C, Mazzini L, Mora G; PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 64. | Wolfson C, Gauvin DE, Ishola F, Oskoui M. Global Prevalence and Incidence of Amyotrophic Lateral Sclerosis: A Systematic Review. Neurology. 2023;101:e613-e623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 65. | Jaiswal MK. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 66. | Chen JJ. Overview of current and emerging therapies for amytrophic lateral sclerosis. Am J Manag Care. 2020;26:S191-S197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Thonhoff JR, Simpson EP, Appel SH. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr Opin Neurol. 2018;31:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 68. | Soares P, Silva C, Chavarria D, Silva FSG, Oliveira PJ, Borges F. Drug discovery and amyotrophic lateral sclerosis: Emerging challenges and therapeutic opportunities. Ageing Res Rev. 2023;83:101790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 69. | Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 861] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 70. | Pineda M, Walterfang M, Patterson MC. Miglustat in Niemann-Pick disease type C patients: a review. Orphanet J Rare Dis. 2018;13:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 71. | Dinkel L, Hummel S, Zenatti V, Malara M, Tillmann Y, Colombo A, Monasor LS, Suh JH, Logan T, Roth S, Paeger L, Hoffelner P, Bludau O, Schmidt A, Müller SA, Schifferer M, Nuscher B, Njavro JR, Prestel M, Bartos LM, Wind-Mark K, Slemann L, Hoermann L, Kunte ST, Gnörich J, Lindner S, Simons M, Herms J, Paquet D, Lichtenthaler SF, Bartenstein P, Franzmeier N, Liesz A, Grosche A, Bremova-Ertl T, Catarino C, Beblo S, Bergner C, Schneider SA, Strupp M, Di Paolo G, Brendel M, Tahirovic S. Myeloid cell-specific loss of NPC1 in mice recapitulates microgliosis and neurodegeneration in patients with Niemann-Pick type C disease. Sci Transl Med. 2024;16:eadl4616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 72. | Beninger P. Miplyffa (arimoclomol). Clin Ther. 2024;46:1089-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 73. | Keam SJ. Arimoclomol: First Approval. Drugs. 2025;85:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 74. | Gong P, Zhang W, He Y, Wang J, Li S, Chen S, Ye Q, Li M. Classification and Characteristics of Mesenchymal Stem Cells and Its Potential Therapeutic Mechanisms and Applications against Ischemic Stroke. Stem Cells Int. 2021;2021:2602871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13048] [Article Influence: 686.7] [Reference Citation Analysis (12)] |
| 76. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] |
| 77. | Purwaningrum M, Jamilah NS, Purbantoro SD, Sawangmake C, Nantavisai S. Comparative characteristic study from bone marrow-derived mesenchymal stem cells. J Vet Sci. 2021;22:e74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 78. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15365] [Article Influence: 569.1] [Reference Citation Analysis (2)] |
| 79. | Zhuo Y, Li SH, Chen MS, Wu J, Kinkaid HY, Fazel S, Weisel RD, Li RK. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. 2010;139:1286-1294, 1294.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Bunnell BA. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells. 2021;10:3433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 81. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy(ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1406] [Article Influence: 108.2] [Reference Citation Analysis (10)] |
| 82. | Guan YT, Xie Y, Li DS, Zhu YY, Zhang XL, Feng YL, Chen YP, Xu LJ, Liao PF, Wang G. Comparison of biological characteristics of mesenchymal stem cells derived from the human umbilical cord and decidua parietalis. Mol Med Rep. 2019;20:633-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Han ZX, Shi Q, Wang DK, Li D, Lyu M. [Basic biological characteristics of mesenchymal stem cells derived from bone marrow and human umbilical cord]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:1248-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Pethe P, Kale V. Placenta: A gold mine for translational research and regenerative medicine. Reprod Biol. 2021;21:100508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci. 2019;20:1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 86. | Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J, Vink R, Gronthos S, Koblar SA. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 87. | Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, Shen P, Lin J. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 88. | Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 89. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 90. | Henschler R, Deak E, Seifried E. Homing of Mesenchymal Stem Cells. Transfus Med Hemother. 2008;35:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 92. | Hwang NS, Zhang C, Hwang YS, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 94. | Xu Q, Hou W, Zhao B, Fan P, Wang S, Wang L, Gao J. Mesenchymal stem cells lineage and their role in disease development. Mol Med. 2024;30:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 95. | Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity-Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics. 2022;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 96. | de Mayo T, Conget P, Becerra-Bayona S, Sossa CL, Galvis V, Arango-Rodríguez ML. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS One. 2017;12:e0177533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 98. | Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, Wilk R, Hudecki J, Los MJ. Novel trends in application of stem cells in skin wound healing. Eur J Pharmacol. 2019;843:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 99. | Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 917] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 100. | Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 297] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 101. | Campos HC, Ribeiro DE, Hashiguchi D, Hukuda DY, Gimenes C, Romariz SAA, Ye Q, Tang Y, Ulrich H, Longo BM. Distinct Effects of the Hippocampal Transplantation of Neural and Mesenchymal Stem Cells in a Transgenic Model of Alzheimer's Disease. Stem Cell Rev Rep. 2022;18:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Gavin C, Meinke S, Heldring N, Heck KA, Achour A, Iacobaeus E, Höglund P, Le Blanc K, Kadri N. The Complement System Is Essential for the Phagocytosis of Mesenchymal Stromal Cells by Monocytes. Front Immunol. 2019;10:2249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 103. | Gavasso S, Kråkenes T, Olsen H, Evjenth EC, Ytterdal M, Haugsøen JB, Kvistad CE. The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS-A Review Focusing on Neuroprotective Properties. Int J Mol Sci. 2024;25:1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 104. | Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8:2002944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (1)] |
| 105. | Nasirishargh A, Kumar P, Ramasubramanian L, Clark K, Hao D, Lazar SV, Wang A. Exosomal microRNAs from mesenchymal stem/stromal cells: Biology and applications in neuroprotection. World J Stem Cells. 2021;13:776-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (2)] |
| 106. | Lei T, Li C, Liu Y, Cui Z, Deng S, Cao J, Yang H, Chen P. Microfluidics-enabled mesenchymal stem cell derived Neuron like cell membrane coated nanoparticles inhibit inflammation and apoptosis for Parkinson's Disease. J Nanobiotechnology. 2024;22:370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 107. | Izquierdo-Altarejos P, Moreno-Manzano V, Felipo V. Pathological and therapeutic effects of extracellular vesicles in neurological and neurodegenerative diseases. Neural Regen Res. 2024;19:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 108. | Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D, Cofano F, Torre ML, Segale L. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact Mater. 2023;29:16-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 109. | Fallahi S, Zangbar HS, Farajdokht F, Rahbarghazi R, Mohaddes G, Ghiasi F. Exosomes as a therapeutic tool to promote neurorestoration and cognitive function in neurological conditions: Achieve two ends with a single effort. CNS Neurosci Ther. 2024;30:e14752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology. 2014;39:169-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 111. | Rudnitsky E, Braiman A, Wolfson M, Muradian KK, Gorbunova V, Turgeman G, Fraifeld VE. Mesenchymal stem cells and their derivatives as potential longevity-promoting tools. Biogerontology. 2025;26:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Song CG, Zhang YZ, Wu HN, Cao XL, Guo CJ, Li YQ, Zheng MH, Han H. Stem cells: a promising candidate to treat neurological disorders. Neural Regen Res. 2018;13:1294-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 113. | Steel K. Alzheimer's disease. N Engl J Med. 2010;362:1844; author reply 1844-1844; author reply 1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3429] [Cited by in RCA: 3741] [Article Influence: 233.8] [Reference Citation Analysis (10)] |
| 114. | Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A, Strittmatter SM. Prion-Protein-interacting Amyloid-β Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. J Biol Chem. 2015;290:17415-17438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 115. | Gaber A, Ahmed OM, Khadrawy YA, Zoheir KMA, Abo-ELeneen RE, Alblihed MA, Elbakry AM. Mesenchymal Stem Cells and Begacestat Mitigate Amyloid-β 25-35-Induced Cognitive Decline in Rat Dams and Hippocampal Deteriorations in Offspring. Biology (Basel). 2023;12:905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Gaber A, Elbakry AM, Aljarari RM, Jaber FA, Khadrawy YA, Sabry D, Abo-ELeneen RE, Ahmed OM. Bone Marrow-Derived Mesenchymal Stem Cells and γ-Secretase Inhibitor Treatments Suppress Amyloid-β25-35-Induced Cognitive Impairment in Rat Dams and Cortical Degeneration in Offspring. Stem Cells Int. 2023;2023:2690949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Xing C, Zhang X, Wang D, Chen H, Gao X, Sun C, Guo W, Roshan S, Li Y, Hang Z, Cai S, Lei T, Bi W, Hou L, Li L, Wu Y, Li L, Zeng Z, Du H. Neuroprotective effects of mesenchymal stromal cells in mouse models of Alzheimer's Disease: The Mediating role of gut microbes and their metabolites via the Microbiome-Gut-Brain axis. Brain Behav Immun. 2024;122:510-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 118. | Hong CG, Chen ML, Duan R, Wang X, Pang ZL, Ge LT, Lu M, Xie H, Liu ZZ. Transplantation of Nasal Olfactory Mucosa Mesenchymal Stem Cells Benefits Alzheimer's Disease. Mol Neurobiol. 2022;59:7323-7336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 119. | Nabil M, Kassem DH, Ali AA, El-Mesallamy HO. Adipose tissue-derived mesenchymal stem cells ameliorate cognitive impairment in Alzheimer's disease rat model: Emerging role of SIRT1. Biofactors. 2023;49:1121-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Lin L, Huang L, Huang S, Chen W, Huang H, Chi L, Su F, Liu X, Yuan K, Jiang Q, Li C, Smith WW, Fu Q, Pei Z. MSC-Derived Extracellular Vesicles Alleviate NLRP3/GSDMD-Mediated Neuroinflammation in Mouse Model of Sporadic Alzheimer's Disease. Mol Neurobiol. 2024;61:5494-5509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 121. | Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 122. | Ebrahim N, Al Saihati HA, Alali Z, Aleniz FQ, Mahmoud SYM, Badr OA, Dessouky AA, Mostafa O, Hussien NI, Farid AS, El-Sherbiny M, Salim RF, Forsyth NR, Ali FEM, Alsabeelah NF. Exploring the molecular mechanisms of MSC-derived exosomes in Alzheimer's disease: Autophagy, insulin and the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2024;176:116836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 123. | Silva RO, Haddad M, Counil H, Zaouter C, Patten SA, Fulop T, Ramassamy C. Exploring the potential of plasma and adipose mesenchymal stem cell-derived extracellular vesicles as novel platforms for neuroinflammation therapy. J Control Release. 2025;377:880-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 124. | Nazari S, Pourmand SM, Motevaseli E, Hassanzadeh G. Mesenchymal stem cells (MSCs) and MSC-derived exosomes in animal models of central nervous system diseases: Targeting the NLRP3 inflammasome. IUBMB Life. 2023;75:794-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 125. | Xu F, Wu Y, Yang Q, Cheng Y, Xu J, Zhang Y, Dai H, Wang B, Ma Q, Chen Y, Lin F, Wang C. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer's Disease Treatment. Adv Mater. 2022;34:e2207107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 126. | Cabrera-Pastor A. Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk. Int J Mol Sci. 2024;25:7041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 127. | Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 128. | Ma S, Zhou X, Wang Y, Li Z, Wang Y, Shi J, Guan F. MG53 protein rejuvenates hUC-MSCs and facilitates their therapeutic effects in AD mice by activating Nrf2 signaling pathway. Redox Biol. 2022;53:102325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 129. | Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22:589-599.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 978] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 130. | Kan I, Barhum Y, Melamed E, Offen D. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev Rep. 2011;7:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 131. | Babaei H, Kheirollah A, Ranjbaran M, Sarkaki A, Adelipour M. Dose-dependent neuroprotective effects of adipose-derived mesenchymal stem cells on amyloid β-induced Alzheimer's disease in rats. Biochem Biophys Res Commun. 2023;678:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 132. | Valipour B, Simorgh S, Mirsalehi M, Moradi S, Taghizadeh-Hesary F, Seidkhani E, Akbarnejad Z, Alizadeh R. Improvement of spatial learning and memory deficits by intranasal administration of human olfactory ecto-mesenchymal stem cells in an Alzheimer's disease rat model. Brain Res. 2024;1828:148764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 133. | Niu Y, Wang X, Li M, Niu B. Exosomes from human umbilical cord Mesenchymal stem cells attenuates stress-induced hippocampal dysfunctions. Metab Brain Dis. 2020;35:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 135. | Li S, Zhang J, Liu X, Wang N, Sun L, Liu J, Liu X, Masoudi A, Wang H, Li C, Guo C, Liu X. Proteomic characterization of hUC-MSC extracellular vesicles and evaluation of its therapeutic potential to treat Alzheimer's disease. Sci Rep. 2024;14:5959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 136. | Wang Y, Jiang J, Fu X, Zhang J, Song J, Wang Y, Duan L, Shao P, Xu X, Zeng L, Zhang F. Fe(3)O(4)@polydopamine nanoparticle-loaded human umbilical cord mesenchymal stem cells improve the cognitive function in Alzheimer's disease mice by promoting hippocampal neurogenesis. Nanomedicine. 2022;40:102507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 137. | Chen S, Sun F, Qian H, Xu W, Jiang J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022;2022:1779346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 138. | Qin C, Li Y, Wang K. Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer's Disease: Inhibition of Neuroinflammation. J Inflamm Res. 2021;14:4761-4775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Visanji NP, Lang AE, Kovacs GG. Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl Neurodegener. 2019;8:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 140. | Xie YX, Naseri NN, Fels J, Kharel P, Na Y, Lane D, Burré J, Sharma M. Lysosomal exocytosis releases pathogenic α-synuclein species from neurons in synucleinopathy models. Nat Commun. 2022;13:4918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 141. | Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1619] [Cited by in RCA: 2016] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 142. | Schiess M, Suescun J, Doursout MF, Adams C, Green C, Saltarrelli JG, Savitz S, Ellmore TM. Allogeneic Bone Marrow-Derived Mesenchymal Stem Cell Safety in Idiopathic Parkinson's Disease. Mov Disord. 2021;36:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 143. | Ramalingam M, Jeong HS, Hwang J, Cho HH, Kim BC, Kim E, Jang S. Autophagy Signaling by Neural-Induced Human Adipose Tissue-Derived Stem Cell-Conditioned Medium during Rotenone-Induced Toxicity in SH-SY5Y Cells. Int J Mol Sci. 2022;23:4193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 144. | Aliakbari F, Marzookian K, Parsafar S, Hourfar H, Nayeri Z, Fattahi A, Raeiji M, Boroujeni NN, Otzen DE, Morshedi D. The impact of hUC MSC-derived exosome-nanoliposome hybrids on α-synuclein fibrillation and neurotoxicity. Sci Adv. 2024;10:eadl3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 145. | Marques CR, Campos J, Sampaio-Marques B, Antunes FF, Dos Santos Cunha RM, Silva D, Barata-Antunes S, Lima R, Fernandes-Platzgummer A, da Silva CL, Sousa RA, Salgado AJ. Secretome of bone marrow mesenchymal stromal cells cultured in a dynamic system induces neuroprotection and modulates microglial responsiveness in an α-synuclein overexpression rat model. Cytotherapy. 2024;26:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 146. | Essawy Essawy A, Abou-ElNaga OA, Mehanna RA, Badae NM, Elsawy ES, Soffar AA. Comparing the effect of intravenous versus intracranial grafting of mesenchymal stem cells against parkinsonism in a rat model: Behavioral, biochemical, pathological and immunohistochemical studies. PLoS One. 2024;19:e0296297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 147. | Peng H, Li Y, Ji W, Zhao R, Lu Z, Shen J, Wu Y, Wang J, Hao Q, Wang J, Wang W, Yang J, Zhang X. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson's Disease. ACS Nano. 2022;16:869-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 148. | Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, Xu Y, Zou G, Jiang P, Tang C, Zheng H, Dai Z. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson's disease therapy. Theranostics. 2018;8:2264-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 149. | Huang W, Zhang T, Li X, Gong L, Zhang Y, Luan C, Shan Q, Gu X, Zhao L. Intranasal Administration of Umbilical Cord Mesenchymal Stem Cell Exosomes Alleviates Parkinson's Disease. Neuroscience. 2024;549:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 150. | Zhang ZX, Zhou YJ, Gu P, Zhao W, Chen HX, Wu RY, Zhou LY, Cui QZ, Sun SK, Zhang LQ, Zhang K, Xu HJ, Chai XQ, An SJ. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson's disease and neuronal damage through inhibition of microglia. Neural Regen Res. 2023;18:2291-2300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 151. | Zhou L, Wang X, Wang X, An J, Zheng X, Han D, Chen Z. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in PD mice via centrally and peripherally suppressing NLRP3 inflammasome-mediated inflammatory responses. Biomed Pharmacother. 2022;153:113535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 152. | Zhuo Y, Li WS, Lu W, Li X, Ge LT, Huang Y, Gao QT, Deng YJ, Jiang XC, Lan ZW, Deng Q, Chen YH, Xiao Y, Lu S, Jiang F, Liu Z, Hu L, Liu Y, Ding Y, He ZW, Tan DA, Duan D, Lu M. TGF-β1 mediates hypoxia-preconditioned olfactory mucosa mesenchymal stem cells improved neural functional recovery in Parkinson's disease models and patients. Mil Med Res. 2024;11:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 153. | He S, Wang Q, Chen L, He YJ, Wang X, Qu S. miR-100a-5p-enriched exosomes derived from mesenchymal stem cells enhance the anti-oxidant effect in a Parkinson's disease model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med. 2023;21:747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 154. | Cai Y, Zhang MM, Wang M, Jiang ZH, Tan ZG. Bone Marrow-Derived Mesenchymal Stem Cell-Derived Exosomes Containing Gli1 Alleviate Microglial Activation and Neuronal Apoptosis In Vitro and in a Mouse Parkinson Disease Model by Direct Inhibition of Sp1 Signaling. J Neuropathol Exp Neurol. 2022;81:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 155. | Yildirim S, Oylumlu E, Ozkan A, Sinen O, Bulbul M, Goksu ET, Ertosun MG, Tanriover G. Zinc (Zn) and adipose-derived mesenchymal stem cells (AD-MSCs) on MPTP-induced Parkinson's disease model: A comparative evaluation of behavioral and immunohistochemical results. Neurotoxicology. 2023;97:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 156. | Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP(+)-Induced Cytotoxicity in vitro. Front Mol Neurosci. 2019;12:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 157. | Park JM, Rahmati M, Lee SC, Shin JI, Kim YW. Effects of mesenchymal stem cell on dopaminergic neurons, motor and memory functions in animal models of Parkinson's disease: a systematic review and meta-analysis. Neural Regen Res. 2024;19:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 158. | Sun Z, Gu P, Xu H, Zhao W, Zhou Y, Zhou L, Zhang Z, Wang W, Han R, Chai X, An S. Human Umbilical Cord Mesenchymal Stem Cells Improve Locomotor Function in Parkinson's Disease Mouse Model Through Regulating Intestinal Microorganisms. Front Cell Dev Biol. 2021;9:808905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 159. | He Y, Li R, Yu Y, Xu Z, Gao J, Wang C, Huang C, Qi Z. HucMSC extracellular vesicles increasing SATB 1 to activate the Wnt/β-catenin pathway in 6-OHDA-induced Parkinson's disease model. IUBMB Life. 2024;76:1154-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 160. | Liu J, Ji Z, He Q, Chen H, Xu X, Mei Q, Hu Y, Zhang H. Direct conversion of human umbilical cord mesenchymal stem cells into dopaminergic neurons for Parkinson's disease treatment. Neurobiol Dis. 2024;201:106683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Jiang Z, Wang J, Sun G, Feng M. BDNF-modified human umbilical cord mesenchymal stem cells-derived dopaminergic-like neurons improve rotation behavior of Parkinson's disease rats through neuroprotection and anti-neuroinflammation. Mol Cell Neurosci. 2022;123:103784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 162. | Wang CC, Hu XM, Long YF, Huang HR, He Y, Xu ZR, Qi ZQ. Treatment of Parkinson's disease model with human umbilical cord mesenchymal stem cell-derived exosomes loaded with BDNF. Life Sci. 2024;356:123014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 163. | Azevedo EM, Fracaro L, Hochuli AHD, Ilkiw J, Bail EL, Lisboa MO, Rodrigues LS, Barchiki F, Correa A, Capriglione LGA, Brofman PRS, Lima MMS. Comparative analysis of uninduced and neuronally-induced human dental pulp stromal cells in a 6-OHDA model of Parkinson's disease. Cytotherapy. 2024;26:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 164. | Lei T, Xiao Z, Zhang X, Cai S, Bi W, Yang Y, Wang D, Li Q, Du H. Human gingival mesenchymal stem cells improve movement disorders and tyrosine hydroxylase neuronal damage in Parkinson disease rats. Cytotherapy. 2022;24:1105-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 165. | Fujii H, Matsubara K, Sakai K, Ito M, Ohno K, Ueda M, Yamamoto A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015;1613:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 166. | Chen YR, Wong CC, Chen YN, Yang BH, Lee PH, Shiau CY, Wang KC, Li CH. Factors derived from human exfoliated deciduous teeth stem cells reverse neurological deficits in a zebrafish model of Parkinson's disease. J Dent Sci. 2024;19:2035-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 167. | Mohamed AS, Abdel-Fattah DS, Abdel-Aleem GA, El-Sheikh TF, Elbatch MM. Biochemical study of the effect of mesenchymal stem cells-derived exosome versus L-Dopa in experimentally induced Parkinson's disease in rats. Mol Cell Biochem. 2023;478:2795-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Lin YY, Chuang DM, Chi CY, Hung SY. Intranasal administration of mesenchymal stem cells overexpressing FGF21 demonstrates therapeutic potential in experimental Parkinson's disease. Neurotherapeutics. 2025;22:e00501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 169. | Alizadeh R, Boroujeni ME, Kamrava SK, Tehrani AM, Bagher Z, Heidari F, Bluyssen HAR, Farhadi M. From Transcriptome to Behavior: Intranasal Injection of Late Passage Human Olfactory Stem Cells Displays Potential in a Rat Model of Parkinson's Disease. ACS Chem Neurosci. 2021;12:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Yang X, Wang X, Xia J, Jia J, Zhang S, Wang W, He W, Song X, Chen L, Niu P, Chen T. Small extracellular vesicles-derived from 3d cultured human nasal mucosal mesenchymal stem cells during differentiation to dopaminergic progenitors promote neural damage repair via miR-494-3p after manganese exposed mice. Ecotoxicol Environ Saf. 2024;280:116569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 171. | Giampà C, Alvino A, Magatti M, Silini AR, Cardinale A, Paldino E, Fusco FR, Parolini O. Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor deficits in the R6/2 mouse model of Huntington's disease. J Cell Mol Med. 2019;23:1581-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 172. | Yu-Taeger L, Stricker-Shaver J, Arnold K, Bambynek-Dziuk P, Novati A, Singer E, Lourhmati A, Fabian C, Magg J, Riess O, Schwab M, Stolzing A, Danielyan L, Nguyen HHP. Intranasal Administration of Mesenchymal Stem Cells Ameliorates the Abnormal Dopamine Transmission System and Inflammatory Reaction in the R6/2 Mouse Model of Huntington Disease. Cells. 2019;8:595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 173. | Ebrahimi MJ, Aliaghaei A, Boroujeni ME, Khodagholi F, Meftahi G, Abdollahifar MA, Ahmadi H, Danyali S, Daftari M, Sadeghi Y. Human Umbilical Cord Matrix Stem Cells Reverse Oxidative Stress-Induced Cell Death and Ameliorate Motor Function and Striatal Atrophy in Rat Model of Huntington Disease. Neurotox Res. 2018;34:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 174. | Elbaz EM, Helmy HS, El-Sahar AE, Saad MA, Sayed RH. Lercanidipine boosts the efficacy of mesenchymal stem cell therapy in 3-NP-induced Huntington's disease model rats via modulation of the calcium/calcineurin/NFATc4 and Wnt/β-catenin signalling pathways. Neurochem Int. 2019;131:104548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 175. | Wenceslau CV, de Souza DM, Mambelli-Lisboa NC, Ynoue LH, Araldi RP, da Silva JM, Pagani E, Haddad MS, Kerkis I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington's Disease 3-NP Rat Model. Cells. 2022;11:1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 176. | Dabrowska S, Turano E, Scambi I, Virla F, Nodari A, Pezzini F, Galiè M, Bonetti B, Mariotti R. A Cellular Model of Amyotrophic Lateral Sclerosis to Study the Therapeutic Effects of Extracellular Vesicles from Adipose Mesenchymal Stem Cells on Microglial Activation. Int J Mol Sci. 2024;25:5707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 177. | Migliarini S, Scaricamazza S, Valle C, Ferri A, Pasqualetti M, Ferraro E. Microglia Morphological Changes in the Motor Cortex of hSOD1(G93A) Transgenic ALS Mice. Brain Sci. 2021;11:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 178. | Tang J, Kang Y, Zhou Y, Chen Q, Lan J, Liu X, Peng Y. Umbilical cord mesenchymal stem cell-conditioned medium inhibits microglial activation to ameliorate neuroinflammation in amyotrophic lateral sclerosis mice and cell models. Brain Res Bull. 2023;202:110760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 179. | Sarikidi A, Kefalakes E, Falk CS, Esser R, Ganser A, Thau-Habermann N, Petri S. Altered Immunomodulatory Responses in the CX3CL1/CX3CR1 Axis Mediated by hMSCs in an Early In Vitro SOD1(G93A) Model of ALS. Biomedicines. 2022;10:2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 180. | Provenzano F, Nyberg S, Giunti D, Torazza C, Parodi B, Bonifacino T, Usai C, Kerlero de Rosbo N, Milanese M, Uccelli A, Shaw PJ, Ferraiuolo L, Bonanno G. Micro-RNAs Shuttled by Extracellular Vesicles Secreted from Mesenchymal Stem Cells Dampen Astrocyte Pathological Activation and Support Neuroprotection in In-Vitro Models of ALS. Cells. 2022;11:3923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 181. | Cudkowicz ME, Lindborg SR, Goyal NA, Miller RG, Burford MJ, Berry JD, Nicholson KA, Mozaffar T, Katz JS, Jenkins LJ, Baloh RH, Lewis RA, Staff NP, Owegi MA, Berry DA, Gothelf Y, Levy YS, Aricha R, Kern RZ, Windebank AJ, Brown RH Jr. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve. 2022;65:291-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 182. | Tavakol-Afshari J, Boroumand AR, Farkhad NK, Adhami Moghadam A, Sahab-Negah S, Gorji A. Safety and efficacy of bone marrow derived-mesenchymal stem cells transplantation in patients with amyotrophic lateral sclerosis. Regen Ther. 2021;18:268-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 183. | Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, Carriero A, Monaco F, Fagioli F. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 184. | Kook MG, Lee S, Shin N, Kong D, Kim DH, Kim MS, Kang HK, Choi SW, Kang KS. Repeated intramuscular transplantations of hUCB-MSCs improves motor function and survival in the SOD1 G(93)A mice through activation of AMPK. Sci Rep. 2020;10:1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 185. | Lan J, Zhou Y, Wang H, Tang J, Kang Y, Wang P, Liu X, Peng Y. Protective effect of human umbilical cord mesenchymal stem cell derived conditioned medium in a mutant TDP-43 induced motoneuron-like cellular model of ALS. Brain Res Bull. 2023;193:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 186. | Huang Y, Yang H, Yang B, Zheng Y, Hou X, Chen G, Zhang W, Zeng X, DU B. Ginsenoside-Rg1 combined with a conditioned medium from induced neuron-like hUCMSCs alleviated the apoptosis in a cell model of ALS through regulating the NF-κB/Bcl-2 pathway. Chin J Nat Med. 2023;21:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 187. | Barczewska M, Grudniak M, Maksymowicz S, Siwek T, Ołdak T, Jezierska-Woźniak K, Gładysz D, Maksymowicz W. Safety of intrathecal injection of Wharton's jelly-derived mesenchymal stem cells in amyotrophic lateral sclerosis therapy. Neural Regen Res. 2019;14:313-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 188. | Krull AA, Setter DO, Gendron TF, Hrstka SCL, Polzin MJ, Hart J, Dudakovic A, Madigan NN, Dietz AB, Windebank AJ, van Wijnen AJ, Staff NP. Alterations of mesenchymal stromal cells in cerebrospinal fluid: insights from transcriptomics and an ALS clinical trial. Stem Cell Res Ther. 2021;12:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 189. | Barczewska M, Maksymowicz S, Zdolińska-Malinowska I, Siwek T, Grudniak M. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: an Original Study. Stem Cell Rev Rep. 2020;16:922-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 190. | Řehořová M, Vargová I, Forostyak S, Vacková I, Turnovcová K, Kupcová Skalníková H, Vodička P, KubinováŠ, Syková E, Jendelová P. A Combination of Intrathecal and Intramuscular Application of Human Mesenchymal Stem Cells Partly Reduces the Activation of Necroptosis in the Spinal Cord of SOD1(G93A) Rats. Stem Cells Transl Med. 2019;8:535-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 191. | Magota H, Sasaki M, Kataoka-Sasaki Y, Oka S, Ukai R, Kiyose R, Onodera R, Kocsis JD, Honmou O. Repeated infusion of mesenchymal stem cells maintain the condition to inhibit deteriorated motor function, leading to an extended lifespan in the SOD1G93A rat model of amyotrophic lateral sclerosis. Mol Brain. 2021;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 192. | Chiarotto GB, Cartarozzi LP, Perez M, Tomiyama ALMR, de Castro MV, Duarte ASS, Luzo ÂCM, Oliveira ALR. Delayed onset, immunomodulation, and lifespan improvement of SOD1(G93A) mice after intravenous injection of human mesenchymal stem cells derived from adipose tissue. Brain Res Bull. 2022;186:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 193. | Zhou J, Li F, Jia B, Wu Z, Huang Z, He M, Weng H, So KF, Qu W, Fu QL, Zhou L. Intranasal delivery of small extracellular vesicles reduces the progress of amyotrophic lateral sclerosis and the overactivation of complement-coagulation cascade and NF-ĸB signaling in SOD1(G93A) mice. J Nanobiotechnology. 2024;22:503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 194. | Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 195. | Bae JS, Han HS, Youn DH, Carter JE, Modo M, Schuchman EH, Jin HK. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;25:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 196. | Bae JS, Furuya S, Shinoda Y, Endo S, Schuchman EH, Hirabayashi Y, Jin HK. Neurodegeneration augments the ability of bone marrow-derived mesenchymal stem cells to fuse with Purkinje neurons in Niemann-Pick type C mice. Hum Gene Ther. 2005;16:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 197. | Bae JS, Furuya S, Ahn SJ, Yi SJ, Hirabayashi Y, Jin HK. Neuroglial activation in Niemann-Pick Type C mice is suppressed by intracerebral transplantation of bone marrow-derived mesenchymal stem cells. Neurosci Lett. 2005;381:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 198. | Lee H, Lee JK, Min WK, Bae JH, He X, Schuchman EH, Bae JS, Jin HK. Bone marrow-derived mesenchymal stem cells prevent the loss of Niemann-Pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing sphingosine-1-phosphate. Stem Cells. 2010;28:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 199. | Lee H, Kang JE, Lee JK, Bae JS, Jin HK. Bone-marrow-derived mesenchymal stem cells promote proliferation and neuronal differentiation of Niemann-Pick type C mouse neural stem cells by upregulation and secretion of CCL2. Hum Gene Ther. 2013;24:655-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 200. | Seo Y, Yang SR, Jee MK, Joo EK, Roh KH, Seo MS, Han TH, Lee SY, Ryu PD, Jung JW, Seo KW, Kang SK, Kang KS. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011;20:1033-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 201. | Bae JS, Carter JE, Jin HK. Adipose tissue-derived stem cells rescue Purkinje neurons and alleviate inflammatory responses in Niemann-Pick disease type C mice. Cell Tissue Res. 2010;340:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 202. | Kang I, Lee BC, Lee JY, Kim JJ, Sung EA, Lee SE, Shin N, Choi SW, Seo Y, Kim HS, Kang KS. Stem cell-secreted 14,15- epoxyeicosatrienoic acid rescues cholesterol homeostasis and autophagic flux in Niemann-Pick-type C disease. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 203. | Yang M, Zhao Y, Li X, Li H, Cheng F, Liu Y, Jia Z, He Y, Lin J, Guan L. Conditioned medium of human menstrual blood-derived endometrial stem cells protects against cell inflammation and apoptosis of Npc1(KO) N2a cells. Metab Brain Dis. 2023;38:2301-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 204. | Svendsen SP, Svendsen CN. Cell therapy for neurological disorders. Nat Med. 2024;30:2756-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 205. | Sawamoto N, Doi D, Nakanishi E, Sawamura M, Kikuchi T, Yamakado H, Taruno Y, Shima A, Fushimi Y, Okada T, Kikuchi T, Morizane A, Hiramatsu S, Anazawa T, Shindo T, Ueno K, Morita S, Arakawa Y, Nakamoto Y, Miyamoto S, Takahashi R, Takahashi J. Phase I/II trial of iPS-cell-derived dopaminergic cells for Parkinson's disease. Nature. 2025;641:971-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 60] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 206. | Tabar V, Sarva H, Lozano AM, Fasano A, Kalia SK, Yu KKH, Brennan C, Ma Y, Peng S, Eidelberg D, Tomishima M, Irion S, Stemple W, Abid N, Lampron A, Studer L, Henchcliffe C. Phase I trial of hES cell-derived dopaminergic neurons for Parkinson's disease. Nature. 2025;641:978-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 207. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1279] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 208. | Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, Ran R, Chen Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci Rep. 2018;8:5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 209. | Choudhery MS, Badowski M, Muise A, Harris DT. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy. 2013;15:330-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 210. | Hori A, Takahashi A, Miharu Y, Yamaguchi S, Sugita M, Mukai T, Nagamura F, Nagamura-Inoue T. Superior migration ability of umbilical cord-derived mesenchymal stromal cells (MSCs) toward activated lymphocytes in comparison with those of bone marrow and adipose-derived MSCs. Front Cell Dev Biol. 2024;12:1329218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 211. | Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 212. | Li J, Xu SQ, Zhao YM, Yu S, Ge LH, Xu BH. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep. 2018;18:4969-4977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 213. | Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, Hirsh A, Igura K, Satoh H, Yokomi I, Nishimura T, Yamaguchi S, Yoshimura K, Rubinstein P, Takahashi TA. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 214. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1299] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 215. | Gao Y, Chi Y, Chen Y, Wang W, Li H, Zheng W, Zhu P, An J, Duan Y, Sun T, Liu X, Xue F, Liu W, Fu R, Han Z, Zhang Y, Yang R, Cheng T, Wei J, Zhang L, Zhang X. Multi-omics analysis of human mesenchymal stem cells shows cell aging that alters immunomodulatory activity through the downregulation of PD-L1. Nat Commun. 2023;14:4373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 216. | Wang X, Ouyang L, Chen W, Cao Y, Zhang L. Efficient expansion and delayed senescence of hUC-MSCs by microcarrier-bioreactor system. Stem Cell Res Ther. 2023;14:284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 217. | De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 371] [Article Influence: 37.1] [Reference Citation Analysis (3)] |
| 218. | Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P, Pérez-Mato M, Vieites-Prado A, Iglesias-Rey R, Rivas J, Planas AM, Sobrino T, Castillo J, Campos F. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep. 2017;7:40758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 219. | Wang M, Liang C, Hu H, Zhou L, Xu B, Wang X, Han Y, Nie Y, Jia S, Liang J, Wu K. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci Rep. 2016;6:30696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 220. | Wei P, Bao R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int J Mol Sci. 2022;24:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 221. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 618] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 222. | Braid LR, Wood CA, Wiese DM, Ford BN. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. 2018;20:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 223. | Scarfe L, Taylor A, Sharkey J, Harwood R, Barrow M, Comenge J, Beeken L, Astley C, Santeramo I, Hutchinson C, Ressel L, Smythe J, Austin E, Levy R, Rosseinsky MJ, Adams DJ, Poptani H, Park BK, Murray P, Wilm B. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res Ther. 2018;9:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 224. | Zhu F, Ji L, Dai K, Deng S, Wang J, Liu C. In situ licensing of mesenchymal stem cell immunomodulatory function via BMP-2 induced developmental process. Proc Natl Acad Sci U S A. 2024;121:e2410579121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 225. | Wang R, Fu J, He J, Wang X, Xing W, Liu X, Yao J, Ye Q, He Y. Apoptotic mesenchymal stem cells and their secreted apoptotic extracellular vesicles: therapeutic applications and mechanisms. Stem Cell Res Ther. 2025;16:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 226. | Yu S, Yu S, Liu H, Liao N, Liu X. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. 2023;14:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 227. | Serrenho I, Ferreira SA, Baltazar G. Preconditioning of MSCs for Acute Neurological Conditions: From Cellular to Functional Impact-A Systematic Review. Cells. 2024;13:845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 228. | Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, Dulong J, Monnier D, Gourmelon P, Gorin NC, Sensebé L; Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 229. | Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends. 2019;13:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/